Abstract
Background
The fraction of persons with influenza virus infection who do not report any signs or symptoms throughout the course of infection is referred to as the asymptomatic fraction.
Methods
We conducted a systematic review and meta-analysis of published estimates of the asymptomatic fraction of influenza virus infections. We found that estimates of the asymptomatic fraction were reported from two different types of studies: first, outbreak investigations with short-term follow-up of potentially exposed persons and virologic confirmation of infections; second, studies conducted across epidemics typically evaluating rates of acute respiratory illness among persons with serologic evidence of infection, in some cases adjusting for background rates of illness from other causes.
Results
Most point estimates from studies of outbreak investigations fell in the range 4%–28% with low heterogeneity (I2=0%) with a pooled mean of 16% (95% CI: 13%, 19%). Estimates from the studies conducted across epidemics without adjustment were very heterogeneous (point estimates 0%–100%; I2=97%), while estimates from studies that adjusted for background illnesses were more consistent with point estimates in the range 65%–85% and moderate heterogeneity (I2=58%). Variation in estimates could be partially explained by differences in study design and analysis, and inclusion of mild symptomatic illnesses as asymptomatic in some studies.
Conclusions
Estimates of the asymptomatic fraction are affected by the study design, and the definitions of infection and symptomatic illness. Considerable differences between the asymptomatic fraction of infections confirmed by virologic versus serologic testing may indicate fundamental differences in the interpretation of these two indicators.
Keywords: influenza, asymptomatic, public health
INTRODUCTION
Influenza virus infections lead to a wide range of clinical manifestations, from severe pneumonia through to mild or even asymptomatic disease (1). Asymptomatic infection is defined as infection without any signs or symptoms of that infection (2). There has been discussion over the proportion of influenza virus infections that are associated with asymptomatic disease, referred to as the asymptomatic fraction. An understanding on the asymptomatic fraction is important in two respects. First, improved estimation of the asymptomatic fraction could aid estimation and prediction of incidence of infection from surveillance data on symptomatic illnesses (3). Second, knowledge of the fraction of infections that are asymptomatic and their infectiousness relative to symptomatic infections would be important in optimizing public health control strategies such as contact tracing and quarantine, and characterizing transmission dynamics using mathematical models (4, 5). However, there is currently no consensus on the value of the AF with different studies typically using values from 20%–50% (4, 6–8). Therefore the objective of our study was to describe and summarize published estimates of the asymptomatic fraction, and to identify factors in study design or analysis that could contribute to differences in estimates of the asymptomatic fraction.
METHODS
Search Strategy
This systematic review was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (9). We identified publications on 11 April 2014 describing the asymptomatic fraction of influenza virus infections in PubMed and Scopus using the following search terms:
asymptomatic[All Fields] AND (“influenza, human”[MeSH Terms] OR (“influenza”[All Fields] AND “human”[All Fields]) OR “human influenza”[All Fields] OR “influenza”[All Fields]). The search was limited to entries created in the database on or before 11 April 2014 but was not limited by publication date. The authors’ own databases of full-text publications were also searched.
Study Selection
The titles of all articles identified by the search strategy were independently screened by two authors (N.H.L.L. and B.J.C.). Only articles written in English were included, and reviews and articles that did not contain empirical data (i.e. collection of clinical samples) on the number of people with any evidence of laboratory-confirmed infection were excluded (the definition of which is given in the next subsection). We then screened abstracts of potentially relevant papers, with studies excluded if 1) abstract or full text was not available, 2) participants were taking antiviral prophylaxis, 3) influenza infections were not laboratory-confirmed, 4) symptoms were not reported or 5) the asymptomatic fraction was undetermined. The full texts of the remaining articles were then reviewed for eligibility. Studies were eligible for inclusion if they provided an estimate of the AF defined as laboratory-confirmed infection without any signs or symptoms, or if not, the number of individuals assessed to be infected with laboratory confirmation together with the number of those who have no evidence of symptomatic illness. Volunteer challenge studies (10) were excluded from the present review, which focused on natural infections, because of the potential for mode of inoculation and infectious dose to affect the probability and severity of symptomatic illness (11, 12). Studies that reported the asymptomatic fraction as the probability of influenza virus infection conditional on asymptomatic illness were also excluded (13–15).
Definition of asymptomatic fraction
The asymptomatic fraction is defined as the probability of illness without any signs and symptoms, or not fulfilling the criteria of illness as defined by the individual studies, conditional on laboratory-confirmed infection. The estimate of asymptomatic fraction was typically reported in the studies as the proportion of individuals without symptoms (or not fulfilling the study-specific case definition) among all individuals with laboratory-confirmed infection. Case definitions of asymptomatic illness included completely asymptomatic (without any symptoms), absence of acute respiratory illness (ARI, usually defined as the presence of respiratory symptoms such as fever/feverish, cough, sore throat, headache, fatigue, muscle pain and runny nose with slight variations across different studies), absence of influenza-like illness (ILI, usually defined as the presence of fever plus cough or sore throat) or absence of fever. Laboratory-confirmed influenza virus infection was defined as an infection that was confirmed by virologic testing either by reverse transcriptase polymerase chain reaction (PCR) or viral culture on a respiratory specimen such as a nasal swab; or an infection indicated by serologic testing by hemagglutination-inhibition (HI), microneutralization (MN), or complement fixation assay (CF), with a ≥4-fold rise in antibody titer in paired sera across an epidemic, or a titer ≥40 in a single serum specimen.
Data extraction
Our principal summary measures were the estimates along with 95% confidence intervals (CI) of the asymptomatic fraction. We extracted whenever available point estimates and 95% CIs of reported asymptomatic fractions, counts of the number of individuals who had laboratory-confirmed infection, and counts of the number of individuals who were asymptomatic among infected, and documented other features of the studies on a standardized form including study design, age range of participants, influenza types/subtypes recovered, laboratory assays used to identify influenza virus infection, the definition of influenza virus infection and of asymptomatic illness, and whether estimates of the asymptomatic fraction were adjusted for background rates of acute respiratory illnesses not due to influenza virus infection. When estimates and 95% CIs of asymptomatic fractions were not reported in the studies they were calculated from the number of individuals infected and the number of those who were asymptomatic, assuming a binomial distribution.
