Abstract
Artificial oocyte activation to overcome failed fertilization after intracytoplasmic sperm injection (ICSI) in human oocytes typically employs Ca2+ ionophores to produce a single cytosolic Ca2+ increase. In contrast, recombinant phospholipase Czeta (PLCζ) causes Ca2+ oscillations indistinguishable from those occurring during fertilization, but remains untested for its efficacy in a scenario of ICSI fertilization failure. Here, we compare PLCζ with other activation stimuli in a mouse model of failed oocyte activation after ICSI, in which heat-treated sperm are injected into mouse oocytes. We show that increasing periods of 56°C exposure of sperm produces a progressive loss of Ca2+ oscillations after ICSI. The decrease in Ca2+ oscillations produces a reduction in oocyte activation and embryo development to the blastocyst stage. We treated such oocytes that failed to activate after ICSI either with Ca2+ ionophore, or with Sr2+ media which causes Ca2+ oscillations, or we injected them with recombinant human PLCζ. All these treatments rescued oocyte activation, although Sr2+ and PLCζ gave the highest rates of development to blastocyst. When recombinant PLCζ was given to oocytes previously injected with control sperm, they developed normally to the blastocyst stage at rates similar to that after control ICSI. The data suggest that recombinant human PLCζ protein is an efficient means of rescuing oocyte activation after ICSI failure and that it can be effectively used even if the sperm already contains endogenous Ca2+ releasing activity.
Keywords: PLCζ, ICSI, fertilization, sperm, oocyte
Introduction
For couples attending in vitro fertilization (IVF) clinics, intracytoplasmic sperm injection (ICSI) is a widely used treatment option (Palermo et al., 1992; Johnson et al., 2013) that is remarkably successful, compared with conventional IVF treatments, for treating many cases of male factor infertility (Palermo et al., 1992; Johnson et al., 2013). However, cases of ICSI where all of the available oocytes from a given collection fail to fertilize (Yanagida, 2004) occur in 1–5% of all ICSI treatment cycles (Liu et al., 1995). In such incidences of ICSI failure, the main cause has been shown to be the lack of oocyte activation (Javed et al., 2010; Vanden Meerschaut et al., 2013a; Neri et al., 2014). The options for couples facing total fertilization failure are limited (Yuzpe et al., 2000; Heindryckx et al., 2005). Total fertilization failure cases are relatively rare, partly because typically ∼10 oocytes are available for sperm injection (Sunkara et al., 2011). This multiplicity of oocytes masks the fact that fertilization after ICSI is less effective when rated per oocyte injected. For example, using sperm and oocytes with apparently normal parameters in cases where tubal blockage was the only evident cause of infertility, only 67% of oocytes were activated after sperm injection (Bukulmez et al., 2000), implying that one in three sperm is ineffective in activating the oocyte. This recurrent failure per oocyte becomes a particular concern when oocytes are limited, in mild ovarian stimulation or natural ovulation cycles (Pelinck et al., 2002; Loutradis et al., 2007).
The activation of mammalian oocytes at fertilization involves an extensive series of Ca2+ transients, known as Ca2+ oscillations. Each Ca2+ spike lasts about 1 min and the Ca2+ transients occur at intervals of 5–30 min (Miyazaki and Ito, 2006; Swann and Lai, 2013). Such Ca2+ oscillations have been observed in human and mouse oocytes after in vitro fertilization and ICSI (Sato et al., 1999). Such Ca2+ oscillations are both necessary and sufficient for oocyte activation (Kurokawa et al., 2004) and may influence subsequent embryo development (Ducibella et al., 2002; Yu et al., 2008). Accumulating scientific and clinical evidence favours the idea that Ca2+ oscillations are triggered after sperm oocyte membrane fusion, which allows the entry of a sperm-specific PLC isoform, termed PLCζ, into the oocyte cytoplasm (Saunders et al., 2002; Nomikos et al., 2005). Injection of PLCζ cRNA or recombinant protein stimulates parthenogenetic activation of mouse and human oocytes, and subsequent development of mouse embryos up to the blastocyst stage at rates similar to those seen after fertilization (Miyazaki, 1995).
