Abstract
Purpose
This cross-sectional study was designed to explore potential factors associated with perceived cognitive impairment (PCI) in breast cancer survivors compared to controls and gain insight into perceived levels of severity for cognitive complaints.
Methods
Women (N=363, 317: breast cancer, 46: healthy controls) completed demographic questionnaire, MD Anderson Symptom Inventory, Attentional Function Index, and Functional Assessment for Cancer Therapy-Cognition. Group classification included pre-chemotherapy, current chemotherapy, and postchemotherapy (≤1, >1–≤2, >2–≤5, >5 years).
Results
A significant group effect was seen for PCI (F6, 355= 7.01, p<0.0001). Controls reported less PCI than all other groups. Neuropathy was inversely correlated with PCI (r= −0.23; p<0.0001) for participants with breast cancer. A significant association was demonstrated between exercise frequency and PCI in women exposed to chemotherapy (F3, 135= 3.78, p<0.05). A multiple linear regression model built using forward selection methods explained 24 % of the variance (adjusted R2) for PCI in breast cancer participants and included group, body mass index (BMI), exercise, fatigue, and distress. Exercise frequency moderated the relationship between BMI and PCI for breast cancer participants (F3, 198=2.4, p= 0.07) and reduced the negative effects of high BMI. The moderating effect of exercise was significant (F3, 133=3.1, p=0.03) when limited to participants exposed to chemotherapy.
Conclusions
PCI decreased for women >5 years postchemotherapy. Overweight survivors who exercised frequently reported less PCI than sedentary survivors. Study results provide support for a relationship between BMI and PCI in breast cancer survivors and exercise as a potential intervention for cognitive complaints. Further investigation of the influence of weight and exercise on cognitive function is warranted.
Keywords: Breast cancer, Cognitive function, Chemotherapy, Neuropathy, Obesity, Exercise patterns
Introduction
Cancer and cancer treatment-related effects on cognitive function are common concerns for breast cancer survivors (National Institute of Health definition of survivorship: from the time of diagnosis through the balance of life) [1]. The majority of breast cancer survivors report some degree of cognitive dysfunction after completion of chemotherapy [2]. A subset of cancer survivors who receive chemotherapy (frequency estimated at 17–34 %) appear to experience long-term cognitive impairment [3]. Long-term cognitive sequelae have been documented as late as 20 years following the completion of therapy for women with breast cancer [4].
Survivors describe cognitive changes such as forgetfulness, absentmindedness, and an inability to focus when performing daily tasks [5]. Complaints also include difficulty with short-term memory, word-finding, reading comprehension, driving/directional sense, and concentration [6]. A variety of mechanisms have been proposed for the development of cognitive impairment, including cytokine-induced inflammatory response, deficits in DNA-repair mechanisms, genetic predisposition [3], chemotherapy-induced anemia, chemotherapy-induced menopause [7], and injury to neural progenitor cells [8]. Cognitive impairment experienced prior to receiving treatment for cancer has been hypothesized to be due to the release of cytokines associated with tissue damage from the tumor [9]. Cognitive impairment perceived prior to treatment for breast cancer also may be influenced by the impact of the cancer diagnosis on mood states (such as anxiety and depression) and the resultant impact on the capacity to direct attention [10]. Results of previous research also suggest relationships between perceived cognitive impairment (PCI) and fatigue, sleep disturbance, and neuropathy for survivors who have received chemotherapy [6, 11].
The potential role of inflammatory cytokines as a causal mechanism for cancer and cancer treatment-related cognitive complaints is intriguing. Chronic inflammation is associated with a negative effect on the neural systems involved in cognition and memory and has been linked to obesity [12, 13]. Obesity is a risk factor for breast cancer, disease recurrence, and poor prognosis [14, 15].Weight gain is common for women receiving chemotherapy for breast cancer [16, 17]. The chronic inflammatory state associated with obesity may contribute to the risk of cognitive changes in this population as has been seen pre-clinically [12] and in populations with other disorders such as the metabolic syndrome that is linked to cardiovascular risk factors [18].
Exercise is associated with improved cognitive function in older adults [19–21] and may be of benefit for individuals suffering from Alzheimer’s disease and dementia [22–24]. Exercise is a strategy employed by some breast cancer survivors to attempt to decrease PCI [6], and evidence is building in support of exercise as an intervention for cancer-associated cognitive complaints [25–32]. The NCCN guidelines recommend regular exercise and strength training for cancer survivors and recently added routine exercise as one of the general strategies for management of cancer-associated cognitive dysfunction [33]. Breast cancer survivors’ patterns of exercise may be predictive of cognitive complaints during and after treatment for breast cancer. A number of factors may contribute to the development and experience of cognitive impairment for breast cancer survivors, including age, education level, menopausal status, endocrine (antiestrogen) therapy, time since radiation therapy, and time since general anesthesia [34, 35, 10, 36, 11, 6].
The purpose of this cross-sectional study was to explore potential factors associated with PCI in breast cancer survivors compared to healthy controls and to gain insight into perceived levels of severity for cognitive complaints. The potential factors of interest included age, education level, group (based upon chemotherapy exposure), time since radiation therapy, time since general anesthesia, antiestrogen therapy, fatigue, sleep disturbance, neuropathy, depression, distress, body mass index, and exercise.
Methods
Recruitment
Upon approval by the University of Kansas Human Subjects Committee, women with breast cancer and healthy controls (from 24 states) were enrolled by recruitment and referral efforts across the USA. IRB approved study synopses, and flyers were used by participating sites, survivorship focused newsletters, and members of Oncology Nursing Society Chapters to recruit or refer eligible women. Eligible participants were required to be adult women able to speak, read, and understand English. All stages of breast cancer, previous and current endocrine (antiestrogen) therapy, and neoadjuvant chemotherapy were accepted. Exclusion criteria included chemotherapy for or a history of a previous malignancy, myeloablative chemotherapy regimens administered in conjunction with bone marrow or stem cell transplant, central nervous system metastases, mental illness, dementia, or Alzheimer’s disease.
