Abstract
Topical preparations of Anemopsis californica have been used by Native American tribes in the southwestern United States and northern Mexico to treat inflammation and infections. We report results of bioassay-guided isolation conducted on a sample of A. californica roots. The furofuran lignans sesamin (1) and asarinin (2) were isolated and shown to have MIC values ranging from 23 to 395 µM against five different species of environmental nontuberculous mycobacteria. These findings are significant given that these bacteria can cause skin, pulmonary, and lymphatic infections. Crude A. californica extracts were analyzed by liquid chromatography - mass spectrometry (LC-MS), and it was determined that sesamin and asarinin were extracted at relatively high levels from roots (1.7–3.1g/kg and 1.1–1.7 g/kg, respectively), but lower levels from leaves (0.13 g/kg for both compounds). Our findings suggest that the majority of activity of crude A. californica root extracts against nontuberculous mycobacteria can be attributed to the presence of sesamin and asarinin. This paper is the first to report isolation of these compounds from a member of the Saururaceae family, and the first to describe their activity against nontuberculous mycobacteria.
Keywords: Anemopsis californica, Saururaceae, nontuberculous mycobacteria, antibacterial, botanical, sesamin, asarinin
Introduction
A new arsenal of antibiotics is needed to address two problems involving treatment of bacterial infections: the emergence of drug-resistance and the existence of bacteria that are innately resistant to most antibiotics. A promising source for new antibacterial compounds is the natural products produced by plants, bacteria, and fungi. It is estimated that 25 to 50 percent of anti-infective agents come from these natural sources [1].
This study focuses on the plant Anemopsis californica (Nutt.) Hook. & Arn. (Saururaceae) as a source of antimicrobial compounds. A. californica, commonly known as "yerba mansa," is native to the southwestern United States and northern Mexico, and its roots, leaves, and stem have been used medicinally by many Native American tribes [2–5]. Despite historical and modern precedent for the use of this plant to treat infection, only a few studies have focused on the chemicals responsible for its anti-infective properties [6–7]. The chemical compounds identified from A. californica thus far are exclusively from the volatile oils of the leaves and roots [8–9]. Volatile oil extracts from A. californica have been shown to inhibit the growth of endometrial, cervical, colon, and breast cancer cells in vitro [8–9], and demonstrated antimicrobial activity against Staphylococcus aureus, Streptococcus pneumoniae, and Geotrichim candidum [6]. Additionally, ethanol and ethyl acetate extracts of various parts of A. californica were shown to inhibit the growth of colon and breast cancer cells, and aqueous A. californica extracts inhibited cell migration and metastasis [10–11]. These studies did not indicate which chemical constituents were responsible for the observed effects.
There are currently no reports of the identities of non-volatile compounds present in A. californica roots and leaves. This is a significant gap in the literature, given that its traditional mode of application of A. californica is a whole plant poultice or decoction. For example, the Shoshoni tribe of Nevada applied boiled and mashed A. californica roots to areas of inflammation and infections [2]. The Pima tribe of Arizona and New Mexico and the Mahuna and Chumash tribes of California used a decoction of leaves and roots to treat wounds [3–4, 12], and the Nevada Paiute and the California Costanoan tribes used decoctions of A. californica roots or leaves to treat pain [2,5].
The ethnobotanical precedent for application of A. californica to treat infections encouraged us to screen extracts from this plant for activity against pathogenic microorganisms. Activity of crude A. californica extracts was noted against several species of nontuberculous mycobacteria. These findings were deemed significant, given the clinical relevance these organisms. Nontuberculous mycobacteria are commonly found in soils, natural waters, and engineered water systems, including household plumbing [13–14], and can cause pulmonary, skin, and lymph node infections [15]. The resultant chronic respiratory or soft tissue infections require long term antibiotic treatment that can have serious side effects [16]. Current estimates report a total number of over 16,000 cases in the United States of nontuberculosis mycobacterial disease per year with a total cost of over $425 million [17]. The objective of this research was to identify compounds from A. californica with potential for the treatment of nontuberculous mycobacterial infections. In addition, we sought to provide insight into the scientific basis for the ethnobotanical use of A. californica to treat bacterial infections.
