Abstract
The Twin Research Registry (TRR) at SRI International is a community-based registry of twins established in 1995 by advertising in local media, mainly on radio stations and in newspapers. As of August 2012, there are 3120 twins enrolled; 86% are 18 or older (mean age 44.9 years, SD 16.9) and 14% less than 18 years of age (mean age 8.9 years, SD 4.5); 67% are female, and 62% are self-reported monozygotic. More than 1375 twins have participated in studies over the last 15 years in collaboration with the University of California Medical Center in San Francisco, the University of Texas MD Anderson Cancer Center, and the Stanford University School of Medicine. Each twin completes a registration form with basic demographic information either online at the TRR website or during a telephone interview. Contact is maintained with members by means of annual newsletters and birthday cards. The managers of the TRR protect the confidentiality of twin data with established policies; no information is given to other researchers without prior permission from the twins and all methods and procedures are reviewed by an Institutional Review Board. Phenotypes studied thus far include those related to nicotine metabolism, mutagen sensitivity, pain response before and after administration of an opioid, and a variety of immunological responses to environmental exposures including second-hand smoke and vaccination for seasonal influenza virus and varicella zoster virus. Twins in the TRR have participated in studies of complex, clinically-relevant phenotypes that would not be feasible to measure in larger samples.
Overview
Investigators within the Center for Health Sciences at SRI International (formerly known as the Stanford Research Institute), an independent, not-for-profit research organization founded in 1946 and headquartered in Menlo Park, California, have a substantial track record of utilizing the twin design to determine the relative proportion of environmental and genetic variance in survey-based and clinically-measured phenotypes. Utilizing twin pairs from the National Academy of Sciences/National Research Council (NAS/NRC) World War II Twin Registry and a more intensively studied subset of twins known as the NHLBI Twin Study, these investigators and their collaborators published biometric studies of the heritability of tobacco smoking behaviors (Carmelli et al., 1992), the co-occurrence of substance use including tobacco, alcohol, and caffeine (Swan et al., 1996; 1997), brain morphology as determined by magnetic resonance imaging (Pfefferbaum et al., 2001; Sullivan et al., 2001; Carmelli et al., 2002; Lessov-Schlaggar et al., 2012) and cognitive functioning as assessed by neuropsychological testing (Swan et al., 1999; Swan & Carmelli, 2002; Lessov-Schlaggar et al., 2007).
Creation, growth, and maintenance of the Twin Research Registry at SRI International
As a follow-up to our 1992 New England Journal of Medicine paper on the genetics of ever smoking, smoking quantity, and smoking cessation (Carmelli et al., 1992), the Twin Research Registry (TRR) at SRI International was created to develop a study sample of adult twins to support more in-depth studies of nicotine dependence and related phenotypes including nicotine metabolism. California laws pertaining to the privacy of birth records and Department of Motor Vehicles files precluded the development of a population-based registry of adult twins. Therefore, we instead elected to create a community-based registry in the San Francisco Bay Area and in 1995 initiated an extensive advertising campaign that included 19 newspapers, San Francisco Bay Area-wide movie theaters, and AM/FM radio stations. Within 2.5 years, this campaign resulted in the enrollment of a total of 1054 individual twins. A five-year anniversary celebration party, held in July 2000, increased enrollment to 1765 individual twins. In 2001, the TRR was expanded to include twins under the age of 18 years. By 2009, as a result of sustained and intensive advertising in local media and TRR member referrals, membership in the TRR increased to 2700 twins. Table 1 provides a description of the basic demographics of the sample of over 3000 twins currently enrolled in the TRR.
Table 1.
Basic demographics of participants on the Twin Research Registry at SRI International
| Registered twins | Self-reported zygosity
|
Gender |
||||
|---|---|---|---|---|---|---|
| Monozygotic | Dizygotic | Unknown | Male | Female | ||
|
|
|
|
|
|
|
|
| Adults | 2,683 (86%) | 1,769 (66%) | 798 (30%) | 116 (4%) | 844 (30%) | 1,839 (70%) |
| Under 18 | 437 (14%) | 159 (36%) | 239 (55%) | 39 (9%) | 199 (46%) | 238 (54%) |
|
| ||||||
| Total | 3,120 | 1,928 (62%) | 1,037 (33%) | 155 (5%) | 1,043 (33%) | 2,077 (67%) |
Contact with twins in the TRR is maintained via annual newsletters and birthday cards. The TRR website is updated periodically (http://www.sri.com/twin) and referrals to the TRR by registered twins are encouraged with a $25 incentive. The TRR also has a presence on Facebook and Twitter. All activities related to recruitment, advertising, and ongoing contact with the twins are reviewed and approved by the Institutional Review Board of SRI International.
