Abstract
Objective
Surgical site infection (SSI) is one of the most common post-operative complications following vascular reconstruction, producing significant morbidity and hospital readmission. In contrast to SSI that develops while patients are still hospitalized, little is known about the cohort of patients that develop SSI following discharge. In this study, we explore the factors that lead to post-discharge SSI, investigate the differences between risk factors for in-hospital versus post-discharge SSI, and develop a scoring system to identify patients that might benefit from post-discharge monitoring of their wounds.
Methods
Patients who underwent major vascular surgery from 2005–2012 for aneurysm and lower extremity occlusive disease were identified from the American College of Surgeons National Surgical Quality Improvement Program Participant Use Files. Patients were categorized as having no SSI, in-hospital SSI, or SSI after hospital discharge. Predictors of post-discharge SSI were determined by multivariable logistic regression and internally validated by bootstrap resampling. Risk scores were assigned to all significant variables in the model. Summative risk scores were collapsed into quartile-based ordinal categories and defined as low-, low/moderate-, moderate/high-, and high-risk. Multivariable logistic regression was used to determine predictors of in-hospital SSI.
Results
Of the 49,817 patients who underwent major vascular surgery, 4,449 (8.9%) were diagnosed with SSI (2.1% in-hospital; 6.9% post-discharge). By multivariable analysis, factors significantly associated with increased odds of post-discharge SSI include female gender, obesity, diabetes, smoking, hypertension, coronary artery disease, critical limb ischemia, chronic obstructive pulmonary disease, dyspnea, neurological disease, prolonged operative time >4 hours, American Society of Anesthesiology classification IV or V, lower extremity revascularization or aortoiliac procedure, and groin anastomosis. The model exhibited moderate discrimination (bias-corrected c-statistic, 0.691) and excellent internal calibration. The post-discharge SSI rate was 2.1% for low-risk patients, 5.1% for low/moderate-risk patients, 7.8% for moderate/high risk patients, and 14% for high-risk patients. In a comparative analysis, comorbidities were the primary driver of post-discharge SSI whereas in-hospital factors (operative time, emergency case status) and complications predicted in-hospital SSI.
Conclusions
The majority of SSIs after major vascular surgery develop following hospital discharge. We have created a scoring system that can select a cohort of patients at high-risk for SSI following discharge. These patients can be targeted for transitional care efforts focused on early detection and treatment with the goal of reducing morbidity and preventing readmission secondary to SSI.
Introduction
Surgical site infection (SSI) is the most common nosocomial infection in surgical patients, accounts for 38% of post-operative complications,1,2 is the leading cause of unplanned and potentially preventable hospital readmission in surgical patients,3–5 and results in approximately 20,000 potentially preventable deaths each year.2,6–8 This complication translates into additional healthcare costs in the United States alone in excess of $3 billion per year.6–8
Recognition of the impact of SSI has led to the development of process measures to prevent SSI in the hospital. One major initiative, the Surgical Infection Prevention (SIP) Project, established SSI prevention measures (such as specified antibiotic schedules) which resulted in a 27% reduction in the incidence of SSI.9,10 These results were translated into the Surgical Care Improvement Project (SCIP), a nationwide effort with the goal of improving surgical care by reducing surgical complications including SSIs.10,11
The foregoing models focus on SSI prevention and assume that wounds post-operatively are monitored directly by surgical and nursing staff during the index hospitalization. However, monitoring virtually ceases once a patient leaves the hospital because the “routine” follow-up visit is usually scheduled 2–3 weeks following hospital discharge.12 This lack of monitoring is a concern, as the majority of patients do not have the experience or expertise to recognize early-stage wound infections.13 Thus, patients often return to the clinic or hospital with an advanced wound infection/complication that requires intensive treatment and, potentially, rehospitalization.11
Risk factors for SSI occurring after hospital discharge have not been extensively studied.8,14–16 To address this gap, we differentiated SSIs that occur pre-versus post-discharge in patients undergoing major vascular procedures. We then determined predictors for post-discharge SSI. Finally, using predictors for post-discharge SSI, we created and internally validated a risk-prediction model to facilitate assignment of risk for post-discharge SSI. Characterizing factors that determine which patients are at high-risk for developing SSI after hospital discharge has the potential to direct transitional care efforts towards the most susceptible patients which may allow early diagnosis and treatment, potentially precluding the need for readmission and reintervention.
Methods
Data acquisition and cohort selection
We analyzed data from 2005–2012 using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) Participant Use Files. The ACS-NSQIP provides outcomes data to participating hospitals for purposes of quality improvement. Data is collected by a trained surgical clinical reviewer on randomly assigned patients from the pre-operative period through 30-days post-operation. Details are recorded on patient demographics, comorbidities, operative variables, and postoperative outcomes by means of medical chart extraction, 30-day interviews and other processes. Clinical reviewer training for participating hospitals and data auditing confirms reliability. The use of NSQIP data does not require patient consent. Details of the database, data collection protocols and variable definitions are available online from the ACS-NSQIP website.17 The Health Services Institutional Review Board at the University of Wisconsin approved this study.
Using current procedural terminology codes we identified all patients who underwent open abdominal aortic aneurysm repair, open aortoiliac repair, and open lower extremity revascularization (codes available in Supplementary Table I, online only).
We defined SSI as a composite measure of four ACS-NSQIP variables: superficial SSI, deep SSI, organ-space SSI, and wound dehiscence/disruption. If a patient had multiple SSI diagnoses, the time to first diagnosis was used to determine whether the SSI occurred in-hospital or after hospital discharge. Patients were excluded from this analysis if they died prior to hospital discharge (2.9%).