Results and Discussion
Bioactivity-guided fractionation of A. californica resulted in the isolation of two compounds, sesamin (1) [22] and its C-7 epimer, asarinin (2). Sesamin was a white solid with a HRESIMS m/z of 355.1175 (calcd for C20H19O6 [M+H]+ m/z 355.1176, , c = 0.0125 g/100 mL, methanol). Asarinin, also called episesamin or isosesamin, was a white solid with a HRESIMS m/z of 355.1167 (calcd for C20H19O6 [M+H]+ m/z 355.1176, , c = 0.0125 g/100 mL, methanol). The 1H and 13C NMR of both sesamin and asarinin were in agreement with literature values [23]. NMR data are included as Supplemental Information (Table S1, Figures S1–S4).
Although sesamin and asarinin are both known compounds, this is the first report of their presence in a member of the Saururaceae plant family. Sesamin was first isolated from sesame seed oil [24–26], while asarinin was first isolated from prickly ash bark [27]. Both sesamin and asarinin have been shown to act as insecticidal synergists with pyrethrins [25–26]. Previous studies have shown that sesamin has moderate activity against S. aureus and no activity against E. coli [28]. In addition, asarinin was shown to be moderately active against S. aureus and B. subtilis, and it was suggested that it inhibits the NorA efflux pump system of S. aureus [29–30].
Sesamin and asarinin demonstrated a range of antimicrobial activities (8 to 140 µg/mL or 22.6 µM to 395 µM) against the five different species of Mycobacterium evaluated (Table 1). This is the first report of activity of sesamin and asarinin against nontuberculous mycobacteria, although both sesamin and asarinin were shown to lack activity against M. tuberculosis [31]. These results are not surprising, given that members of the M. tuberculosis complex do not share the same susceptibilities to anti-mycobacterial antibiotics with the nontuberculous mycobacteria [16].
Table 1.
Minimum inhibitory concentrations (MIC) of crude extracts of A. californica, sesamin, and asarinin against Mycobacterium species
| Sample | MIC (µg/mL) Against: | ||||
|---|---|---|---|---|---|
| M. smegmatis | M. abscessus | M. chelonae | M. marinum | M. avium A5 | |
| Sample 1a | 125 | >250 | >250 | 250 | 125 |
| Sample 2a | >250 | >250 | >250 | >250 | >250 |
| Sample 3b | 125 | >250 | 250 | >250 | >250 |
| Sesamin | 8 | >130 | 65 | 13 | 8 |
| Asarinin | 35 | >140 | 140 | 35 | 35 |
| Rifampinc | 25 | 0.8 | 0.15 | 0.8 | 2 |
Samples 1 and 2 are for root and aerial extracts-respectively from the same A. californica plant.
Sample 3 represents a large batch root extract that was subjected to bioactivity-directed fractionation, resulting in the isolation of sesamin and asarinin.
The antibiotic rifampin is included as a positive control. Negative control (vehicle, 2% DMSO) caused no significant growth inhibition.
Crude A. californica extracts were tested against representative Mycobacterium species (Table 1). Samples 1 and 3 (root extracts) demonstrated MIC values ranging from 125 to >250 µg/mL, while sample 2 (aerial extract) demonstrated weak or no inhibition (MIC >250 µg/mL). These differences in activity were likely due to the higher levels of sesamin and asarinin present in roots as compared to leaves (Table 2).
Table 2.