Statistical Analysis
We constructed a forest plot of the estimates and 95% CIs of the asymptomatic fraction using the estimates reported in the studies or calculated from the counts of number of individuals infected and counts of number of infected individuals who were asymptomatic. Estimates of the asymptomatic fraction were classified by type of study and heterogeneity was estimated using the I2 statistic with a random-effects model (16). I2 is interpreted as the proportion of total variation in the effect estimates that is due to heterogeneity between studies, with an I2 of 0% indicating that all variability is due to sampling error within studies and I2 values of 25%, 50% and 75% indicating low, medium and high degrees of heterogeneity respectively (17, 18). Pooled estimates of the asymptomatic fraction would only be made if there was low heterogeneity. All analyses were conducted with R version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria) and the meta for package (19).
RESULTS
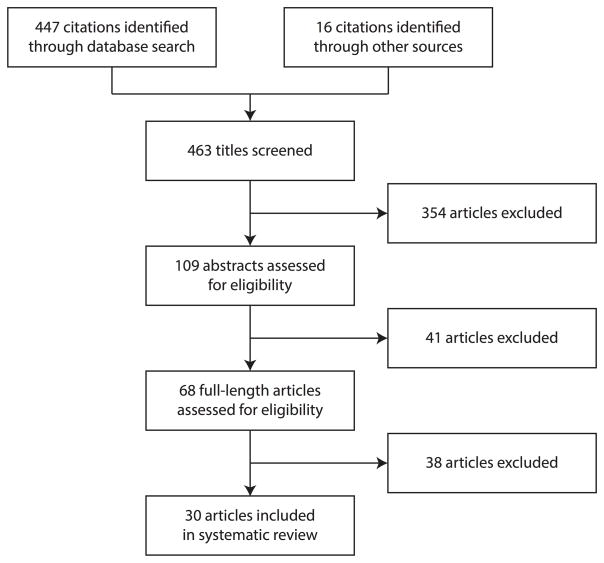
We identified 463 titles in the first step. We then reviewed 109 abstracts and 68 full-length articles, and eventually selected 30 articles for inclusion in this review (Figure 1). The articles could be classified into two types of study design: outbreak investigations (11 studies) and trans-epidemic studies (19 studies). The characteristics of the 30 included studies are summarized in Table 1.
Figure 1.
Flow diagram of the process and results of study selection.
Table 1.
Summary of characteristics of studies included in the review of the asymptomatic fraction (AF) of influenza virus infections
| Reference | Study Period | Study design | Laboratory method | Infection Definition | Asymptomatic Definition |
Influenza types/subtypes | Age | Adjustment/Comparison with background rates |
Remarks |
|---|---|---|---|---|---|---|---|---|---|
| Aho 201043 | Nov 2009 – Dec 2009 | Serologic studies | HI | single serum HI titer ≥ 40 | No ARI | H1N1pdm09 | 20 – 28y | Unadjusted | Reported the estimate of the AF as 50% (=84/169) based on HI titer ≥10 as definition for confirmed infection; Estimate of the AF became 51.9% (=40/77) if based on HI titer ≥40 as definition for confirmed infection. Therefore we extracted the estimate of the AF as 40/77 (51.9%) |
| Cowling 201021 | Jul 2009 – Aug 2009 | Outbreak/epidemic investigations | PCR | PCR +ve | No ARI | H1N1pdm09, H3N2 | All ages | Unadjusted | - |
| Gill 197646 | Apr 1974 – Oct 1974 | Serologic studies | HI | paired sera HI titer ≥ 4-fold rise | No ARI | H3N2 | 4 – 83y | Unadjusted | - |
| Gill 197747 | Apr 1976 – Oct 1976 | Serologic studies | HI | paired sera HI titer ≥ 4-fold rise | No symptoms | H3N2 | 14 – 68y | Unadjusted | Individual-level data on symptoms and age were provided, therefore age-specific AF could be estimated as follows: 0/6 (0%) for 14–19y, 3/26 (11.5%) for 20–49y, 1/17 (5.9%) for ≥50y |
| Gill 198548 | 1977/78 and 1980/81 influenza seasons | Serologic studies | HI | paired sera HI titer ≥ 4-fold rise | No symptoms | H1N1(1980/81), H3N2 (1977/78) | 11 – 90y | Unadjusted | - |
| Hall 197349 | Nov 1965 – May 1969 | Serologic studies | CF | paired sera CF titer ≥ 4-fold rise | No ARI | B | ≥ 1.5y | Compared | Also reported the estimate of the AF in non-seroconverted individuals was 21/33 (64%) |
| Hayward 201432 | Oct 2006 – Jul 2011 | Serologic studies | HI | paired sera HI titer ≥ 4-fold rise | No ARI | H1N1pdm09, H1N1, H3N2, B | ≥ 5y | Adjusted | Denominator based on person-season of follow-up, with the AF adjusted for background illness: 192 respiratory illnesses over 280 person-seasons of follow-up from 327 participants with serological evidence of infection, versus 623 respiratory illnesses over 1423 person-seasons of follow-up from 1742 participants without serological evidence of infection, i.e. rate of respiratory illness attributable to influenza was 23 (95% CI 13–34) illnesses per 100 person-seasons; Reported insufficient power to test whether the AF varied by age or subtype |
| Horby 201235 | Dec 2007 – Apr 2010 | Serologic studies | PCR or HI | PCR +ve; OR paired sera HI titer ≥ 4-fold rise; OR single serum HI titer ≥ 160 (seasonal influenza) or ≥ 80 (H1N1pdm09 in <40y) |
No ILI | H1N1pdm09, H1N1, H3N2, B | ≥ 5y | Unadjusted | Denominator based on person-season of follow-up: 62 respiratory illnesses over 438 person-seasons of follow-up with evidence of infection, from a total of 1,793 person-seasons of follow up from a cohort of 932 participants |
| Jackson 201122 | May 2009 – Jun 2009 | Outbreak/epidemic investigations | HI or MN | paired sera HI/MN titer ≥ 4-fold rise; OR single serum HI titer ≥ 20 and single serum MN titer ≥ 40 | No ARI | H1N1pdm09 | All ages | Unadjusted | Each of the 4 asymptomatic infected individuals belonged to each of the 4 age groups (0–9y, 10–18y, 19–54y, ≥55y) |
| Jaeger 201138 | Mar 2009 – May 2009 | Serologic studies | HI or MN | paired sera HI/MN titer ≥ 4-fold rise | No ARI | H1N1pdm09 | 19 – 74y | Unadjusted | Among the 9 seropositive individuals, 6 were identified by seroconversion ≥4-fold, and the remaining 3 were identified by a MN titer of ≥40 plus a HI titer of ≥20 |
| Khaokham 201336 | Feb 2006 – Oct 2009 | Serologic studies | PCR or HI | PCR +ve; OR paired sera HI titer ≥ 4-fold rise |
No ARI | H1N1pdm09 | ≥ 19y | Compared | Among the 142 confirmed infections, 123 were identified by HI, 16 by PCR, and 3 by both HI and PCR; The reported estimate of the AF was adjusted for age, sex, race, service, rank and shipboard location of exposure to ill persons; Also reported the estimate of the AF in non-infected indidividuals was 69% (95% CI 64–74%) |
| LaForce 200744 | 2000/01 influenza season | Serologic studies | PCR or culture or HI | PCR +ve; OR culture +ve; OR paired sera HI titer ≥ 4-fold rise |
No ARI | H1N1, H3N2, B | ≥ 12y | Unadjusted | Among the 52 confirmed infections, 51 were identified by seroconversion and the remaining case by viral culture in respiratory specimen |
| Lau 201023 | Jan 2008 – Sep 2008 | Outbreak/epidemic investigations | PCR | PCR +ve | No symptoms | H1N1, H3N2, B | All ages | Unadjusted | - |
