Abstract
Objective
To obtain normative data for the canine cornea and conjunctiva using high-resolution Time- and Fourier-Domain optical coherence tomography (TD-OCT and FD-OCT) and ultrasound pachymetry (USP).
Animals
One hundred sixty-eight eyes of 133 healthy, young, intact laboratory beagles.
Procedures
The cornea and conjunctiva of 16 eyes of 8 healthy young intact female intact beagles were imaged using FD-OCT. Corneal thickness was measured with FD-OCT and USP, while corneal and conjunctival epithelial thickness was measured with FD-OCT. The central corneal thickness (CCT) was determined in 152 eyes of 125 healthy young adult intact female (35) and male (90) beagles using TD-OCT. Mixed effects linear regression was used for statistical analysis.
Results
The CCT was (mean ± standard deviation) 497.54 ± 29.76, 555.49 ± 17.19 and 594.81 ± 33.02 μm as measured by FD-OCT, USP and TD-OCT, respectively. The central, superior paraxial, superior perilimbal corneal epithelial thickness and superior bulbar conjunctival epithelial thickness was 52.38 ± 7.27, 56.96 ± 6.47, 69.06 ± 8.84 and 42.98 ± 6.17 μm, respectively. When comparing techniques used for measuring CCT (USP versus FD-OCT and FD-OCT versus TD-OCT), USP and TD-OCT generated significantly greater values in comparison to FD-OCT (both P < 0.001). For all dogs CCT increased with increasing age and body weight (both P < 0.001) and was higher in intact males versus females using TD-OCT (P = 0.034).
Conclusion
High-resolution FD-OCT and TD-OCT provide detailed non-invasive evaluation of in vivo canine anterior segment structures. Normative values of the canine cornea and conjunctiva are reported.
Keywords: Cornea, conjunctiva, optical coherence tomography, canine, laboratory beagle, ultrasound pachymetry
INTRODUCTION
Dogs are widely used in ophthalmic drug and device development programs and as experimental animal models in vision science studies. Furthermore, dogs are the most common species with spontaneous ocular disease seen in veterinary practice and serve as important models for several ophthalmic diseases in humans.(1-4)
Optical coherence tomography (OCT) is a noninvasive technique for high-resolution and cross-sectional tomographic imaging of tissue by measuring optical reflections.(5-7) It enables quantitative and qualitative evaluation of the corneal microstructure. In addition, OCT images can be digitally stored and compared. In human patients, OCT has provided significant advances in the diagnosis of glaucoma and vitreoretinal diseases, such as macular degeneration and macular edema secondary to diabetic retinopathy in humans.(8-10) Fourier-Domain OCT (FD-OCT; also known as Spectral-Domain) is increasingly preferred over Time-Domain OCT (TD-OCT) because of the higher acquisition speeds and greater resolution of the acquired images.(11) FD-OCT increases axial resolution 2- to 3-fold and scan speed 60- to 110-fold compared with TD-OCT.(12) However, TD-OCT units have been commercially available longer than FD-OCT units, are generally less expensive and provide images with greater penetration into the eye, albeit with lower resolution.
Anterior segment imaging is a rapidly advancing field and is increasingly being utilized in physician-based ophthalmic practices for a number of purposes including planning for photorefractive surgery, monitoring corneal diseases including keratoconus, measuring anterior chamber depth, and assessing narrowing of the iridocorneal angle (ICA).(8-10, 13-23) Anterior segment imaging using OCT has recently emerged in canine research and is expected to be useful in clinical patients as well.(24, 25) A recent case series confirmed that this technique is applicable to the evaluation of the canine and feline cornea in healthy and pathological conditions.(24) FD-OCT and TD-OCT have both recently been used for imaging and quantification of corneal, limbal, and bulbar conjunctival epithelial thickness in healthy humans.(13, 15, 26) One of these studies(26) showed that humans with ocular disease, such as chronic glaucoma (medically managed) or keratoconjunctivitis sicca (KCS), having thickened conjunctiva compared with healthy individuals and it was concluded that conjunctival thickness measurement represents a promising new endpoint for evaluation and grading of the severity of ocular surface disease.(26) Fourier-Domain OCT (FD-OCT) has also been used for diagnosing and monitoring other anterior segment conditions such as conjunctivochalasis and been used for assessing the tear meniscus of humans.(14, 27, 28) In veterinary ophthalmology, OCT has been primarily used in canine retinal and glaucoma research(29-32) and there are currently limited data available on OCT values of the canine anterior segment.(25, 33-36) Studies have found SD-OCT to be a reliable tool for measuring CCT in dogs.(35, 36)
Ultrasonic pachymetry (USP) is widely used for measuring corneal thickness in animals and humans. Several studies in humans have demonstrated good correlation between corneal thickness measurements obtained by USP and OCT pachymetry.(37, 38) To date, there are only a few publications on USP and OCT corneal pachymetry in dogs.(24, 25, 35, 36, 39) The aims of this study were to obtain images of the cornea using FD-OCT and TD-OCT and conjunctiva using FD-OCT of healthy laboratory beagles, and to compare corneal FD-OCT pachymetry and USP in 3 different locations (superior perilimbal, superior paraxial, and central cornea). We present normative data for CCT in a large population of normal laboratory beagles using TD-OCT and compare the results with the FD-OCT CCT measurements.