Quantity of sesamin and asarinin in extracts prepared from roots or leaves/stems of A. californica
| Sample Name |
Plant part | Sesamin Concentrationa | Asarinin Concentration | ||
|---|---|---|---|---|---|
| yieldb (ppt) ± SD |
% in extractc ± SD |
yield (ppt) ± SD |
% in extract ± SD |
||
| Sample 1 | Root | 3.1 ± 0.3 | 8.8 ± 1.0 | 1.7 ± 0.1 | 4.7 ± 0.4 |
| Sample 2 | Leaf/stem | 0.13 ± 0.02 | 0.95 ± 0.14 | 0.13 ± 0.02 | 0.9 ± 0.2 |
| Sample 3 | Root | 1.7 ± 0.2 | 8.8 ± 0.9 | 1.1 ± 0.1 | 5.9 ± 0.2 |
Concentrations were determined by LC - MS analysis of extracts prepared from the relevant plant parts. Standard deviations are for triplicate analyses of the same extract. Extract concentration was calculated based on linear regression analysis of 6 point calibration curves of peak area versus concentration with slope (m) = 587881 ± 4628, intercept (b) = −20512 ± 10554, and R2 = 0.9998 for sesamin and a slope (m) = 647726 ± 16450, intercept (b) = −15594 ± 37516, and R2 = 0.9974 for asarinin. Extracts were diluted so that the concentrations tested fell within the linear range of the calibration curve.
Yield is reported as parts per thousand or mass of pure compound (g) per mass of original plant material (kg).
% in extract is reported as mass (g) of sesamin or asarinin per mass of solid extract (g) × 100.
The % in extract values are provided for the purpose of comparison with biological data.
The root extracts (samples 1 and 3) were significantly less active compared to the pure compounds. However, these extracts are crude mixtures, and sesamin and asarinin constituted only a fraction of their content (Table 2). Accounting for this difference, it appeared that the antimycobacterial activity of the crude extracts could be largely attributed to the presence of sesamin and asarinin. For example, sample 1, a root extract of A. californica, demonstrated an MIC of 125 µg/mL against M. smegmatis (Table 1). Sample 1 contained 8.8 ± 1.0 % sesamin and 4.7 ± 0.4 % asarinin (Table 2); thus, the MIC of 125 µg/mL (expressed as mass of crude extract/volume media) is equivalent to 16.8 µg/mL (expressed as mass of sesamin and asarinin, combined, per volume of media). This value is within the range of the reported MICs for sesamin and asarinin alone against M. smegmatis (8 and 35 µg/mL, respectively).
In conclusion, the results of the quantitative analysis suggest that the majority of activity of A. californica root extracts against nontuberculous mycobacteria can be attributed to the presence of relatively high levels of sesamin and asarinin. Importantly, the presence of anti-mycobacterial compounds in A. californica roots supports the traditional use of this plant as a treatment for infection, although follow up studies would be necessary to evaluate the in vivo relevance of these findings. The higher levels of sesamin and asarinin in roots over leaves suggest that root extracts would be more effective than leaf extracts for treatment of mycobacterial infections.
Materials and Methods
Plant Material
Cultivated Anemopsis californica plant material was obtained from two sites, Horizon Herbs in Williams, OR (42°12' 17.21 "N, 123°19' 34.61"W; voucher number NCU592735, identified by Richard A. Cech) and Apache Creek Ranch in Santa Fe, NM (35°35' 56.40"N, 105°50' 27.22"W; voucher number NCU602027, identified by Amy Brown). Vouchers are retained at the University of North Carolina Herbarium. Harvested plant material was separated into three different portions, a root sample (sample #1, 9.8 g dry weight), a leaf/stem sample from the same plant (sample #2, 7.5 g dry weight, both harvested from Horizon Herbs in April, 2010), and a large batch of roots/rhizomes to facilitate isolation work (sample #3, 520 g dry weight) harvested from Apache Creek Ranch in November 2010. All plant material was air dried prior to extraction.