Explanatory Variables
Primary exposure variables included NSQIP-provided patient demographics and comorbidities. Secondary exposure variables included perioperative characteristics and post-operative complications. Peripheral vascular disease is defined as a history of revascularization or amputation for atherosclerotic disease. We created composite variables as previously described18 for coronary artery disease (CAD) (angina, myocardial infarction, percutaneous coronary intervention or cardiac surgery) and neurological disease (cerebrovascular accident or stroke with or without neurological deficit, transient ischemic attack, hemiplegia, paraplegia, quadriplegia, CNS tumor, or impaired sensorium). Post-operative complications were classified as infectious (pneumonia, urinary tract infection), respiratory (reintubation, failure to wean from ventilator within 48 hours), renal (progressive renal insufficiency, acute renal failure), central nervous system (cerebral vascular accident, stroke with neurological deficit, coma > 24 hours, peripheral nerve injury), cardiac (cardiac arrest requiring CPR, myocardial infarction), DVT/PE (thrombophlebitis, deep vein thrombosis, pulmonary embolism), sepsis (sepsis, septic shock), transfusion for bleeding and graft failure. Post-operative complications occurring after hospital discharge and/or after development of a SSI were excluded. We defined a prolonged length of stay (LOS) as a post-operative in-hospital stay ≥ 2-weeks (i.e. the 90th percentile for postoperative LOS).
A subset of health history variables (i.e. history of surgically treated peripheral vascular disease, rest pain or gangrene, alcohol abuse) was missing in 21% of observations. Following methodology tested by Hamilton et al,19 and others,4 we created missing value indicators as part of the respective dummy variables. This method can be highly explanatory and also advantageous compared to statistical imputation, particularly if there is substantial variation in missing data amongst hospitals.19 These missing value indicators for respective variables were included in the multivariable analyses.
Outcome
We defined the primary outcome of interest as a SSI developing between hospital discharge and prior to 30-days post surgery. The secondary outcome of interest was SSI developing prior to hospital discharge. Patients with in-hospital SSI remained eligible for development of a post-discharge SSI.
Statistical analysis
First, we analyzed unadjusted associations of 30-day post-discharge SSI with primary and secondary exposures using chi-square testing. A multivariable logistic regression model with backward selection (P3>3.15 as exit criterion) was then used to evaluate variables associated with the risk of post-discharge SSI. During model fitting, we ensured inclusion of variables age, gender, race, body mass index (BMI), diabetes, smoking, and graft failure because of previously described association to SSI.14,18,20,21
This “final” model is subject to overfitting, and therefore may overestimate its own predictive power.22 To correct for this, we generated 200 random bootstrap samples with replacement from the original data set.23 Each bootstrapped sample had the same number of records as the original dataset. The same backward selection algorithm was used to fit the multivariable logistic regression model on each bootstrap sample. This model was “frozen” and then applied on the original data set.24 The difference between the c-statistic generated from the two models was used to calculate the statistical optimism. The mean statistical optimism was then calculated using the 200 bootstrap samples and was used to adjust the final model c-statistic to produce a bias-corrected estimate of the model’s reliability. The fit of the model was assessed using the Hosmer and Lemeshow goodness-of-fit test.22,25 A value of P <.05 was considered significant.
Risk Prediction Score
From the final model we created a simplified scoring system based on the regression coefficients. To derive the score, the beta coefficient of each risk factor was divided by the absolute value of the lowest coefficient and then rounded to the nearest integer, a methodology that has been previously described.26,27 The summative risk score for a patient developing a post-discharge SSI is the total of all points for each individual risk factor. We then divided the cohort based on risk score quartiles and computed respective post-discharge SSI risks by averaging the model-predicted risks for all patients in that quartile. The internal calibration of the scoring system was checked by comparing the predicted post-discharge SSI prevalence with the observed post-discharge SSI prevalence.
Secondary Analysis
To determine significant predictors of in-hospital SSI, the same backward selection multivariable logistic regression method described above was used; though for this model we excluded prolonged length of stay as a covariate.
Results
Patient Characteristics
The sample consisted of 49,817 patients who underwent major vascular surgery (open abdominal aortic aneurysm repair: n=8,866; open aortoiliac repair: n=13,248; open lower extremity revascularization: n=27,703). The majority of the patients were male (65%) and white (78%). The mean age was 67 years (standard deviation: 11 years). Vascular-related comorbidities were common, with many patients having hypertension (82%), CAD (29%), critical limb ischemia (34%), and peripheral vascular disease (42%). Almost half (46%) of patients were smokers. Many (62%) patients were overweight or obese.
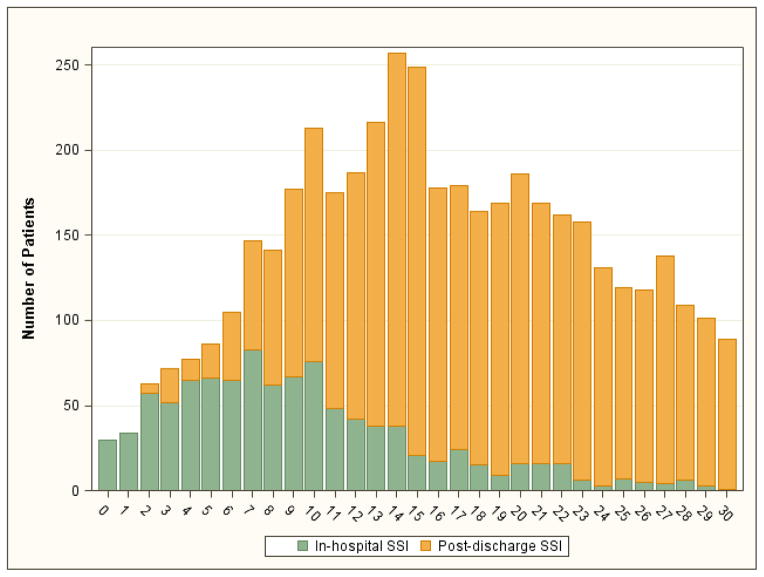
Out of all patients analyzed, 4,449 (8.9%) were diagnosed with an SSI (2.1% prior to discharge; 6.9% after hospital discharge). Rates of SSI by procedure and timing are displayed in Table I. The distribution of the number of days from surgery to a diagnosis of SSI (either in-hospital SSI or post-discharge SSI) is displayed in Figure 1. Only 24 (0.1%) patients were diagnosed with both in-hospital and post-discharge SSI.