METHODS
All aspects of the study were approved by the Institutional Animal Care and Use Committees of the University of California-Davis and Covance and were performed according to the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. All dogs included were laboratory beagles. Prior to study entry, all dogs underwent a complete physical examination and only systemically healthy dogs were included in the study. All dogs also received a detailed ophthalmic examination, including slit lamp biomicroscopy and indirect ophthalmoscopy; only dogs assessed as having normal eyes were used. None of the dogs had received topical eye medications prior to entry into the study.
Dogs were sedated with midazolam (0.1 mg/kg) and butorphanol (0.3 mg/kg) or dexmedetomidine (2.5-5 μg/kg) intramuscularly (or intravenously for dexmedetomidine) as needed for sedation prior to imaging. Dogs were then placed in sternal recumbency for all imaging, and balanced salt solution (BSS; Akorn Inc., Buffalo Grove, IL, USA) or 1.0% carboxymethylcellulose (Refresh Liquigel®; Allergan, Irvine, California, USA) was applied topically as necessary throughout the sedation and subsequent procedures to prevent corneal desiccation. A Barraquer eyelid speculum was used to keep the eyes open on the sedated dogs when needed (this was not needed for the TD-OCT imaging). All measurements were performed by the same person (ARS) to minimize variability.
FD-OCT & USP
Eight healthy female dogs (16 eyes) with a mean age of 0.52 ± 0.12 years and weight of 6.83 ± 1.40 kg were examined by FD-OCT and USP. FD-OCT imaging (RTVue® 100, software version 6.1; Optovue Inc., Fremont, California, USA; 26000 A scan/sec, 5 μm axial resolution, 840 nm superluminescent diode) of the cornea and conjunctiva was performed. The FD-OCT system with a corneal adaptor module was used for corneal pachymetry on both eyes of each dog in the central, superior paraxial, and superior perilimbal cornea, and for superior bulbar conjunctival epithelial thickness measurements (measured approximately 1 mm posterior to the anterior border of the limbus). Three repeat measurements in close proximity to each other were manually obtained in each location from images stored in the RTVue unit using the RTVue software measuring tool. The RTVue measuring tool was used to measure thickness of the cornea (full and and epithelial thickness) and conjunctival epithelium. Ultrasound pachymetry (USP; Accupach VI; Accutome Ultrasound Inc., Malvern, PA, USA) was performed on both eyes of each dog in the central, superior paraxial, and superior perilimbal cornea typically following OCT measurements. The ultrasound probe was manually placed as perpendicularly as possible to the cornea after applying a drop of 0.5% proparacaine (Alcon Inc., Fort Worth, TX, USA) to the cornea. Three consecutive corneal thickness measurements in each location were obtained and averaged automatically by the instrument to obtain a mean value. Three mean measurements were saved for each location. Two dogs did not have complete measurements (they did not have 3 mean measurements in each location and one of them had no measurements done in the superior paraxial region); values that were available for these two dogs were included in the analysis.
TD-OCT
TD-OCT data consisted of scans from 152 eyes of 125 dogs. Specifically, both eyes of 27 dogs, 58 right eyes (OD) and 40 left eyes (OS) were examined. Thirty-five intact females and 90 intact males were included. The mean age and body weight of dogs were 1.10 ± 0.31 years and 8.78 ± 1.71 kg (females), and 0.97 ± 0.25 years and 11.78 ± 2.24 kg (males), respectively. Imaging of the cornea using TD-OCT (Visante™ 1000, software version 3.0.0.139, Carl Zeiss Meditec, Dublin, California, USA; 2048 A scan/sec, 18 μm axial resolution, 1310 nm superluminescent diode) was performed. A scale bar was saved on each image, and ImageJ (free software, version 1.46; NIH, Bethesda, Maryland, USA) was used to measure the CCT on each scan after calibration to the scale on the image. One measurement was manually obtained in the central corneal location on each scan.
Statistics
Mixed effects linear regression was used to evaluate the main and interaction effects of eye, weight, and when appropriate corneal location and method of measurement. The individual dog was treated as a random effect; all other variables were considered as fixed effects. The descriptive measurements for both eyes were averaged unless there was a significant difference between eyes. Analyses were performed using Stata/IC 12.1 (StataCorp LP, College Station, Texas, USA). All measurements were expressed as mean ± standard deviation. For all analyses, values of P ≤ 0.05 were considered significant.
RESULTS
High quality, high-resolution in vivo images of the canine conjunctiva (FD-OCT), and cornea (FD-OCT and TD-OCT) were obtained (Figures 1–3). Specifically, the central cornea (Figure 1), superior paraxial cornea, superior perilimbal cornea, and superior bulbar conjunctival epithelium (Figure 2) were examined with FD-OCT. The central cornea was examined with TD-OCT (Figure 3). Please note the orderly arrangement of collagen fibers and clear distinction of the epithelium from the stroma on the FD-OCT images (Figure 1 and 2). No significant differences were noted between replicates for any measurement obtained. Unless otherwise mentioned in the following, no significant differences were noted between eyes for measurements obtained.