Extraction and LC-MS
Three batches of A. californica plant material were cut and ground, and then was macerated in methanol for 24 hours. The marc for each extract was subsequently soaked in methanol a total of three times, and the methanol was decanted and combined. The methanol extracts were evaporated to dryness with a rotary evaporator and subjected to liquid-liquid partitioning using published methods [18]. Briefly, the methanol extract was defatted by partitioning between a 1:1 ratio of hexane to methanol, and the latter fraction was then dried down and further partitioned between 4:1:5 of chloroform:methanol:water. The chloroform fraction was evaporated to dryness and its antimicrobial activity was tested using a broth microdilution assay. The yields from the chloroform fraction of samples 1, 2, and 3 were 343 mg, 104 mg, and 10 g, respectively.
For the isolation of the active compounds, the chloroform extract (10 g, sample #3, roots and rhizomes) was subjected to two stages of normal-phase chromatography on a CombiFlash®Rf ISCO using 120 g RediSep Rf Gold® Silica Columns (20 – 40 µm particle size, Teledyne ISCO, Lincoln, NE, USA). The first stage of normal-phase chromatography was performed with a hexane/chloroform/methanol gradient on silica gel (eluent chloroform and methanol, flow rate 18 mL/min) and the eluate was pooled into 12 fractions. Fraction VI (360–450 mL, 645 mg) was subjected to a second stage of separation with a hexane/acetone/methanol gradient on silica gel (eluent acetone and methanol, flow rate 18 ml/min) and pooled into 10 fractions. Fraction V (250–320 mL, 420 mg), the active fraction, was then subjected to further purification with two successive stages of isocratic separation with reversed-phase preparative HPLC on a C18 column (Phenomenex, Gemini-NX, 5 µm, 250 × 21.2 mm, flow rate 21 mL/min). The first stage of reversed-phase separation employed a 50:50 acetonitrile:water isocratic mobile phase composition, and the eluate was pooled into 5 fractions. Fractions II and III from this separation (200–300 mL and 320–400 mL, 75 mg and 80 mg, respectively) were then combined and subjected to a second reversed-phase separation with an isocratic mobile phase composition of 75:25 methanol:water. Sesamin (1) (0.012 % yield, 60 mg, 98.3% purity) eluted at 15 min and asarinin (2) (0.0096% yield, 60 mg, 98.5% purity) eluted at 16.5 min.
Test Bacteria, Chemicals, Biochemicals, and MIC
Mycobacterium marinum (ATCC strain 927), M. smegmatis strain mc2155 (ATCC strain 700084), M. abscessus strain AAy-P-1, M. chelonae strain EO-P-1, M. intracellulare strain TMC 1406T (ATCC 13950), and M. avium strain A5 [19–20] were grown in Middlebrook 7H9 broth medium containing 0.5 % (v/v) glycerol and 10 % (v/v) oleic acid-albumin with aeration (120 rpm) for 7 days at 37°C or 30°C (only M. marinum). Minimal inhibitory concentration (MIC) of each fraction or compound was measured by broth microdilution with a starting inoculum of 0.5–1.0 ×105 CFU/mL [20–21]. The plates were incubated for 4 days at 37°C or 30° C (only M. marinum) and the turbidity measured (absorbance 580 nm). Extracts were tested over a concentration range of 0.12 to 250 µg/mL by two-fold dilutions, in the presence of a constant DMSO concentration (2%). The MIC was defined as the lowest concentration completely inhibiting bacterial growth. Rifampin (Sigma, St. Louis, MO purity ≥ 97%) served as the control.
Quantitative Analysis
Sesamin and asarinin were identified in the crude A. californica extracts by matching retention time and fragmentation patterns with those of the isolated standard compounds. The concentrations of these compounds were then measured using selective reaction monitoring (SRM) on a triple quadrupole mass spectrometer (TSQ Access; Thermo Scientific, Waltham, MA, USA) with an electrospray ionization source in the positive ion mode. Transitions of 337.1 to 203.1 and 337.1 to 289.2 were employed for the isomeric compounds. The mass spectrometer was coupled to a reversed phase high performance liquid chromatograph (HPLC) (Agilent HP1200; Santa Clara, CA, USA) with a PFP column (5 µm, 150 × 4.6 mm; Phenomenex, Torrance, CA, USA). An acetonitrile (1% formic acid):water (1% formic acid) gradient was employed at 1.0 mL/min with HPLC grade solvents. A calibration curve (concentration range of 0.05 to 5.0 µg/mL) of concentration versus average peak area for triplicate injections was employed for quantitative analysis. Extracts were diluted so that sesamin and asarinin concentrations fell within the linear range of the calibration curve.