Instruments
Participants were given the option to complete an electronic or hard copy questionnaire (identical forms) comprised of demographic information, the MD Anderson Symptom Inventory (MDASI) [37], the Functional Assessment of Cancer Therapy for Cognition (FACT-COG) version 3 [38], and the Attentional Function Index (AFI) [39]. These instruments have been tested in the oncology population and are psychometrically sound [39, 37, 38].
The MDASI symptom severity scale items for fatigue, disturbed sleep, feelings of distress, numbness, or tingling (neuropathy), and feeling sad (depression) were included in the analysis. These items are ranked from 0 (not present) to 10 (as bad as you can imagine). Three of the four scales were scored from the FACT-COG including perceived cognitive impairments (PCI-18 items; 0=never, 4=several times a day), perceived cognitive abilities (PCA-7 items; 0=not at all, 4=very much), and impact on quality of life (QOL-4 items; 0=not at all, 4=very much). Higher scores on the FACT-COG equate to higher perceived cognitive function (due to reverse coding). Use of the AFI provides assessment of perceived losses in the capacity to direct attention [39]. Individual items are ranked from 0 (not at all) to 10 (extremely well). The mean overall score for the AFI was used as an additional measure of cognitive function for the study (attentional fatigue). Participants also were asked to rate difficulty driving, difficulty with reading comprehension, balance, and coordination on a 0–4 scale (0=never, 4=several times a day) based on issues raised by participants in previous research and the lack of items addressing these concerns on available validated instruments [6]. Demographic information included participants’ self-report of height and weight (used to calculate BMI), age, menopausal status, education level, work status, marital status, ethnicity, race, previous or current antiestrogen therapy, history of general anesthesia and radiation therapy, and current exercise patterns (type, frequency, and duration). Participants also reported type and duration of chemotherapy regimen, including date of completion (used to calculate time since chemotherapy). Participants were classified into seven groups based on exposure to chemotherapy: control, pre-chemotherapy, current chemotherapy, and postchemotherapy (≤1, >1 to 2, >2 to 5, >5 years).We chose this classification system based on research results indicating that a subset of survivors continue to experience cognitive complaints past 1–2 years of completing chemotherapy [40]. Our goal was to try to capture (albeit cross-sectionally) a sense of the trajectory of cognitive improvement over time.
Statistical analyses
This study was powered for the primary outcome of PCI: A sample size of 224 subjects (32 per cohort) was required to detect an effect size of 0.52 for PCI using a one-way analysis of variance model with 80 % power at the 5 % level of significance. All other analyses were considered hypothesis-generating. Baseline demographic and clinical characteristics are described using means and standard deviations for continuous variables (or in the presence of skewness or outliers, by medians and interquartile ranges (IQR)); for categorical variables, frequencies and percentages are reported. To test for differences in PCI among groups, ANOVA with weighted least squares was used to account for the heterogeneity in variances observed between groups (Levene’s test p<0.05). All pairwise comparisons of PCI between groups were tested using Fisher’s least significant difference method. Weighted least squares regression was used to test for an association between time-since-chemotherapy (continuous) and PCI scores in chemo-exposed breast cancer participants. Bivariate associations between continuous variables were examined using Pearson’s product-moment correlation coefficient. If skewness was identified, Spearman’s rank-sum correlation coefficient was substituted. Pearson’s chi-square test was used to identify significant bivariate associations between categorical variables. Fisher’s exact test was used for situations where cell counts were too small for valid inferences based on the chi-square statistic. Bivariate associations between categorical and continuous outcomes were assessed using ANOVA (or Wilcoxon tests, if assumptions were violated). Forward step-wise regression procedures were used to build a model for PCI from a set of potential covariates. Maximized adjusted R-square and minimized Akaike information criterion (AIC) and Bayes information criterion (BIC) were used to select the best model. Because the amount of missing data was small relative to the size of the study (1 case in 363), case-wise deletion was used for missing data for the primary aim of the study. A description of the missingness rates for other variables is included in Table 1. No significant differences between subjects with complete versus incomplete data were found. All analyses were performed using SAS® version 9.4.
Table 1.