Mouse oocytes have been frequently used as a model system for studying human IVF and ICSI. The mouse oocyte has also been used to directly test the ability of human sperm to cause oocyte activation or Ca2+ oscillations after cross-species ICSI (Araki et al., 2004; Vanden Meerschaut et al., 2013a). Injection of human sperm into mouse oocytes can trigger oocyte activation, but sperm from failed clinical ICSI cycles generally show a reduced ability to activate mouse oocytes after injection (Araki et al., 2004; Vanden Meerschaut et al., 2013a). Furthermore, injecting human sperm into mouse oocytes also causes a series of Ca2+ oscillations. The frequency of these human sperm-induced Ca2+ oscillations is often higher than observed at fertilization in mouse oocytes (Yoon et al., 2012; Nikiforaki et al., 2014). This is probably because human PLCζ has a greater intrinsic potency than mouse PLCζ and is able to cause the same pattern of Ca2+ oscillations at ∼10 times lower concentrations than mouse PLCζ (Yu et al., 2008). It has been reported that sperm from men with repeated fertilization failure, or with mutations in PLCζ protein, show a markedly reduced ability to cause Ca2+ oscillations after ICSI into mouse oocytes (Yoon et al., 2008; Heytens et al., 2009; Kashir et al., 2011, 2012). However, it was noteworthy that even for men who have had successful ICSI cycles, only ∼55% of normal sperm cause high-frequency Ca2+ oscillations in mouse oocytes (Vanden Meerschaut et al., 2013a; Nikiforaki et al., 2014). These data suggest that in cases of human ICSI failure, there might be either a deficiency, or relative lack of Ca2+ oscillations.
In cases of failed or poor rates of fertilization after ICSI, the only available treatment option is the use of artificial oocyte activation agents. This usually consists of treating oocytes with Ca2+ ionophores, such as A23187 or ionomycin, a procedure that has been successfully used to overcome fertilization failure in many cases (Kyono et al., 2012). Despite its apparent utility there are few controlled studies on the efficacy of Ca2+ ionophores as a means of rescuing failed oocyte activation and development. Ca2+ ionophore application, as currently used in most clinics, only causes a single large Ca2+ increase that does not mimic the series of Ca2+ oscillations seen at fertilization (Rinaudo et al., 1997). Stimuli that elicit multiple Ca2+ transients are known to be a more effective means of activating mammalian oocytes (Ozil and Swann, 1995; Ducibella et al., 2002). Sr2+ media can be used to cause such repetitive Ca2+ oscillations in mouse oocytes, but Sr2+ has not been shown to trigger Ca2+ release in human oocytes (Rogers et al., 2004). PLCζ remains the only physiological agent that has been repeatedly shown to produce a prolonged series of Ca2+ oscillations in all mammalian oocytes studied, including human oocytes (Ito et al., 2011; Nomikos et al., 2013a, b; Kashir et al., 2014).
In this study, we have investigated whether recombinant human PLCζ protein can be used to rescue cases of failed fertilization or to improve poor development rates after ICSI. We use a mouse model of failed oocyte activation after ICSI by applying mild heat treatment of mouse sperm. We find that heat-treated sperm display a reduced ability to generate Ca2+ oscillations in mouse oocytes, consistent with consequent observations of reduced preimplantation development. We also show that fertilization failure and poor embryo development after ICSI with heat-treated sperm can be rescued by subsequent microinjection of recombinant human PLCζ protein, which along with Sr2+ media, is more effective than Ca2+ ionophore. Moreover, we demonstrate that microinjection of PLCζ after standard ICSI with normal, untreated sperm does not impair embryo development. These data provide the basis for future studies to examine the potential use of recombinant human PLCζ as a biological therapeutic to rescue human oocytes from failed fertilization after ICSI.
Materials and Methods
All chemicals were obtained from Sigma Aldrich UK unless stated otherwise and were of embryo grade where available. M2 media was used for handling oocytes outside the incubator. KSOM was obtained from Merk-Millipore (Watford, UK), or made as previously described (Summers et al., 1995). The microinjection buffer was 100 mM KCl in 20 mM Hepes, pH 7.2 (Swann, 1990).
Gametes and embryos
Mouse oocytes were obtained from superovulated female MF1 mice as described previously (Yu et al., 2008). Cumulus-oocyte masses from oviducts were incubated in hyaluronidase in M2 media, and then isolated oocytes were washed in M2 media alone. Mouse embryos were cultured in KSOM media (Summers et al., 1995) under mineral oil in an incubator at 37°C gassed with 5% CO2. Mouse sperm were collected from the cauda epididymis of euthanized male mice (C57xCBA F1 hybrid), released into T6 media (Jones et al., 1995) and in some cases, sperm were frozen in the same media (without cryopreservation) in a −80°C freezer. For heat treatment, sperm were incubated for the specified times in a water bath at 56°C then sonicated for ∼10 s before being added to drops containing the oocytes. For Sr2+ activation after ICSI as described by Yoshida and Perry (2007) using heat inactivated sperm for 30 min, oocytes were allowed to recover for 30 min in M2. Oocytes were then incubated afterwards in calcium-free HKSOM supplemented with 5 mM Sr2+ for 2 h after which they are washed in M2 then cultured in KSOM under 5% CO2 in air at 37°C.
