Abstract
Chronic caffeine exerts negligible effects on learning and memory in normal adults, but it is unknown whether this is also true for children and adolescents. The hippocampus, a brain region important for learning and memory, undergoes extensive structural and functional modifications during pre-adolescence and adolescence. As a result, chronic caffeine may have differential effects on hippocampus-dependent learning in pre-adolescents and adolescents compared with adults. Here, we characterized the effects of chronic caffeine and withdrawal from chronic caffeine on hippocampus-dependent (contextual) and hippocampus-independent (cued) fear conditioning in pre-adolescent, adolescent, and adult mice. The results indicate that chronic exposure to caffeine during pre-adolescence and adolescence enhances or impairs contextual conditioning depending on concentration, yet has no effect on cued conditioning. In contrast, withdrawal from chronic caffeine impairs contextual conditioning in pre-adolescent mice only. No changes in learning were seen for adult mice for either the chronic caffeine or withdrawal conditions. These findings support the hypothesis that chronic exposure to caffeine during pre-adolescence and adolescence can alter learning and memory and as changes were only seen in hippocampus-dependent learning, this suggests that the developing hippocampus may be sensitive to the effects of caffeine.
Keywords: caffeine, development, cognition, learning, memory, adenosine
1. Introduction
Caffeine, a non-specific adenosine receptor antagonist (Fredholm et al., 1999), is the only licit psychoactive drug available to minors in all 50 United States (Luebbe & Bell, 2009). Approximately 75-95% of children and adolescents consume caffeine on a regular basis (Frary, Johnson, & Wang, 2005; James, Kristjánsson, & Sigfúsdóttir, 2011). In addition, the number of young people who consume caffeine and the quantities consumed are increasing (Temple, 2009) and yet there is limited information on how caffeine affects the pre-adolescent and adolescent brain (Temple, 2009).
Chronic caffeine has been shown to reverse cognitive deficits induced by aging, sleep deprivation, and in animal models of disrupted cognition (e.g. Alzheimer's disease models and attention deficit disorder models) (for review see Cunha & Agostinho, 2010), but chronic caffeine does not appear to affect learning and memory in normal adult humans (Herz, 1999; Koppelstaetter et al., 2008; Warburton, 1995) or rodents (Alhaider, Aleisa, Tran, Alzoubi, & Alkadhi, 2010; Alzoubi et al., 2013; Corodimas, Stieg, & Pruitt, 2000). Likewise, although withdrawal from chronic caffeine can produce negative affective states (e.g., irritability) and have adverse effects on cognition (e.g., difficulty concentrating; see Juliano & Griffiths, 2004 for a review of caffeine withdrawal), withdrawal has little effect on memory in adults (Addicott & Laurienti, 2009; Comer, Haney, Foltin, & Fischman, 1997; Lane & Phillips-Bute, 1998). Thus, in adults, chronic caffeine has limited effects on learning and memory, and withdrawal from chronic caffeine alters cognition but leaves memory processes largely intact.
The effects of chronic caffeine and withdrawal from chronic caffeine on learning and memory during pre-adolescence and adolescence are not well understood. Habitual caffeine consumption by adolescents is associated with attention deficit hyperactivity disorder (ADHD)- like symptoms (Dosh et al., 2010). In addition, children who habitually consume caffeine show impaired performance on tests of attention 24 h after caffeine cessation (Bernstein et al., 1998) and negative academic outcomes (James et al., 2011). Thus, caffeine consumption during childhood may impair attention and/or produce deficits in learning and memory. However, few studies have attempted to address the causal relationship between exposure to chronic caffeine during pre-adolescence and adolescence and changes in learning and memory.
Exposing rats to caffeine during pre-adolescence (between postnatal days 25 and 38) impairs discriminatory ability in the novel object recognition task in adulthood (i.e. when animals are 63-70 days old) (Pires, Pamplona, Pandolfo, Prediger, & Takahashi, 2010), yet continuing caffeine treatment throughout pre-adolescence, adolescence, and adulthood (between postnatal days 21 and 90) enhances novel object recognition memory when the rats are tested while remaining on caffeine (Abreu, Silva-Oliveira, Moraes, Pereira, & Moraes-Santos, 2011). Furthermore, Ardais and colleagues (2014) demonstrated that exposure to chronic caffeine during adolescence enhances novel object recognition when animals are tested with caffeine on board later in adolescence. This raises the possibility that pre-adolescents and adolescents are sensitive to the effects of chronic caffeine on learning, but the reason for the opposite effects in the different studies is unclear.
Although findings imply that caffeine has age-dependent effects on learning and memory, the specific memory systems modulated by caffeine exposure during different developmental periods are not well understood. The hippocampus undergoes structural and functional changes during adolescence (Pokorný & Yamamoto, 1981; Seress & Ribak, 1995; Zehr, Nichols, Schulz, & Sisk, 2008; Pyapali, Turner, Wilson, & Swartzwelder, 1999; Swartzwelder, Wilson, & Tayyeb, 1995a, 1995b; White & Swartzwelder, 2004). In addition, the adolescent hippocampus and associated learning processes are sensitive to nicotine. These considerations led us to hypothesize that chronic caffeine exposure would have particularly marked effects on learning mediated by the hippocampus in adolescent animals.
Chronic exposure to other psychostimulants has a more pronounced effect on hippocampus-dependent conditioning in pre-adolescent and adolescent animals than adults. For example, Portugal and colleagues (2012) demonstrated that exposing mice to chronic nicotine during pre-adolescence and adolescence enhanced contextual fear conditioning, yet exposure to chronic nicotine in adulthood had no effect on contextual conditioning. Furthermore, in the same study, adolescent mice showed deficits in contextual conditioning when withdrawn from a dose of chronic nicotine that did not produce deficits in adult mice. Chronic nicotine had no effect on cued conditioning in any age group. Given that the hippocampus is essential for contextual fear conditioning in rodents, but not cued fear conditioning (Phillips & LeDoux, 1992; Rudy, 1993; Kim & Fanselow, 1992; Logue, Paylor, & Wehner, 1997), these results suggest that hippocampus-dependent memory systems are more vulnerable to psychostimulant exposure during pre-adolescence and adolescence than in adulthood.
In sum, it is unclear if exposure to chronic caffeine during pre-adolescence or adolescence specifically affects hippocampus-dependent learning and memory as opposed to learning and memory in general. Thus, our goal here was to investigate the age-dependent effects of chronic caffeine and withdrawal from chronic caffeine on contextual and cued fear conditioning. We hypothesized that chronic caffeine would enhance contextual conditioning in pre-adolescent and adolescent mice, but not adult mice due to previous findings that chronic exposure to caffeine starting in pre-adolescence and continuing through testing enhances novel object recognition (Abreu et al., 2011; Ardais et al., 2014), and that chronic exposure to the psychostimulant nicotine enhances contextual conditioning in pre-adolescent mice, but not adult mice (Portugal et al., 2012). In addition, we hypothesized that withdrawal from chronic caffeine would impair contextual conditioning in pre-adolescent and adolescent mice because the developing hippocampus could be more sensitive to withdrawal effects. Finally, we investigated the age-dependent effects of chronic caffeine on anxiety-related behavior as a control for changes in anxiety because chronic caffeine increases anxiety-related behavior in adolescent rats (Ardais et al., 2014) and anxiety can influence the expression of fear (Davis, Walker, & Lee, 1997; Helmstetter, 1993).