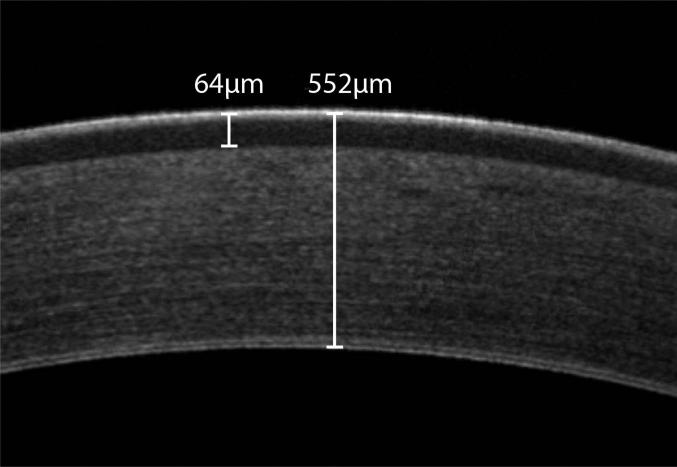
Figure 1.
In vivo FD-OCT of the normal cornea in an 8-month-old intact female beagle; central corneal thickness was 552 μm and central corneal epithelial thickness was 64 μm. Note that the epithelium and an orderly arrangement of the collagen fibrils within the stroma can be observed.
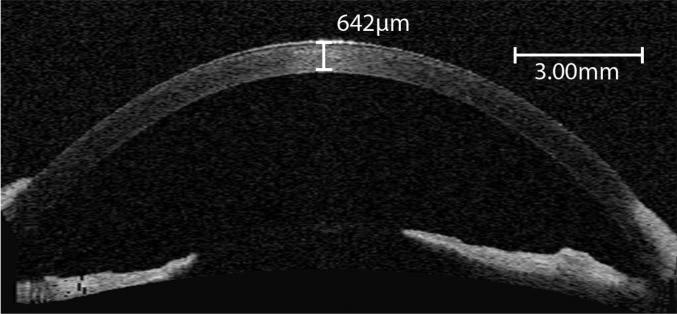
Figure 3.
In vivo Visante™ OCT scan of the left cornea in a young healthy beagle; the central corneal thickness was 642 μm. Note that the cornea has a homogenous appearance and specific corneal layers cannot be observed.
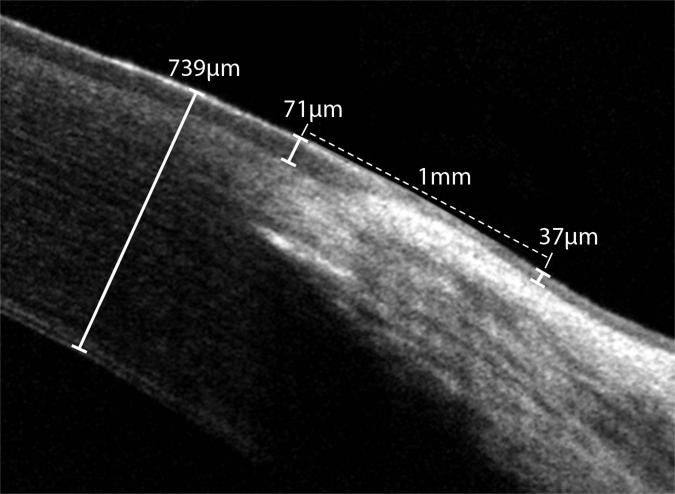
Figure 2.
In vivo FD-OCT of the normal limbus in an 8-month-old intact female beagle; superior perilimbal corneal thickness was 739 μm and perilimbal corneal epithelial thickness was 71 μm. The superior bulbar conjunctival epithelial thickness 1.01 mm posterior to the anterior border of the limbus was 37 μm thick.
Corneal and conjunctival epithelial thickness (FD-OCT)
Using FD-OCT, values obtained for all locations differed significantly with the superior bulbar conjunctival, central corneal, superior paraxial corneal, and superior perilimbal corneal epithelial thickness measured at 42.98 ± 6.17, 52.38 ± 7.27, 56.96 ± 6.47, and 69.06 ± 8.84 μm, respectively.
Corneal full thickness (FD-OCT versus USP)
The cornea was thickest peripherally and thinnest axially (see Table 1) with values obtained at all locations and techniques used differing significantly (P ≤ 0.05) from each other. No significant interaction was noted between technique and location (i.e., all USP and FD-OCT values were significantly different from each other in each location when comparing the two techniques; P <0.001 for the CCT and superior perilimbal region and P = 0.0055 for the superior paraxial region). A statistically significant difference was detected between the left and right eyes in the superior perilimbal region using FD-OCT pachymetry (P < 0.001; OD: 664.58 ± 64.61 μm, OS: 627.58 ± 50.58 μm). No significant difference was noted between eyes in the superior paraxial location (P = 0.133) and central cornea (P = 0.478).