Supplementary Material
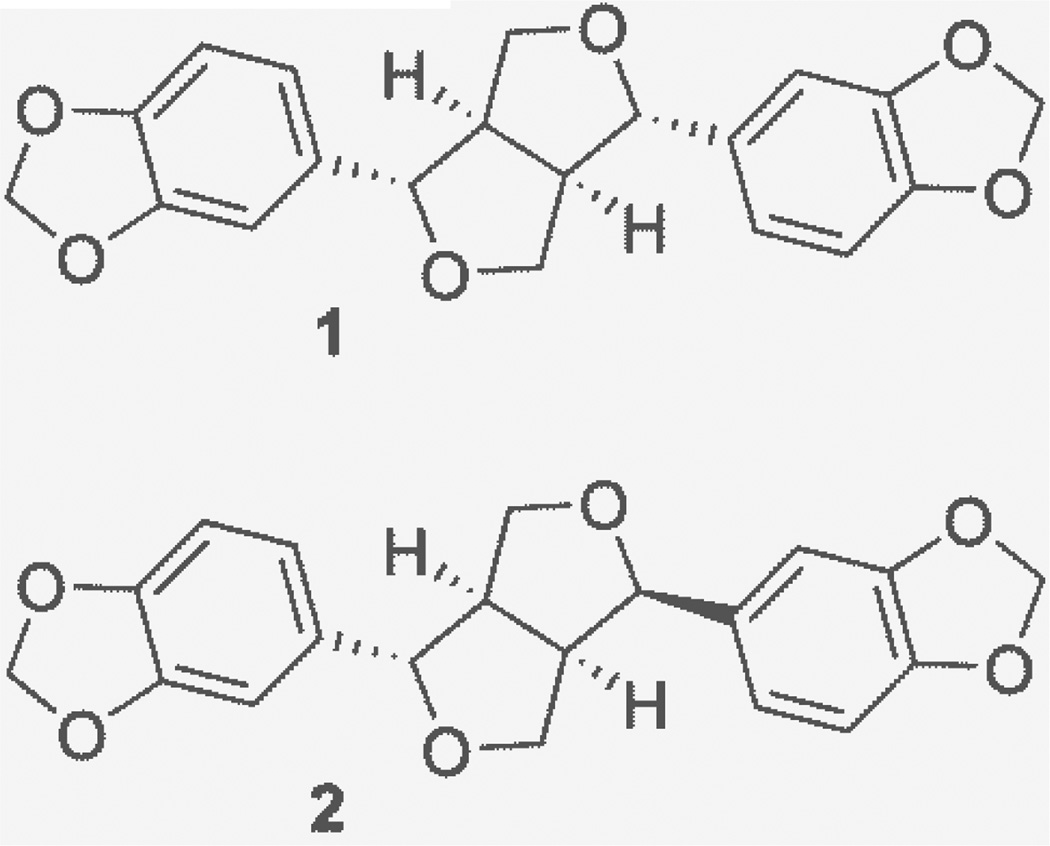
Fig 1.
Structures of furofuran lignans sesamin (1) and asarinin (2) isolated from A. californica.
Acknowledgements
We are grateful to Richard Cech (Horizon Herbs) and Amy Brown (Apache Creek Herbs) for providing A. californica plants. Thanks also to Carol Ann McCormick for preservation of voucher specimens. The authors acknowledge the technical skill of Ms. Myra D. Williams (Virginia Tech) for measurement of anti-mycobacterial activity of fractions, Dr. Brandie Ehrmann (UNC Greensboro) for assistance with mass spectrometry experiments, and Tyler Graf (UNC Greensboro) for assistance with isolation work. Funding was provided by the National Center for Complementary and Alternative Medicine, a component of the National Institutes of Health (R15 AT005005). MS data were collected in the Triad Mass Spectrometry Facility.