| Li 201140 | Sep2009 – Oct 2009 | Serologic studies | HI and MN | single serum HI titer ≥ 40 and single serum MN titer ≥ 20 | No symptoms | H1N1pdm09 | 15 – 21y | Unadjusted | Among the 505 confirmed infections, all were identified by serology except 2 by PCR in throat swab; With 156 asymptomatic patients, in the abstract reported the “asymptomatic infection rate (risk) was 9.9%” with the denominator based on 1570 students who have participated in the survey; but in the full text reported 30.9% with the denominator based on 505 students who have confirmed infection. Therefore we extracted the estimate of the AF as 156/505 (30.9%) |
| Loeb 201228 | Dec 2007 – Jun 2010 | Outbreak/epidemic investigations | PCR | PCR +ve | No symptoms | H1N1, H1N1pdm09, H3N2, B | All ages | Unadjusted | Denominator based on person-season of follow-up: 23 asymptomatic viral shedding episodes among 238 viral shedding episodes from 208 participants; Reported the AF for H3N2 infections was statistically significantly lower than other subtypes (H3N2: 0%, H1N1: 15%, H1N1pdm09: 12%, B: 5%) |
| Monto 198534 | 1976/77 and 1977/78 influenza seasons | Serologic studies | HI | paired sera HI titer ≥ 4-fold rise | No symptoms | H3N2 (1977/78), B (1976/77) | All ages | Adjusted | Reported “pathogenicity index”, ie. illness rate in seroconverted individuals minus that in non-seroconverted individuals: 15.1% for influenza A/H3N2 and 33.7% for influenza B. Therefore we extracted the estimates of the AFs as 1–15.1%=84.9% for influenza A/H3N2 and 1–33.7%=66.3% for influenza B. Also reported the age-specific pathogencity index, and we converted it to AF as below: A/H3N2: 92.9% (0–4y), 75.6% (5–19y), 93.5% (20–49y), 68.6% (≥50y) B: 90.3% (0–4y), 64.1% (5–19y), 58.6% (20–49y), 86.7% (≥50y) |
| Mutsch 200550 | Jan 1998 – Mar 2000 | Serologic studies | HI | paired sera HI titer ≥ 4-fold rise | No fever | H1N1, H3N2, B | 12 – 83y | Unadjusted | Reported “32/40 (80%) were asymptomatic”, but the 40 cases were based on 18 confirmed (paired sera HI titer ≥4-fold rise) and 22 probable (paired sera HI titer 2–3.9 fold rise) cases. Therefore we extracted the estimate of the AF as 5/18 (27.8%) |
| Neatherlin 201337 | Apr 2009 – Jun 2009 | Serologic studies | HI and MN | single serum HI titer ≥ 20 and single serum MN titer ≥ 40 | No ARI | H1N1pdm09 | All ages | Unadjusted | - |
| Papenburg 201024 | May 2009 – July 2009 | Outbreak/epidemic investigations | PCR | PCR +ve | No symptoms | H1N1pdm09 | All ages | Unadjusted | Reported the estimate of the AF as 5/53 (9.4%) based on the result from MN in paired sera or/and PCR in nasopharyngeal swabs, but only individuals ≥7y provided paired sera while all individuals provided swabs. Therefore we extracted the estimate of the AF based on PCR-confirmed infection only which was 3/45 (6.7%); The estimate of the AF based on seroconversion was 3/36 (8.3%) |
| Riley 201139 | Jul 2009 – Feb 2010 | Serologic studies | MN | paired sera MN titer ≥ 4-fold rise | No ARI | H1N1pdm09 | ≥ 3y | Compared | Also reported the age-specific AF: 10/41 (24.4%) for 0–18y, 19/45 (42.2%) for ≥19y; Also reported the estimate of the AF in non-seroconverted individuals: 28/57 (49.1%) for 0 – 18y, 423/627 (67.5%) for ≥19y, i.e. 451/684 (65.9%) for all ages |
| Sagrera 200229 | Feb 1999 – Oct 1999 | Outbreak/epidemic investigations | culture | culture +ve | No symptoms | A (subtype not reported) | <1y | Unadjusted | - |
| Simmerman 201125 | Apr 2008 – Aug 2009 | Outbreak/epidemic investigations | PCR or HI | PCR +ve; OR paired sera HI titer ≥ 4-fold rise |
No symptoms | H1N1, H1N1pdm09, H3N2, B | All ages | Unadjusted | Among the 343 confirmed infections, 309 were identified by PCR and 34 by HI; Also reported symptomatic cases (mean age 30y) were significantly older than asymptomatic cases (38y) |
| Smit 201230 | Aug 2009 – early 2010 | Outbreak/epidemic investigations | PCR or HI | PCR +ve; OR paired sera HI titer ≥ 4-fold rise |
No ARI | H1N1pdm09 | ≥ 18y | Unadjusted | Among 3 seroconverted non-vaccinated individuals, 2 were asymptomatic. The remaining symptomatic individual was also positive by PCR, did not meet the criteria for influenza-like illness but reported some influenza-like signs and symptoms (cough, headache and malaise), therefore we extracted the estimate of the AF as 2/3 (66.7%) using no ARI as the definition for asymptomatic instead of no ILI as was used in the study |
| Suess 201026 | Apr 2009 – Aug 2009 | Outbreak/epidemic investigations | PCR | PCR +ve | No symptoms | H1N1pdm09 | All ages | Unadjusted | - |
| Suess 201220 | Jan 2008 – Apr 2011 | Outbreak/epidemic investigations | PCR | PCR +ve | No ILI | H1N1, H1N1pdm09, H3N2, B | All ages | Unadjusted | Also reported the age-specific AF: 0/21 (0%) for children <14y, 9/44 (21%) for adults ≥14y |
| Tandale 201045 | Sep 2009 – Oct 2009 | Serologic studies | HI | single serum HI titer ≥ 40 | No ILI | H1N1pdm09 | All ages | Unadjusted | Reported “Almost 90% pandemic H1N1 infections were asymptomatic or mild” |
| Thai 201427 | Sep – Dec 2009 | Outbreak/epidemic investigations | PCR | PCR +ve | No ARI | H1N1pdm09 | All ages | Unadjusted | This study included a subset of the subjects from Horby et al. AJE 2012, but in the present study only PCR-confirmed infection (but not by seroconversion) was used for the estimation of the AF |
| Toyokawa 201141 | Jun 2009 – Jul 2009 | Serologic studies | HI | single serum HI titer ≥ 40 | No fever | H1N1pdm09 | 29 – 49y | Unadjusted | Reported most of the seropositive HCWs were asymptomatic (only 1 had fever out of the 14 seropositive), but 8 out of the 14 seropositive had received chemoprophylaxis including the individual with fever. Therefore we extracted the estimate of the AF as 6/6 (100%) |
| Wang 201033 | 2005/06 influenza season | Serologic studies | HI | paired sera HI titer ≥ 4-fold rise | No ILI | H1N1, H3N2 | 9 – 11y | Adjusted | The estimate of the AF was adjusted by log-linear binomial regression model accounting for the probability of not showing symptoms because of other pathogens |
| Williams 201042 | Jan 2007 – Mar 2007 | Serologic studies | HI | paired sera HI titer ≥ 4-fold rise | No ARI | H1N1, H3N2, B | Adults | Unadjusted | - |
ARI indicates acute respiratory illness; CF, complement fixation assay; HI, hemagglutination-inhibition assay; ILI, influenza-like illness; MN, microneutralization assay; PCR, polymerase chain reaction; +ve, detection of viral RNA (by PCR) or infectious virions (by viral culture).