Table 1.
Corneal thickness as measured by FD-OCT, TD-OCT, ± USP in young healthy laboratory beagles (Mean ± SD)
| Corneal location | Superior perilimbal (μm) | Superior paraxial (μm) | Central (μm) |
|---|---|---|---|
| USP (16 eyes, F) | 617.59 ± 32.98*,‡ | 585.41 ± 27.83*,‡ | 555.49 ± 17.19*,‡ |
| FD-OCT (16 eyes, F) | 646.08 ± 60.37*,‡ | 561.06 ± 55.66*,‡ | 497.54 ± 29.76*,‡,† |
| TD-OCT (152 eyes, M & F) | 594.81 ± 33.02 | ||
| TD-OCT (41 eyes, F) | 580.32 ± 29.24†,§ | ||
| TD-OCT (111 eyes, M) | 600.16 ± 32.84§ |
FD-OCT = Fourier Domain Optical Coherence Tomography.
TD-OCT = Time Domain Optical Coherence Tomography.
USP = Ultrasound pachymetry.
“*”,“†” or “§” = Statistically significant difference in corneal thickness between US pachymetry versus FD-OCT (*), FD- versus TD-OCT (†), or males versus females (§)(p ≤ 0.05).
Statistically significant difference in corneal thickness between locations (p ≤ 0.05).
F = Intact females.
M = Intact males.
Central corneal thickness (TD-OCT and TD- versus FD-OCT)
The mean CCT for the 152 eyes imaged as measured by TD-OCT was 594.81 ± 33.02 μm. No significant effect of eye (P = 0.820) was noted. A significant effect of sex was noted (P = 0.034), with CCT in intact males (600.16 ± 32.84 μm) significantly greater versus intact females (580.32 ± 29.24 μm) using TD-OCT. For all TD-OCT data the central corneal thickness significantly increased with increasing month of age (13.15 ± 3.49 μm, P < 0.001) and kg body weight (16.36 ± 3.97 μm, P < 0.001), although a significant negative interaction (-1.13 ± 0.33 μm, P = 0.001) between age and body weight was noted (the positive “effect” of one of these variables declines as the other variable's value increases. That is, at lower weights the effect of age leads to increased corneal thickness, but at higher weights this age effect will be diminished. The same finding holds for the effect of weight at lower versus older ages).
Since the dogs imaged with FD-OCT in this study consisted of female intact beagles only, only data from intact females from the TD-OCT was used for comparison with FD-OCT: When controlling for body weight and age in these intact females, the effect of technique was significant with CCT measured by TD-OCT significantly greater (P < 0.001) than CCT measured by FD-OCT.
DISCUSSION
At present, there are only a few publications describing in vivo imaging of the ocular surface epithelium in humans (corneal and conjunctival epithelium), dogs, and cats (central corneal epithelium only), and no OCT data on ocular surface epithelium in other species exists to our knowledge.(13, 15, 24, 26, 40) Similar to previous studies in humans the present study demonstrated the canine corneal epithelium was thinnest centrally and thickest peripherally.(15, 26) In addition, central corneal epithelial thickness obtained from canines in the present study (52.38 ± 7.27 μm) was similar to results obtained in recent studies in humans (48.3 ± 2.9 μm(26), 52.5 ± 2.4 μm(41) and 54.7 ± 1.9 μm(15)), dogs (50-60 μm)(24), and cats (55-65 μm)(24). Conjunctival epithelial thickness values obtained in the present study (42.98 ± 6.17 μm) were similar to previous reports in humans published by Feng et al (44.9 ± 3.4 μm), Francoz et al (middle-aged control group, superior quadrant 43.6 ± 11.7 μm), and two studies by Zhang et al (42.4 ± 7.4 μm and 47.3 ± 8.4 μm).(13, 15, 26, 40) To the best of our knowledge, this is the first report of conjunctival epithelial thickness measurement in the canine species using FD-OCT. Similar to in people, it is expected that FD-OCT imaging of canine corneal and conjunctival epithelium represents a promising tool for monitoring and grading severity of ocular surface disease.(26) The dog is an excellent model for ocular surface diseases such as KCS in humans.(42, 43) Recently, it has been reported that human patients with KCS have increased corneal and conjunctival epithelial thickness. (26) Thus, it would be interesting to compare in vivo imaging data of the cornea and conjunctiva in normal and KCS-affected dogs.