2. Methods
2.1. Subjects
Male C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME). C57BL/6J mice were selected because robust fear conditioning has been demonstrated in this strain (Balogh & Wehner, 2003; Bolivar, Pooler, & Flaherty, 2001). Housing rooms were illuminated on a 12:12 h light/dark cycle with lights on at 7:00 AM. Mice received ad libitum food and water. Mice were housed 2 per cage. All behavioral procedures were performed between 11:00 AM and 5:00 PM. The experiments were approved by the Temple University Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Mice arrived one week prior to the start of experiments to acclimate to the colony room. On the day of arrival, pre-adolescent mice were postnatal day (PND) 16, adolescent mice were PND 31, and adult mice were PND 64. PND 16 were shipped with Dams and weaned at PND 21. At the start of all experiments, pre-adolescent mice were PND 21, adolescent mice were PND 38, and adult mice were PND 70. The neurodevelopmental and behavioral changes that occur in humans are analogous to those that occur in rodents (Schneider, 2013; Spear, 2000). For example, at approximately PND 30, male mice exhibit signs of puberty, increased risk-seeking, impulsivity, and reward sensitivity, and begin exhibiting social behavior that is more characteristic of adult mice (Schneider, 2013; Terranova, Laviola, de Acetis, & Alleva, 1998). In mice, early adolescence occurs between PND 21 and 28, middle-adolescence occurs between PND 34 and 46, late adolescence occurs between PND 46 and 59, and adulthood has been described as PND 60 and beyond (Hefner & Holmes, 2007; Laviola, Macrı, Morley-Fletcher, & Adriani, 2003; Spear, 2000).
2.2. Apparatus
2.2.1. Fear conditioning
Training and testing of contextual fear conditioning occurred in four identical chambers (17.78 cm × 19.05 cm × 38.10 cm) that were housed in sound attenuating boxes (Med-Associates, St. Albans, VT). The front and back walls of the training chambers were constructed from Plexiglas and the side walls were aluminum. The bottoms of the training chambers were composed of stainless steel grid floors (rods were 2 mm in diameter and spaced 1 cm apart) connected to a scrambled shock generator. Background noise (69 dB) during training and testing was provided by ventilation fans. Stimulus administration during training and testing was controlled by a computer running Med-PC software. Cued fear conditioning was tested in an altered context, which consisted of four identical chambers with different dimensions than the training context (20.32 cm × 22.86 cm × 17.78 cm). The front and back walls of the altered context chamber were constructed from Plexiglas, the side walls were constructed from aluminum, and the floors were constructed from smooth plastic. The altered context chambers were located in a different room from the training chambers. A vanilla scent was used to further distinguish the altered context from the training context. Thus, the altered context chambers differed from the training chambers in size, construction, tactile cues, visual cues, and olfactory cues. The training and testing chambers used in the present study were previously described by Davis and colleagues (2005).
2.2.2. Zero maze
The zero maze was constructed of white Plexiglas and consisted of a 5.5 cm wide circular track with an inside diameter of 34 cm, a mid-track circumference of 121 cm, and an elevation of 40 cm (Tarantino, Gould, Druhan, & Bucan, 2000). The maze has two open quadrants with a raised 2 mm edge and two closed quadrants with walls 11 cm high. Lighting was held at 40 lux. White noise (69 dB) was generated by a fan in the room.
2.3. Behavioral Procedures
2.3.1. Fear Conditioning
The procedure for fear conditioning has been used in previous studies (Davis, James, Siegel, & Gould, 2005; Gould & Higgins, 2003). Training was initiated by placing mice in the training context and activating the house lights. Baseline freezing was then scored for 120 s. Then, a 30 s white noise conditioned stimulus (CS, 85 dB) was activated that co-terminated with a 2 s 0.62 mA footshock unconditioned stimulus (US). After 120 s, a second CS-US pairing was administered. The end of the training session consisted of a 30 s interval that terminated when the house lights turned off. The training session lasted a total of 5.5 min.
A separate group of mice was also trained using a reduced conditioning protocol as a control. They were trained with one 15 s white noise CS that co-terminated with a 2 s 0.62 mA footshock US as was described previously (Gould et al., 2004). Freezing was defined as the absence of all movement except respiration (Blanchard & Blanchard, 1969) and was scored using a time-sampling procedure for 1 s every 10 s. Freezing was the dependent variable and the measure of learning and memory for all fear conditioning experiments.
Contextual fear conditioning was tested 24 h after training by returning mice to the training chambers, activating the house lights, and scoring freezing for 5 min. Cued fear conditioning was tested 1 h after contextual fear testing by placing mice in the altered context, activating the house lights, and scoring freezing for 6 min. During the first 3 min, freezing was scored in the absence of the CS. During the last 3 min, freezing was scored in the presence of the CS. At the end of the 6 min session, the house lights were turned off. A solution of 70% ethanol was used to clean the conditioning chambers after training and testing.
2.3.2. Shock sensitivity
Mice were tested for shock sensitivity in the same chambers used for fear conditioning, as described in previous studies (Gulick & Gould, 2009; Kenney, Wilkinson, & Gould, 2010). In brief, after 3 min of acclimation to the chambers, mice were exposed to a range of 2 s foot shocks (0.10-0.80 mA), which escalated by 0.10 mA over a testing period that lasted approximately 20 min. There were three presentations at each shock intensity, with a 20 s inter-stimulus interval and a 90 s inter-trial interval. Movement was scored during each shock presentation (0 = no response; 1 = hop; 2 = jump; 3 = run; 4 = horizontal jump; 5 = vertical jump) as described previously (Gulick & Gould, 2009). A solution of 70% ethanol was used to clean the chambers after the end of each session.
2.3.3. Zero Maze
The effects of chronic caffeine on anxiety-related behavior were tested using a zero maze. Mice were placed in a closed quadrant and allowed to explore the zero maze for 5 min. During this time mice were video recorded and the distance traveled was tracked using PanLab Smart software. Videos were manually scored for anxiety-related variables (i.e., time spent in both open and closed quadrants, number of transitions between quadrants, and rearing) as described elsewhere (Shepherd, Grewal, Fletcher, Bill, & Dourish, 1994; Tarantino, Gould, Druhan, & Bucan, 2000). An entry into an arm was defined when the back legs of each mouse had crossed into that quadrant. Rearing was defined as the mouse raising upright on its hind legs. A solution of 70% ethanol was used to clean the zero maze after the end of each session.
2.4. Drug Administration and Experimental Design
For all experiments, caffeine (C0750; Sigma-Aldrich, St. Louis, MO) was dissolved in filtered water and administered through drinking bottles at 0, 1.0, or 3.0 mg/mL. The selection of chronic caffeine concentrations was based on previous work (Dall'Igna et al., 2003; Jaszyna, Gasior, Shoaib, Yasar, & Goldberg, 1998; Rossi et al., 2009). A preliminary dose analysis indicated that higher concentrations of caffeine were not well tolerated. Bottles were changed every 2-3 days as described previously (Boeck et al., 2009; da Silva et al., 2003).
2.4.1. The effects of chronic caffeine on fear conditioning
To investigate the effects of chronic caffeine on fear conditioning, PND 21, 38, and 70 mice were administered 0, 1.0, or 3.0 mg/mL caffeine through drinking bottles for 14 days. Mice were trained in fear conditioning on day 13 of chronic treatment and tested for contextual and cued conditioning on day 14 of chronic treatment (n = 8 per age and treatment group). A separate cohort of PND 21 mice were administered chronic caffeine at 0 or 1.0 mg/mL for 14 days, trained using a reduced 1 CS-US fear conditioning protocol on day 13 of chronic treatment, and tested for contextual and cued conditioning on day 14 of chronic treatment (n = 9-11 per treatment group).
2.4.2. The effects of withdrawal from chronic caffeine on fear conditioning
To examine the effects of withdrawal from chronic caffeine on fear conditioning, PND 21, 38, and 70 mice were administered 0, 1.0, or 3.0 mg/mL caffeine through drinking bottles for 12 days. All bottles were changed and filled with water on day 12. On day 13, the mice were trained in fear conditioning and, on day 14, they were tested for contextual and cued conditioning (n = 8). On day 21, the mice were re-tested for contextual and cued recall. Prior work indicates mice experience caffeine withdrawal at the timepoints examined here (i.e., 24-48 h after cessation of chronic caffeine treatment, see Sukhotina, Zvartau, Danysz, & Bespalov, 2004; Kaplan, Greenblatt, Kent, & Cotreau-Bibbo, 1993).