Sample description
| Study group | Missing (n, %) |
p value | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 46) |
Pre-chemo (n = 42) |
Current chemo (n = 47) |
≤1 year (n = 72) |
>1 to 2 years (n = 45) |
>2 to 5 years (n = 58) |
>5 years (n = 53) |
|||||
| Age in years (mean, SD) | 49.4 (9.8) | 52 (11.8) | 53.1 (8.6) | 52.8 (11.3) | 53.6 (9.6) | 54 (8.8) | 62.3 (10) | 1 (0.3) | <0.01 | ||
| BMI (mean, SD) | 27.3 (5.9) | 29 (6.9) | 29.8 (7.1) | 27.8 (5.7) | 28.1 (6.2) | 29.2 (6.3) | 27.2 (4.7) | 2 (0.6) | 0.22 | ||
| Exercise frequency | None (n, %) | 9 (19.6) | 19 (45.2) | 23 (49.0) | 19 (26.4) | 12 (26.7) | 20 (34.5) | 20 (34.5) | 0 (0) | 0.18 | 122 |
| Monthly (n, %) | 2 (4.4) | 3 (7.1) | 1 (2.1) | 0 (0) | 1 (2.2) | 5 (8.6) | 1 (1.9) | 13 | |||
| 2–3×/week (n, %) | 19 (41.3) | 7 (16.7) | 14 (29.8) | 29 (40.3) | 15 (33.3) | 17 (29.3) | 15 (28.3) | 116 | |||
| 4–5×/wk (n, %) | 10 (41.3) | 7 (16.7) | 5 (10.6) | 16 (22.2) | 9 (20.0) | 10 (17.2) | 11 (20.8) | 68 | |||
| Daily (n, %) | 6 (13.0) | 61 (14.3) | 4 (8.5) | 8 (11.1) | 8 (17.8) | 6 (10.3) | 6 (11.3) | 44 | |||
| Menopause status | Pre (n, %) | 20 (43.5) | 11 (26.2) | 9 (19.2) | 9 (12.5) | 4 (8.9) | 3 (5.2) | 0 (0) | 1 (0.3) | <0.01 | 56 |
| Peri (n, %) | 5 (10.9) | 0 (0) | 4 (8.5) | 7 (9.7) | 0 (0) | 1 (1.7) | 1 (1.9) | 18 | |||
| Post (n, %) | 21 (45.7) | 31 (73.9) | 34 (72.3) | 56 (77.8) | 41 (91.1) | 53 (91.4) | 52 (98.1) | 288 | |||
| Anti-estrogen current (n, %) | – | 5 (12.0) | 4 (8.5) | 33 (45.8) | 24 (53.3) | 37 (63.8) | 16 (30.2) | 0 (0) | 119 | ||
| Education | Grade school (n, %) | 1 (2.2) | 0 (0) | 0 (0) | 1 (1.4) | 0 (0) | 1 (1.7) | 1 (1.9) | 1 (0.3) | 0.09 | 4 |
| High school (n, %) | 2 (4.4) | 11 (26.2) | 14 (29.8) | 14 (9.4) | 8 (17.8) | 12 (20.7) | 8 (17.8) | 69 | |||
| College (n, %) | 19 (41.3) | 22 (52.4) | 19 (40.4) | 37 (51.4) | 24 (53.3) | 34 (58.6) | 28 (52.8) | 183 | |||
| Grad school (n, %) | 24 (52.2) | 9 (21.4) | 14 (29.8) | 19 (26.4) | 13(28.9) | 11 (19.0) | 16 (30.2) | 106 | |||
| Hispanic or Latino ethnicity (n, %) | 2 (4.4) | 1 (2.4) | 4 (8.5) | 1 (1.4) | 0 (0) | 1 (1.4) | 2 (3.8) | 3 (0.8) | 0.26 | 11 | |
| Race | Caucasian (n, %) | 33 (71.7) | 36 (85.7) | 41 (87.2) | 63 (87.5) | 39 (86.7) | 48 (82.7) | 47 (88.7) | 3 (0.8) | <0.01 | 307 |
| African-American (n, %) | 1 (2.2) | 4 (9.5) | 1 (2.1) | 5 (6.9) | 6 (13.3) | 6 (10.3) | 1 (1.9) | 24 | |||
| American Indian/Alaskan Native (n, %) | 0 (0) | 1 (2.4) | 1 (2.1) | 0 (0) | 0 (0) | 3 (5.2) | 1 (1.9) | 6 | |||
| Asian (n, %) | 9 (19.6) | 1 (2.4) | 0 (0) | 2 (2.8) | 0 (0) | 0 (0) | 2 (3.8) | 14 | |||
| Pacific Islander (n, %) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 | |||
| Two or more races (n, %) | 3 (6.5) | 0 (0) | 1 (2.1) | 2 (2.8) | 0 (0) | 1 (1.7) | 1 (1.9) | 8 | |||
| Stage of breast cancer | I (n, %) | – | 17 (40.5) | 8 (17.0) | 20 (27.8) | 12 (26.7) | 19 (32.8) | 10 (18.9) | 20 (5.5) | <0.01 | 86 |
| II (n, %) | – | 16 (38.1) | 14 (29.8) | 29 (40.1) | 22 (48.9) | 26 (44.8) | 26 (49.1) | 133 | |||
| III (n, %) | – | 6 (14.3) | 11 (23.4) | 17 (23.6) | 5 (11.1) | 9 (15.5) | 8 (15.1) | 56 | |||
| IV (n, %) | – | 0 (0) | 11 (23.4) | 3 (4.2) | 4 (8.9) | 2 (3.5) | 2 (3.8) | 22 | |||
| Radiation therapy (n, %) | 0 (0) | 3 (7.1) | 11 (23.4) | 49 (68.1) | 34 (75.6) | 43 (74.1) | 34 (64.2) | 0 (0) | <0.01 | 174 | |
Exercise definition: physical activity that is planned, structured, and repetitive for the purpose of conditioning any part of the body
Results
Sample
Our sample was comprised of 363 women, including 317 diagnosed with breast cancer and 46 healthy controls (Table 1). No significant differences were seen between the seven groups with the exception of age and BMI. Participants >5 years out from completion of chemotherapy were 8–12 years older than participants in all other groups (p<0.05). The majority of participants were Caucasian (92 %), married (69 %), postmenopausal (79 %), educated at the college level or above (80 %), and worked full time (78%). The majority of participants with breast cancer were diagnosed with stage II disease (n=133).Women receiving chemotherapy had, on average, higher BMI (mean BMI=29.8) than those who were no more than 1 year removed from chemotherapy (mean BMI 27.4 kg/m2; mean difference 2.4; 95 % CI 0.04–4.7; p<0.05) and >5 years (mean BMI 27.2 kg/m2; mean difference 2.6 kg/m2; 95 % CI 0.12–5.2; p<0.05) from completion of chemotherapy.