In the present study, corneal thickness measurements obtained by FD-OCT and USP were compared at the central, superior paraxial, and superior perilimbal regions in 8 young and healthy laboratory beagles. Some variability was present and could have been partially attributed to the inability to perform USP in the same area as FD-OCT pachymetry. It has been suggested that discrepancy between the two techniques (USP and OCT) can be due to USP being more user dependent.(44) Ultrasound pachymetry requires delicate probing of the cornea, which increases the risk of corneal injury in fragile corneas and can be poorly tolerated by some animals. Its reliability can be limited by several factors, such as patient compliance, because the USP probe needs to be manually placed as perpendicularly as possible to the center of the cornea. In a study of 80 eyes of 40 healthy human subjects it was reported repeated measurements of CCT by FD-OCT had less variability than those obtained by USP, are more reproducible, possibly more reliable, and may better represent actual central corneal thickness (CCT).(45) Specifically, we obtained significantly lower values for CCT with FD-OCT compared with USP at 497.54 ± 29.76 and 555.49 ± 17.19 μm, respectively. We suspect this was most likely due to the inability to hold the ultrasound probe perfectly perpendicular to the surface of the cornea; therefore, true CCT was slightly overestimated. This was similar to data found in previous studies in humans and a recent study in dogs comparing the two techniques.(25, 44-46) In addition, acoustic wave propagation velocities can differ between species for several ocular tissues (47-51), and thus may contribute to inaccuracies obtained with USP in dogs since the velocity used to determine thickness (1640 m/s) is derived from the human cornea.(52) In contrast to the CCT findings, significantly greater corneal thickness values were obtained with FD-OCT versus USP in the superior perilimbal region. Consistent with human studies, this study demonstrated that the cornea was thicker peripherally compared with centrally when measured by each technique.(53) It is likely that placement of the ultrasound probe occurred slightly further away from/anterior to the limbus despite attempts to place the probe at or very close to the limbus, which could have caused the US measurements to be less than the OCT measurements in this region as the cornea is thinner axially in dogs.(39) In contrast, FD-OCT allows direct visualization of the limbus and superior corneal perilimbal FD-OCT measurements were obtained immediately adjacent to it (Figure 2). Unfortunately, the FD-OCT images obtained in the limbal region were not large enough to consistently allow measurements 1 or more mm anterior to the limbus, and because most of the dogs went on to participate in another study shortly after imaging, repeat-measurements in different locations were not possible to test this hypothesis in this population of dogs. While no statistically significant differences were noted between eyes using both techniques in the two other locations measured (central and superior paraxial cornea), a significant difference was noted between eyes in the superior perilimbal region using FD-OCT pachymetry (P < 0.001; OD: 664.58 ± 64.61 μm, OS: 627.58 ± 50.58 μm, difference 37 μm). This, combined with the relative high standard deviations in the superior perilimbal measurements, suggests that OCT measurements obtained in this location were less accurate in the sedated dogs. A limitation of the FD-OCT and USP measurements reported is the relatively low number of dogs (8 dogs, 16 eyes) imaged in this arm of the study.
As in people, CCT has been reported to increase with age in dogs.(39, 54) Gilger et al found the mean corneal thickness as measured by USP to increase by 14.23 ± 2.26 μm/month of age in dogs and 1.83 ± 0.38 μm/kg bodyweight. After adjusting for age and weight, he found females to have significantly thinner corneas than males (22.43 ± 11.03 μm).(39) Similarly, we found a significant effect of sex in the present study with males having a significantly greater CCT with TD-OCT in comparison with females. Furthermore, we also found a significant increase in CCT with increase in age and body weight from the TD-OCT data and a negative interaction between these two variables was noted. Thus, the effect of age or body weight became smaller as the other variable increases in the present study.
We compared CCT, as measured by FD- versus TD-OCT, in intact female laboratory beagles, after adjusting for body weight and age and found that significantly higher values were obtained by TD-OCT. While the FD- and TD-OCT measurements in the present study were performed in two different dog populations, we note that we only compared the intact females within each population. Furthermore, adjustments were made for factors known to influence corneal thickness including age and body weight to rule out effect of these variables. Although additional confounding variables such as genetic and environmental factors may have played a role, it was most likely that the majority of the difference in CCT was a result of the technique used. The FD-OCT used in the current study (RTVue 100) possesses an increased axial resolution of 3.6-fold and scan speed of 12.7-fold (A scan/sec) compared with the TD-OCT used (Visante™ 1000. The higher resolution and higher speed (which minimizes motion artifacts) of FD-OCT should allow for more accurate measurements.(12) In contrast to FD-OCT where measurement tools within the software was used to measure central corneal thickness, the use of an indirect tool (ImageJ) for TD-OCT measurements, following calibration of the scale on the image, may have introduced additional variability.
While the variation between techniques in the present study was statistically significant, the differences in values obtained by the FD-OCT and USP techniques in the superior perilimbal and paraxial regions (28.49 and 24.35 μm, respectively) was unlikely to result in clinically relevant differences under most circumstances in veterinary patients. The difference between techniques in the central corneal region may be more clinically relevant (USP versus FD-OCT: 57.95 μm, and FD- versus TD-OCT: 82.78 μm) especially for canine surgical models were precise depth measurements are required. Based on these results, it is recommended that the same technique be utilized when comparing and monitoring corneal thickness of laboratory beagles over time. Further studies are indicated to obtain normative data in other canine breeds.
In conclusion, we have generated normative data of the cornea and conjunctiva of the laboratory beagle using TD-OCT, FD-OCT & USP. High-resolution OCT enabled detailed noninvasive in vivo evaluation of canine cornea and conjunctiva in sedated dogs. This study provides normative values with which to evaluate data obtained from laboratory beagles with spontaneous corneal disease, induced models of anterior segment disease and in evaluation of test compound effects in drug development programs. Since the techniques used yielded significantly different results, we recommend using the same technique when monitoring changes over time or comparing subjects.