2.4.3. The effects of chronic caffeine on anxiety-related behavior and shock-sensitivity
To investigate the effects of chronic caffeine on anxiety-related behavior and shock-sensitivity, PND 21, 38, and 70 mice were administered 0, 1.0, or 3.0 mg/mL caffeine through drinking bottles for 14 days. On day 13 of treatment, the mice were tested in the zero maze and on day 14 of treatment they were tested for shock sensitivity (n = 8-12 age and treatment group). As a control for the withdrawal fear conditioning experiments, a separate group of PND 21 mice were administered 0 or 3.0 mg/mL caffeine through drinking bottles for 12 days. Twenty-four hours after treatment cessation (i.e., day 13 of the experiment), the mice were tested in the zero maze and on day 14 they were tested for shock sensitivity (n = 7-8 per treatment group).
2.5 Data Analysis
One-way ANOVAs were used to analyze treatment effects on each fear conditioning measure (i.e., freezing during baseline, context, pre-CS, and CS) and each zero maze measure (i.e., time spent in open quadrants, number of transitions between quadrants, and rearing) when more than two treatment groups were tested. One-way ANOVAs were followed by Dunnett's post-hoc tests using the water-treated group as the reference group when a significant treatment effect was detected. Unpaired t-tests were used to analyze treatment effects on each fear conditioning and zero maze measure when fewer than three treatment groups were tested (i.e., in control experiments). Regression analyses between context freezing and pre-CS freezing scores were run to aid in interpretation of seemingly concomitant changes in context freezing and pre-CS freezing. Two-way (3 treatment groups × 8 shock levels) repeated measures ANOVAs were used to analyze the effect of treatment on shock sensitivity within each age group. Two-way ANOVAs were followed by Bonferonni post-hoc tests when a significant interaction was detected, or planned t-tests when a significant main effect was detected. Excel was used to run regression analyses and Graph Pad Prism 5.0F (GraphPad, San Diego, CA, USA) was used for all other statistical analyses.
3. Results
3.1. The age-dependent effects of chronic caffeine on fear conditioning
The effects of exposure to caffeine (at 0, 1.0, or 3.0 mg/mL) for 14 days on hippocampus-dependent (contextual) and hippocampus-independent (cued) fear conditioning (Phillips & LeDoux 1992; Logue, Paylor, & Wehner, 1997; Kim and Fanselow, 1992) were investigated in 3 age groups of mice (Figure 1). A significant effect of caffeine treatment was found in pre-adolescent and adolescent mice for contextual conditioning (pre-adolescent: [F(2, 21) = 38.49, p < 0.001]; adolescent: [F(2, 21) = 19.35, p < 0.001]). Post-hoc tests revealed that pre-adolescent and adolescent mice exposed to 1.0 mg/mL chronic caffeine exhibited enhanced contextual conditioning relative to water-treated controls within each respective age group (p < 0.05). In addition, pre-adolescent and adolescent mice exposed to 3.0 mg/mL caffeine exhibited decreased contextual conditioning relative to water-treated controls within each respective age group (p < 0.05). No significant effect of treatment on contextual conditioning was found in adult mice [F(2, 21) = 1.94, p > 0.05]. Thus, chronic caffeine has concentration-dependent effects on contextual conditioning in pre-adolescent and adolescent mice, but not adult mice.
Fig. 1.
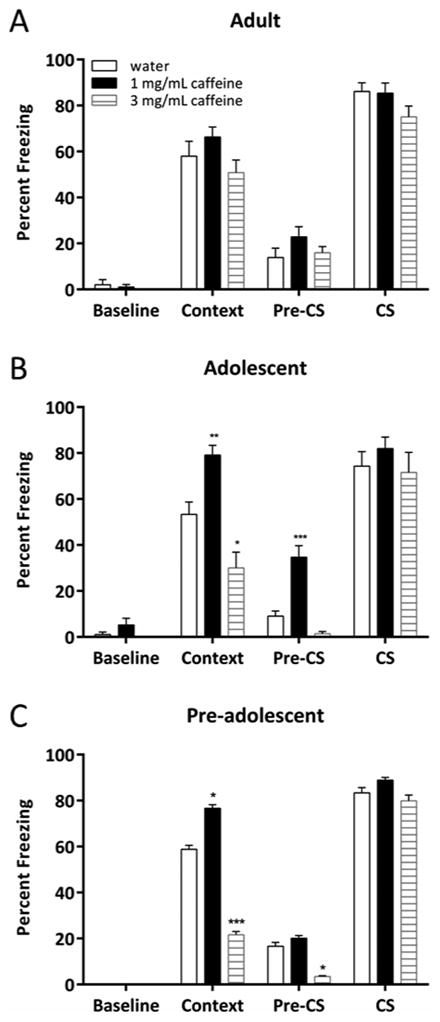
The age-dependent effects of chronic caffeine on fear conditioning (n = 8). No concentration of chronic caffeine tested affected baseline or CS freezing in any age group (A-C). In adolescent and pre-adolescent mice only, chronic caffeine at 1.0 mg/mL enhanced contextual fear conditioning, yet chronic caffeine at 3.0 mg/mL produced deficits in contextual fear conditioning (B and C). Chronic caffeine at 1.0 mg/mL increased pre-CS freezing in adolescent mice only (B) and chronic caffeine at 3.0 mg/mL decreased pre-CS freezing in pre-adolescent mice only (C). Error bars indicate SEM, (*) indicates p < 0.05, (**) indicates p < 0.01, and (***) indicates p < 0.001 compared to water treated mice from each respective age group.
Caffeine induced changes in pre-CS freezing that were age-dependent as well. Although caffeine had no effect on pre-CS freezing in adult mice [F(2, 21) = 1.57, p > 0.05], caffeine altered pre-CS freezing in pre-adolescent [F(2, 21) = 7.16, p < 0.01] and adolescent mice [F(2, 21) = 29.72, p < 0.001]. Post-hoc tests revealed that pre-adolescent mice treated with chronic caffeine at 3.0 mg/mL froze less in the altered context during the pre-CS period compared with water-treated control mice. In contrast, adolescent mice treated with chronic caffeine at 1.0 mg/mL froze more in the altered context during the pre-CS period compared with water-treated control mice (all p's < 0.05). A significant association was found between context freezing and pre-CS freezing in pre-adolescent mice treated with chronic caffeine at 1.0 mg/mL (r = 0.76, p < 0.05). However, there was no correlation between context freezing and pre-CS freezing in pre-adolescent mice treated with chronic caffeine at 3.0 mg/mL (r = 0.41, p > 0.05), adolescent mice treated with chronic caffeine at 1.0 mg/mL (r = 0.53, p > 0.05), or adolescent mice treated with chronic caffeine at 3.0 mg/mL (r = 0.11, p > 0.05).
No significant effects of caffeine on baseline freezing or cued fear conditioning were observed in any age group (adult baseline: [F(2, 21) = 0.53, p > 0.05]; adolescent baseline: [F(2, 21) = 2.38, p > 0.05]; adult cued: [F(2, 21) = 2.10, p > 0.05]; adolescent cued: [F(2, 21) = 0.62, p > 0.05]; pre-adolescent cued: [F(2, 21) = 0.61, p > 0.05]), which suggests that treatment with chronic caffeine had no effect on non-specific freezing behavior or hippocampus-independent conditioning.
3.2. The effect of decreasing the level of conditioning on chronic caffeine-induced enhancement of contextual conditioning vs. cued conditioning
We next sought to determine if the lack of effect of chronic caffeine on cued conditioning was due to a ceiling effect (Figure 2). Thus, we conditioned pre-adolescent mice with a single CS-US pairing and a shorter duration CS to reduce levels of conditioned freezing. Pre-adolescent mice treated with chronic caffeine at 1.0 mg/mL showed enhanced contextual conditioning [t(18) = 2.70, p < 0.05], yet decreasing the number of CS-US pairings and the duration of the CS did not result in caffeine-enhanced cued conditioning [t(18) = 0.64, p > 0.05]. In addition, chronic caffeine had no effect on pre-CS freezing [t(18) = 1.66, p > 0.05]. Therefore, the lack of a treatment effect on cued conditioning was not due to a ceiling effect.