Table I.
Rates of 30-day surgical site infection by operative procedure.
| Open AAA Repair (n=8,866) | Open Aortoiliac Repair (n=13,248) | Lower Extremity Revascularization (n=27,703) | |
|---|---|---|---|
| In-Hospital SSI, n (%) | 214 (2.4%) | 314 (2.4%) | 534 (1.9%) |
| Post-discharge SSI, n (%) | 171 (1.9%) | 820 (6.2%) | 2420 (8.7%) |
| Overall SSI, n (%) | 383 (4.3%) | 1128 (8.5%) | 2938 (11%) |
AAA, Abdominal Aortic Aneurysm
Figure 1. Distribution of the number of days from major vascular surgery procedure to diagnosis of SSI for all patients diagnosed with SSI.
A diagnosis occurring while in-hospital is represented in green; a diagnosis occurring after hospital discharge is represented in yellow.
SSI, Surgical site infection
Comparative univariate analyses
The characteristics of patients who developed SSI after discharge are displayed in Supplementary Table II. Patients who developed SSI after discharge, relative to those who did not, were more likely female (43% vs. 35%, p <.0001), obese (43% vs. 26%, p <.0001), insulin dependent diabetic (26% vs. 16%, p <.0001), to smoke (49% vs. 46%, p=.0077) or to have a diagnosis of critical limb ischemia (45% vs. 34%, p <.0001). Furthermore, these patients underwent reoperation within 30-days of the initial procedure at more than 3 times the rate of patients without post-discharge SSI (39% vs. 12%, p <.0001).
Multivariable analysis
Adjusted odds ratios (OR) and 95% confidence intervals (CI) for SSI occurring after hospital discharge are summarized in Table II. Aortoiliac repair or lower extremity revascularization was associated with higher odds of post-operative SSI relative to open AAA repair (OR, 2.4; 95% CI, 1.9–2.9 and OR, 3.1; 95% CI, 2.5–3.8 respectively). Being obese was also strongly associated with higher odds of SSI after discharge (OR, 2.3; 95% CI, 2.1–2.5). Other chronic conditions that were associated with elevated odds of developing SSI after discharge included insulin dependent diabetes (OR, 1.3; 95% CI, 1.2–1.4), critical limb ischemia (OR, 1.2; 95% CI, 1.1–1.3) and hypertension (OR, 1.2; 95% CI, 1.1–1.4). Additional risks factors included female gender (OR, 1.4; 95% CI, 1.3–1.5), smoking (OR, 1.2; 95% CI, 1.1–1.3), groin anastomosis (OR, 1.2; 95% CI, 1.0–1.4) and operative time >6 hours (OR, 1.5; 95% CI, 1.4–1.7). Certain variables were associated with reduced odds of post-discharge SSI with the most protective, unsurprisingly, being a post-operative length of stay ≥ 14 days (OR, 0.4; 95% CI, 0.3–0.5). Other patient characteristics that were protective include black race (OR, 0.8; 95% CI, 0.7–0.9) and totally dependent functional status (OR, 0.6; 95% CI, 0.4–0.9). Renal and sepsis complications were also protective (OR, 0.6; 95% CI, 0.4–0.9 and OR, 0.7; 95% CI, 0.5–0.9 respectively).
Table II.
Results of multivariable logistic regression analysis for predictors of 30-day post-discharge surgical site infection.