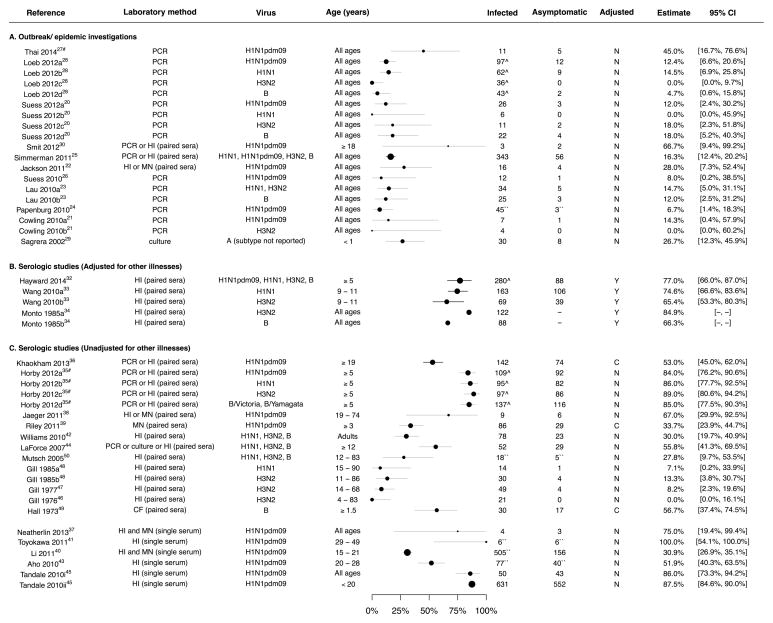
Studies in the group of outbreak/epidemic investigations included eight household transmission studies (20–27) and three studies in other settings (28–30). In these studies, identification of initial laboratory-confirmed cases was followed by intense follow-up of exposed persons that included repeated collection of respiratory specimens or sera regardless of symptomatic illness. The asymptomatic fraction could then be estimated among exposed persons (excluding the initial cases) based on the proportion of laboratory-confirmed infections without symptomatic illness. Point estimates of the asymptomatic fraction from the studies in this group fell within the range 4%–28% or had wide confidence intervals extending into this range (Figure 2A). Heterogeneity measured by the I2 statistic was low (0%) with a pooled mean of 16% (95% confidence interval, CI: 13%, 19%). Loeb et al. reported that the asymptomatic fraction was lower for H3N2 infections compared to infections with H1N1 and B (28), while there were no differences between subtypes in some other studies (20, 31).
Figure 2.
Forest plot of estimates of the asymptomatic fraction (‘Estimate’), stratified by study design. Panel A: estimates from outbreak investigations in which potentially exposed individuals were followed intensively for a short time and infections were typically confirmed by virologic methods. Panel B and C: estimates from cohort studies in which individuals were followed across entire influenza seasons, and numbers of illnesses assessed in individuals with serologic evidence of infection. Estimates in Panel B were adjusted for rates of symptomatic illness in uninfected persons, and not adjusted in Panel C.
Footnotes: The values for 95% confidence interval (“95% CI”) were either supplied from the articles (black) or derived from the point estimates (grey). We cannot derive the 95% CI for Monto et al. (34) as the number of individuals who were asymptomatic among infected was not provided. If individual estimates for different subtypes of influenza A virus (a–d) or populations (i–ii) from the same study were provided, they were presented separately. Studies by Thai et al. (27) and Horby et al. (35) were conducted in the same cohort of subjects (#). For some of the studies estimates of the asymptomatic fractions and counts were extracted differently from what was reported (``) and justifications were given in Table 1. Some studies reported estimates of the asymptomatic fractions with denominator based on person-season of follow up (^). The column “Adjusted” indicates whether estimate of the asymptomatic fraction was adjusted (Y) for rates of symptomatic illness in uninfected persons or not (N), or although not adjusted a separate estimate of the asymptomatic fraction was reported for individuals without evidence of laboratory-confirmed influenza virus infections (C). Remarks for each individual study are included in Table 1.
Abbreviations. PCR: reverse transcriptase polymerase chain reaction; HI: hemagglutination-inhibition assay; MN: microneutralization assay; CF: complement fixation assay; culture: viral culture; paired sera: the corresponding serologic assay (HI, MN or CF as indicated) was conducted in baseline and convalescent sera; single serum: the corresponding serologic assay was conducted in a single serum specimen; +ve: positive.
The other 19 studies could be grouped together as serologic studies where individuals were followed up across entire epidemics, and testing of single or paired sera was used to identify infections, rarely in combination with virologic testing (32–50). Illness reports in the same individuals could then be used to infer how many influenza virus infections might have been symptomatic. The earliest study we identified was published in 1973 (49). Overall, point estimates of the asymptomatic fraction from this group of studies were spread over a wide range of 0%–100% with very high heterogeneity (I2=97%) (Figure 2B and 2C).