PCI
PCI was found to be highly positively associated with time since chemotherapy in breast cancer participants with chemotherapy (t=2.77, p=0.0062).A significant overall group effect was found for PCI (F6, 355=7.01, p<0.0001). Mean PCI scores were significantly better for healthy controls than any other group (Table 2). Even participants classified as pre-chemotherapy showed a 7.4 point deficit in PCI scores, on average, when compared to healthy controls (95 % CI=0.4, 14.4). This deficit in PCI scores increased for breast cancer subjects who had been or currently were being treated with chemotherapy, but the difference appeared to decrease for subjects who were 5 or more years postchemotherapy. Further statistically significant differences in mean PCI were identified between breast cancer subjects pre-chemotherapy and (1) those 1 or fewer years out from chemotherapy and (2) those >1 to 2 years out from chemotherapy, with pre-chemotherapy subjects scoring 8.3 points higher, on average, than the former (95 % CI=2, 14.7) and 12 points higher, on average, than the latter (95 % CI=5.3, 18.7). Similarly, attentional function index (overall AFI score—higher scores equate to less attentional fatigue) scores were higher for the healthy control group, although significant differences were noted only for participants currently receiving chemotherapy, 1 or fewer years out from chemotherapy, and >2 years to ≤5 years of completion of chemotherapy.
Table 2.
Mean differences in perceived cognitive impairment (95 % confidence intervals) from weighted least squares estimation for study group comparisons identified as significant at the 5 % level
| Group 1 | Group 2 | Mean difference (group 1–2) |
95 % CI on difference |
|---|---|---|---|
| Control | Pre-chemo | 7.4 | 0.4, 14.4 |
| Current chemo | 12.7 | 5.9, 19.5 | |
| ≤1 year | 15.7 | 9.5, 21.9 | |
| >1 to ≤2 years | 12.4 | 5.6, 19.3 | |
| >2 to ≤5 years | 19.4 | 12.9, 25.9 | |
| >5 years | 10.7 | 4, 17.3 | |
| Pre-chemo | ≤1 year | 8.3 | 2, 14.7 |
| >2 to ≤5 years | 12 | 5.3, 18.7 | |
| Current chemo | >2 to ≤5 years | 6.7 | 0.2, 13.1 |
| >1 to ≤2 years | >2 to ≤5 years | 6.9 | 0.4, 13.5 |
| >2 to ≤5 years | >5 years | −8.7 | −15, −2.5 |
PCA and QOL
Significant overall group effects also were found for PCA and QOL (PCA: F6, 354=3.10, p=0.006; QOL: F6, 354=2.3, p= 0.04). Healthy controls’ mean PCA scores did not differ from participants scores prior to receiving chemotherapy but were significantly higher than participants who were ≤1 year or within 2 to 5 years of completing chemotherapy (see Table 3). Participants who were >5 years from completing chemotherapy scored higher on the PCA scale than those ≤1 year or between 2 and 5 years of completing therapy. Significantly better QOL was reported for healthy controls compared to participants within 1 year or between 2 to 5 years of completing chemotherapy (see Table 3). Better QOL was demonstrated prior to chemotherapy than for participants who were within 2 to 5 years of having completed therapy. Participants who were >5 years from completing chemotherapy reported better QOL than those who were within 2 to 5 years.
Table 3.
Group means (standard deviations) for perceived cognitive impairment, perceived cognitive abilities, and quality of life
| Group | PCI | PCA | QOL |
|---|---|---|---|
| Control | 61.1 (9.4) | 19.1 (8.8) | 13.2 (4.8) |
| Pre-chemo | 53.7 (14.9) | 20.2 (6.9) | 12.3 (4.3) |
| Current chemo | 48.4 (16.9) | 17.6 (7.2) | 11.1 (5.2) |
| ≤1 year | 45.4(18.2) | 15.5(6.8) | 11.3 (5.1) |
| >1 to ≤2 years | 48.6 (17.2) | 17.6 (7.2) | 11.1 (5.4) |
| >2 to ≤5 years | 41.7 (18.3) | 15.9 (6.8) | 9.7 (5.1) |
| >5 years | 50.4 (18.2) | 19.0 (6.9) | 11.7 (4.9) |
PCI perceived cognitive impairment, PCA perceived cognitive abilities, QOL quality of life
Additional instrument items
Participants were asked to rank four symptom items not included in the validated study instruments related to directional sense and concentration when driving, reading comprehension, balance, and coordination. Significant associations with PCI were seen for each of these items: difficulty with direction (F1, 354=23.49, p<0.0001), difficulty with driving (F1, 354= 11.89, p<0.0006), difficulty with reading comprehension (F1, 354=237.92, p<0.0001), and balance (F1, 354=16.39, p<0.0001). The association with coordination was not significant.
Bivariate associations of symptoms with perceived cognitive impairment
Prior to building the final regression model, bivariate associations among factors and outcomes were investigated across study cohorts to identify multicollinearity among factors, determine the factors most highly correlated with PCI, and reduce the number of candidates for the model building process. Significant correlation was demonstrated between neuropathy and PCI (r=−0.23; p<.0001) for participants with breast cancer. More specifically, correlation between neuropathy and PCI was noted for pre-chemotherapy participants (r=−0.48, p<0.001) and participants who were receiving or had completed chemotherapy (r=−0.19, p<0.01). Participants who reported receiving taxanes (n=214) reported more neuropathy (F=7.7, p<0.01) than those who did not receive taxanes (n=149). Participants who received taxanes reported significantly worse PCI (F=33.1, p<0.01). Significant correlations were seen for both PCI and AFI with fatigue (r=−0.41, p<0.001; r=−0.32, p<0.001), sleep disturbance (r=−0.32, p<0.0001; r=−0.32, p<0.0001), and distress (r=−0.40, p<0.0001; r=−0.40, p<0.0001) for participants who were receiving or had completed chemotherapy.
Demographic and behavioral correlations
Age inversely correlated with PCI (r=−0.30, p<0.05) for healthy controls. Age positively correlated with PCI (r=0.30, p<0.0001) for participants receiving or finished with chemotherapy. BMI correlated significantly with stage of breast cancer for participants who were receiving or had completed chemotherapy (r=0.14, p<0.05). BMI was found to be significantly different between subjects reporting regular exercise (at least two to three times per week) and those who did not (p<0.01). The average BMI among those who reported no regular exercise was 30.8 (SE=0.6), compared to 26.9 (SE= 0.4) for those who reported exercising at least two to three times per week. Exercise frequency was positively correlated with PCI (more frequent exercise correlated with better self-reports of cognitive function, F=3.78, p<0.05).