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Demographics | |||
| Female gender | 1.4 | (1.3, 1.5) | <.0001 |
| Age ≥ 65 | 0.9 | (0.9, 1.0) | .0426 |
| Race: | |||
| White | Reference | ||
| Black | 0.8 | (0.7, 0.9) | .0002 |
| Other | 0.9 | (0.8,1.0) | .2041 |
| Comorbidities | |||
| BMI | |||
| Normal | Reference | ||
| Overweight | 1.3 | (1.2,1.5) | <.0001 |
| Obese | 2.3 | (2.1, 2.5) | <.0001 |
| Underweight | 0.8 | (0.7, 1.1) | .1323 |
| Diabetes | |||
| No Diabetes | Reference | ||
| Non-insulin (PO) | 1.2 | (1.1, 1.3) | .0019 |
| Insulin | 1.3 | (1.2, 1.4) | <.0001 |
| Smoker | 1.2 | (1.1, 1.3) | <.0001 |
| Hypertension | 1.2 | (1.1, 1.4) | .0002 |
| Coronary Artery Disease | 1.1 | (1.0, 1.2) | .0307 |
| Critical Limb Ischemia | 1.2 | (1.1, 1.3) | <.0001 |
| Functional status | |||
| Independent | Reference | ||
| Partially dependent | 1.1 | (1.0, 1.2) | .0856 |
| Totally dependent | 0.5 | (0.4, 0.8) | .0066 |
| COPD | 1.1 | (1.1, 1.2) | .0327 |
| Dyspnea | |||
| None | Reference | ||
| Moderate exertion | 1.2 | (1.1, 1.3) | .0004 |
| At rest | 1.2 | (0.9, 1.6) | .1457 |
| Neurological Disease | 1.1 | (1.0, 1.3) | .0088 |
| Bleeding disorder | 1.1 | (1.0, 1.2) | .1389 |
| Loss of weight | 0.7 | (0.5, 1.0) | .0627 |
| Perioperative Characteristics | |||
| Emergency Case | 1.1 | (1.0, 1.3) | .1055 |
| Operation time | |||
| <4 hours | Reference | ||
| 4–6 hours | 1.4 | (1.3, 1.5) | <.0001 |
| >6 hours | 1.5 | (1.4, 1.7) | <.0001 |
| Operative Procedure | |||
| AAA | Reference | ||
| Open Aortoiliac | 2.4 | (1.9, 2.9) | <.0001 |
| Lower extremity revascularization | 3.1 | (2.5, 3.8) | <.0001 |
| Groin anastamosis | 1.2 | (1.0, 1.4) | .0110 |
| Graft | |||
| None | Reference | ||
| Vein | 0.9 | (0.7, 1.1) | .4533 |
| Other than vein | 0.8 | (0.7, 1.0) | .0717 |
| ASA class: | |||
| I or II | Reference | ||
| III | 1.1 | (0.9, 1.3) | .4209 |
| IV or V | 1.2 | (1.0, 1.4) | .0411 |
| Complications | |||
| Renal | 0.6 | (0.4, 0.9) | .0270 |
| Graft Loss | 0.2 | (0.0, 1.5) | .1242 |
| Sepsis | 0.7 | (0.5, 0.9) | .0118 |
| Other Adverse Outcomes | |||
| Prolonged LOS (≥14-days) | 0.4 | (0.3, 0.5) | <.0001 |
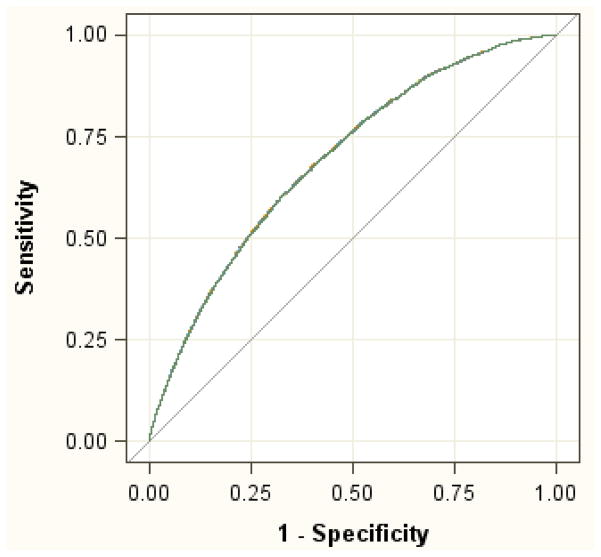
The unadjusted area under the receiver operator curve (ROC) of the model (c-statistic) was 0.694 (95% CI: 0.682 – 0.706). The adjusted ROC of the model was 0.691 (95% CI: 0.679 – 0.703).
OR, odds ratio; CI, confidence interval; LOS, Length of Stay; ASA, American Society of Anesthesiologists.
Bootstrap Analysis and Model Fit
The unadjusted c-statistic of the final model was 0.694 (95% CI: 0.682 – 0.706) (Figure 2). The adjusted c-statistic after bootstrap correction for model optimism was 0.691 (95% CI: 0.679 – 0.703). These similar values suggest there was only small bias due to overfitting and indicate fair predictive discrimination of the model. The Hosmer–Lemeshow goodness-of-fit test produced a χ2 value of 4.636 (df=8, P-value=0.7957) indicating excellent model internal calibration.
Figure 2.
The receiver operator curve (ROC) of the final model.
Risk Prediction Model
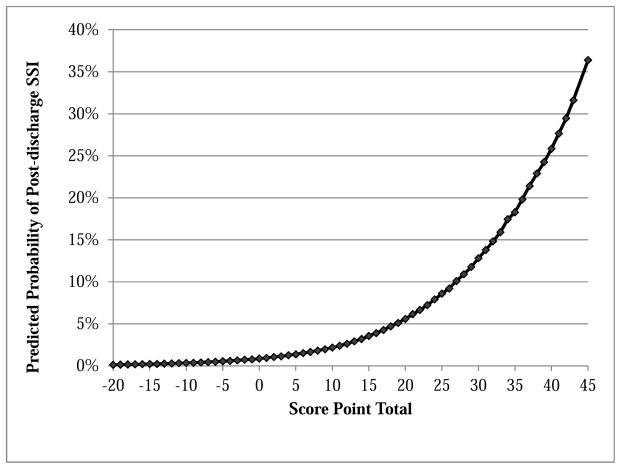
Overall, scores of significant predictors for post-discharge SSI ranged from −9 for prolonged length of stay to 12 for patients undergoing an open lower extremity revascularization. Total risk scores were generated for patients by adding the score from individual risk factors (Table III). For example, a female (+4) who is obese (+9), hypertensive (+2), totally dependent at baseline (−6), and experienced a prolonged operative case (+5) has a total risk score of 14. These summated risk scores ranged from −20 to 45 with the majority of scores (>90%) between 0 and 30. The 30-day post-discharge SSI risk associated with the total risk score represents the average risk amongst all patients having the same total score (Figure 3). The median risk score was 21 with a corresponding predicted post-discharge SSI probability of 6.1%. The sensitivity of the model was approximately 90% for a predicted risk threshold of 4% or greater, and approximately 50% for a threshold of 9% or greater. Further sensitivity and specificity values at varying predictive risk thresholds are displayed in Table IV.