In one early study, Monto et al. defined the “pathogenicity index” as the excess rate of illnesses in individuals with serologic evidence of infection compared to those without (34). In their study, Monto et al. subtracted illness rates in individuals without rises in paired titers from illness rates in individuals with titer rises, stratifying by age and then calculating the weighted mean. Assuming that the risk of influenza virus infection was independent of the rate of non-influenza illnesses, the authors estimated that at least 15.1% of influenza A(H3N2) and 33.7% of influenza B virus infections led to symptomatic illness (34). Most studies did not adjust for rates of illness from other non-influenza causes in this way, while one study used a similar approach to the pathogenicity index described above (32), and another study used a regression method (33). The five adjusted estimates of the asymptomatic fraction (Figure 2B) were in the range 65%–85% and were higher than most of the unadjusted estimates (Figure 2C). There was less heterogeneity among the studies that reported adjusted estimates, with I2 statistics of 58% for adjusted versus 97% for unadjusted estimates.
While most studies defined the asymptomatic fraction as infection completely without symptoms, some studies presented estimates of the asymptomatic fraction in terms of the proportion of infected persons that did not have febrile illness (41, 50), or the proportion of infected persons that did not have an illness which fulfilled a case definition for influenza-like illness that included fever (Table 1) (20, 30, 33, 35, 45).
Most of the studies (24/30) did not report data on age-specific asymptomatic fractions (Table 1), while in two studies the estimates of the asymptomatic fraction did not allow stratification by age because either all or none of the cases was asymptomatic (41, 46). In the remaining four studies where the age group-specific AFs (20, 34, 39) or data at individual level (47) were reported, the estimates of the asymptomatic fraction for influenza A tended to be higher in adults than in children or elderly, but Monto et al. reported that the pathogenicity index was highest in adults with influenza B virus infection after adjusting for other illnesses (34).
A few of the excluded studies are worthy of mention. Three studies presented the probability of influenza virus infection among asymptomatic persons, which is quite different to the asymptomatic fraction as we defined it above and strongly depends on the prevalence of infection (13–15). We excluded one study that determined laboratory-confirmed cases from both the recovery of viral RNA from intense follow up and from serologic evidence of infection across an epidemic, without providing a breakdown (51). One study measured the prevalence of influenza virus infection among inbound international airline travelers with symptomatic and asymptomatic illness (52), allowing inference on the fraction of infections associated with asymptomatic or pre-symptomatic virus shedding although such an estimate was not reported. Another study investigated asymptomatic infection among re-infected individuals, and reported that occurrence of symptoms was prevented during reinfection with a closely related virus even five years later (53).
DISCUSSION
Estimates of the asymptomatic fraction are affected by the study design, and the definitions of infection and symptomatic illness. Estimates of the asymptomatic fraction based on outbreak investigations and household transmission studies appeared to provide more homogeneity in estimates of the asymptomatic fraction, with most point estimates in the range 4%–28% and a pooled mean of 16% (95% CI: 13%, 19%) (Figure 2A). Advantages of outbreak investigations and household transmission studies in determining the asymptomatic fraction include the reduced risk of recall bias in symptom reporting with intense prospective follow-up, and the ability to identify the time of infection within a short time frame. However, determining infections based on polymerase chain reaction (PCR) may under-ascertain some infections, since it has been reported that some exposed persons can have serologic evidence of infection without PCR-confirmed infection or symptomatic disease. For example, a study in Hong Kong reported that 6/19 (32%) of exposed persons with 4-fold or greater rises in antibody titer did not have PCR-confirmed infection and did not report symptoms (21). In addition, studies of this type might underestimate the asymptomatic fraction if symptomatic illnesses not due to influenza virus infection were misattributed to influenza.
We identified considerable variability in estimates of the asymptomatic fraction based on cohort studies with point estimates from 0%–100% (Figure 2B and 2C). It is unclear whether this heterogeneity is indicative of real differences in the asymptomatic fraction in different studies and settings. It is possible that infections acquired in the community are milder on average than secondary cases in outbreaks in households or other confined settings, because of the less intense exposure from the community so that lower infection dose might lead to milder illness (10, 54). Infection indicated by serology could be an indicator of adaptive protection which would lead to more asymptomatic infections in individuals with prior exposures or older age (53). On the other hand, it is possible to consider a number of reasons why the heterogeneity might be artefacts of the study design, including variation in the degree of under-reporting of illnesses, and varying definitions of serologic evidence of infection and asymptomatic infection. Regarding the definitions of serologic evidence of infection, most studies used ≥4-fold rise in antibody titer in paired sera to indicate infection, but some studies used less stringent (55) or more stringent (35) criteria. The use of seropositivity in a single serum specimen to indicate infection during the study period could have led to misclassification of some infections in some studies, as individuals might have different baseline titers prior to the study period. Regarding the definitions of “asymptomatic”, many of the studies did not define the asymptomatic fraction explicitly. Some studies presented estimates of the asymptomatic fraction using a definition that included symptomatic illnesses in the numerator, as individuals not fulfilling the specified case definitions (e.g. influenza-like illness) were considered asymptomatic (33, 38, 41, 45, 50). However, a considerable proportion of persons with influenza virus infection have afebrile but symptomatic disease (24, 36, 39, 42) which could have led to overestimation of the asymptomatic fraction.
A few studies adjusted for symptomatic illnesses not caused by influenza (32–34), and some other studies compared rates of disease in persons with versus without evidence of infection without making a single adjusted estimate of the asymptomatic fraction (36, 39, 49). The adjusted estimates (32–34) (Figure 2B) were more consistent with point estimates in the range 65%–85%. Such approaches require the assumption that the risk of non-influenza illnesses is independent of the risk of influenza virus infections, which might not always hold (5, 31, 56). The idea of non-independence is not new (56) and the implication for estimation of the asymptomatic fraction was explicitly discussed by Monto et al. who wrote that their approach might underestimate the pathogenicity of the virus in question, “because influenza may replace another illness during a limited time period” (34). This remains controversial.
In the outbreak studies, a reduced asymptomatic fraction among H3N2 infections would be consistent with greater seriousness of H3N2 compared to H1N1 and B infections (28, 57). Some studies could not identify significant differences in the asymptomatic fraction between types/subtypes (21, 33, 35), and some reported lower estimates of the asymptomatic fraction for H1N1 (48) and B (34).