Zygosity assessment
Originally, twins were asked to complete a short registration form consisting of the following questions (Cederlof et al., 1961; Sarna et al., 1978): 1) “As far as you know, are you and your twin: fraternal, identical or don’t know?”; 2) “During your entire life, how close do you feel that you and your twin have been compared with your impression of closeness between ordinary siblings: less close, as close as, somewhat closer, or much closer than ordinary siblings?”; 3) “How far in miles do you live from your twin now?” 4) “How frequently do you and your twin get together now: almost daily, 1–4 times per week, 1–3 times per month, occasionally during the year, less than once per year?” Responses to the first questions were then used to assign preliminary zygosity status. More recently, a revised questionnaire was mailed to all registered twins to collect responses to a series of questions developed at Washington University in St. Louis to estimate twin zygosity. The classification algorithm assigns weights to responses to items concerning physical similarity, whether parents, teachers or strangers ever mistook one twin for the other, whether blood test verification was ever obtained, and self-reported zygosity (Heath et al., 2003).
For those twins participating in one of our studies, self-reported zygosity is confirmed based on genotyping of short tandem repeat (STR) regions of genomic DNA (Edwards et al., 1991) and the twins are notified of the results. We have learned that this information is an important motivator for twins to participate in research studies. For those twins who have indicated in surveys that they have had their zygosity confirmed by laboratory testing, we have asked that they send in their results so we can add them to our database. So far we have received 39 such reports.
Projects involving the Twin Research Registry at SRI International
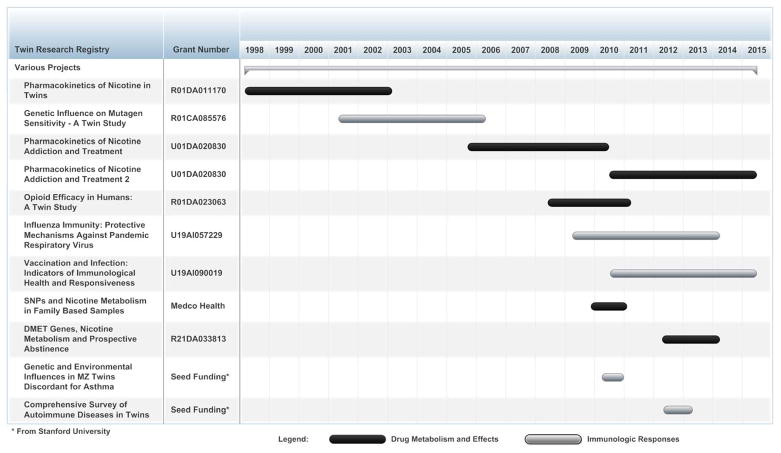
Since 1998, the TRR at SRI International has supported at least 10 distinct, funded studies. These studies fall into two broad categories: those that are focused on drug metabolism and related phenotypes such as subjective effects and dependence and those that are focused on the effects of a variety of agents that influence the human immunological response repertoire. Figure 1 provides a timeline for each study and Table 2 summarizes the phenotypes described in published papers that have been examined in twin participants recruited from the TRR.
Figure 1.
A timeline of funded studies that have utilized data from participants in the Twin Research Registry at SRI International
Table 2.