Regression modeling
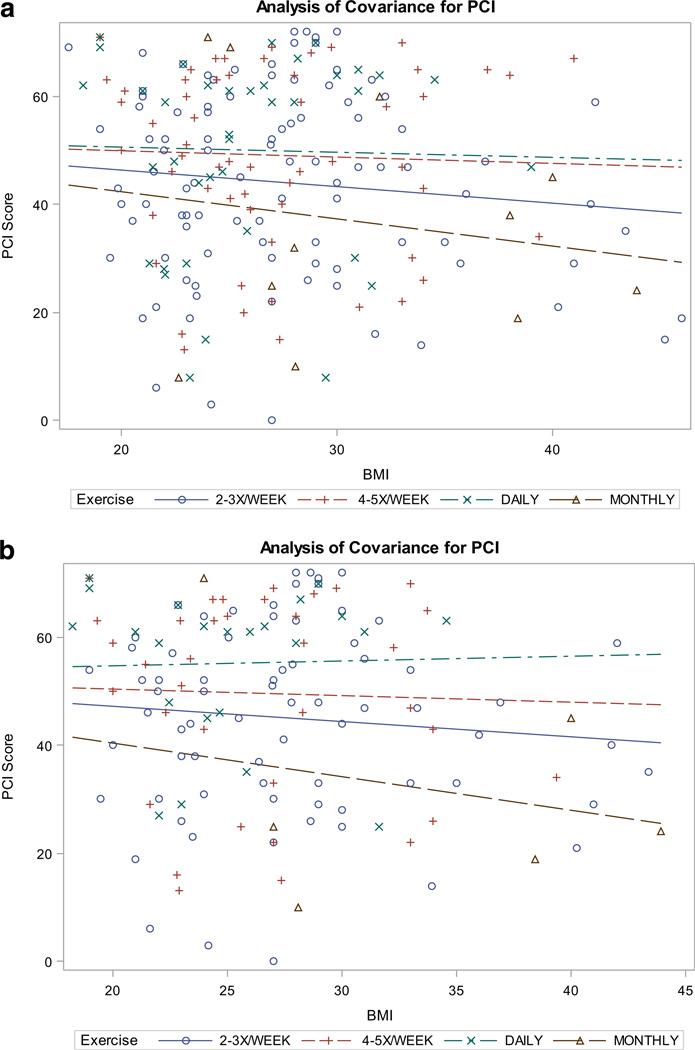
The multiple linear regression model for PCI was built, limited to breast cancer participants only, with forward selection methods identifying the most parsimonious and relevant model. Education level, menopausal status, time since general anesthesia, and stage of disease were not identified as important covariates to model. The final model explained 24 % of the variance (adjusted R2) and included the following predictors of PCI: group (time since chemotherapy), BMI, exercise, fatigue, and distress (see Table 4). Further analysis was conducted to explore an interaction between BMI and exercise frequency related to PCI for women with breast cancer. In breast cancer participants (pre-chemo, current chemo, and postchemo), exercise appeared to reduce the negative effects of high BMI on PCI; that is, as exercise frequency increased, the relationship between BMI and PCI disappeared (F3, 198= 2.4, p=0.07, Fig. 1a). Breast cancer survivors who reported relatively sedentary lifestyles (monthly exercise) saw greater declines in PCI scores (worse perception of cognitive function) as BMI increased than those who reported regular exercise (daily). When limited to chemo-exposed breast cancer survivors, this moderating effect of exercise became more apparent (F3, 133=3.1, p=0.03, Fig. 1b). This relationship was not seen in healthy controls (F3, 32=0.6, p=0.6).
Table 4.
Multivariable linear regression model for perceived cognitive impairment (r2adj = 0.24, r2 = 0.27)
| Model parameter | Estimate (SE) | p value | |
|---|---|---|---|
| Study groupa | Current | −3.8 (3.4) | 0.3 |
| ≤1 year | −8.7 (3.2) | 0.007 | |
| >1 to ≤2 years | −4.5 (3.4) | 0.2 | |
| >2 to ≤5 years | −10.3 (3.2) | 0.001 | |
| >5 years | −6.4 (3.3) | 0.05 | |
| Fatigue | −1.8 (0.4) | <0.0001 | |
| Distress | −1.4 (0.4) | 0.0006 | |
| BMI | −0.19 (0.15) | 0.2 | |
| Exerciseb | Monthly | −15.1 (5) | 0.003 |
| 2–3×/week | −6.1 (2.3) | 0.008 | |
| 4–5×/week | −3.7 (2.6) | 0.16 | |
| Daily | −2.8 (3.1) | 0.4 |
Pre-chemo is reference group
None is reference group
Fig. 1.
a Interaction effect for BMI, exercise, and PCI in breast cancer survivors. b Interaction effect for BMI, exercise, and PCI in breast cancer survivors exposed to chemotherapy
Discussion
The study results provide further evidence for the presence of cognitive complaints prior to receiving chemotherapy for breast cancer, an increase in cognitive complaints during treatment and an overall decrease in cognitive complaints over time. Mean PCI scores for women >5 years out from completion of chemotherapy were not statistically different than women prior to receiving chemotherapy, although scores remained worse than healthy controls. The study results are consistent with recent work published by Sanford et al. (2014) that indicated that PCI scores less than 59 (out of the total of 72 points for the PCI subscale) were indicative of significant PCI [41].
A significant negative rating for quality of life was demonstrated within 5 years of receiving chemotherapy. However, mean QOL scores for women who were >5 years out from chemotherapy did not differ significantly from the healthy controls. The cross-sectional design of the study prevents making individual longitudinal assessments of PCI and QOL but does provide some indication of risk factors associated with PCI and longer term sequelae. Variables of particular interest included neuropathy, BMI, and exercise patterns.