Footnotes
Conflict of Interest
The authors report no conflict of interest.
References
- 1.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Train P, Henrichs JR, Archer WA. Medicinal uses of plants by Indian tribes of Nevada -in Bioactive plants. Lawrence: Quarterman Publications; 1978. pp. 19–20. [Google Scholar]
- 3.Curtin LSM. By the prophet of the earth: Ethnobotany of the Pima. Tucson: University of Arizona Press; 1984. p. 156. [Google Scholar]
- 4.Romero JB. The Botanical lore of the California Indians. New York: Vintage Press, INC; 1954. p. 15. [Google Scholar]
- 5.Bocek BR. Ethnobotany of Costanoan indians, California, based on collections by John P. Harrington. Econ Bot. 1984;38:240–255. [Google Scholar]
- 6.Medina AL, Lucero ME, Holguin FO, Estell RE, Posakony JJ, Simon J, O'Connell MA. Composition and antimicrobial activity of Anemopsis californica leaf oil. J Agric and Food Chem. 2005;53:8694–8698. doi: 10.1021/jf0511244. [DOI] [PubMed] [Google Scholar]
- 7.Dimayuga RE, Garcia SR. Antimicrobial screening of medicinal plants from Baja California Sur, Mexico. J Ethnopharmacol. 1991;31:181–192. doi: 10.1016/0378-8741(91)90004-w. [DOI] [PubMed] [Google Scholar]
- 8.Medina-Holguin AL, Micheletto S, Holguin FO, Rodriguez J, O'Connell MA. Environmental influences on essential oils in roots of Anemopsis californica. Hortscience. 2007;42:1578–1583. [Google Scholar]
- 9.Medina-Holguin AL, Holguin FO, Micheletto S, Goehle S, Simon JA, O'Connell MA. Chemotypic variation of essential oils in the medicinal plant, Anemopsis californica. Phytochemistry. 2008;69:919–927. doi: 10.1016/j.phytochem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaminski CN, Ferrey SL, Lowrey T, Guerra L, Van Slambrouck S, Steelant WFA. In vitro anticancer activity of Anemopsis californica. Oncol Lett. 2010;1:711–715. doi: 10.3892/ol_00000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels AL, Van Slambrouck S, Lee RK, Arguello TS, Browning J, Pullin MJ, Kornienko A, Steelant WF. Effects of extracts from two Native American plants on proliferation of human breast and colon cancer cell lines in vitro. Oncol Rep. 2006;15:1327–1331. [PubMed] [Google Scholar]
- 12.Timbrook J. Virtuous herbs: Plants in Chumash medicine. J Ethnobiol. 1987;7:171–180. [Google Scholar]
- 13.Falkinham JO., III Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerging Infectious Diseases. 2011;17:419–424. doi: 10.3201/eid1703.101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Groote MA, Pace NR, Fulton K, Falkinham JO., III Relationships between Mycobacterium isolates from patients with pulmonary mycobacterial infection and potting soils. Appl Environ Microbiol. 2006;72:7602–7606. doi: 10.1128/AEM.00930-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billinger ME, Olivier KN, Viboud C, Montes de Oca R, Steiner C, Holland SM, Prevots DR. Nontuberculous mycobacteria–associated lung disease, United States in hospitalized persons, 1998–2005. Emerging Infectious Diseases. 2009;15:1562–1569. doi: 10.3201/eid1510.090196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K, Subcommittee AMD. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Amer J Resp Critical Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 17.Collier SA, Stockman LJ, Hicks LA, Garrison LE, Zhou FJ, Beach MJ. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol Infect. 2012;140:2003–2013. doi: 10.1017/S0950268811002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu JQ, Graf TN, Lee D, Chai HB, Mi Q, Kardono LB, Setyowati FM, Ismail R, Riswan S, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kroll DJ, Falkinham JO, III, Wall ME, Wani MC, Kinghorn AD, Oberlies NH. Cytotoxic and antimicrobial constituents of the bark of Diospyros maritima collected in two geographical locations in Indonesia. J Nat Prod. 2004;67:1156–1161. doi: 10.1021/np040027m. [DOI] [PubMed] [Google Scholar]
- 19.Beggs ML, Crawford JT, E'isenach KD. Isolation and sequencing of the replication region of Mycobacterium avium plasmid pLR7. J Bacteriol. 1995;177:4836–4840. doi: 10.1128/jb.177.17.4836-4840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falkinham JO, Macri RV, Maisuria BB, Actis ML, Sugandhi EW, Williams AA, Snyder AV, Jackson FR, Poppe MA, Chen L, Ganesh K, Gandour RD. Antibacterial activities of dendritic amphiphiles against nontuberculous mycobacteria. Tuberculosis. 2011;92:173–181. doi: 10.1016/j.tube.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Sugandhi EW, Macri RV, Williams AA, Kite BL, Slebodnick C, Falkinham JO, III, Esker AR, Gandour RD. Synthesis, critical micelle concentrations, and antimycobacterial properties of homologous, dendritic amphiphiles. Probing intrinsic activity and the "cutoff" effect. J Med Chem. 2007;50:1645–1650. doi: 10.1021/jm061240d. [DOI] [PubMed] [Google Scholar]
- 22.Bussey RO, III, Cech NB, Sy-Cordero AA, Falkinham JO, Oberlies NH. Sesamin from the roots of Yerba mansa (Anemposis californica) Planta Med. 2012;78:1111. doi: 10.1055/s-0034-1368352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CY, Chow TJ, Wu TS. The epimerization of sesamin and asarinin. J Nat Prod. 2005;68:1622–1624. doi: 10.1021/np050106d. [DOI] [PubMed] [Google Scholar]
- 24.Tocher JF. Isolation of another substance from sesame oil. Amer J Pharm. 1891;63:143–146. [Google Scholar]
- 25.Haller HL, LaForge FB, Sulivan WN. Some compounds related to sesamin: their structures and their synergistic effect with pyrethrum insecticides. J Org Chem. 1942;7:185. [Google Scholar]
- 26.Nissanka APK, Karunaratne V, Bandara BMR, Kumar V, Nakanishi T, Nishi M, Inada A, Tillekeratne LMV, Wijesundara DSA, Gunatilaka AAL. Antimicrobial alkaloids from Zanthoxylum tetraspermum and caudatum. Phytochemistry. 2001;56:857–861. doi: 10.1016/s0031-9422(00)00402-7. [DOI] [PubMed] [Google Scholar]
- 27.Eberhardt EG. Prickly Ash Bark. Am J Pharm. 1890;71:231–236. [Google Scholar]
- 28.Christov R, Bankovaa V, Tsvetkova I, Kujumgiev A, Delgado Tejerac A. Antibacterial furfuran lignans from Canary Islands propolis. Fitoterapia. 1999;70:89–92. [Google Scholar]
- 29.Yang GZ, Hu Y, Yang B, Chen Y. Lignans from the bark of Zanthoxylum planispinum. Helv Chim Acta. 2009;92:1657–1664. [Google Scholar]
- 30.Zloh M, Gibbons S. The role of small molecule-small molecule interactions in overcoming biological barriers for antimicrobial drug action. Theor Chem Acc. 2007;117:231–238. [Google Scholar]
- 31.Rangkaew N, Suttisri R, Moriyasu M, Kawanishi K. A new acyclic diterpene acid and bioactive compounds from Knema glauca. Arch Pharmacal Res. 2009;32:685–692. doi: 10.1007/s12272-009-1506-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.