Representative phenotypes examined in the Twin Research Registry at SRI International (published only)
| Project/Phenotype |
|---|
| Pharmacokinetics of nicotine in twins |
|
|
| Hepatic--nicotine and cotinine clearance, the conversion of nicotine to cotinine, nicotine metabolic ratio in plasma and urine |
| Glucuronidation--nicotine-glucuronide/nicotine, cotinine-glucuronide/cotinine, 3 hydroxycotinine-glucuronide/3 hydroxycotinine, nicotine-glucuronide/nicotine area under the curve, cotinine-glucuronide/cotinine area under the curve |
| Renal--total clearance nicotine and cotinine, glomerular filtration rate, net secretory/reabsorptive clearance of nicotine and cotinine |
| Pharmacodynamic--heart rate acceleration and deceleration during and after infusion of a fixed dose of nicotine |
|
|
| Genetic influence on mutagen sensitivity |
|
|
| Cancer susceptibility markers--mutagen sensitivity (mean breaks per cell) in response to bleomycin, benzo[a]pyrene diol epoxide, gamma radiation, 4-nitroquinoline-1-oxide; mitochondrial DNA content |
|
|
| Opioid efficacy in humans |
|
|
| Experimental pain--threshold and tolerance to heat and cold pain before and after infusion of alfentanil |
| Non-analgesic effects--sedation, respiratory depression, nausea, pruritis, positive affective response before and after infusion of alfentanil |
Studies focused on drug metabolism and related phenotypes
The study “Pharmacokinetics of Nicotine in Twins” (DA011170) involved 139 twin pairs (110 monozygotic [MZ] and 29 dizygotic [DZ] pairs) who participated in an in-hospital fixed dose infusion of radio-labeled nicotine and cotinine protocol. The pharmacokinetics of nicotine metabolism in both plasma and urine were subsequently determined using techniques developed by Benowitz, Jacob, and colleagues (Jacob et al., 1988; Jacob et al., 1991; Benowitz & Jacob, 1994; Jacob et al., 2002). Data from this study, the world’s largest existing twin study of nicotine metabolism, have resulted in several reports on biometric estimates of genetic and environmental contributions to different aspects of nicotine metabolism (Swan et al., 2004; Swan et al., 2005; Swan et al., 2009; Benowitz et al., 2008; Conti et al., 2009; Swan & Lessov-Schlaggar, 2009; Lessov-Schlaggar et al., 2009) as well as measured genetic and environmental influences on metabolism itself (Benowitz, Lessov-Schlaggar et al., 2006; Benowitz, Swan et al., 2006; Al Koudsi et al., 2006; Mwenifumbo et al., 2008).
Data from the study described above contributed to the first and second generations of the project “Pharmacokinetics of Nicotine Addiction and Treatment” (U01DA020830). In the first generation of this project, DNA samples from the twin study as well as those from a family study of nicotine metabolism (University of California Tobacco-Related Disease Research Program, 7PT2000-1004) were integrated with a collection of DNA samples from eight randomized clinical trials of various smoking cessation treatments for genotyping or sequencing and analysis of selected pharmacodynamic and pharmacokinetic loci. Analysis of pharmacodynamic candidate genes resulted in the identification of genetic associations (both common and rare variants) with responsiveness to treatment (Conti et al., 2008, Swan et al., 2011, Lee et al., 2011, 2012) and with nicotine dependence (Bergen et al., 2009; Wessel et al, 2010; Falcone et al., 2011). The second generation of this project (ongoing) will examine the extent to which individual variation in the nicotine metabolic ratio (NMR; 3′hydroxycotinine/cotinine ratio) with known heritability [Swan et al., 2009]) mediates responsiveness to medications commonly used to treat nicotine dependence in a randomized trial design (Lerman et al., 2006; Swan et al., 2010).
The most recent studies involving DNA samples collected from the original twin study of nicotine metabolism utilize data from the DMET™ Plus Assay from Affymetrix that was used to interrogate 1936 markers at 236 drug metabolizing and transporter genes for their association with the NMR in the twin dataset and in the previously mentioned family study of nicotine metabolism dataset. The initial work of collecting and analyzing this genotype data, “Metabolic SNPs and Nicotine and Cotinine Metabolism in Two Family Based Samples” was funded by a Collaboration Agreement among Medco Health Solutions, Affymetrix, and SRI International (Bergen et al., 2010), and the work will be extended using funding from NIH in the project “DMET Genes, Nicotine Metabolism, and Prospective Abstinence” (DA033813). The initial analysis of genotype data identified variation at several pharmacokinetic genes associated with nicotine metabolism, including classical genes and genes not previously associated with nicotine metabolism (Bergen et al., 2010). Future work will take a unique approach to gene discovery by using analysis of the two family based datasets, where associations identified in the twin dataset (Bergen et al., 2010) are being confirmed in the family study dataset (Bergen, Wacholder et al., 2012; Bergen, Javitz et al., 2012). Those SNP associations common to both datasets will then be examined for their significance in relation to abstinence outcomes in the previously described collection of 8 clinical trials. This approach should determine in a more rapid fashion those SNPs which have potential for translation to the clinical setting as biomarkers for association with nicotine metabolism-related phenotypes such as cigarettes per day and prospective abstinence.