Table III.
Risk scores for 30-day post-discharge surgical site infection derived from the multivariate logistic regression final model. The scores were generated as described in the methods.
| Variables | Points |
|---|---|
| Lower extremity revascularization | 12 |
| Aortoiliac procedure | 9 |
| Obese | 9 |
| Operative time >6 hours | 5 |
| Female gender | 4 |
| Overweight | 3 |
| Operative time 4–6 hours | 3 |
| Insulin dependent DM | 3 |
| Non-insulin dependent DM | 2 |
| Smoker | 2 |
| Hypertension | 2 |
| Critical Limb Ischemia | 2 |
| Dyspnea with moderate exertion | 2 |
| Groin anastomosis | 2 |
| ASA class IV or V | 2 |
| COPD | 1 |
| Coronary Artery Disease | 1 |
| Neurological Disease | 1 |
| Age ≥ 65 years | −1 |
| Black race | −2 |
| Sepsis complication | −4 |
| Renal complication | −6 |
| Totally dependent functional status | −6 |
| Prolonged LOS (≥14-days) | −9 |
DM, Diabetes Mellitus; COPD, Chronic Obstructive Pulmonary Disorder; ASA, American Society of Anesthesiologists; LOS, Length of Stay.
Figure 3. Predicted probability of post-discharge surgical site infection by total point score.
SSI, Surgical site infection
Table IV.
Sensitivity and specificity of final predictive model at varying predictive risk thresholds.a
| Predicted Risk | Sensitivity (%) | 95% CI (%) | Specificity (%) | 95% CI (%) |
|---|---|---|---|---|
| ≥1% | 99 | 99–100 | 3.5 | 3.4–3.7 |
| ≥2% | 98 | 97–98 | 14 | 13–14 |
| ≥3% | 95 | 94–95 | 21 | 21–22 |
| ≥4% | 90 | 89–91 | 31 | 30–31 |
| ≥5% | 82 | 81–84 | 42 | 41–42 |
| ≥6% | 73 | 72–75 | 53 | 52–53 |
| ≥7% | 64 | 63–66 | 62 | 62–63 |
| ≥8% | 57 | 55–58 | 70 | 70–71 |
| ≥9% | 49 | 47–51 | 76 | 76–77 |
| ≥10% | 42 | 40–43 | 81 | 81–82 |
| ≥12% | 30 | 29–32 | 88 | 88–89 |
| ≥15% | 17 | 16–18 | 95 | 94–95 |
| ≥20% | 5.5 | 4.9–6.5 | 99 | 98–99 |
| ≥25% | 1.6 | 1.2–2.0 | 99 | 99–100 |
| ≥30% | 0.5 | 0.2–0.7 | 100 | 99–100 |
Confidence intervals do not account for the uncertainty derived from development of the final model.
CI, confidence interval
To simplify the risk scores, we collapsed all patients into 4 ordinal categories based upon quartiles defined as low (<16 points), low-moderate (16–20 points), moderate-high (21–25 points), and high-risk (>25 points) groups. The respective post-discharge SSI predicted probabilities for these groups were 2.1%, 5.1%, 7.8% and 14% respectively (Table V). There was excellent agreement between the predicted and observed post-discharge SSI rates by risk category (R2=0.99) (Supplementary Figure 1, online only).
Table V.
Risk score group classification with predicted and actual risk.
| Risk category | Points | Predicted risk | Actual risk | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Low | < 16 | 2.1% | 2.1% | 97% | 14% |
| Low/Moderate | 16 – 20 | 5.1% | 5.2% | 82% | 43% |
| Moderate/High | 21 – 25 | 7.8% | 7.5% | 58% | 69% |
| High | > 25 | 14% | 14% | 21% | 93% |
Sensitivity and specificity percentages correspond to predicted risk thresholds.
Secondary outcomes
Significant variables associated with SSI occurring in-hospital are displayed in Supplemental table III. Demographic variables associated with higher odds of in-hospital SSI include female gender (OR, 1.3; 95% CI, 1.2–1.5) and “other” race vs. white race (OR, 1.4; 95% CI, 1.2–1.7). Comorbid factors associated with the greatest odds include totally dependent functional status (OR, 2.3; 95% CI, 1.6–3.2) and obesity (OR, 1.7; 95% CI, 1.5–2.0). Perioperative characteristics including emergency case (OR, 1.7; 95% CI, 1.4–2.1) and prolonged operative time (OR, 2.1; 95% CI, 1.8–2.6) were also were associated with increased odds. Post-operative complications were associated with increased odds of in-hospital SSI, with the most significant being sepsis preceding SSI (OR, 3.7; 95% CI, 3.0–4.5) and respiratory complications (OR, 2.6; 95% CI, 2.1–3.2).