Fig. 2.
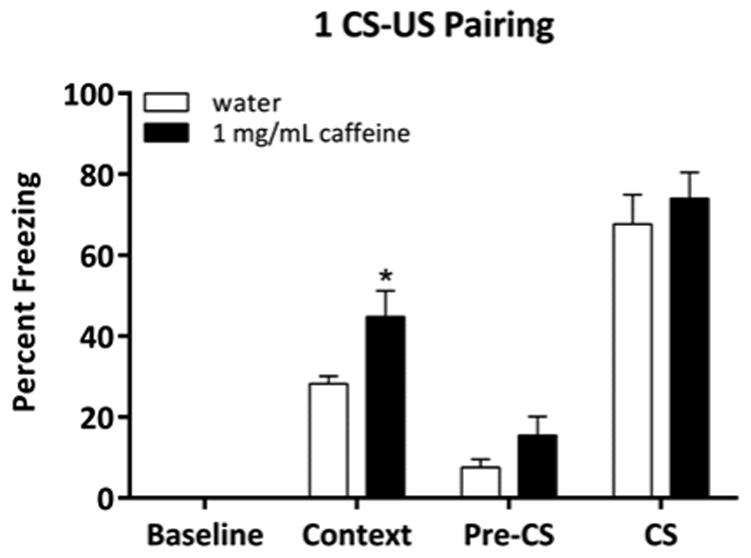
The effect of chronic caffeine on hippocampus-dependent (contextual) and -independent (cued) conditioning using a reduced fear conditioning protocol (n = 9-11). Chronic caffeine at 1.0 mg/mL had no effect on pre-CS or CS freezing in pre-adolescent mice using a reduced conditioning protocol. In contrast, caffeine at 1.0 mg/mL enhanced hippocampus-dependent contextual conditioning using the same protocol. Error bars indicate SEM, (*) indicates p < 0.05 compared with water treated mice.
3.3. Caffeine and shock sensitivity
To rule out potential non-specific effects of chronic caffeine treatment on the response to shock, shock sensitivity during chronic caffeine treatment was tested in all age groups. A significant main effect of shock level was found within each age group (adult: [F(7, 154) = 155.8, p < 0.0001]; adolescent: [F(7, 147) = 171.8, p < 0.0001]; pre-adolescent: [F(7, 147) = 136.4, p < 0.0001] (data not shown). In addition, a significant main effect of treatment was observed in adult [F(2, 154) = 4.60, p < 0.05], but not adolescent [F(2, 147) = 3.38, p > 0.05] or pre-adolescent [F(2, 147) = 2.60, p > 0.05] mice. Planned comparisons revealed that adult mice treated with chronic caffeine at 1.0 mg/mL showed an increased response to shock compared with adult mice treated with chronic caffeine at 3.0 mg/mL [t(134) = 2.55, p < 0.05]; however, neither caffeine treated group was significantly different from the control group that consumed water (water vs. 1.0 mg/mL: [t(126) = 1.05, p > 0.05]; water vs. 3.0 mg/mL: [t(134) = 1.47, p > 0.05]). Given the lack of shock × treatment interaction for all age groups, and the lack of a significant difference observed between caffeine and water treated adult mice, it was concluded that the observed chronic caffeine-induced changes in fear conditioning could not be attributed to differences in response to shock.
3.4. The age-dependent effects of withdrawal from chronic caffeine on fear conditioning
To explore the age-dependent effects of withdrawal from chronic caffeine on contextual and cued conditioning, pre-adolescent, adolescent, and adult mice were treated with chronic caffeine for 12 days and withdrawn from treatment 24 h prior to fear conditioning training (Figure 3A-C). A significant effect of treatment on contextual conditioning in pre-adolescent mice was found [F(2, 21) = 20.07, p < 0.001]. Post-hoc tests revealed that withdrawal from chronic caffeine at both 1.0 mg/mL and 3.0 mg/mL produced deficits in contextual conditioning in pre-adolescent mice (all p's < 0.05). A control experiment conducted on a separate group of pre-adolescent mice found that withdrawal from chronic caffeine at 3.0 mg/mL had no effect on shock sensitivity (main effect of treatment: [F(1, 13) = 2.39, p > 0.05]; main effect of shock level [F(7, 91) = 85.16, p < 0.0001]) (data not shown). No other significant effects of withdrawal from chronic caffeine were observed for any other fear conditioning measure in any age group (adult context: [F(2, 21) = 2.42, p > 0.05]; adolescent context:[F(2, 21) = 1.79, p > 0.05], adult pre-CS:[F(2, 21) = 2.96, p > 0.05]; adolescent pre-CS:[F(2, 21) = 0.77, p > 0.05]; pre-adolescent pre-CS:[F(2, 21) = 0.28, p > 0.05]); adult CS: [F(2, 21) = 0.04, p > 0.05]; adolescent CS: [F(2, 21) = 2.27, p > 0.05]; pre-adolescent CS: [F(2, 21) = 2.48, p > 0.05]). Thus, withdrawal from chronic caffeine disrupted contextual conditioning in pre-adolescent mice, but had no effect on contextual conditioning in adolescent or adult mice.
Fig. 3.
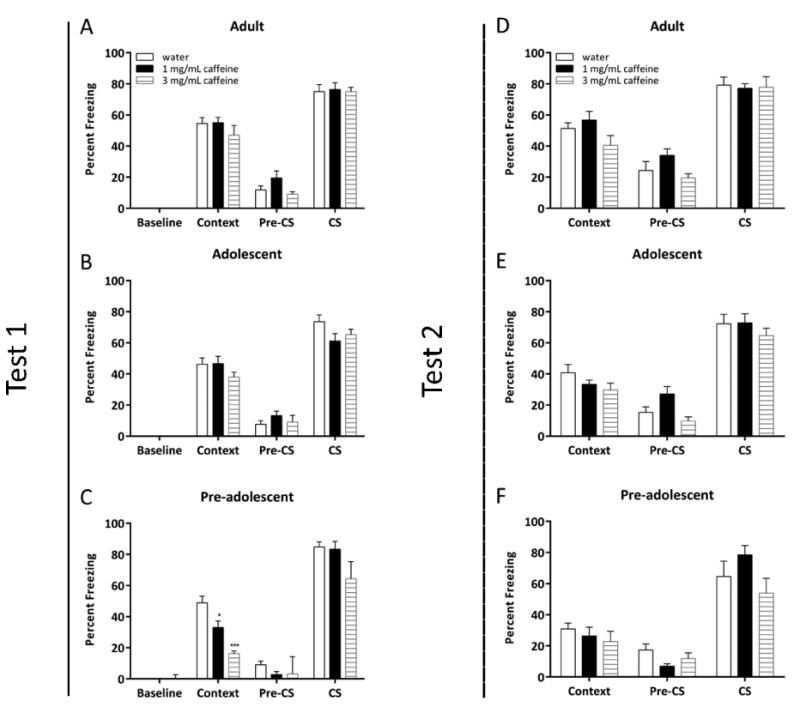
The age-dependent effects of withdrawal from chronic caffeine on fear conditioning (n = 8). Withdrawal from chronic caffeine had no effect on baseline, context, pre-CS, or CS freezing in adolescent or adult mice (A and B). Pre-adolescent mice withdrawn from chronic caffeine at both 1.0 mg/mL and 3.0 mg/mL showed deficits in contextual fear conditioning, but not cued fear conditioning (C). Withdrawal had no effect on recall at Test 2 in any age group (D-F). Error bars indicate SEM, (*) indicates p < 0.05 and (***) indicates p < 0.001 compared to water treated mice from each respective age group.