The latest project in the group of studies focused on drug effects, “Opioid Efficacy in Humans” (DA023063), recruited 81 MZ pairs and 31 DZ pairs from the TRR to participate in a computer-controlled infusion of the mu-opioid agonist alfentanil or placebo in a single occasion, randomized cross-over study of pain sensitivity and analgesic effects (Angst et al., 2010). In this unique study, experimental heat and cold pressor pain models were examined both before and after administration of alfentanil. Analyses determined the relative proportion of genetic and environmental factors in pain tolerance and threshold (Angst et al, 2012) and, in a separate analysis, subsequent analgesic and drug side effects (Angst et al., 2012). Ruau et al (2012) recently used a bioinformatics approach to identify candidate genes associated with pain ratings of several medical conditions. A number of identified candidates were subsequently genotyped using DNA from twin participants who had been prospectively assessed for experimentally-induced pain phenotypes as a step in the validation process.
Studies focused on the human immunological response repertoire
Mutagen sensitivity is an in vitro short term lymphocyte culture assay that gauges host susceptibility by measuring induced chromatid breaks following exposure to an array of mutagens. A series of previous studies indicated that mutagen sensitivity is a promising environmentally-related cancer risk predictor. The assay was expanded by replacing the initial test mutagen bleomycin with 4-nitroquinoline-1-oxide (4-NQO, an ultraviolet light (UV) mimetic agent), gamma-radiation, and benzo(alpha)pyrene diolepoxide (BPDE, a metabolic product of benzo(alpha)pyrene, which is a component of tobacco smoke) to measure risk of other cancers. At the time this study was conducted (“Genetic Influence on Mutagen Sensitivity”, CA085576) the relative contributions of genetic and environmental factors to indicators of mutagen sensitivity were unknown. One hundred forty eight pairs of MZ twins, 57 pairs of DZ twins, and 50 siblings were recruited from the TRR to provide peripheral blood lymphocytes for measurement of in vitro mutagen sensitivity. Subsequent published reports provided estimates for genetic and environmental influence on several markers of mutagen sensitivity (Wu et al., 2006), genotype-phenotype correlations between mutagen sensitivity and genetic variants in the nucleotide excision repair pathway (Lin et al., 2007), and the heritability of mitochondrial DNA content, a risk factor for renal cell carcinoma (Xing et al., 2008).
The field of immunology is currently in a state of rapid discovery with the advent of increased understanding of the extent to which human immunological processes and mechanisms underlie common and rare conditions. Because the discovery of immunological markers of disease in humans is still in its early stages, the extent to which many of these markers are influenced by genetic and/or environmental sources of variation remains to be determined. The use of the twin design is a cost-effective way to determine the relative proportion of genetic and environmental influence both on the occurrence of conditions of known or suspected immunologic etiology as well as on biomarkers that are either associated with or predictive of these conditions (Krishnan et al., 2012). A series of twin studies are being conducted with members of Stanford University Medical School’s Institute for Immunity, Transplantation, and Infection (ITI) and the Human Immune Monitoring Core. As a group, these projects utilize MZ and DZ twin pairs from the TRR as a way to determine the relative contribution of genetic and environmental factors to innate and adaptive immunological responses to vaccination for seasonal influenza virus (“Influenza Immunity: Protective Mechanisms Against Pandemic Respiratory Virus”, U19AI057229) and for varicella zoster virus (“Vaccination and Infection: Indicators of Immunological Health and Responsiveness”, U19AI090019). In the influenza vaccine study, twins of both zygosities are recruited on a seasonal basis to receive the U.S. Centers for Disease Control-approved vaccine and then followed on a repeated basis to provide blood samples for immunological assay of markers of response. As of the date of this write-up, 74 MZ pairs and 28 DZ pairs of a variety of ages have participated in this protocol. In the second study, the twin design will be used to examine sources of variability in the response to the FDA-approved vaccine for varicella zoster, a viral agent with substantial morbidity and mortality outcomes in older adults (Pickering & Leplege, 2011). For this study, 40 MZ twin pairs over the age of 50 who have had chicken pox in the past will be recruited to participate in a study of the immune response to the Zostavax© vaccine. In addition, 10 pairs of MZ twins of age 40–49 who have had chicken pox in the past are being recruited to participate in a cross-sectional study designed to assess immune responses to the naturally-acquired varicella zoster virus. These studies are aimed at learning more about age-related differences in immune function. To date three pairs have completed the cross-sectional study and five pairs the vaccine study.