Given that disease severity is known to vary by age (58–60), and that immunity changes substantially with age (61, 62), it would be reasonable to hypothesize changes in the asymptomatic fraction with age. However, most of the studies that we reviewed did not provide sufficient data to allow stratification of the estimates of the asymptomatic fraction by age (Table 1). Most studies also did not report on the vaccination status of the infected individuals who did not report symptoms, although given the timing of studies conducted during the first wave of H1N1pdm09, participants in those studies would not have been vaccinated against H1N1pdm09. More data on factors that might affect the estimates of the asymptomatic fraction would be valuable, such as larger studies that permit assessment of age-specific asymptomatic fractions.
Knowledge of the asymptomatic fraction is important from two perspectives: (1) the fraction of cases that are infected but asymptomatic is important for assessing the severity and the burden of disease; and (2) the fraction of cases that are infectious but asymptomatic is important for optimizing public health control measures. For example, the potential impact to humans of emerging infectious diseases with zoonotic origin and limited human-to-human transmission depends on the fraction of exposed individuals with symptomatic illness (63). On the other hand, entry screening for infectious diseases at borders using health declaration forms and infrared thermal scanners is predicated on the idea that the asymptomatic fraction of diseases of interest is low (64), and isolation is only a useful measure if most infectious patients will be symptomatic. The two broad types of studies described above may provide information on each of these interpretations of the asymptomatic fraction. Some individuals with detectable influenza virus shedding do not subsequently have serologic evidence of infection (27, 56, 65), while other individuals with serologic evidence of infection do not have detectable virus shedding (21, 66), suggesting one should exercise caution on the interchangeability and the interpretation of the estimates of asymptomatic fraction based on different definitions. Estimates from serologic studies, with a denominator based on serologic evidence of infection, may be more relevant in understanding the severity of illness. Estimates of the asymptomatic fraction from outbreak investigations, where the denominator is infections with detectable virus shedding, may be more relevant in understanding the transmission potential of asymptomatic versus symptomatic infections.
Our review was subject to some limitations. First, our search may have missed some published estimates of the asymptomatic fraction, and broadening the search would have substantially increased workload. However we believe including any such studies would not change our conclusions substantially. We previously reviewed household transmission studies of H1N1pdm09 (67), and few such studies were conducted before 2009, therefore only a minimal number of such studies might have been missed. On the other hand, inclusion of additional serologic studies would not have changed our conclusions as well since the existing studies of this type have already demonstrated a high heterogeneity in estimates of the asymptomatic fraction, and including more studies would only increase the heterogeneity further.
Second, we did not formally assess the risk of bias in each study, but we did consider how features in the design and analysis of studies could contribute to bias in the estimates of the asymptomatic fraction (Figure 1). Selection bias may have affected estimates of the asymptomatic fraction if patients included in cohorts or transmission studies were not generalizable to infections in other settings, and this was explicitly discussed above as a potential explanation (i.e. a difference in the intensity of the exposure) for the difference between the estimates of the asymptomatic fraction in transmission studies and cohort studies (Figure 2A versus Figure 2B). The cohort studies are particularly likely to be prone to information biases in both assessment of infection and assessment of symptomatic disease. Finally, we did not identify sufficient estimates of the asymptomatic fraction to permit meta-regression analysis of the influence of study design characteristics and other factors on the estimates of the asymptomatic fraction.
In conclusion, the true asymptomatic fraction of influenza virus infections may depend on how infections are identified, and we found quite different estimates of the asymptomatic fraction in two different types of studies. In outbreak investigations where infections were virologically confirmed, we found a pooled mean of 16% (95% CI: 13%, 19%) of infections were asymptomatic, whereas in longitudinal studies in which infections were identified using serology the point estimates of the asymptomatic fraction adjusted for illness from other causes fell in the range 65%–85%. We could not fully explain the differences in the scale of estimates from these two types of studies, although features of the respective analyses would have led to under- and over-estimation of the asymptomatic fraction respectively. A study in Vietnam did include both of these strategies, estimating the asymptomatic fraction as 45% (17%–77%) in outbreak investigations versus 86% (82%–89%) in the longitudinal serologic analysis (27, 35). One potential approach to resolve these differences would be a hybrid study, where intensive follow-up with frequent virologic testing regardless of illness throughout an influenza season is used to ascertain all infections and illnesses in a cohort.
Acknowledgments
FUNDING
This project was supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), and the Area of Excellence Scheme of the University Grants Committee of Hong Kong (grant no. AoE/M-12/06), and a commissioned grant from the Health and Medical Research Fund from the Government of the Hong Kong Special Administrative Region. The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
The authors thank Vicky Fang for technical support, and Tim Tsang and Lincoln Lau for helpful discussions.
Footnotes
CONFLICTS OF INTEREST
DKMI has received research funding from F. Hoffmann-La Roche Ltd. BJC has received research funding from MedImmune Inc. and Sanofi Pasteur, and consults for Crucell NV. The authors report no other potential conflicts of interest.