Preliminary qualitative work conducted by Myers [6] involved interviews for 18 breast cancer survivors within 6 to 12 months of completing chemotherapy who reported cognitive impairment. Eight of these participants had experienced neuropathy as a result of the chemotherapy and five noted residual numbness at the time of the interviews. In the current study, significant correlation was seen between neuropathy and participants’ reports of worse cognitive function (lower mean PCI scores indicate more cognitive complaints) for participants with breast cancer over all as well as prior to, during, and following chemotherapy. Neuropathy also was correlated with fatigue, sleep disturbance, and distress. As expected, neuropathy was associated with taxane therapy; however, we also demonstrated that women who received taxanes reported worse PCI. Neuropathy was not included in the regression model due to redundancy with fatigue and distress once sleep disturbance was dropped from the model. However, the strong correlation of neuropathy with PCI in the current study is intriguing and warrants further study in future longitudinal research. Exploration of potential mechanisms associated with peripheral neurotoxicity may be of interest. Neuropathy reported by healthy controls and women with breast cancer prior to receiving chemotherapy presumably was due to comorbid conditions such as diabetes.
Significant associations with PCI were seen for the non-validated items related to difficulty with direction, driving, reading comprehension, and balance. Further expansion of the FACT-COG items has since led to the development of two Patient Reported Outcomes Measurement and Information System (PROMIS) v1.0-Applied Cognition-Abilities and Applied Cognition-Concerns [42]. The PROMIS item bank now includes an item related to following driving directions. Further investigation of items concerning reading comprehension, the mechanics of driving, and balance may be warranted.
Exercise frequency was shown to correlate with participants’ mean PCI scores. Women who were exposed to chemotherapy (currently receiving or completed) who exercised more frequently reported less severe cognitive complaints. Interaction effects were examined for BMI and exercise frequency with PCI. Interestingly, a moderating effect for exercise frequency was demonstrated for the impact of BMI on PCI. These results are hypothesis generating for the impact that obesity has on cognitive function for women with breast cancer and particularly those who receive chemotherapy. Additionally, these results lend support to the potential benefit of regular exercise as an intervention to mitigate the cognitive complaints associated with breast cancer and chemotherapy.
The study results were unexpected in a few areas. The inverse relationship between age and PCI seen in healthy controls (younger participants reported better cognitive function) was contrasted by the positive relationship between age and PCI seen for women with breast cancer (younger women reported worse cognitive function). Cognitive decline is well documented for elderly adults [43] and is consistent with the study results demonstrated here. However, fewer cognitive complaints were noted for older women with breast cancer. A similar result has been seen in previous studies and the authors’ speculated that the subtle cognitive changes associated with treatment for breast cancer are more noticeable for younger women who are still working and faced with balancing multiple tasks as compared to older women who may be retired and facing less cognitively challenging activities or less demanding life roles, or better able to cope with stressors due to life experience [10]. Other potential explanations may be the general recovery over time that was demonstrated for study participants who were further out from completion of chemotherapy or the response shift phenomenon that may occur for individuals who experience a health state change [44].
A number of study limitations must be acknowledged. Information was not collected regarding the presence of comorbid conditions (such as diabetes) beyond questions relating to the exclusion criteria for dementia, Alzheimer’s disease, or central nervous system metastases. Interpretation of results is limited by the cross-sectional study design. The study was appropriately powered to answer the primary aim, which was to explore potential predictors of perceived cognitive impairment (PCI) for breast cancer survivors compared to healthy controls and to gain insight into perceived levels of severity for cognitive complaints. All other analyses were exploratory. Prospective, longitudinal studies will provide stronger evidence than a cross-sectional examination of participants’ PCI ratings for specific time points prior to, during, and following chemotherapy. This study was based upon participants’ self-report of cognitive function as opposed to conducting neurocognitive tests. However, due to the limited sensitivity of available neurocognitive tests for cancer and cancer therapy-related cognitive impairment [45], and the evidence that survivors’ reports of cognitive changes precede objective evidence by neuroimaging [46], survivors’ perceptions of cognitive function provide valuable data and are an important study endpoint. Our study was limited to survivors’ perceptions of global cognitive functioning. Future examination of survivors’ perceptions of functioning in specific cognitive domains also would be of interest. BMI was calculated from participants’ self-report of height and weight. Thus, the potential for underestimation or overestimation of BMI exists. However, results of previous research indicate that correlation between self-reported height and weight and clinical measurements is high (above 90 %) [47]. Perhaps a more important study limitation is the lack of a measure of visceral fat (such as waist to hip ratio or sagittal abdominal diameter). Visceral fat has been shown to be a stronger predictor of the chronic inflammation associated with obesity and metabolic syndrome than BMI and thus may be more pertinent to assess in research investigating cognitive function. A recent publication of preliminary research indicated a significant correlation between visceral abdominal fat and deficits in verbal learning and memory for healthy adult women of reproductive age [48].
Our study results are consistent with previous research indicating the presence of perceived cognitive impairment prior to treatment for breast cancer, worsening of PCI during treatment, and some improvements in PCI over time following treatment. Additionally, our results provide support for a relationship between BMI and PCI in breast cancer survivors and suggest that exercise may mitigate the impact of BMI on cognition. Further investigation of the influence of weight and exercise on cognitive function is warranted as is the examination of potential mechanisms underlying the relationship between obesity and cognitive function in breast cancer survivors. Investigation of the influence of chronic inflammation associated with obesity and with the body’s response to cancer and cancer therapy may lend support to relevant interventions to manage this problem.