Ethical approval
All procedures using animals were approved by Cardiff University Animals Ethics Committee and carried out under a UK Home Office Project Licence.
Microinjection
Mouse oocytes were microinjected with sperm using custom-made ICSI pipettes (Yoshida and Perry, 2007). The micropipettes containing the sperm were advanced through the oocyte plasma membrane using a piezo-pulse delivered by a Prime Tech piezo manipulation system (Intracel, Royston, UK). Recombinant PLCζ was injected using a fine tip micropipette that was inserted into the oocyte using pressure pulses (Picopump, World Precision Instruments, USA), as described previously (Saunders et al., 2002).
Ca2+ measurements
Cytosolic free Ca2+ concentrations were measured in individual mouse oocytes by monitoring the fluorescence of PE3 or Rhod dextran (Takahashi et al., 1999). The PE3 dye was loaded into oocytes by incubating for 30 min in 10 μM of PE3-AM (also known as fura2LeakRes-AM) dissolved in M2 media. PE3 is a similar dye to fura2 except that it does not undergo compartmentalization or extrusion from the cytosol and hence can be used for longer-term recordings of Ca2+ (Takahashi et al., 1999). Oocytes were then washed free of the AM dye and allowed to equilibrate for another ∼30 min before making fluorescence measurements. Rhod dextran was injected into oocytes before the start of experiments. The measurements of fluorescence were made by placing oocytes in drops of HKSOM media on a heated stage of a Nikon TiU epifluorescence microscope. For PE3 based recordings, oocytes were exposed to fluorescence excitation at 380 nm and emission measured at 510–550 nm (Takahashi et al., 1999). The fluorescence ratio of the signals at 350 and 380 nm was then plotted against time as a measure of relative cytosolic Ca2+ concentrations. In a similar manner for Rhod dextran, the fluorescence was plotted against time with excitation at 550 nm and emission collected at 580–620 nm (Swann and Lai, 2013).
Recombinant protein expression and purification
Human PLCζ was expressed as a NusA-6xHis-tagged fusion protein and purified by affinity chromatography on Ni-NTA resin using standard procedures (Qiagen) and elution with 275 mM imidazole, as described previously (Nomikos et al., 2013b). NusA tagged human PLCζ was tested for its ability to cause Ca2+ oscillations in oocytes prior to use and was injected at a pipette concentration of 0.01 mg/ml (Nomikos et al., 2013a, b; Nikiforaki et al., 2014). Control NusA protein was purified in a similar manner and when injected separately was used at a pipette concentration of 0.1 mg/ml in KCl/Hepes buffer.
Statistical analysis
Data on the number of Ca2+ transients and rate of embryo development from control and experimental groups were analysed using ANOVA or pairwise Student's t-tests. Differences at P < 0.05 were considered significant.
Results
Sperm treatment and Ca2+ oscillations after ICSI
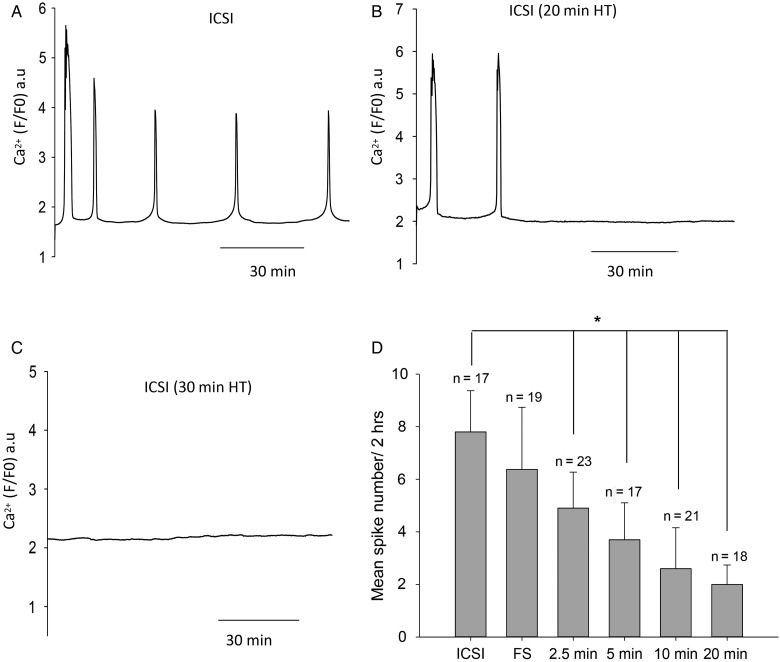