A second series of studies with collaborators and support from Stanford University’s ITI have recruited 27 MZ twin pairs who are either concordant or discordant for asthma. These twin pairs were identified by mailed survey of the entire TRR. Of the approximately 1350 responses received, 76 pairs were subsequently identified and contacted for possible participation in a clinical study in which medical history, lung function measures, and blood samples were collected for immunological assay. Thus far, 21 MZ twin pairs discordant for asthma have been examined for differences in epigenetic modifications of T cells, mediators of the inflammatory responses linked to asthma and the extent to which such modifications are associated with exposure to second-hand smoke (Runyon et al., 2012). Because of the successful approach taken to identify twin pairs with and without a history of asthma, a second, more comprehensive survey of members of the TRR for a wide variety of conditions of interest to immunologists will be conducted in 2012–2013.
Conclusions
As described above, biometric studies of twins continue to provide clues with regard to genetic and environmental contributors to a wide variety of phenotypes associated with important health outcomes. The inclusion of genotypic status in quantitative models of variation in MZ and DZ twins provides insight into the amount of variation a single gene or set of genes may contribute to the estimate of total additive genetic influence in selected phenotypes (Zaitlen & Kraft, 2012). The study of MZ twins discordant for a particular trait or for an environmental exposure provides insight into epigenetic effects that may explain the twin discordance (Bell & Spector, 2011). The TRR at SRI International, while relatively modest in size compared to other, much larger population-based registries described in this issue of Twin Research and Human Genetics, will continue to contribute to ground-breaking findings concerning the etiologies of complex phenotypes that are not feasible to assess on a larger scale.
Acknowledgments
The authors wish to thank: Mary McElroy for her efforts in the development of the Registry and the coordination of projects; Jill Rubin for her efforts in recruitment and maintenance of the Registry; Dee Campbell for telephone contact with twins during registration; and to the twins of the Registry for their continuing participation and dedication to research.
Footnotes
The Twin Research Registry at SRI International can be contacted at: twin@sri.com; telephone: 1-800-SRI-TWIN; Facebook: https://www.facebook.com/TwinSRI; and Twitter: @twinSRI.
References
- Al Koudsi N, Mwenifumbo JC, Sellers EM, Benowitz NL, Swan GE, Tyndale RF. Characterization of the novel CYP2A6*21 allele using in vivo nicotine kinetics. European Journal of Clinical Pharmacology. 2006;62:481–484. doi: 10.1007/s00228-006-0113-3. [DOI] [PubMed] [Google Scholar]
- Angst MS, Lazzeroni LC, Phillips NG, Drover DR, Tingle M, Ray A, Swan GE, Clark JD. Aversive and Reinforcing Opioid Effects: A Pharmacogenomic Twin Study. Anesthesiology. 2012;117:22–37. doi: 10.1097/ALN.0b013e31825a2a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Phillips NG, Drover DR, Tingle M, Galinkin JL, Christians U, Swan GE, Lazzeroni LC, Clark JD. Opioid pharmacogenomics using a twin study paradigm: methods and procedures for determining familial aggregation and heritability. Twin Research and Human Genetics. 2010;13:412–425. doi: 10.1375/twin.13.5.412. [DOI] [PubMed] [Google Scholar]
- Angst MS, Phillips NG, Drover DR, Tingle M, Ray A, Swan GE, Lazzeroni LC, Clark JD. Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain. 2012;153:1397–1409. doi: 10.1016/j.pain.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JT, Spector TD. A twin approach to unraveling epigenetics. Trends In Genetics. 2011;27:116–125. doi: 10.1016/j.tig.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clinical Pharmacology and Therapeutics. 1994;56:483–493. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clinical Pharmacology and Therapeutics. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Swan GE, Jacob P, 3rd, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clinical Pharmacology and Therapeutics. 2006;80:457–467. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE. Genetic influences in the variation in renal clearance of nicotine cotinine. Clinical Pharmacology and Therapeutics. 2008;84:243–247. doi: 10.1038/clpt.2008.54. [DOI] [PubMed] [Google Scholar]