Acknowledgments
The authors wish to acknowledge the following for their assistance with recruitment or referrals to the study: University of Kansas Breast Cancer Survivorship Center, Kansas City, KS; University of Kansas Cancer Center, Westwood, KS; HCA MidAmerica Division, Kansas City KS and MO; Penrose Medical Center, Colorado Springs, CO; Memorial Cancer Center, Colorado Springs, CO; Dorcy Cancer Center at Sr. Mary Corwin Hospital, Pueblo, CO; Midwest Cancer Alliance, Kansas City KS; St. Joseph Medical Center, Kansas City, MO; Saint Mary’s Medical Center, Blue Springs, MO; Loma Linda Veterans Administration, Loma Linda, CA; Oncology Nursing Society National Survivorship, Quality of Life, and Rehabilitation Special Interest Group; Oncology Nursing Society Greater Kansas City and Pikes Peak/Southeast Colorado Chapters; CURE Magazine; Kansas City Nursing Research Consortium. A portion of Dr. Wick’s effort on this study was supported by the National Cancer Institute, Grant 1 P30 CA168524-01. A portion of Dr. Myers’ effort was supported by the National Institute of Nursing Research (T32TNR011972A).
Footnotes
Conflict of interest Jamie Myers, Jo Wick, and Jennifer Klemp declare that they have no conflict of interest. We have full control of all primary data and agree to allow the journal to review our data if requested.
Informed consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants for being included in the study.
References
- 1.National Institutes of Health Office of Cancer Survivorship. 2015 http://cancercontrol.cancer.gov/ocs/statistics/definitions.html.
- 2.Collins B, MacKenzie J, Tasca GA, Scherling C, Smith AD. Cognitive effects of chemotherapy in breast cancer patients: a dose-response study. Psychooncology. 2013;7:1517–1527. doi: 10.1002/pon.3163. doi: 10.1002/pon.3163. [DOI] [PubMed] [Google Scholar]
- 3.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 5.Hess LM, Insel KC. Chemotherapy-related change in cognitive function: a conceptual model. Oncol Nurs Forum. 2007;24:981–994. doi: 10.1188/07.ONF.981-994. [DOI] [PubMed] [Google Scholar]
- 6.Myers JS. Chemotherapy-related cognitive impairment: the breast cancer experience. Oncol Nurs Forum. 2012;39:E31–E40. doi: 10.1188/12.ONF.E31-E40. doi: 10.1188/12.OONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- 7.Jansen C, Miaskowski C, Dodd M, Dowling G, Kramer J. Potential mechanisms for chemotherapy-induced impairments in cognitive function. Oncol Nurs Forum. 2005;32:1151–1163. doi: 10.1188/05.ONF.1151-1163. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:1–23. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleeland CS, Bennett G, Dantzer R, Dougherty PM, Dunn AJ, Meyers C, Miller AH, Payne R, Reuben JM, Wang XS, Lee BN. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 10.Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14:70–78. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 11.Myers JS, Sousa VD, Donovan HS. Predictors of self-reported memory problems in patients with ovarian cancer who have received chemotherapy. Oncol Nurs Forum. 2010;37:596–603. doi: 10.1188/10.ONF.596-603. [DOI] [PubMed] [Google Scholar]
- 12.Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014;34:2618–2631. doi: 10.1523/JNEUROSCI.4200-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain aging. Nat Rev Neurosci. 2012;13:209–216. doi: 10.1038/nrn3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Cunha PA, de Carlos Back LK, Sereia AF, Kubelka C, Ribeiro MC, Fernandes BL, de Souza IR. Interaction between obesity-related genes, FTO and MC4R, associated to an increase of breast cancer risk. Mol Biol Rep. 2013;40:6657–6664. doi: 10.1007/s11033-013-2780-3. [DOI] [PubMed] [Google Scholar]
- 15.Dalamaga M. Obesity, insulin resistance, adipocytokines and breast cancer: new biomarkers and attractive therapeutic targets. World J Exp Med. 2013;3(3):34–42. doi: 10.5493/wjem.v3.i3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lankester KJ, Phillips E, Lawton PA. Weight gain during adjuvant and neoadjuvant chemotherapy for breast cancer: an audit of 100 women receiving FEC or CMF chemotherapy. Clin Oncol (R Coll Radiol) 2002;14:64–67. doi: 10.1053/clon.2001.0014. [DOI] [PubMed] [Google Scholar]
- 17.Rock CL, Flatt SW, Newman V, Caan BJ, Haan MN, Stefanick ML, Faerber S, Pierce J. Factors associated with weight gain in women after diagnosis of breast cancer. J Am Diet Assoc. 1999;99:1212–1221. doi: 10.1016/s0002-8223(99)00298-9. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AG. The metabolic syndrome, inflammation, and risk of cognitive decline. J Am Med Assoc. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 19.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A: Biol Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 20.Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 21.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. J Am Med Assoc. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 22.de Andrade L, Gobbi L, Coelho F, Christofoletti G, Costa JL, Stella F. Benefits of multimodal exercise intervention for postural control and frontal cognitive functions in individuals with Alzheimer's disease: a controlled trial. J Am Geriatr Soc. 2013;61:1919–1926. doi: 10.1111/jgs.12531. [DOI] [PubMed] [Google Scholar]
- 23.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. J Am Med Assoc. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 24.Vidoni ED, Van Sciver A, Johnson DK, He J, Honea R, Haines B, Goodwin J, Laubinger MP, Anderson HS, Kluding PM, Donnelly JE, Billinger SA, Burns JM. A community-based approach to trials of aerobic exercise in aging and Alzheimer's disease. Contemp Clin Trials. 2012;33:1105–1116. doi: 10.1016/j.cct.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumann FT, Drosselmeyer N, Leskaroski A, Knicker A, Krakowski-Roosen H, Zopf EM, Bloch W. 12-Week resistance training with breast cancer patients during chemotherapy: effects on cognitive abilities. Breast Care. 2011;6:142–143. [Google Scholar]