To create a clinical scenario mimicking poor fertilization after ICSI, we exposed mouse sperm to heat treatment since previous studies have shown that the oocyte activating sperm factor is sensitive to mild heat treatment (Perry et al., 1999). We incubated mouse sperm at 56°C for time intervals varying from 2.5 to 30 min. Figure 1A–C shows the recordings of Ca2+ oscillations observed in mouse oocytes after ICSI with either fresh (control) sperm or with sperm that had been heat treated for various times such as 20 or 30 min. ICSI using fresh sperm results in a series of Ca2+ oscillations during the first 2 h. In contrast, any heat treatment of sperm resulted in a significantly decreased number of Ca2+ spikes over the 2 h recording period. ICSI with sperm that had been heat treated for shorter durations such as 2.5 min produced some Ca2+ spikes (Fig. 1D), but significantly fewer than with control sperm. ICSI with frozen and thawed sperm caused slightly fewer Ca2+ oscillations in the 2 h recording period than freshly prepared sperm (Fig. 1D). Figure 1D also shows that the mean number of Ca2+ spikes is progressively reduced as the heat treatment is increased from 2.5 min, with a complete loss of Ca2+ oscillations after ICSI seen with a 30 min treatment. These data suggest that exposure of sperm at 56°C for increasing time periods is associated with a progressive reduction in the ability of sperm to trigger Ca2+ oscillations.
Figure 1.
Intracellular Ca2+ measured in mouse oocytes after ICSI. Ca2+ levels are plotted as fluorescence of the Ca2+-sensitive dye, Rhod dextran. In (A) is shown an example where an oocyte was injected with control fresh sperm (from n = 17 recordings), in (B) an oocyte was injected with sperm that has been heat treated for 20 min (n = 16), in (C) the oocyte was injected with sperm heat treated for 30 min (n = 13). In (D), the mean and standard deviation of the number of Ca2+ spikes after ICSI is plotted for control sperm and for sperm exposed to different times of heat treatments. One-way ANOVA shows a significant difference between the control sperm and each of the heat-treated sperm samples (P = 0.0092) which is indicated by the * above the bars. Error bars are SDs.
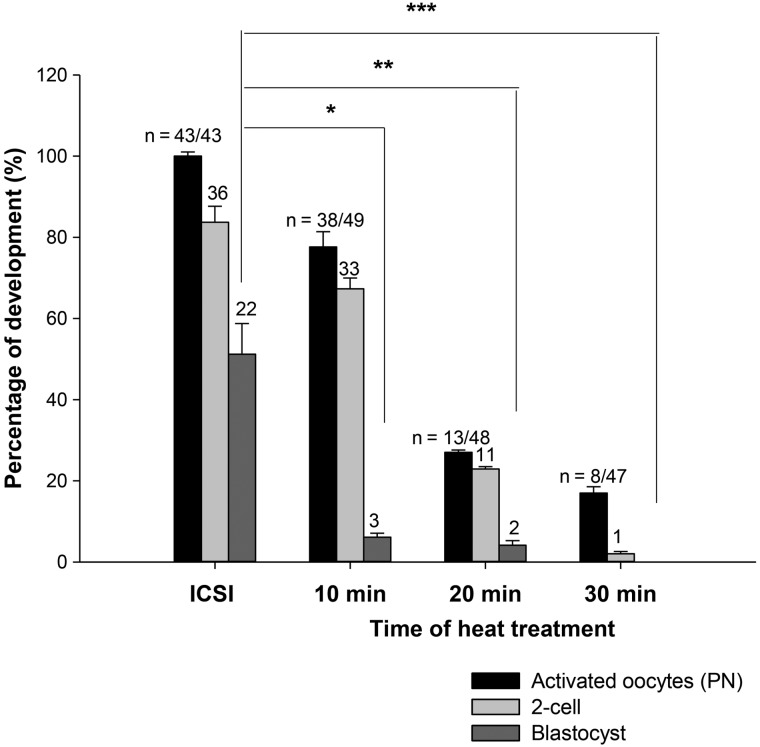
We further examined the effect of heat-treated ICSI sperm upon the oocyte activation rate and subsequent development to the blastocyst stage. We used MF1 oocytes for this work where control rates of development in vitro to the blastocyst stage after fertilization are around 50% (Ozil and Swann, 1995; Ducibella et al., 2002). Figure 2 and Supplementary Table S1 show that the rate of oocyte activation, as defined by pronuclear formation, was progressively reduced by heat treating sperm for 2.5–30 min. Similarly, embryonic development to blastocysts was also reduced in embryos resulting from ICSI with heat-treated sperm. Figure 2 indicates that an increase in time of sperm exposure to 56°C is consistent with a progressive decrease in the number of ICSI embryos that develop to the 2-cell or blastocyst stage. These data suggest that mild heat treatment of mouse sperm leads to loss in the capacity of sperm to support both Ca2+ oscillations and preimplantation development after ICSI. The observed thermal impairment of sperm function mimics a clinical scenario of oocyte activation failure and poor development after IVF/ICSI.