- Bergen AW, Conti DV, Van Den Berg D, Lee W, Liu J, Li D, Guo N, Mi H, Thomas PD, Lessov-Schlaggar CN, Krasnow R, He Y, Nishita D, Jiang R, McClure JB, Tildesley E, Hops H, Tyndale RF, Benowitz NL, Lerman C, Swan GE. Dopaminergic genes and nicotine dependence in treatment seeking and community smokers. Neuropsychopharmacology. 2009;34:2252–2264. doi: 10.1038/npp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Michel M, Krasnow R, Nishita D, Lessov-Schlaggar CN, Sanders CL, Markward NJ, Stanek EJ, Freuh FW, Hockett RD, Kaufman J, Benowitz NL, Swan GE. Metabolic SNPs and nicotine and cotinine metabolism. Abstract. Annual Meeting of the American Society of Human Genetics; Washington, DC: American Society of Human Genetics; 2010. [Google Scholar]
- Bergen AW, Javitz HS, Michel M, Krasnow R, Nishita D, Lessov-Schlaggar CN, Hops H, Markward NJ, Hall S, Baker T, Conti DV, Tyndale RF, Benowitz NL, Swan GE. Drug metabolizing enzyme genes and nicotine and cotinine metabolism. Abstract. Annual Meeting of the American Society of Human Genetics; San Francisco, CA: American Society of Human Genetics; 2012. [Google Scholar]
- Bergen AW, Wacholder A, Michel M, Nishita D, Krasnow R, Javitz HS, Swan GE. Genome-wide association studies on smoking behavior and nicotine dependence. Abstract. Annual Meeting of the Society for Research on Nicotine and Tobacco; Houston, TX: Society for Research on Nicotine and Tobacco; 2012. [Google Scholar]
- Carmelli D, Swan GE, DeCarli C, Reed T. Quantitative genetic modeling of regional brain volumes and cognitive performance in older male twins. Biological Psychology. 2002;61:139–155. doi: 10.1016/s0301-0511(02)00056-x. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking--a study of male twins. New England Journal of Medicine. 1992;327:829–833. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- Cederlof R, Friberg L, Jonsson E, Kaij L. Studies on similarity diagnosis in twins with the aid of mailed questionnaires. Acta Genetica (Basel) 1961;11:338–362. doi: 10.1159/000151168. [DOI] [PubMed] [Google Scholar]
- Conti DV, Lee W, Li D, Liu J, Van Den Berg D, Thomas PD, Bergen AW, Swan GE, Tyndale RF, Benowitz NL, Lerman C for the Pharmacogenetics of Nicotine Addiction Treatment Consortium. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Humam Molecular Genetics. 2008;17:2834–2848. doi: 10.1093/hmg/ddn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti DV, Lewinger JP, Swan GE, Tyndale RF, Benowitz NL, Thomas PD. National Cancer Institute, Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine use and Dependence, Tobacco Control Monograph No. 20. Chapter 12. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009. Using ontologies in hierarchical modeling of genes and exposure in biologic pathways. NIH Publication No. 08-6366. [Google Scholar]
- Edwards A, Civitello A, Hammond HA, Caskey CT. DNA typing and genetic mapping with trimeric and tetrameric tandem repeats. American Journal of Human Genetics. 1991;49:746–756. [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Jepson C, Benowitz N, Bergen AW, Pinto A, Wileyto EP, Baldwin D, Tyndale RF, Lerman C, Ray R. Association of the nicotine metabolite ratio and CHRNA5/CHRNA3 polymorphisms with smoking rate among treatment-seeking smokers. Nicotine & Tobacco Research. 2011;13:498–503. doi: 10.1093/ntr/ntr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Nyholt DR, Neuman R, Madden PA, Bucholz KK, Todd RD, Nelson EC, Montgomery GW, Martin NG. Zygosity diagnosis in the absence of genotypic data: an approach using latent class analysis. Twin Research. 2003;6:22–26. doi: 10.1375/136905203762687861. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Benowitz NL, Shulgin A. Synthesis of optically pure deuterium labeled nicotine, nornicotine and cotinine. Journal of Labelled Compounds and Radiopharmaceuticals. 1988;25:1117–1128. [Google Scholar]