REFERENCES
- 1.Kaswan R. Characteristics of a canine model of KCS: effective treatment with topical cyclosporine. Advances in experimental medicine and biology. 1994;350:583–94. doi: 10.1007/978-1-4615-2417-5_99. [DOI] [PubMed] [Google Scholar]
- 2.Tsai S, Miller PE, Struble C, Howard S, Almazan A, Burke JA, et al. Topical application of 0.005% latanoprost increases episcleral venous pressure in normal dogs. Veterinary ophthalmology. 2012;15(Suppl 1):71–8. doi: 10.1111/j.1463-5224.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 3.Bentley E, Murphy CJ, Li F, Carlsson DJ, Griffith M. Biosynthetic corneal substitute implantation in dogs. Cornea. 2010;29(8):910–6. doi: 10.1097/ICO.0b013e3181c846aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AVMA US Pet Ownership & Demographic Sourcebook. 2012 edition. Schaumburg, IL: 2012. [Google Scholar]
- 5.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254(5035):1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal EZ, Williams JM, Weinreb RN, Girkin CA, Berry CC, Zangwill LM. Reproducibility of nerve fiber layer thickness measurements by use of optical coherence tomography. Ophthalmology. 2000;107(12):2278–82. doi: 10.1016/s0161-6420(00)00341-9. [DOI] [PubMed] [Google Scholar]
- 7.Chen TC, Cense B, Pierce MC, Nassif N, Park BH, Yun SH, et al. Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Archives of ophthalmology. 2005;123(12):1715–20. doi: 10.1001/archopht.123.12.1715. [DOI] [PubMed] [Google Scholar]
- 8.Schuman JS, Hee MR, Puliafito CA, Wong C, Pedut-Kloizman T, Lin CP, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Archives of ophthalmology. 1995;113(5):586–96. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 9.Menke MN, Dabov S, Knecht P, Sturm V. Reproducibility of retinal thickness measurements in patients with age-related macular degeneration using 3D Fourier-domain optical coherence tomography (OCT) (Topcon 3D-OCT 1000). Acta ophthalmologica. 2011;89(4):346–51. doi: 10.1111/j.1755-3768.2009.01692.x. [DOI] [PubMed] [Google Scholar]
- 10.Jittpoonkuson T, Garcia PM, Rosen RB. Correlation between fluorescein angiography and spectral-domain optical coherence tomography in the diagnosis of cystoid macular edema. The British journal of ophthalmology. 2010;94(9):1197–200. doi: 10.1136/bjo.2009.170589. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim MA, Sepah YJ, Symons RC, Channa R, Hatef E, Khwaja A, et al. Spectral- and time-domain optical coherence tomography measurements of macular thickness in normal eyes and in eyes with diabetic macular edema. Eye (Lond) 2012;26(3):454–62. doi: 10.1038/eye.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuman JS. Spectral domain optical coherence tomography for glaucoma (an AOS thesis). Transactions of the American Ophthalmological Society. 2008;106:426–58. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Li Q, Liu B, Zhou H, Wang H, Zhang Z, et al. In vivo cross-sectional observation and thickness measurement of bulbar conjunctiva using optical coherence tomography. Investigative ophthalmology & visual science. 2011;52(10):7787–91. doi: 10.1167/iovs.11-7749. [DOI] [PubMed] [Google Scholar]
- 14.Gumus K, Crockett CH, Pflugfelder SC. Anterior segment optical coherence tomography: a diagnostic instrument for conjunctivochalasis. American journal of ophthalmology. 2010;150(6):798–806. doi: 10.1016/j.ajo.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, Simpson TL. Corneal, limbal, and conjunctival epithelial thickness from optical coherence tomography. Optometry and vision science. 2008;85(9):E880–3. doi: 10.1097/OPX.0b013e318185272d. [DOI] [PubMed] [Google Scholar]
- 16.Doors M, Berendschot TT, de Brabander J, Webers CA, Nuijts RM. Value of optical coherence tomography for anterior segment surgery. Journal of cataract and refractive surgery. 2010;36(7):1213–29. doi: 10.1016/j.jcrs.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Konstantopoulos A, Kuo J, Anderson D, Hossain P. Assessment of the use of anterior segment optical coherence tomography in microbial keratitis. American journal of ophthalmology. 2008;146(4):534–42. doi: 10.1016/j.ajo.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Konstantopoulos A, Hossain P, Anderson DF. Recent advances in ophthalmic anterior segment imaging: a new era for ophthalmic diagnosis? The British journal of ophthalmology. 2007;91(4):551–7. doi: 10.1136/bjo.2006.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Shekhar R, Huang D. Corneal pachymetry mapping with high-speed optical coherence tomography. Ophthalmology. 2006;113(5):792–9. doi: 10.1016/j.ophtha.2006.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Meisler DM, Tang M, Lu AT, Thakrar V, Reiser BJ, et al. Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology. 2008;115(12):2159–66. doi: 10.1016/j.ophtha.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang M, Li Y, Avila M, Huang D. Measuring total corneal power before and after laser in situ keratomileusis with high-speed optical coherence tomography. Journal of cataract and refractive surgery. 2006;32(11):1843–50. doi: 10.1016/j.jcrs.2006.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radhakrishnan S, Goldsmith J, Huang D, Westphal V, Dueker DK, Rollins AM, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Archives of ophthalmology. 2005;123(8):1053–9. doi: 10.1001/archopht.123.8.1053. [DOI] [PubMed] [Google Scholar]