Mutual and Unique Risk Factors
Significant factors associated with both in-hospital and post-discharge SSI include aortoiliac surgery, female gender, obesity, critical limb ischemia, American Society of Anesthesiology (ASA) class IV/V, and prolonged operative time. Significant unique factors associated with inhospital SSI include demographics and comorbid conditions: race, congestive heart failure, partially dependent functional status, dialysis, co-existing open or infected wound, and chronic steroid use; but also included perioperative characteristics and complications: emergency case status, ventilator-use prior to surgery, wound class, and respiratory, bleeding, graft-loss and sepsis complications. Significant unique factors associated with post-discharge SSI mainly include demographics and comorbid conditions: race, diabetes, smoking, hypertension, CAD, COPD, dyspnea, neurological disease, and lower extremity revascularization procedure. (Supplementary Table III, online only)
Discussion
By distinguishing between in-hospital versus post-discharge SSI, we define characteristics that may uniquely predispose vascular surgery patients to develop SSI after discharge. This distinction is essential because protocols to monitor and manage SSI occurring in the hospital must be different from those designed to monitor and manage SSI following discharge. As such, we determined which risk factors are significant in predicting post-discharge SSI and subsequently generated a simple and accurate scoring system that can assign a risk for post-discharge SSI. Furthermore, we have demonstrated that predictors of SSI occurring in-hospital indeed differ from those occurring after discharge for vascular surgery patients.
Historically, SSI is a well-known and common complication of patients undergoing vascular surgery with some studies citing wound complications in more than 30% of patients undergoing open vascular reconstruction.28,29 Using the ACS-NSQIP data, we found a SSI rate of 8.9% with approximately 77% of these having occurred after discharge from the hospital. It is important to note that in this analysis we have focused on patients undergoing open vascular reconstruction versus endovascular and have excluded patients treated with carotid endarterectomy (CEA) because the incidence of wound infection following CEA is very low.
In the current study, a multitude of factors ranging from those known prior to admission to those determined during the hospitalization were used in a multivariable logistic regression to identify variables associated with post-discharge SSI. Factors that were associated with higher odds of post-discharge SSI include female gender, procedure type (with operations involving the lower extremities and groin anastomosis at increased risk), obesity, diabetes, smoking, hypertension, critical limb ischemia, COPD, CAD, neurological disease, and ASA class IV/V. The majority of these risk factors have previously been described as significant risk factors for SSI in vascular patients including female gender,29,30 obesity,28,31,32, diabetes,29,33 CAD,29 smoking,21 COPD,18,29,34 prolonged operative time,18,34 and infrainguinal incisions.29 However, none of the prior studies explicitly determined whether these factors were unique to patients that develop SSI following hospital discharge. The timing of the development of SSI has been previously examined in a broad population of surgical patients. In a population-based retrospective cohort study of over 600,000 patients undergoing various elective surgical procedures, Daneman and colleagues reported independent risk factors for post-discharge SSI including female gender, increased duration of surgery, diabetes and obesity.15 In a separate prospective cohort study of 1,506 patients undergoing general surgery procedures, Delgado-Rodríguez and colleagues found the greatest independent risk factor for post-discharge SSI to be elevated BMI.16 Our results reinforce the pronounced impact of obesity on development of SSI after discharge.
We were able to demonstrate differences in characteristics and independent predictors between patients developing SSI in-hospital versus after discharge.14–16 SSIs occurring after discharge are predominantly associated with a patient’s comorbidity burden whereas those that develop inhospital are largely associated with perioperative factors. Specifically, we found that SSI occurring after hospital discharge is more common for patients with comorbid conditions such as diabetes, smoking, hypertension, CAD, and COPD. Although risk factors for SSI occurring inhospital also include select patient comorbidities, more dominant predictors are perioperative characteristics and complications such as emergency case status, wound class, and respiratory, bleeding and sepsis complications. These results suggest that patient comorbidity is the primary driver of infection and poor wound healing following discharge.
Proven transitional care interventions focused on wound surveillance are lacking; the majority of trialed transitional care models have only been tested or implemented for medical or combined medical/surgical patients.3,35–37 Moving forward, effective transitional care models will need to be customized for the surgical population, with an emphasis on wound monitoring. One established intervention that could be adapted is pre-discharge counseling followed post-discharge phone contact, which has been demonstrated by Kind et al36 to decrease readmissions in a Veterans Affairs population by one-third. An alternative method that has been suggested by Fernandes-Taylor and colleagues is to have rapid in-person follow-up within the first week after surgery for a wound examination in order to detect SSI at an early stage.12 And yet another alternative combines continuous contact with a patient along with early “virtual” follow-up by means of smartphone technology: patients take and transmit secured images of their wounds and symptom-related questions daily for clinician review.38 The implementation of effective transitional care focused on wound management and monitoring holds promise to decrease the burden of severe SSI in vulnerable patients.
We developed a prediction model for risk of SSI after hospital discharge and a corresponding risk scoring system that we believe with further validation can be useful for clinicians and patients for a number of reasons. A scoring system will allow the identification of high-risk individuals using readily available patient information. Additionally, use of a scoring system can assist clinicians inform selected patients of their risk for developing a wound infection and employ their assistance in wound care. Lastly, a scoring system can define for researchers the patient cohort at high-risk for wound infection allowing the design of patient specific protocols that might be effective in preventing this complication. Nevertheless, whereas our scoring system has been developed on one of the most representative and best-standardized surgical outcomes databases currently available for the U.S. population, we caution against any use in clinical practice until it can be validated through the use of external alternative data sources and independent analyses.
We found 5 factors associated with lower odds of developing post-discharge SSI. These findings were surprising and we can only speculate as why these factors are protective. There may be a selection effect among patients of black race as many African-American patients tend to undergo primary amputation without an antecedent attempt at revascularization;39 this speculation is substantiated as the protective effect of race remained when examining only patients with lower extremity occlusive disease, but was not present when analyzing only AAA patients. Sepsis and renal complications were also protective for post-discharge SSI; these patients are usually under close surveillance after discharge and likely have early follow-up, decreasing the chance of wound infection. Patients who are totally dependent at baseline were protected from post-discharge SSI; these patients, once out of hospital, are also likely to have close follow-up or existing caretakers that can avert wound complications.