To investigate the effects of withdrawal from chronic caffeine on later recall of a fear memory acquired during withdrawal, mice were retested (Test 2) for contextual and cued memory 7 days after their first test (Test 1). One-way ANOVAs did not reveal any significant differences in recall of contextual or cued conditioning between treatment groups within each age group (adult context: [F(2, 21) = 2.42, p > 0.05]; adolescent context: [F(2, 21) = 1.79, p > 0.05]; pre-adolescent context: [F(2, 21) = 0.54, p .0.05]; adult cued: [F(2, 21) = 0.04, p > 0.05]; adolescent cued: F(2, 21) = 0.69, p > 0.05]; pre-adolescent cued: [F(2, 21) = 2.78, p > 0.05]) (Figure 3D-F). A significant effect of treatment on pre-CS freezing was observed in adolescent mice [F (2, 21) = 5.52, p < 0.05]; however, post-hoc tests were n.s. (p > 0.05).
3.5. The age-dependent effects of chronic caffeine on anxiety-related behavior in the zero maze
To examine the age-dependent effects of caffeine on anxiety-related behavior, the time spent in the open quadrants of the zero maze was analyzed in pre-adolescent, adolescent, and adult mice treated with chronic caffeine for 13 days (at 0, 1.0, or 3.0 mg/mL) (Figure 4A). A significant effect of treatment on time spent in the open quadrants was found in pre-adolescent mice [F (2, 32) = 4.14, p < 0.05]. Post-hoc tests revealed that pre-adolescent mice treated with chronic caffeine at 3.0 mg/mL spent more time in the open quadrants of the zero maze (all p's < 0.05). A significant effect of treatment on time spent in open quadrants was found in adolescent mice [F (2, 32) = 4.63, p < 0.05], but post-hoc tests were n.s. (p's > 0.05). There was no effect of treatment on time spent in open quadrants in adult mice [F (2, 32) = 0.75, p > 0.05]. Thus, chronic caffeine at 3.0 mg/mL decreased anxiety-related behavior in pre-adolescent mice, yet had no effect on anxiety-related behavior in adult mice. In contrast, a control experiment conducted on a separate group of pre-adolescent mice found that withdrawal from chronic caffeine at 3.0 mg/mL had no effect on time spent in open quadrants [t(13) = 0.88, p > 0.05] (data not shown).
Fig. 4.
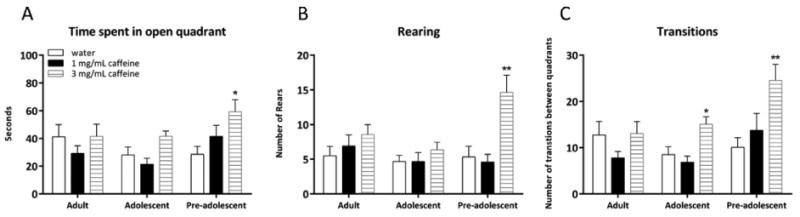
The age-dependent effects of chronic caffeine on anxiety-related behavior in the zero maze. Caffeine treatment altered the time spent in the open quadrant of the zero maze in adolescent and pre-adolescent mice, but not adult mice (A). Chronic caffeine at 3.0 mg/mL increased rearing in pre-adolescent mice, and increased transitions in both adolescent and pre-adolescent mice, but not adult mice (B and C). Error bars indicate SEM, (*) indicates p < 0.05, (**) indicated p < 0.01 compared with water treated mice from each respective age group.
The effect of treatment on rearing and transitions between quadrants was examined in the zero maze as well (see Tarantino et al., 2000) (Figure 4B and C). There was a significant effect of treatment on rearing in pre-adolescent mice [F(2, 32) = 9.72, p < 0.001], but not adolescent [F(2, 32) = 0.75, p > 0.05] or adult mice [F(2, 32) = 1.14, p > 0.05]. Post-hoc tests revealed that pre-adolescent mice treated with chronic caffeine at 3.0 mg/mL reared significantly more than the water-treated control mice (p < 0.05). In addition, a significant effect of treatment on transitions was found in both pre-adolescent and adolescent mice (pre-adolescent: [F(2, 32) = 5.60, p < 0.05]; adolescent: [F(2, 32) = 7.57, p < 0.001]), but not adult mice [F(2, 32) = 1.43, p > 0.05]. Post-hoc tests revealed that pre-adolescent and adolescent mice treated with chronic caffeine at 3.0 mg/mL were more active in the zero maze than water treated control mice (all p's < 0.05). Together, the zero maze results indicate that chronic caffeine at 3.0 mg/mL decreases anxiety-related behavior and/or increases activity in pre-adolescent mice, and that chronic caffeine increases activity in adolescent mice. Finally, pre-adolescent mice treated with chronic caffeine at 3.0 mg/mL displayed increased rearing whereas adolescent mice did not, suggesting that caffeine-induced changes in behavior differ between these age groups. A separate group of pre-adolescent mice that were withdrawn from chronic caffeine at 3.0 mg/mL displayed behavior in the zero maze that was comparable to age-matched water-treated control mice (rearing: [t(13) = 1.33, p > 0.05]; transitions [t(13) = 0.52, p > 0.05]) (data not shown), which suggests cessation of chronic caffeine returns anxiety-related behavior and/or activity to water-treated control levels.
4. Discussion
In the present study, the effects of chronic caffeine and withdrawal from chronic caffeine on hippocampus-dependent (contextual) and hippocampus-independent (cued) fear conditioning were investigated in pre-adolescent, adolescent, and adult mice. Chronic caffeine had concentration-dependent effects on contextual conditioning in pre-adolescent and adolescent mice, but not adult mice. Specifically, chronic caffeine at 1.0 mg/mL enhanced contextual conditioning in pre-adolescent and adolescent mice, whereas chronic caffeine at 3.0 mg/mL produced deficits in contextual conditioning in pre-adolescent and adolescent mice. In contrast, withdrawal from both concentrations of chronic caffeine impaired contextual conditioning in pre-adolescent, but not adolescent or adult mice. Cued conditioning was not affected by chronic caffeine or withdrawal from chronic caffeine in any age group. Together, our results demonstrate that chronic caffeine and withdrawal from chronic caffeine have age-dependent effects on hippocampus-dependent learning but not on hippocampus-independent learning. This difference in the effects of caffeine on cued versus contextual fear conditioning is consistent with the hypothesis that caffeine alters the neural circuitry underlying contextual fear conditioning differently than the neural circuitry underlying cued fear conditioning. It is unlikely that the lack of effect of chronic caffeine on cued conditioning was due to a ceiling effect, because freezing to the CS was not altered by caffeine following training that produced lower levels of conditioning (i.e., a one shock presentation). Thus, our data suggest that chronic caffeine may be acting on the hippocampus, or afferent or efferent structures that modulate hippocampal function, to alter contextual conditioning in pre-adolescent and adolescent mice.
There are several explanations for why hippocampus-dependent learning and memory may be more affected by caffeine exposure during pre-adolescence and adolescence compared with adulthood. First, the hippocampus undergoes structural (Pokorný & Yamamoto, 1981; Seress & Ribak, 1995; Zehr, Nichols, Schulz, & Sisk, 2008) and functional (Pyapali, Turner, Wilson, & Swartzwelder, 1999; Swartzwelder, Wilson, & Tayyeb, 1995a, 1995b; White & Swartzwelder, 2004) modifications during adolescence. Second, caffeine is a non-specific adenosine receptor antagonist (Fredholm et al., 1999), and although the hippocampus expresses high levels of inhibitory adenosine A1 receptors (A1Rs), yet lower levels of facilitatory adenosine A2A receptors (A2ARs) throughout life (Costenla et al., 2010; Cunha et al., 1995; Fastbom et al., 1987; Fredholm et al., 2005; Rosin & Robeva, 1998); the adenosinergic system changes throughout development. For example, between late adolescence (postnatal day 42) and adulthood (postnatal day 180) the density of A1Rs decrease and the density of A2ARs increase in the hippocampus (Cunha et al., 1995). Therefore, caffeine may have age-dependent effects on hippocampal excitability due to age-related differences in adenosine receptor levels. In fact, Costenla and colleagues (2011) found that selective A1R blockade increases LTP magnitude in young adult rats, but has no effect on middle-aged or aged rats. Moreover, selective A2AR blockade attenuated LTP more in old rats than middle-aged and young adult rats. Therefore, age-related differences in adenosine receptor levels could contribute to the effect of caffeine on hippocampal-dependent learning.