References
- 1.Punpanich W, Chotpitayasunondh T. A review on the clinical spectrum and natural history of human influenza. Int J Infect Dis. 2012;16(10):e714–23. doi: 10.1016/j.ijid.2012.05.1025. [DOI] [PubMed] [Google Scholar]
- 2.Kalter SS. Inapparent Infections with Influenza Viruses. Exp Biol Med. 1951;76(3):570–1. doi: 10.3181/00379727-76-18560. [DOI] [PubMed] [Google Scholar]
- 3.Lee VJ, Chen MI, Yap J, et al. Comparability of different methods for estimating influenza infection rates over a single epidemic wave. Am J Epidemiol. 2011;174(4):468–78. doi: 10.1093/aje/kwr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson NM, Cummings DA, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437(7056):209–14. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 5.Reich NG, Lessler J, Chu H, et al. Identification of the asymptomatic ratio. Epidemiology. 2011;22(3):333–5. doi: 10.1097/EDE.0b013e31821092b1. [DOI] [PubMed] [Google Scholar]
- 6.Halloran ME, Ferguson NM, Eubank S, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proc Natl Acad Sci U S A. 2008;105(12):4639–44. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longini IM, Jr, Nizam A, Xu S, et al. Containing pandemic influenza at the source. Science. 2005;309(5737):1083–7. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 8.Milne GJ, Kelso JK, Kelly HA, et al. A small community model for the transmission of infectious diseases: comparison of school closure as an intervention in individual-based models of an influenza pandemic. PLoS ONE. 2008;3(12):e4005. doi: 10.1371/journal.pone.0004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Brit Med J. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775–85. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 11.Little JW, Douglas RG, Jr, Hall WJ, et al. Attenuated influenza produced by experimental intranasal inoculation. J Med Virol. 1979;3(3):177–88. doi: 10.1002/jmv.1890030303. [DOI] [PubMed] [Google Scholar]
- 12.Alford RH, Kasel JA, Gerone PJ, et al. Human influenza resulting from aerosol inhalation. P Soc Exp Biol Med. 1966;122(3):800–4. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 13.Fabbiani M, Sali M, Di Cristo V, et al. Prospective evaluation of epidemiological, clinical, and microbiological features of pandemic influenza A (H1N1) virus infection in Italy. J Med Virol. 2011;83(12):2057–65. doi: 10.1002/jmv.22231. [DOI] [PubMed] [Google Scholar]
- 14.Huo X, Zu R, Qi X, et al. Seroprevalence of avian influenza A (H5N1) virus among poultry workers in Jiangsu Province, China: an observational study. BMC Infect Dis. 2012;12:93. doi: 10.1186/1471-2334-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell TJ, Fox A, Peng Y, et al. Identification of H5N1-Specific T-Cell Responses in a High-risk Cohort in Vietnam Indicates the Existence of Potential Asymptomatic Infections. J Infect Dis. 2011;205(1):20–7. doi: 10.1093/infdis/jir689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Brit Med J. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 20.Suess T, Remschmidt C, Schink SB, et al. Comparison of shedding characteristics of seasonal influenza virus (sub)types and influenza A(H1N1)pdm09; Germany, 2007–2011. PLoS ONE. 2012;7(12):e51653. doi: 10.1371/journal.pone.0051653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362(23):2175–84. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson ML, France AM, Hancock K, et al. Serologically confirmed household transmission of 2009 pandemic influenza A (H1N1) virus during the first pandemic wave--New York City, April–May 2009. Clin Infect Dis. 2011;53(5):455–62. doi: 10.1093/cid/cir437. [DOI] [PubMed] [Google Scholar]
- 23.Lau LL, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis. 2010;201(10):1509–16. doi: 10.1086/652241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papenburg J, Baz M, Hamelin ME, et al. Household transmission of the 2009 pandemic A/H1N1 influenza virus: elevated laboratory-confirmed secondary attack rates and evidence of asymptomatic infections. Clin Infect Dis. 2010;51(9):1033–41. doi: 10.1086/656582. [DOI] [PubMed] [Google Scholar]
- 25.Simmerman JM, Suntarattiwong P, Levy J, et al. Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok, Thailand. Influenza Other Respi Viruses. 2011;5(4):256–67. doi: 10.1111/j.1750-2659.2011.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suess T, Buchholz U, Dupke S, et al. Shedding and transmission of novel influenza virus A/H1N1 infection in households--Germany, 2009. Am J Epidemiol. 2010;171(11):1157–64. doi: 10.1093/aje/kwq071. [DOI] [PubMed] [Google Scholar]
- 27.Thai PQ, Mai LQ, Welkers MR, et al. Pandemic H1N1 virus transmission and shedding dynamics in index case households of a prospective Vietnamese cohort. J Infect. 2014;68(6):581–90. doi: 10.1016/j.jinf.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeb M, Singh PK, Fox J, et al. Longitudinal study of influenza molecular viral shedding in Hutterite communities. J Infect Dis. 2012;206(7):1078–84. doi: 10.1093/infdis/jis450. [DOI] [PubMed] [Google Scholar]
- 29.Sagrera X, Ginovart G, Raspall F, et al. Outbreaks of influenza A virus infection in neonatal intensive care units. Pediatr Infect Dis J. 2002;21(3):196–200. doi: 10.1097/00006454-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Smit PM, Mulder JW, Ahdi M, et al. Low attack rate of novel influenza A (H1N1) virus infection among healthcare workers: a prospective study in a setting with an elaborated containment plan. Int Arch Occup Environ Health. 2012;85(2):163–70. doi: 10.1007/s00420-011-0652-5. [DOI] [PubMed] [Google Scholar]
- 31.Cowling BJ, Ng S, Ma ESK, et al. Protective efficacy against pandemic influenza of seasonal influenza vaccination in children in Hong Kong: a randomized controlled trial. Clin Infect Dis. 2012;55(5):695–702. doi: 10.1093/cid/cis518. [DOI] [PubMed] [Google Scholar]
- 32.Hayward AC, Fragaszy EB, Bermingham A, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014;2(6):445–54. doi: 10.1016/S2213-2600(14)70034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TE, Lin CY, King CC, et al. Estimating pathogen-specific asymptomatic ratios. Epidemiology. 2010;21(5):726–8. doi: 10.1097/EDE.0b013e3181e94274. [DOI] [PubMed] [Google Scholar]
- 34.Monto AS, Koopman JS, Longini IM., Jr Tecumseh study of illness. XIII. Influenza infection and disease, 1976–1981. Am J Epidemiol. 1985;121(6):811–22. doi: 10.1093/oxfordjournals.aje.a114052. [DOI] [PubMed] [Google Scholar]
- 35.Horby P, Maile Q, Fox A, et al. The epidemiology of interpandemic and pandemic influenza in Vietnam, 2007–2010: the Ha Nam household cohort study I. Am J Epidemiol. 2012;175(10):1062–74. doi: 10.1093/aje/kws121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khaokham CB, Selent M, Loustalot FV, et al. Seroepidemiologic investigation of an outbreak of pandemic influenza A H1N1 2009 aboard a US Navy Vessel-San Diego, 2009. Influenza Other Respi Viruses. 2013;7(5):791–8. doi: 10.1111/irv.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neatherlin J, Cramer EH, Dubray C, et al. Influenza A(H1N1)pdm09 during air travel. Travel Med Infect Dis. 2013;11(2):110–8. doi: 10.1016/j.tmaid.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaeger JL, Patel M, Dharan N, et al. Transmission of 2009 pandemic influenza A (H1N1) virus among healthcare personnel-Southern California, 2009. Infect Control Hosp Epidemiol. 2011;32(12):1149–57. doi: 10.1086/662709. [DOI] [PubMed] [Google Scholar]