We explored the relationship of reoperation to the development of SSI and found that reoperation within 30-days after surgery occurred more than 3 times as often in patients developing SSI after discharge compared to those without post-discharge SSI. Unfortunately in the majority of the patient cohort we were not able to determine the timing of infection versus reoperation, except in 2012 (the final year of data used in our analysis) when this information is available. For patients from this year, the diagnosis of SSI preceded or was made on the same day as reoperation in 75% of patients. In only 25% of patients did the diagnosis of SSI follow reoperation. Thus, it appears that both factors may be in play: reoperation for any reason likely leads to the development of SSI as reopening, remanipulation and possible contamination of the wound certainly has the potential to increase the incidence of infection; and alternatively, reoperation might have been required to treat a wound infection. As NSQIP continues to collect details on “time-to” reoperation, future reanalysis may further enhance a predictive model.
The results of this analysis should be interpreted in the context of several limitations, particularly those related to the database. Even though NSQIP samples from a very large number of hospitals, participation in NSQIP is voluntary and thus may not be representative of outcomes for all U.S. hospitals. Geographic or site-specific information are unavailable; therefore, hospital-type and procedure-volume cannot be examined. Furthermore, certain variables are not captured by NSQIP (e.g. seroma or use of prophylactic antibiotics) and therefore may limit the predictive power of our model. Our identified risk factors for SSI after hospital discharge may not apply to all surgical procedures because we studied only major vascular procedures; these patients have been shown to have exceptionally high rates of SSI and are not representative of the overall surgical patient population.
In conclusion, our risk score prediction model is the first to use known factors at the time of discharge to predict SSI in patients undergoing major vascular surgery. Identifying those patients who require ongoing monitoring after discharge and ensuring that they receive appropriate transitional care may uniquely stem the burden of SSI in this high-risk population.
Supplementary Material
Acknowledgments
Sources of Funding
Jason T. Wiseman is supported by an NIH research training grant (T32 HL110853). K. Craig Kent and Sara Fernandes-Taylor are supported by an AHRQ research grant (R21 HS023395). The project described was supported by the Clinical and Translational Science Award program, through grant UL1TR000427 from the NIH National Center for Advancing Translational Sciences.
Footnotes
This study was accepted for presentation at the 2015 Vascular Annual Meeting of the Society for Vascular Surgery, Chicago, Mass, June 17-20, 2015.
Conflicts of Interest: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Am J Infect Control. 1999 Apr;27(2):97–134. [PubMed] [Google Scholar]
- 2.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004 Dec;32(8):470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 3.Wiseman J, Guzman A, Fernandes-Taylor S, Engelbert T, Saunders R, Kent K. General and vascular surgery readmissions: A systematic review. J Am Coll Surg. 2014;219(13):552–69. doi: 10.1016/j.jamcollsurg.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta PK, Fernandes-Taylor S, Ramanan B, Engelbert TL, Kent KC. Unplanned readmissions after vascular surgery. J Vasc Surg Society for Vascular Surgery. 2013 Nov 15;315:1–10. doi: 10.1016/j.jvs.2013.09.002. in 2011. [DOI] [PubMed] [Google Scholar]
- 5.Engelbert TL, Fernandes-Taylor S, Gupta PK, Kent KC, Matsumura J. Clinical characteristics associated with readmission among patients undergoing vascular surgery. J Vasc Surg Society for Vascular Surgery. 2014 Dec 21;59(5):1349–55. doi: 10.1016/j.jvs.2013.10.103. [DOI] [PubMed] [Google Scholar]
- 6.De Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009 Jun;37(5):387–97. doi: 10.1016/j.ajic.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Kirkland K, Briggs J, Trivette S. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20(11):725–30. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 8.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003 Feb;9(2):196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellinger EP, Hausmann SM, Bratzler DW, Johnson RM, Daniel DM, Bunt KM, et al. Hospitals collaborate to decrease surgical site infections. Am J Surg. 2005 Jul;190(1):9–15. doi: 10.1016/j.amjsurg.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006 Aug 1;43(3):322–30. doi: 10.1086/505220. [DOI] [PubMed] [Google Scholar]
- 11.Surgical Care Improvement Project [Internet] The Joint Commission. 2014 Available from: http://www.jointcommission.org/surgical_care_improvement_project/
- 12.Saunders RS, Fernandes-Taylor S, Rathouz PJ, Saha S, Wiseman JT, Havlena J, et al. Outpatient follow-up versus 30-day readmission among general and vascular surgery patients: a case for redesigning transitional care. Surgery Elsevier Inc. 2014 Oct;156(4):949–56. doi: 10.1016/j.surg.2014.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitby M, McLaws M-L, Collopy B, Looke DFL, Doidge S, Henderson B, et al. Post-discharge surveillance: can patients reliably diagnose surgical wound infections? J Hosp Infect. 2002 Nov;52(3):155–60. doi: 10.1053/jhin.2002.1275. [DOI] [PubMed] [Google Scholar]