The ability of chronic caffeine at 1.0 mg/mL to enhance contextual conditioning in pre-adolescent and adolescent mice may be related to the development of tolerance. Acute caffeine is known to specifically affect hippocampus-dependent learning and memory in adults in a dose-dependent manner. For example, low doses of caffeine (e.g., 0.3 mg/kg) enhance retention and retrieval in the Morris water maze task (Angelucci et al., 2002), and high doses of caffeine (e.g., 30 mg/kg) disrupt acquisition and retrieval of contextual fear conditioning (Corodimas et al., 2000). However, in adults chronic caffeine has no effect on performance in the radial arm water maze (Alzoubi et al., 2013; Alhaider et al., 2010), object recognition (Botton et al., 2010), inhibitory avoidance (Sallaberry et al., 2013), or contextual fear conditioning (Corodimas et al., 2000) when administered in a wide range of doses and for different periods (0.3 mg/mL for 3 months, 0.3 mg/mL for 4 weeks, 10 mg/kg/day for 4 days, 1 mg/mL for 30 days, or through 5-25 mg s.c. pellets for 7 days, respectively). Therefore, it is possible that pre-adolescent and adolescent mice do not develop the same degree of tolerance to the effects of caffeine on hippocampus-dependent learning and memory as adults.
Chronic caffeine at 3.0 mg/mL may exert effects beyond the adenosinergic system to produce deficits in contextual conditioning. In support, higher doses of caffeine that produce higher than average plasma levels of caffeine act on different molecular substrates (e.g. phosphodiesterases and GABAA receptors) than lower doses of caffeine (Fredholm et al., 1999). Furthermore, higher doses of caffeine act on different brain regions and exert different behavioral effects than do lower doses. For example, higher doses of caffeine can produce deficits in cognition and increase glucose utilization in the shell of the nucleus accumbens (Fredholm et al., 1999; Hyman, Malenka, & Nestler, 2006; Kaminer, 2010; Nehlig, 1999; Nehlig, Armspach, & Namer, 2010). Therefore, chronic caffeine at 3.0 mg/mL could disrupt contextual conditioning by acting on the nucleus accumbens and/or the hippocampus. In support, lesions to the nucleus accumbens disrupt contextual fear conditioning, but not cued fear conditioning (Levita, Dalley, & Robbins, 2002). Future studies are needed to determine if chronic caffeine has age-dependent effects on the hippocampus, nucleus accumbens, and/or other brain regions involved in contextual conditioning.
An alternative explanation for the contextual conditioning deficits observed in pre-adolescent and adolescent mice treated with chronic caffeine at 3.0 mg/mL is that caffeine-induced hyperactivity interfered with the freezing response. However, our results suggest caffeine-induced hyperactivity is not responsible for decreased freezing during contextual testing. First, mice treated with chronic caffeine at 3.0 mg/mL did not show decreased freezing during the CS. If hyperactivity caused decreased freezing, we would have expected freezing to be muted for all fear conditioning measures. Second, there was no association between contextual freezing and pre-CS freezing in mice treated with chronic caffeine at 3.0 mg/mL, again suggesting that hyperactivity cannot explain the conditioning deficits we observed.
The effects of withdrawal from chronic caffeine on cognition have received little attention. Therefore, we also examined the effects of withdrawal from chronic caffeine on contextual and cued conditioning in pre-adolescent, adolescent, and adult mice. Withdrawal from chronic caffeine specifically disrupted contextual conditioning in pre-adolescent mice regardless of concentration, yet had no effect on contextual or cued conditioning in adolescent or adult mice. Thus, while pre-adolescent mice show enhanced contextual conditioning during treatment with chronic caffeine at 1.0 mg/mL, they show deficits during withdrawal from the same concentration. We demonstrated that pre-adolescent mice withdrawn from the highest concentration of caffeine tested (i.e., 3.0 mg/mL) display normal behavior in the zero maze and normal response to shock, which supports the conclusion that withdrawal from chronic caffeine during pre-adolescence causes cognitive deficits.
Withdrawal from chronic caffeine is associated with a variety of symptoms in humans including fatigue, decreased alertness, difficulty concentrating, irritability, and depressed mood (Juliano & Griffiths, 2004). In addition, negative symptoms associated with withdrawal from chronic caffeine have been reported in children (Bernstein et al., 1998), adolescents (Bernstein, Carroll, Thuras, Cosgrove, & Roth, 2002), and adults (Juliano & Griffiths, 2004) alike. Therefore, withdrawal from chronic caffeine is associated with negative symptoms in all age groups; however, our data suggest that only pre-adolescent animals show withdrawal-induced learning and memory deficits. Interestingly, Luebbe and colleagues (2011) found that children who experience more intense withdrawal symptoms after caffeine cessation tend to drink more caffeine rather than less. Thus, the findings that chronic caffeine may have positive effects on cognition in pre-adolescent animals, whereas withdrawal from chronic caffeine may have negative effects on cognition in pre-adolescent animals is consistent with the possibility that children may be more vulnerable to developing caffeine dependence than adolescents or adults. Furthermore, the findings that both chronic and withdrawal from chronic caffeine at 3.0 mg/mL impaired contextual conditioning in pre-adolescent mice may suggest that exposure to chronic caffeine at 3.0 mg/mL produces disruptive effects on hippocampus-dependent learning and memory that continue after caffeine cessation.
Again, the effects of caffeine on learning were most likely not due to peripheral effects because we did not find chronic caffeine-induced changes in response to footshock. To determine if chronic caffeine affected anxiety, which could have an effect on unconditioned fear responding (Davis, Walker, & Lee, 1997; Helmstetter, 1993), we analyzed anxiety-related behavior in the zero maze. Chronic caffeine at 1.0 mg/mL did not decrease time spent in the open quadrant of the zero maze, a measure of anxiety, in any age group, suggesting that any change in freezing observed at this dose was not due to changes in shock sensitivity or anxiety.
Although chronic caffeine at 3.0 mg/mL did not produce deficits in cued fear conditioning in pre-adolescent or adolescent mice, it did alter behavior in the zero maze. Specifically, exposure to chronic caffeine at 3.0 mg/mL during adolescence increased transitions in the zero maze, but had no effect on time spent in the open arm, suggesting an increase in activity. In contrast, exposure to chronic caffeine at 3.0 mg/mL during pre-adolescence increased both transitions and rearing, as well as time spent in the open quadrant of the zero maze, suggesting an increase in activity and/or a decrease in anxiety. It should be noted that an increase in exploratory behavior, including increased activity (i.e. increased transitions), in anxiety paradigms has been interpreted as a release of exploratory inhibition (Bailey & Crawley, 2009), and has been observed after animals are treated with anxiolytic drugs (Crawley & Goodwin, 1980). Therefore, exposure to chronic caffeine at 3.0 mg/mL during pre-adolescence and adolescence could increase activity and/or decreases anxiety-related behavior. While it would be reasonable to conclude that increased activity levels may be responsible for the observed contextual conditioning deficits in pre-adolescent and adolescent mice treated with chronic caffeine at 3.0 mg/mL, this treatment did not affect cued conditioning, which suggests that chronic caffeine at 3.0 mg/mL specifically impairs contextual conditioning rather than reducing freezing as a result of an increase in activity levels.
In conclusion, we found age-dependent differences in the effects of chronic caffeine on fear conditioning. Chronic caffeine at 1.0 mg/mL enhanced hippocampus-dependent contextual conditioning in pre-adolescent and adolescent mice. In contrast, chronic caffeine at 3.0 mg/mL impaired hippocampus-dependent contextual conditioning in pre-adolescent and adolescent mice. Furthermore, withdrawal from chronic caffeine at both concentrations tested impaired contextual conditioning in pre-adolescent mice only. No effects of chronic caffeine or withdrawal from chronic caffeine were observed in adult mice. Overall, our results indicate that chronic caffeine and withdrawal from chronic caffeine exert age-dependent effects on hippocampus-dependent learning and memory, but not hippocampus-independent learning and memory. These effects may be due to age-related differences in hippocampus structure and function, the adenosinergic system, or chronic caffeine-induced changes in other brain regions that modulate hippocampus-dependent learning and memory.