- Jacob P, 3rd, Wilson M, Yu L, Mendelson J, Jones RT. Determination of 4-hydroxy-3-methoxyphenylethylene glycol 4-sulfate in human urine using liquid chromatography-tandem mass spectrometry. Analytical Chemistry. 2002;7:5290–5296. doi: 10.1021/ac020101a. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biological Mass Spectrometry. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- Krishnan E, Lessov-Schlaggar CN, Krasnow RE, Swan GE. Nature versus nurture in gout: a twin study. American Journal of Medicine. 2012;125:499–504. doi: 10.1016/j.amjmed.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Lee W, Bergen AW, Swan GE, Li D, Liu J, Thomas P, Tyndale RF, Benowitz NL, Lerman C, Conti DV. Gender-stratified gene and gene-treatment interactions in smoking cessation. Pharmacogenomics Journal. 2011 doi: 10.1038/tpj.2011.30. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Ray R, Bergen AW, Swan GE, Thomas P, Tyndale RF, Benowitz NL, Lerman C, Conti DV. DRD1 associations with smoking abstinence across slow and normal nicotine metabolizers. Pharmacogenetics & Genomics. 2012;22:551–554. doi: 10.1097/FPC.0b013e3283539062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, Kaufmann V, Restine S, Hawk L, Niaura R, Berrettini W. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology. 2006;31:231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- Lessov-Schlaggar CN, Swan GE, Reed T, Wolf PA, Carmelli D. Longitudinal genetic analysis of executive function in elderly men. Neurobiology of Aging. 2007;28:1759–1768. doi: 10.1016/j.neurobiolaging.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Lessov-Schlaggar CN, Benowitz NL, Jacob P, 3rd, Swan GE. Genetic influences on individual differences in nicotine glucoronidation. Twin Research and Human Genetics. 2009;12:507–513. doi: 10.1375/twin.12.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessov-Schlaggar CN, Swan GE, DeCarli C, Krasnow RE, Reed T, Wolf PA, Carmelli D. Longitudinal genetic analysis of brain volumes in normal elderly males: the NHLBI twin study. Neurobiology of Aging. 2012;33:636–644. doi: 10.1016/j.neurobiolaging.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Swan GE, Shields PG, Benowitz NL, Gu J, Amos CI, de Andrade M, Spitz MR, Wu X. Mutagen sensitivity and genetic variants in nucleotide excision repair pathway: genotype-phenotype correlation. Cancer Epidemiology Biomarkers and Prevention. 2007;16:2065–2071. doi: 10.1158/1055-9965.EPI-06-1041. [DOI] [PubMed] [Google Scholar]
- Mwenifumbo JC, Lessov-Schlaggar CN, Zhou Q, Krasnow RE, Swan GE, Benowitz NL, Tyndale RF. Identification of Novel CYP2A6*1B Variants: The CYP2A6*1B Allele is Associated With Faster In Vivo Nicotine Metabolism. Clinical Pharmacology and Therapeutics. 2008;83:115–121. doi: 10.1038/sj.clpt.6100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Genetic regulation of regional microstructure of the corpus callosum in late life. Neuroreport. 2001;12:1677–1681. doi: 10.1097/00001756-200106130-00032. [DOI] [PubMed] [Google Scholar]
- Pickering G, Leplege A. Herpes zoster pain, postherpetic neuralgia, and quality of life in the elderly. Pain Practice. 2011;11:397–402. doi: 10.1111/j.1533-2500.2010.00432.x. [DOI] [PubMed] [Google Scholar]
- Ruau D, Dudley JT, Chen R, Phillips NG, Swan GE, Lazzeroni LC, Clark JD, Butte AJ, Angst MS. Integrative approach to pain genetics identifies pain sensitivity loci across diseases. PLoS Computional Biology. 2012;8:e1002538. doi: 10.1371/journal.pcbi.1002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon R, Rajeshuni N, Cachola L, Swan GE, Nadeau K. The effects of smoking on the immune response: Gene methylation and T-cell-based responses in twins discordant for smoking. American Journal of Respiratory Critical Care Medicine. 2012;185:A3878. [Google Scholar]