- 23.Rosas Salaroli CH, Li Y, Zhang X, Tang M, Branco Ramos JL, Allemann N, et al. Repeatability of laser in situ keratomileusis flap thickness measurement by Fourier-domain optical coherence tomography. Journal of cataract and refractive surgery. 2011;37(4):649–54. doi: 10.1016/j.jcrs.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Famose F. Assessment of the use of spectral domain optical coherence tomography (SD-OCT) for evaluation of the healthy and pathological cornea in dogs and cats. Veterinary ophthalmology. 2014;17(1):12–22. doi: 10.1111/vop.12028. [DOI] [PubMed] [Google Scholar]
- 25.Alario AF, Pirie CG. Central corneal thickness measurements in normal dogs: a comparison between ultrasound pachymetry and optical coherence tomography. Veterinary ophthalmology. 2014;17(3):207–11. doi: 10.1111/vop.12074. [DOI] [PubMed] [Google Scholar]
- 26.Francoz M, Karamoko I, Baudouin C, Labbe A. Ocular surface epithelial thickness evaluation with spectral-domain optical coherence tomography. Investigative ophthalmology & visual science. 2011;52(12):9116–23. doi: 10.1167/iovs.11-7988. [DOI] [PubMed] [Google Scholar]
- 27.Gumus K, Pflugfelder SC. Increasing prevalence and severity of conjunctivochalasis with aging detected by anterior segment optical coherence tomography. American journal of ophthalmology. 2013;155(2):238–42. e2. doi: 10.1016/j.ajo.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Li Y, Lu AT, Liu P, Tang M, Yiu SC, et al. Reproducibility of tear meniscus measurement by Fourier-domain optical coherence tomography: a pilot study. Ophthalmic surgery, lasers & imaging : the official journal of the International Society for Imaging in the Eye. 2009;40(5):442–7. doi: 10.3928/15428877-20090901-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panzan CQ, Guven D, Weiland JD, Lakhanpal RR, Javaheri M, de Juan E, Jr., et al. Retinal thickness in normal and RCD1 dogs using optical coherence tomography. Ophthalmic surgery, lasers & imaging : the official journal of the International Society for Imaging in the Eye. 2004;35(6):485–93. [PubMed] [Google Scholar]
- 30.Grahn BH, Sandmeyer LL, Breaux C. Retinopathy of Coton de Tulear dogs: clinical manifestations, electroretinographic, ultrasonographic, fluorescein and indocyanine green angiographic, and optical coherence tomographic findings. Veterinary ophthalmology. 2008;11(4):242–9. doi: 10.1111/j.1463-5224.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 31.Grozdanic SD, Matic M, Betts DM, Sakaguchi DS, Kardon RH. Recovery of canine retina and optic nerve function after acute elevation of intraocular pressure: implications for canine glaucoma treatment. Veterinary ophthalmology. 2007;10(Suppl 1):101–7. doi: 10.1111/j.1463-5224.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 32.Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene therapy. 2007;14(4):292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- 33.Rosolen SG, Riviere ML, Lavillegrand S, Gautier B, Picaud S, LeGargasson JF. Use of a combined slit-lamp SD-OCT to obtain anterior and posterior segment images in selected animal species. Veterinary ophthalmology. 2012;15(Suppl 2):105–15. doi: 10.1111/j.1463-5224.2012.01037.x. [DOI] [PubMed] [Google Scholar]
- 34.Almazan A, Tsai S, Miller PE, Lee SS, Vilupuru AS, Burke JA, et al. Iridocorneal angle measurements in mammalian species: normative data by optical coherence tomography. Veterinary ophthalmology. 2013;16(2):163–6. doi: 10.1111/j.1463-5224.2012.01030.x. [DOI] [PubMed] [Google Scholar]
- 35.Alario AF, Pirie CG. A spectral-domain optical coherence tomography device provides reliable corneal pachymetry measurements in canine eyes. The Veterinary record. 2013;172(23):605. doi: 10.1136/vr.101530. [DOI] [PubMed] [Google Scholar]
- 36.Alario AF, Pirie CG. Reliability of manual measurements of corneal thickness obtained from healthy canine eyes using spectral-domain optical coherence tomography (SD OCT). Can J Vet Res. 2014;78(3):221–5. [PMC free article] [PubMed] [Google Scholar]