-
▪
Chronic caffeine enhances contextual conditioning in adolescent and pre-adolescent mice.
-
▪
Withdrawal from chronic caffeine impairs contextual conditioning in pre-adolescent mice.
-
▪
Chronic caffeine has no effect on contextual conditioning in adult mice.
Acknowledgments
This work was supported by grant from the National Institute on Drug Abuse (NIDA, DA017949 TJG) and the Center for Scholars Fund, College of Liberal Arts Temple University (DB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu RV, Silva-Oliveira EM, Moraes MFD, Pereira GS, Moraes-Santos T. Chronic coffee and caffeine ingestion effects on the cognitive function and antioxidant system of rat brains. Pharmacology, Biochemistry, and Behavior. 2011;99(4):659–64. doi: 10.1016/j.pbb.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Addicott MA, Laurienti PJ. A comparison of the effects of caffeine following abstinence and normal caffeine use. Psychopharmacology. 2009;207(3):423–31. doi: 10.1007/s00213-009-1668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaider IA, Aleisa AM, Tran TT, Alzoubi KH, Alkadhi KA. Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep. 2010;33(4):437–44. doi: 10.1093/sleep/33.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzoubi KH, Abdul-Razzak KK, Khabour OF, Al-Tuweiq GM, Alzubi MA, Alkadhi K. Caffeine prevents cognitive impairment induced by chronic psychosocial stress and/or high fat-high carbohydrate diet. Behavioural Brain Research. 2013;237:7–14. doi: 10.1016/j.bbr.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Angelucci MEM, Cesário C, Hiroi RH, Rosalen PL, Da Cunha C. Effects of caffeine on learning and memory in rats tested in the Morris water maze. Brazilian Journal of Medical and Biological Research. 2002;35(10):1201–8. doi: 10.1590/s0100-879x2002001000013. [DOI] [PubMed] [Google Scholar]
- Ardais AP, Borges MF, da Rocha AS, Sallaberry C, Cunha RA, Porciúncula LO. Caffeine triggers behavioral and neurochemical alterations in adolescent rats. Neuroscience. 2014 Apr 1–13; doi: 10.1016/j.neuroscience.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Bailey K, Crawley J. Methods of Behavior Analysis in Neuroscience. Taylor & Francis Group, LLC; 2009. Anxiety-related behaviors in mice. [Google Scholar]
- Balogh SA, Wehner JM. Inbred mouse strain differences in the establishment of long-term fear memory. Behavioural Brain Research. 2003;140(1-2):97–106. doi: 10.1016/s0166-4328(02)00279-6. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Carroll ME, Dean NW, Crosby RD, Perwien AR, Benowitz NL. Caffeine withdrawal in normal school-age children. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37(8):858–65. doi: 10.1097/00004583-199808000-00016. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Carroll ME, Thuras PD, Cosgrove KP, Roth ME. Caffeine dependence in teenagers. Drug and Alcohol Dependence. 2002;66(1):1–6. doi: 10.1016/s0376-8716(01)00181-8. [DOI] [PubMed] [Google Scholar]
- Blanchard R, Blanchard D. Passive and active reactions to fear-eliciting stimuli. Journal of Comparative and Physiological Psychology. 1969;68(1):129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Boeck CR, Marques VB, Valvassori SS, Constantino LC, Rosa DVF, Lima FF, Quevedo J. Early long-term exposure with caffeine induces cross-sensitization to methylphenidate with involvement of DARPP-32 in adulthood of rats. Neurochemistry International. 2009;55(5):318–22. doi: 10.1016/j.neuint.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Pooler O, Flaherty L. Inbred strain variation in contextual and cued fear conditioning behavior. Mammalian Genome : Official Journal of the International Mammalian Genome Society. 2001;12(8):651–6. doi: 10.1007/s003350020039. [DOI] [PubMed] [Google Scholar]
- Botton PH, Costa MS, Ardais AP, Mioranzza S, Souza DO, Rocha JBT, Porciuncula LO. Caffeine prevents disruption of memory consolidation in the inhibitory avoidance and novel object recognition tasks by scopolamine in adult mice. Behavioral Brain Research. 2010;214:254–259. doi: 10.1016/j.bbr.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Comer SD, Haney M, Foltin RW, Fischman MW. Effects of caffeine withdrawal on humans living in a residential laboratory. Experimental and Clinical Psychopharmacology. 1997;5(4):399–403. doi: 10.1037//1064-1297.5.4.399. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, Stieg JM, Pruitt JC. Acute exposure to caffeine selectively disrupts context conditioning in rats. Psychopharmacology. 2000;152(4):376–382. doi: 10.1007/s002130000557. [DOI] [PubMed] [Google Scholar]
- Costenla AR, Cunha RA, de Mendonça A. Caffeine, adenosine receptors, and synaptic plasticity. Journal of Alzheimer's Disease. 2010;20(Suppl 1):S25–34. doi: 10.3233/JAD-2010-091384. [DOI] [PubMed] [Google Scholar]
- Costenla AR, Diógenes MJ, Canas PM, Rodrigues RJ, Nogueira C, Maroco J, Agostinho PM, Ribeiro JA, Cunha RA, de Mendonça A. Enhanced role of adenosine A(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. The European Journal of Neuroscience. 2011;34(1):12–21. doi: 10.1111/j.1460-9568.2011.07719.x. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin F. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology Biochemistry Behavior. 1980;13(2):167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Agostinho PM. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. Journal of Alzheimer's Disease. 2010;20:S95–116. doi: 10.3233/JAD-2010-1408. [DOI] [PubMed] [Google Scholar]
- Cunha R, Constantino M, Sebastião A, Ribeiro J. Modification of A1 and A2A adenosine receptor binding in aged striatum, hippocampus and cortex of the rat. NeuroReport. 1995;6:1583–88. doi: 10.1097/00001756-199507310-00029. [DOI] [PubMed] [Google Scholar]
- Da Silva RS, Bruno AN, Battastini AMO, Sarkis JJF, Lara DR, Bonan CD. Acute caffeine treatment increases extracellular nucleotide hydrolysis from rat striatal and hippocampal synaptosomes. Neurochemical Research. 2003;28(8):1249–54. doi: 10.1023/a:1024292831762. [DOI] [PubMed] [Google Scholar]
- Dall'Igna OP, Da Silva AL, Dietrich MO, Hoffmann A, de Oliveira RV, Souza DO, Lara DR. Chronic treatment with caffeine blunts the hyperlocomotor but not cognitive effects of the N-methyl-D-aspartate receptor antagonist MK-801 in mice. Psychopharmacology. 2003;166(3):258–63. doi: 10.1007/s00213-002-1362-1. [DOI] [PubMed] [Google Scholar]
- Davis Ja, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2005;25(38):8708–13. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Annal of the New York Academy of Science. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Dosh T, Helmbrecht T. A comparison of the associations of caffeine and cigarette use with depressive and ADHD symptoms in a sample of young adult smokers. Journal of Addiction Medicine. 2010;4(1):52–54. doi: 10.1097/ADM.0b013e3181b508ec.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fastbom J, Pazos A, Probst A, Palacios JM. Adenosine A1 receptors in the human brain: a quantitative autoradiographic study. Neuroscience. 1987;22(3):827–39. doi: 10.1016/0306-4522(87)92962-9. [DOI] [PubMed] [Google Scholar]
- Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. Journal of the American Dietetic Association. 2005;105(1):110–3. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological Reviews. 1999;51(1):83–133. [PubMed] [Google Scholar]
- Fredholm B, Chen J, Cunha R, Svenningsson P, Vaugeois J. Adenosine and brain function. International Review of Neurobiology. 2005;63:192–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Gould T, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiology of Learning and Memory. 2003;80:147–157. doi: 10.1016/S1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behavioral Brain Research. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. The hippocampus and cingulate cortex differentially mediate the effects of nicotine on learning versus on ethanol-induced learning deficits through different effects at nicotinic receptors. Neuropsychopharmacology. 2009;34(9):2167–79. doi: 10.1038/npp.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behavioural Brain Research. 2007;176(2):210–5. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ. Stress-induced hypoalgesia and defensive freezing are attenuated by application of diazepam to the amygdala. Pharmacology Biochemistry Behavior. 1993;44(2):433–8. doi: 10.1016/0091-3057(93)90487-e. [DOI] [PubMed] [Google Scholar]