Figure 2.
The percentage of embryo developmental to the blastocyst stage after ICSI is compared with that after ICSI using sperm that had been heat inactivated for various times. A significant difference for development to blastocyst was seen from control ICSI for the 10, 20, 30 min heat treatments (Student's t-test pairwise between the control and each heat treatment, P = 0.0038, 0.0023, and 0.000, respectively). Error bars are SEMs.
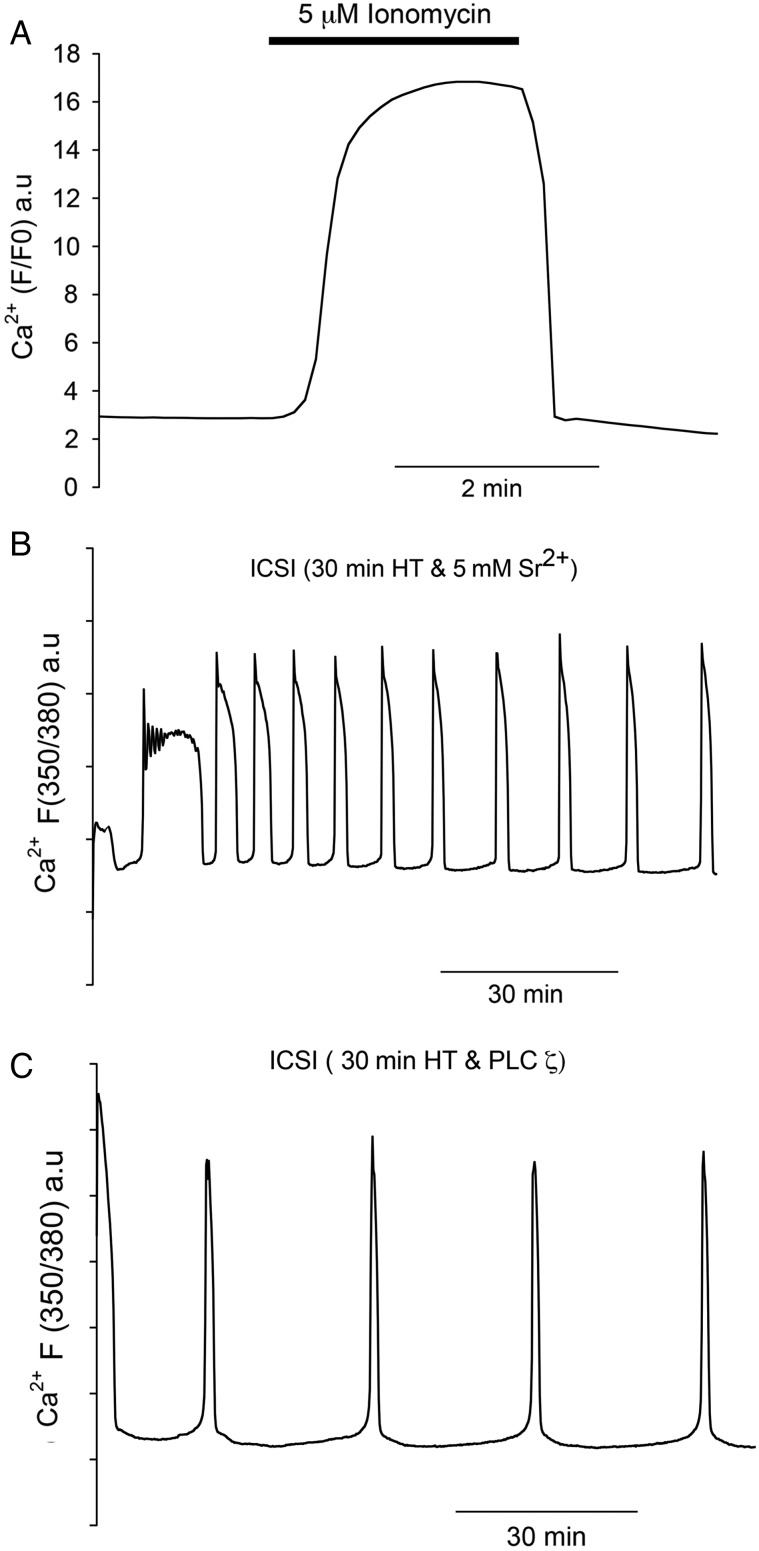
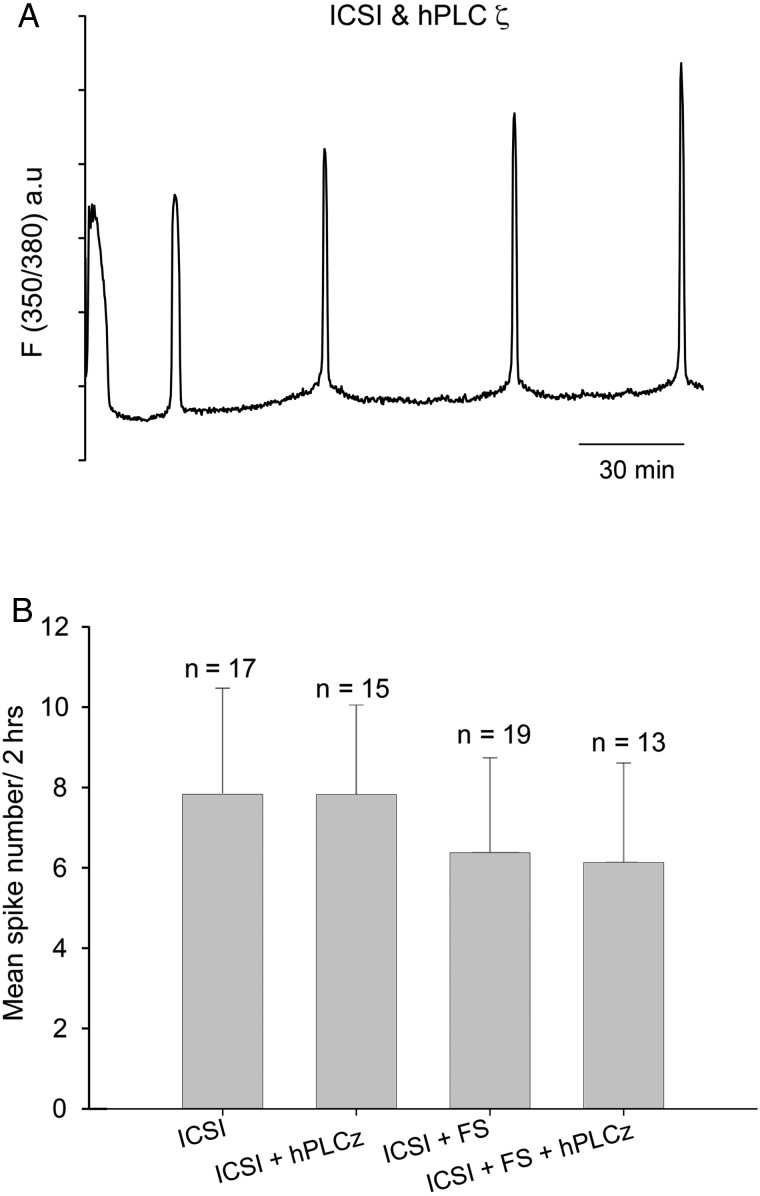
The most common treatment for failed fertilization after ICSI is to expose oocytes to Ca2+ ionophores (Nasr-Esfahani et al., 2010). Figure 3A shows that oocytes injected with sperm heat-treated for 30 min responded with a single large Ca2+ increase after exposure to ionomycin. For Fig. 3A the ionomycin was washed out after 5 min in order to mimic the duration of the Ca2+ transient seen in human oocytes exposed to ionophore (Rinaudo et al., 1997). We then wanted to compare these stimuli to those that are known to cause Ca2+ oscillations. First we used Sr2+ media which, as shown in Fig. 3B, causes a series of Ca2+ oscillations (mean of 7.91 Ca2+ spikes in 2 h, with a standard deviation of 2.64, n = 15) after ICSI in a manner previously described (Perry et al., 1999). However, in order to generate the Ca2+ oscillations in a way that would be effective in human oocytes, we used recombinant PLCζ protein. After ICSI with 30 min heat-treated sperm, oocyte Ca2+ oscillations could be rescued by microinjection of recombinant human PLCζ (Fig. 3C). The amount of PLCζ protein we used is comparable to that used in previous experiments and was designed to generate a pattern of Ca2+ oscillations similar to those seen after ICSI with control sperm. Importantly, we also performed double injection experiments by injecting PLCζ protein into oocytes that had been through ICSI with control (fresh) sperm (Fig. 4A). For these double-injected oocytes, there were also Ca2+ oscillations after PLCζ injection, although we cannot distinguish the relative contribution to these oscillations from the PLCζ injection and the injected sperm. It was, however, notable that the frequency of Ca2+ oscillations in these experiments was similar to that in control ICSI, or with ICSI using frozen–thawed sperm (see Fig. 4B).