- 14.Gibson A, Tevis S, Kennedy G. Readmission after delayed diagnosis of surgical site infection: a focus on prevention using the American College of Surgeons National Surgical Quality Improvement Program. Am J Surg Elsevier Inc. 2014 Oct 10;207(6):832–9. doi: 10.1016/j.amjsurg.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daneman N, Lu H, Redelmeier Da. Discharge after discharge: predicting surgical site infections after patients leave hospital. J Hosp Infect. 2010 Jul;75(3):188–94. doi: 10.1016/j.jhin.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Delgado-Rodríguez M, Gomez-Ortega A, Sillero-Arneas M, Llorca J. Epidemiology of Surgical-site Infections Diagnosed After Hospital Discharge: A Prospective Cohort Study. Infect Control Hosp Epidemiol. 2001;22(1):24–30. doi: 10.1086/501820. [DOI] [PubMed] [Google Scholar]
- 17.American College of Surgeons - National Surgical Quality Improvement Program [Internet] Available from: http://site.acsnsqip.org/
- 18.Greenblatt DY, Rajamanickam V, Mell MW. Predictors of surgical site infection after open lower extremity revascularization. J Vasc Surg Elsevier Inc. 2011 Aug;54(2):433–9. doi: 10.1016/j.jvs.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton BH, Ko CY, Richards K, Hall BL. Missing data in the American College of Surgeons National Surgical Quality Improvement Program are not missing at random: implications and potential impact on quality assessments. J Am Coll Surg Elsevier Inc. 2010 Feb;210(2):125–39.e2. doi: 10.1016/j.jamcollsurg.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Bandyk DF. Vascular surgical site infection: risk factors and preventive measures. Semin Vasc Surg. 2008 Sep;21(3):119–23. doi: 10.1053/j.semvascsurg.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Neumayer L, Hosokawa P, Itani K, El-Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007 Jun;204(6):1178–87. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Harrell FE. Regression Modeling Strategies: with applications to linear models, logistic regression, and survival analysis. Springer; 2001. [Google Scholar]
- 23.Steyerberg E, FH Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 24.Harrell FEH, Lee KL, Mark DB. Tutorial in biostatistics multivariable prognostic models. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Shtatland E, Cain E, Barton M. The perils of stepwise logistic regression and how to escape them using information criteria and the output delivery system. Proc from 26th Annu SAS Users Gr Int Conf. 2001:222–6. [Google Scholar]
- 26.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg Elsevier Inc. 2010 Sep;52(3):539–48. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 27.McPhee JT, Nguyen LL, Ho KJ, Ozaki CK, Conte MS, Belkin M. Risk prediction of 30-day readmission after infrainguinal bypass for critical limb ischemia. J Vasc Surg Society for Vascular Surgery. 2013 Jun;57(6):1481–8. doi: 10.1016/j.jvs.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 28.Kent KC, Bartek S, Kuntz KM, Anninos E, Skillman JJ. Prospective study of wound complications in continuous infrainguinal incisions after lower limb arterial reconstruction: incidence, risk factors, and cost. Surgery. 1996 Apr;119(4):378–83. doi: 10.1016/s0039-6060(96)80135-8. [DOI] [PubMed] [Google Scholar]
- 29.Turtiainen J, Hakala T. Surgical wound infections after peripheral vascular surgery. Scand J Surg. 2013;(0):1–6. doi: 10.1177/1457496913514384. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen LL, Brahmanandam S, Bandyk DF, Belkin M, Clowes AW, Moneta GL, et al. Female gender and oral anticoagulants are associated with wound complications in lower extremity vein bypass: an analysis of 1404 operations for critical limb ischemia. J Vasc Surg. 2007 Dec;46(6):1191–7. doi: 10.1016/j.jvs.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giles K, Hamdan A, Pomposelli F. Body Mass Index: Surgical Site Infections and Mortality After Lower Extremity Bypass from the National Surgical Quality Improvement Program 2005–2007. Ann Vasc Surg. 2010;24(1):1–15. doi: 10.1016/j.avsg.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davenport DL, Xenos ES, Hosokawa P, Radford J, Henderson WG, Endean ED. The influence of body mass index obesity status on vascular surgery 30-day morbidity and mortality. J Vasc Surg The Society for Vascular Surgery. 2009 Jan;49(1):140–7. 147.e1. doi: 10.1016/j.jvs.2008.08.052. discussion 147. [DOI] [PubMed] [Google Scholar]
- 33.Virkkunen J, Heikkinen M, Lepäntalo M, Metsänoja R, Salenius J-P. Diabetes as an independent risk factor for early postoperative complications in critical limb ischemia. J Vasc Surg. 2004 Oct;40(4):761–7. doi: 10.1016/j.jvs.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 34.Haridas M, Malangoni Ma. Predictive factors for surgical site infection in general surgery. Surgery. 2008 Oct;144(4):496–501. doi: 10.1016/j.surg.2008.06.001. discussion 501–3. [DOI] [PubMed] [Google Scholar]
- 35.Hansen L, Young R. Interventions to Reduce 30-Day Rehospitalization: A Systematic Review. Ann Intern Med. 2011;155:520–8. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 36.Kind AJH, Jensen L, Barczi S, Bridges A, Kordahl R, Smith Ma, et al. Low-cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood) 2012 Dec;31(12):2659–68. doi: 10.1377/hlthaff.2012.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman EA, Parry C, Chalmers S, Min S. The Care Transitions Intervention. 2014. p. 166. [DOI] [PubMed] [Google Scholar]
- 38.Wiseman J, Fernandes-Taylor S, Engelbert T, Saunders R, Kent K. A Novel Approach to Postoperative Wound Surveillance: Using Available Smartphone Technology to Improve Transitional Care. J Surg Res. 2014;186(2) [Google Scholar]
- 39.Goodney PP, Holman K, Henke PK, Lori L, Dimick JB, Stukel TA, et al. Regional intensity of vascular care and lower extremity amputation rates. J Vasc Surg. 2013;57(6):1471–80. doi: 10.1016/j.jvs.2012.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.