- Sarna S, Kaprio J, Sistonen P, Koskenvuo M. Diagnosis of twin zygosity by mailed questionnaire. Human Heredity. 1978;28:241–254. doi: 10.1159/000152964. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Swan GE, Carmelli D. Heritability of hippocampal size in elderly twin men: equivalent influence from genes and environment. Hippocampus. 2001;11:754–762. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D. Evidence for genetic mediation of executive control: a study of aging male twins. Journals of Gerontology Series B Psychological Sciences and Social Sciences. 2002;57:133–143. doi: 10.1093/geronb/57.2.p133. [DOI] [PubMed] [Google Scholar]
- Swan GE, Javitz HS, Jack LM, Wessel J, Michel M, Hinds DA, Stokowksi RP, McClure JB, Catz SL, Richards J, Zbikowski SM, Deprey M, McAfee T, Conti DV, Bergen AW. Varenicline for smoking cessation: nausea severity and variation in nicotinic receptor genes. Pharmacogenomics Journal. 2011;12:349–358. doi: 10.1038/tpj.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, Deprey M, Richards J, McAfee TA. Behavioral counseling and varenicline treatment for smoking cessation. American Journal of Preventive Medicine. 2010;38:482–490. doi: 10.1016/j.amepre.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Reed T, Jack LM, Miller BL, Markee T, Wolf PA, DeCarli C, Carmelli D. Differential genetic influence for components of memory in aging adult twins. Archives of Neurology. 1999;56:1127–1132. doi: 10.1001/archneur.56.9.1127. [DOI] [PubMed] [Google Scholar]
- Swan GE, Benowitz NL, Jacob P, 3rd, Lessov CN, Tyndale RF, Wilhelmsen K, Krasnow RE, McElroy MR, Moore SE, Wambach M. Pharmacogenetics of nicotine metabolism in twins: Methods and procedures. Twin Research. 2004;7:435–448. doi: 10.1375/1369052042335269. [DOI] [PubMed] [Google Scholar]
- Swan GE, Benowitz NL, Lessov CN, Jacob P, 3rd, Tyndale RF, Wilhelmsen K. Nicotine metabolism: The impact of CYP2A6 on estimates of additive genetic influence. Pharmacogenetics & Genomics. 2005;15:115–125. doi: 10.1097/01213011-200502000-00007. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D, Cardon LR. Heavy consumption of alcohol, cigarettes, and coffee in male twins. Journal of Studies in Alcohol. 1997;58:182–190. doi: 10.15288/jsa.1997.58.182. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D, Cardon LR. The consumption of tobacco, alcohol, and coffee in Caucasian male twins: A multivariate genetic analysis. Journal of Substance Abuse. 1996;8:19–31. doi: 10.1016/s0899-3289(96)90055-3. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN, Bergen AW, He Y, Tyndale RF, Benowitz NL. Genetic and environmental influences on the ratio of 3′hydroxycotinine to cotinine in plasma and urine. Pharmacogenetics & Genomics. 2009;19:388–398. doi: 10.1097/FPC.0b013e32832a404f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. Tobacco addiction and pharmacogenetics of nicotine metabolism. Journal of Neurogenetics. 2009;23:262–271. doi: 10.1080/01677060802572903. [DOI] [PubMed] [Google Scholar]
- Wessel J, McDonald SM, Hinds DA, Stokowski RP, Javitz HS, Kennemer M, Krasnow R, Dirks W, Hardin J, Pitts SJ, Michel M, Jack L, Ballinger DG, McClure JB, Swan GE, Bergen AW. Resequencing of nicotinic acetylcholine receptor genes and association of common and rare variants with the Fagerstrom test for nicotine dependence. Neuropsychopharmacology. 2010;35:2392–2402. doi: 10.1038/npp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Spitz MR, Amos CI, Lin J, Shao L, Gu J, de Andrade M, Benowitz NL, Shields PG, Swan GE. Mutagen sensitivity has high heritability: Evidence from a twin study. Cancer Research. 2006;66:5993–5996. doi: 10.1158/0008-5472.CAN-06-1007. [DOI] [PubMed] [Google Scholar]
- Xing J, Chen M, Wood CG, Lin J, Spitz MR, Ma J, Amos CI, Shields PG, Benowitz NL, Gu J, deAndrade M, Swan GE, Wu X. Mitochondrial DNA content: its genetic heritability and association with renal cell carcinoma. Journal of the National Cancer Institute. 2008;100:1104–1112. doi: 10.1093/jnci/djn213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitlen N, Kraft P. Heritability in the genome-wide association era. Human Genetics. 2012 doi: 10.1007/s00439-012-1199-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]