- 26.Fardell JE, Vardy J, Winocur G. Chemotherapy and cognitive impairment: treatment options. Nature. 2011;90:366–376. doi: 10.1038/clpt.2011.112. [DOI] [PubMed] [Google Scholar]
- 27.Galantino ML, Greene L, Daniels L, Dooley B, Muscatello L, O'Donnell L. Longitudinal impact of yoga on chemotherapy-related cognitive impairment and quality of life in women with early stage breast cancer: a case series. Explore. 2012;8:127–135. doi: 10.1016/j.explore.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Korstjens I, Mesters I, van der Peet E, Gijsen B, van den Borne B. Quality of life of cancer survivors after physical and psychosocial rehabilitation. Eur J Cancer Prev. 2006;15:541–547. doi: 10.1097/01.cej.0000220625.77857.95. [DOI] [PubMed] [Google Scholar]
- 29.Oh B, Butow PN, Mullan BA, Clarke SJ, Beale PJ, Pavlakis N, Lee MS, Rosenthal DS, Larkey L, Vardy J. Effect of medical qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Support Care Cancer. 2012;20:1235–1242. doi: 10.1007/s00520-011-1209-6. doi: 10.1007/s00520-011-1209-6. [DOI] [PubMed] [Google Scholar]
- 30.Reid-Arndt AA, Matsuda S, Cox CR. Tai chi effects on neuropsychological, emotional, and physical functioning following cancer treatment: a pilot study. Complement Ther Clin Pract. 2012;18:26–30. doi: 10.1016/j.ctcp.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Sprod LK, Mohile SG, Demark-Wahnefried W, Janelsins MC, Peppone LJ, Morrow GR, Lord R, Gross H, Mustian KM. Exercise and cancer treatment symptoms in 408 newly diagnosed older cancer patients. J Geriatr Oncol. 2012;3:90–97. doi: 10.1016/j.jgo.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crowgey T, Peters KB, Hornsby WE, Lane A, McSherry F, Herndon JE, West MJ, Williams CL, Jones LW. Relationship between exercise behavior, cardiorespiratory fitness, and cognitive function in early breast cancer patients treated with doxoubicin-containing chemotherapy: a pilot study. Appl Physiol Nutr Metab. 2014;39:724–729. doi: 10.1139/apnm-2013-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denlinger CS, Ligibel JA, Are M, Baker KS, Demark-Wahefried W, Dizon D, Friedman DL, Goldman M, Jones L, King A, Kvale E, Langbaum TS, Leonardi-Warren K, McCabe MS, Melisko M, Montoya JG, Mooney KH, Morgan MA, Moslehi JJ, O'Conner T, Overholser L, Paskett ED, Peppercorn J, Raza M, Rodriquez MA, Syrjala KL, Wakabayashi MT, Zee P, McMillian NR, Freedman-Cass DA. Survivorship: healthy lifestyles, version 2.2014. J Natl Compr Cancer Netw. 2014;12:1222–1237. doi: 10.6004/jnccn.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahles TA, Saykin AJ. Cognitive effects of standard-dose chemotherapy in patients with cancer. Cancer Investig. 2001;19:812–820. doi: 10.1081/cnv-100107743. [DOI] [PubMed] [Google Scholar]
- 35.Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, Paraska KK, Ryan CM. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 36.Merriman JD, Jansen C, Koetters T, West C, Dodd M, Lee K, Paul SM, Aouizerat BE, Cooper B, Swift PS, Wara W, Miaskowski C. Predictors of the trajectories of self-reported attentional fatigue in women with breast cancer undergoing radiation therapy. Oncol Nurs Forum. 2010;37:423–432. doi: 10.1188/10.ONF.423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC. Assessing symptom distress in cancer patients: the M.D. Anderson symptom inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 38.Wagner L, Sweet J, Butt Z, Lai J, Cella D. Measuring patient self-reported cognitive function: development of the functional assessment of cancer therapy-cognitive function instrument. J Support Oncol. 2009;7:W32–W39. [Google Scholar]
- 39.Cimprich B, Visovatti M, Ronis DL. The attentional function index- A self-report cognitive measure. Psychooncology. 2011;20:194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 40.Ahles TA, Saykin A. Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer. 2002;3(Suppl 3):S84–S90. doi: 10.3816/cbc.2002.s.018. [DOI] [PubMed] [Google Scholar]
- 41.Sanford SD, Beaumont JL, Butt Z, Sweet J, Cella D, Wagner L. Prospecrive longitudinal evaluation of a symptom cluster in breast cancer. J Pain Symptom Manag. 2014;47:721–730. doi: 10.1016/j.jpainsymman.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Lai J, Wagner L, Jacobsen PB, Cella D. Self-reported cognitive concerns and abilities: two sides of one coin? Psychooncology. 2014 doi: 10.1002/pon.3522. doi: 10.1002/pon.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirk-Sanchez NJ, McGough EL. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging. 2014;9:51–62. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz CE. Applications of response shift theory and methods to participation measurement: a brief history of a young field. Arch Phys Med Rehabil. 2010;91(Suppl 1):S38–S43. doi: 10.1016/j.apmr.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 45.Jansen CE, Miaskowski CA, Dodd MJ, Dowling GA. A meta-analysis of the sensitivity of various neuropsychological tests used to detect chemotherapy-induced cognitive impairment in patients with breast cancer. Oncol Nurs Forum. 2007;34:997–1005. doi: 10.1188/07.ONF.997-1005. [DOI] [PubMed] [Google Scholar]
- 46.Saykin A, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stommel M, Schoenborn CA. Accuracy and usefulness of BMI measures based on self-reported weigt and height: findings from the NHANES & NHIS 2001–2006. BMC Public Health. 2009;9:421. doi: 10.1186/1471-2458-9-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bove RM, Brick DJ, Healy BC, Mancuso SM, Gerweck AV, Bredella MA, Sherman JC, Miller KK. Metabolic and endocrine correlates of cognitive function in healthy young women. Obesity. 2013;21:1343–1349. doi: 10.1002/oby.20212. [DOI] [PMC free article] [PubMed] [Google Scholar]