- 39.Riley S, Kwok KO, Wu KM, et al. Epidemiological characteristics of 2009 (H1N1) pandemic influenza based on paired sera from a longitudinal community cohort study. PLoS Med. 2011;8(6):e1000442. doi: 10.1371/journal.pmed.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li T, Liu Y, Di B, et al. Epidemiological investigation of an outbreak of pandemic influenza A (H1N1) 2009 in a boarding school: serological analysis of 1570 cases. J Clin Virol. 2011;50(3):235–9. doi: 10.1016/j.jcv.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Toyokawa T, Sunagawa T, Yahata Y, et al. Seroprevalence of antibodies to pandemic (H1N1) 2009 influenza virus among health care workers in two general hospitals after first outbreak in Kobe, Japan. J Infect. 2011;63(4):281–7. doi: 10.1016/j.jinf.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Williams CJ, Schweiger B, Diner G, et al. Seasonal influenza risk in hospital healthcare workers is more strongly associated with household than occupational exposures: results from a prospective cohort study in Berlin, Germany, 2006/07. BMC Infect Dis. 2010;10:8. doi: 10.1186/1471-2334-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aho M, Lyytikainen O, Nyholm JE, et al. Outbreak of 2009 pandemic influenza A(H1N1) in a Finnish garrison--a serological survey. Euro Surveill. 2010;15(45) [PubMed] [Google Scholar]
- 44.LaForce C, Man CY, Henderson FW, et al. Efficacy and safety of inhaled zanamivir in the prevention of influenza in community-dwelling, high-risk adult and adolescent subjects: a 28-day, multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2007;29(8):1579–90. doi: 10.1016/j.clinthera.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Tandale BV, Pawar SD, Gurav YK, et al. Seroepidemiology of pandemic influenza A (H1N1) 2009 virus infections in Pune, India. BMC Infect Dis. 2010;10:255. doi: 10.1186/1471-2334-10-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill PW, Murphy AM. Naturally acquired immunity to influenza type A: a clinical and laboratory study. Med J Aust. 1976;2(9):329–33. doi: 10.5694/j.1326-5377.1976.tb130219.x. [DOI] [PubMed] [Google Scholar]
- 47.Gill PW, Murphy AM. Naturally acquired immunity to influenza type A: a further prospective study. Med J Aust. 1977;2(23):761–5. doi: 10.5694/j.1326-5377.1977.tb99276.x. [DOI] [PubMed] [Google Scholar]
- 48.Gill PW, Murphy AM. Naturally acquired immunity to influenza type A. Lessons from two coexisting subtypes. Med J Aust. 1985;142(2):94–8. doi: 10.5694/j.1326-5377.1985.tb133042.x. [DOI] [PubMed] [Google Scholar]
- 49.Hall CE, Cooney MK, Fox JP. The Seattle virus watch. IV. Comparative epidemiologic observations of infections with influenza A and B viruses, 1965–1969, in families with young children. Am J Epidemiol. 1973;98(5):365–80. doi: 10.1093/oxfordjournals.aje.a121566. [DOI] [PubMed] [Google Scholar]
- 50.Mutsch M, Tavernini M, Marx A, et al. Influenza virus infection in travelers to tropical and subtropical countries. Clin Infect Dis. 2005;40(9):1282–7. doi: 10.1086/429243. [DOI] [PubMed] [Google Scholar]
- 51.Yan L, Gao Y, Zhang Y, et al. Epidemiological and virological characteristics of pandemic influenza A (H1N1) school outbreaks in China in 2009. PLoS ONE. 2012;7(9):e45898. doi: 10.1371/journal.pone.0045898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Priest PC, Jennings LC, Duncan AR, et al. Effectiveness of border screening for detecting influenza in arriving airline travelers. Am J Public Health. 2013;103(8):1412–8. doi: 10.2105/AJPH.2012.300761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonoguchi T, Sakoh M, Kunita N, et al. Reinfection with influenza A (H2N2, H3N2, and H1N1) viruses in soldiers and students in Japan. J Infect Dis. 1986;153(1):33–40. doi: 10.1093/infdis/153.1.33. [DOI] [PubMed] [Google Scholar]
- 54.Gottfredsson M, Halldorsson BV, Jonsson S, et al. Lessons from the past: familial aggregation analysis of fatal pandemic influenza (Spanish flu) in Iceland in 1918. Proc Natl Acad Sci U S A. 2008;105(4):1303–8. doi: 10.1073/pnas.0707659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarabbo M, Lapa D, Castilletti C, et al. Retrospective investigation of an influenza A/H1N1pdm outbreak in an Italian military ship cruising in the Mediterranean Sea, May–September 2009. PLoS ONE. 2011;6(1):e15933. doi: 10.1371/journal.pone.0015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordan WS, Jr, Denny FW, Jr, Badger GF, et al. A study of illness in a group of Cleveland families. XVII. The occurrence of Asian influenza. Am J Hyg. 1958;68(2):190–212. doi: 10.1093/oxfordjournals.aje.a119962. [DOI] [PubMed] [Google Scholar]
- 57.Frank AL, Taber LH, Wells JM. Comparison of infection rats and severity of illness for influenza A subtypes H1N1 and H3N2. J Infect Dis. 1985;151(1):73–80. doi: 10.1093/infdis/151.1.73. [DOI] [PubMed] [Google Scholar]
- 58.Presanis AM, De Angelis D, et al. New York City Swine Flu Investigation T. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 2009;6(12):e1000207. doi: 10.1371/journal.pmed.1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong JY, Kelly H, Ip DK, et al. Case fatality risk of influenza A (H1N1pdm09): a systematic review. Epidemiology. 2013;24(6):830–41. doi: 10.1097/EDE.0b013e3182a67448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong JY, Wu P, Nishiura H, et al. Infection fatality risk of the pandemic A(H1N1)2009 virus in Hong Kong. Am J Epidemiol. 2013;177(8):834–40. doi: 10.1093/aje/kws314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahnke YD, Saqr A, Hazenfeld S, et al. Age-related changes in durability and function of vaccine-elicited influenza-specific CD4(+) T-cell responses. Vaccine. 2011;29(47):8606–14. doi: 10.1016/j.vaccine.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frasca D, Diaz A, Romero M, et al. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10(3):330–5. doi: 10.1016/j.arr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Z, Liu H, Lu J, et al. Asymptomatic, mild, and severe influenza A(H7N9) virus infection in humans, Guangzhou, China. Emerg Infect Dis. 2014;20(9):1535–40. doi: 10.3201/eid2009.140424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Priest PC, Duncan AR, Jennings LC, et al. Thermal image scanning for influenza border screening: results of an airport screening study. PLoS ONE. 2011;6(1):e14490. doi: 10.1371/journal.pone.0014490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrie JG, Ohmit SE, Johnson E, et al. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis. 2011;203(9):1309–15. doi: 10.1093/infdis/jir015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110(1):145–60. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lau LL, Nishiura H, Kelly H, et al. Household transmission of 2009 pandemic influenza A (H1N1): a systematic review and meta-analysis. Epidemiology. 2012;23(4):531–42. doi: 10.1097/EDE.0b013e31825588b8. [DOI] [PMC free article] [PubMed] [Google Scholar]