Figure 3.
Intracellular Ca2+ measured in mouse oocytes treated with artificial stimuli after ICSI. Conditions are the same as in Fig. 1 except that PE3 was used to record Ca2+ in (B) and (C) where fluorescence is in arbitrary (ratio) units (a.u.). In (A) is shown an example of oocyte treated with ionomycin for 5 min (n = 21 recordings) where the bar indicates the time for which ionomycin was applied. In (B) is an example of an oocyte injected with heat treatment (30 min) inactivated sperm and then treated with Sr2+ media (n = 12). In (C) is shown an example of an oocyte injected with heat treatment (30 min) inactivated sperm and then subsequently injected with purified recombinant PLCζ protein (n = 15).
Figure 4.
Intracellular Ca2+ measured in oocytes after ICSI. Ca2+ levels are plotted as a fluorescence ratio of the Ca2+ sensitive dye PE3. In (A) is a representative of oocytes injected with fresh sperm followed by an injection of hPLCζ. (B) The mean frequency of Ca2+ oscillations after injecting hPLCζ protein into oocytes that had also been injected with control fresh or frozen sperm. One-way ANOVA showed no significant difference between the groups (P = 0.169). Error bars are SDs.
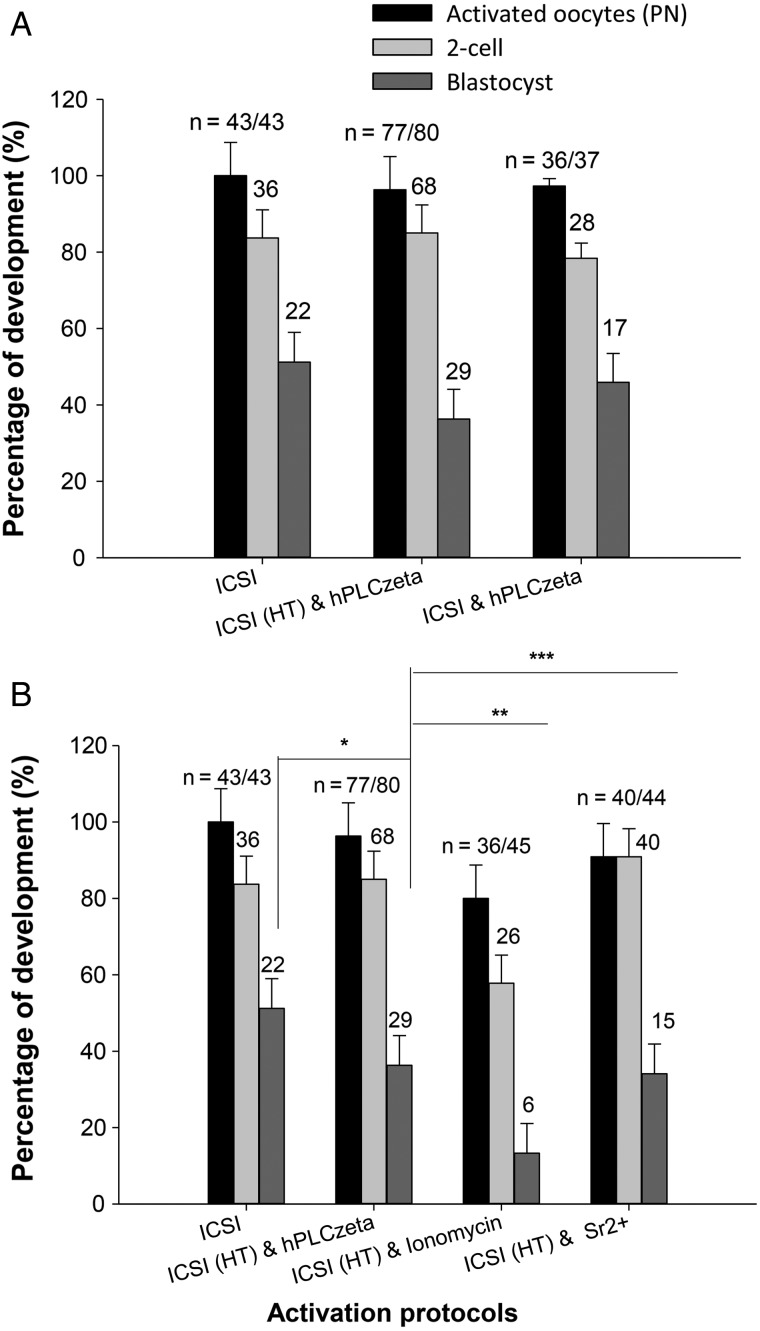
The above experiments show that Ca2+ signals could be restored by the use of ionomycin, Sr2+ media, or by introducing recombinant PLCζ protein, in oocytes injected with heat-treated sperm. We monitored oocyte activation and subsequent development in such oocytes. Figure 5 shows that following ICSI with sperm treated for 30 min and then incubated with Ca2+ ionophore, oocytes could be activated but only 13.3% formed blastocysts (see also Supplementary Table S2). In contrast, when we used the same procedure but replaced ionophore activation with Sr2+ media, or PLCζ injection, not only were oocytes activated but more than 34–36% developed to the blastocysts stage. This is significantly more than with ionophore treatment. We found that the use of frozen sperm was less effective than fresh sperm in triggering development up to the blastocyst stage, but that injection of PLCζ could effectively rescue these oocytes and give rise to developmental rates similar to those of fresh sperm (Supplementary Table S2). These data suggest that agents that induce Ca2+ oscillations, such as PLCζ protein injection, provide an effective means of rescuing oocyte activation failure or poor development after ICSI. We also noted that injecting either PLCζ protein after ICSI using control sperm also led to oocyte activation and that the rate of 2-cell and blastocyst formation was similar to that seen with control sperm ICSI alone (Fig. 5 and Supplementary Table S2). These data show that the injection of PLCζ, in addition to a normal Ca2+ oscillations stimulus from a control sperm, does not appear to impair preimplantation development to the blastocyst stage.