- 37.Barkana Y, Gerber Y, Elbaz U, Schwartz S, Ken-Dror G, Avni I, et al. Central corneal thickness measurement with the Pentacam Scheimpflug system, optical low-coherence reflectometry pachymeter, and ultrasound pachymetry. Journal of cataract and refractive surgery. 2005;31(9):1729–35. doi: 10.1016/j.jcrs.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 38.Nam SM, Im CY, Lee HK, Kim EK, Kim TI, Seo KY. Accuracy of RTVue optical coherence tomography, Pentacam, and ultrasonic pachymetry for the measurement of central corneal thickness. Ophthalmology. 2010;117(11):2096–103. doi: 10.1016/j.ophtha.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Gilger BC, Whitley RD, McLaughlin SA, Wright JC, Drane JW. Canine corneal thickness measured by ultrasonic pachymetry. American journal of veterinary research. 1991;52(10):1570–2. [PubMed] [Google Scholar]
- 40.Zhang X, Li Q, Xiang M, Zou H, Liu B, Zhou H, et al. Bulbar conjunctival thickness measurements with optical coherence tomography in healthy Chinese subjects. Investigative ophthalmology & visual science. 2013 doi: 10.1167/iovs.12-11003. [DOI] [PubMed] [Google Scholar]
- 41.Duma SM, Bisplinghoff JA, Senge DM, McNally C, Alphonse VD. Eye injury risk from water stream impact: biomechanically based design parameters for water toy and park design. Current eye research. 2012;37(4):279–85. doi: 10.3109/02713683.2011.626911. [DOI] [PubMed] [Google Scholar]
- 42.Barabino S, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Investigative ophthalmology & visual science. 2004;45(6):1641–6. doi: 10.1167/iovs.03-1055. [DOI] [PubMed] [Google Scholar]
- 43.Schrader S, Mircheff AK, Geerling G. Animal models of dry eye. Developments in ophthalmology. 2008;41:298–312. doi: 10.1159/000131097. [DOI] [PubMed] [Google Scholar]
- 44.Correa-Perez ME, Lopez-Miguel A, Miranda-Anta S, Iglesias-Cortinas D, Alio JL, Maldonado MJ. Precision of high definition spectral-domain optical coherence tomography for measuring central corneal thickness. Investigative ophthalmology & visual science. 2012;53(4):1752–7. doi: 10.1167/iovs.11-9033. [DOI] [PubMed] [Google Scholar]
- 45.Vollmer L, Sowka J, Pizzimenti J, Yu X. Central corneal thickness measurements obtained with anterior segment spectral domain optical coherence tomography compared to ultrasound pachymetry in healthy subjects. Optometry. 2012;83(5):167–72. [PubMed] [Google Scholar]
- 46.Ishibazawa A, Igarashi S, Hanada K, Nagaoka T, Ishiko S, Ito H, et al. Central corneal thickness measurements with Fourier-domain optical coherence tomography versus ultrasonic pachymetry and rotating Scheimpflug camera. Cornea. 2011;30(6):615–9. doi: 10.1097/ICO.0b013e3181d00800. [DOI] [PubMed] [Google Scholar]
- 47.Gorig C, Varghese T, Stiles T, van den Broek J, Zagzebski JA, Murphy CJ. Evaluation of acoustic wave propagation velocities in the ocular lens and vitreous tissues of pigs, dogs, and rabbits. American journal of veterinary research. 2006;67(2):288–95. doi: 10.2460/ajvr.67.2.288. [DOI] [PubMed] [Google Scholar]
- 48.Schiffer SP, Rantanen NW, Leary GA, Bryan GM. Biometric study of the canine eye, using A-mode ultrasonography. American journal of veterinary research. 1982;43(5):826–30. [PubMed] [Google Scholar]
- 49.Silverman RH, Patel MS, Gal O, Sarup A, Deobhakta A, Dababneh H, et al. Effect of corneal hydration on ultrasound velocity and backscatter. Ultrasound in medicine & biology. 2009;35(5):839–46. doi: 10.1016/j.ultrasmedbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang J, Liu, Jun Variance of Speed of Sound and Correlation with Acoustic Impedance in Canine Corneas. Ultrasound in medicine & biology. 2001;37(10):1714–21. doi: 10.1016/j.ultrasmedbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Chan T, Payor S, Holden BA. Corneal thickness profiles in rabbits using an ultrasonic pachometer. Investigative ophthalmology & visual science. 1983;24(10):1408–10. [PubMed] [Google Scholar]
- 52.Sarvazyan AP, Urban MW, Greenleaf JF. Acoustic waves in medical imaging and diagnostics. Ultrasound in medicine & biology. 2013;39(7):1133–46. doi: 10.1016/j.ultrasmedbio.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haque S, Jones L, Simpson T. Thickness mapping of the cornea and epithelium using optical coherence tomography. Optometry and vision science : official publication of the American Academy of Optometry. 2008;85(10):E963–76. doi: 10.1097/OPX.0b013e318188892c. [DOI] [PubMed] [Google Scholar]
- 54.Rufer F, Schroder A, Bader C, Erb C. Age-related changes in central and peripheral corneal thickness: determination of normal values with the Orbscan II topography system. Cornea. 2007;26(1):1–5. doi: 10.1097/01.ico.0000240095.95067.3f. [DOI] [PubMed] [Google Scholar]