- Herz R. Caffeine effects on mood and memory. Behaviour Research and Therapy. 1999;37 doi: 10.1016/s0005-7967(98)00190-9. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- James JE, Kristjánsson AL, Sigfúsdóttir ID. Adolescent substance use, sleep, and academic achievement: evidence of harm due to caffeine. Journal of Adolescence. 2011;34(4):665–73. doi: 10.1016/j.adolescence.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Jaszyna M, Gasior M, Shoaib M, Yasar S, Goldberg SR. Behavioral effects of nicotine, amphetamine and cocaine under a fixed-interval schedule of food reinforcement in rats chronically exposed to caffeine. Psychopharmacology. 1998;140(3):257–71. doi: 10.1007/s002130050766. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Griffiths RR. A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology. 2004;176(1):1–29. doi: 10.1007/s00213-004-2000-x. [DOI] [PubMed] [Google Scholar]
- Kaminer Y. Problematic use of energy drinks by adolescents. Child and Adolescent Psychiatric Clinics of North America. 2010;19(3):643–50. doi: 10.1016/j.chc.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Greenblatt DJ, Kent MA, Cotreau-Bibbo MM. Caffeine treatment and withdrawal in mice: relationships between dosage, concentrations, locomotor activity and A1 adenosine receptor binding. Journal of Pharmacology and Experimental Therapeutics. 1993;266(3):1563–72. [PubMed] [Google Scholar]
- Kenney JW, Wilkinson DS, Gould TJ. The enhancement of contextual fear conditioning by ABT-418. Behavioural Pharmacology. 2010;21(3):246–9. doi: 10.1097/FBP.0b013e32833a5b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Fanselow M. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behavioral Neuroscience. 1992;106(4):591–96. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- Koppelstaetter F, Poeppel TD, Siedentopf CM, Ischebeck a, Verius M, Haala I, Krause BJ. Does caffeine modulate verbal working memory processes? An fMRI study. NeuroImage. 2008;39(1):492–9. doi: 10.1016/j.neuroimage.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Lane J, Phillips-Bute B. Caffeine deprivation affects vigilance performance and mood. Physiology & Behavior. 1998;65(1):171–5. doi: 10.1016/s0031-9384(98)00163-2. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macrı S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neuroscience & Biobehavioral Reviews. 2003;27(1-2):19–31. doi: 10.1016/S0149-7634(03)00006-X. [DOI] [PubMed] [Google Scholar]
- Levita L, Dalley JW, Robbins TW. Disruption of Pavlovian contextual conditioning by excitotoxic lesions of the nucleus accumbens core. Behavioral Neuroscience. 2002;116(4):539–552. doi: 10.1037//0735-7044.116.4.539. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behavioral Neuroscience. 1997;111(1):104–13. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Luebbe AM, Bell DJ. Mountain Dew or mountain don't?: a pilot investigation of caffeine use parameters and relations to depression and anxiety symptoms in 5th- and 10th-grade students. The Journal of School Health. 2009;79(8):380–7. doi: 10.1111/j.1746-1561.2009.00424.x. [DOI] [PubMed] [Google Scholar]
- Luebbe A. Child and adolescent anxiety sensitivity, perceived subjective effects of caffeine and caffeine consumption. Journal of Caffeine Research. 2011;1(4) doi: 10.1089/caf.2011.0020. [DOI] [Google Scholar]
- Nehlig A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neuroscience and Biobehavioral Reviews. 1999;23(4):563–76. doi: 10.1016/s0149-7634(98)00050-5. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Armspach J, Namer I. SPECT assessment of brain activation induced by caffeine: no effect on areas involved in dependence. Dialogues in Clinical Neuroscience. 2010:255–263. doi: 10.31887/DCNS.2010.12.2/anehlig. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pires VA, Pamplona FA, Pandolfo P, Prediger RDS, Takahashi RN. Chronic caffeine treatment during prepubertal period confers long-term cognitive benefits in adult spontaneously hypertensive rats (SHR), an animal model of attention deficit hyperactivity disorder (ADHD) Behavioural Brain Research. 2010;215(1):39–44. doi: 10.1016/j.bbr.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Research Bulletin. 1981;7(2):113–20. doi: 10.1016/0361-9230(81)90075-7. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiology of Learning and Memory. 2012;97(4):482–94. doi: 10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyapali G, Turner D, Wilson W, Swartzwelder H. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19(2):107–11. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Rosin D, Robeva A. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. The Journal of Comparative Neurology. 1998;401:163–186. [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Mataluni G, Sacchetti L, Siracusano A, Centonze D. Caffeine drinking potentiates cannabinoid transmission in the striatum: interaction with stress effects. Neuropharmacology. 2009;56(3):590–7. doi: 10.1016/j.neuropharm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Rudy J. Contextual conditioning and auditory cue conditioning dissociate during development. Behavioral Neuroscience. 1993;107(5):887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Sallaberry C, Nunes F, Costa MS, Fioreze GT, Ardais AP, Botton PH, Klaudat B, Forte T, Souza DO, Elisabetsky E. Chronic caffeine prevents changes in inhibitory avoidance memory and hippocampal BDNF immunocontent in middle-aged rats. Neuropharmacology. 2013;64:153–59. doi: 10.1016/j.neuropharm.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Schneider M. Adolescence as a vulnerable period to alter rodent behavior. Cell and Tissue Research. 2013;354(1):99–106. doi: 10.1007/s00441-013-1581-2. [DOI] [PubMed] [Google Scholar]
- Seress L, Ribak C. Postnatal development of CA3 pyramidal neurons and their afferents in the Ammon's horn of rhesus monkeys. Hippocampus. 1995;231:217–31. doi: 10.1002/hipo.450050308. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher a, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology. 1994;116(1):56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sukhotina IA, Zvartau EE, Danysz W, Bespalov AY. Caffeine withdrawal syndrome in social interaction test in mice: effects of the NMDA receptor channel blockers, memantine and neramexane. Behavioral Pharmacology. 2004;15(3):207–14. [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcoholism, Clinical and Experimental Research. 1995a;19(6):1480–5. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcoholism, Clinical and Experimental Research. 1995b;19(2):320–3. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Tarantino L, Gould T, Druhan J, Bucan M. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mammalian Genome. 2000;564:555–564. doi: 10.1007/s003350010107. [DOI] [PubMed] [Google Scholar]
- Temple JL. Caffeine Use in Children: What we know, what we have left to learn, and why we should worry. Neuroscience Biobehavioral Reviews. 2009;33(6):793–806. doi: 10.1016/j.neubiorev.2009.01.001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, de Acetis L, Alleva E. A description of the ontogeny of mouse agonistic behavior. Journal of Comparative Psychology. 1998;112(1):3–12. doi: 10.1037/0735-7036.112.1.3. [DOI] [PubMed] [Google Scholar]
- Warburton DM. Effects of caffeine on cognition and mood without caffeine abstinence. Psychopharmacology. 1995;119(1):66–70. doi: 10.1007/BF02246055. [DOI] [PubMed] [Google Scholar]
- White A, Swartzwelder H. Hippocampal function during adolescence. Annals New York Academy of Sciences. 2004;7:206–20. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Nichols LR, Schulz KM, Sisk CL. Adolescent development of neuron structure in dentate gyrus granule cells of male Syrian hamsters. Developmental Neurobiology. 2008;68(14):1517–26. doi: 10.1002/dneu.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]