Figure 5.
Embryo developmental to blastocyst stage after various activation protocols. In (A) embryo developmental rates are shown after control ICSI, ICSI using heat inactivated sperm (HI) followed by PLCζ injection and ICSI with control sperm followed by PLCζ injection. One-way ANOVA reveals no significant difference in development with a P-value of 0.097. In (B), embryo development is shown after control ICSI and ICSI using heat inactivated sperm (as in A), and this is compared with ICSI using heat inactivated sperm (HI) followed by treatment with ionomycin or Sr2+ media. The rates of blastocyst development after treatment with ionomycin were significantly lower when compared with treatment with hPLCζ (t-test pairwise comparisons; *P = 0.079 for control ICSI versus hPLCζ, **P = 0.008 for hPLCζ versus Ionomycin, ***P = 0.093 for hPLCζ versus Sr2+). Error bars are SEMs.
Discussion
Fertilization failure in an oocyte after ICSI occurs both sporadically in many treatment cycles and can sometimes occur in all oocytes. Whilst there may be many reasons for fertilization failure, the most common cause is failure of oocyte activation (Javed et al., 2010; Nasr-Esfahani et al., 2010). In this study, we showed that mild heat treatment of mouse sperm leads to a reduction in its ability to trigger Ca2+ oscillations in mouse oocytes after ICSI. It is known that exposing mouse sperm to temperatures of ∼90°C results in DNA damage, but that heat treatment at lower temperatures (56°C) does not cause DNA damage nor impair embryo development when an independent oocyte activation stimulus is used (Perry et al., 1999). Our present data confirm that sperm contain a heat labile factor that triggers Ca2+ oscillations and this sperm-borne factor is sensitive to relatively mild heat treatment of 56°C for 30 min. This heat treatment is similar to that previously used to inactivate SOAF (sperm oocyte activating factor) in earlier reports of mouse ICSI (Perry et al., 1999). Previous ICSI studies also demonstrated that the SOAF, which is present in the sperm perinuclear theca, is PLCζ (Perry et al., 1999), as identified by Saunders et al. (2002). PLCζ is presently the only sperm-specific protein confirmed to have an intrinsic ability to cause Ca2+ oscillations in mammalian oocytes by multiple independent research teams (Saunders et al., 2002; Kurokawa et al., 2004; Rogers et al., 2004; Miyazaki and Ito, 2006; Nomikos et al., 2014). Therefore, it is probable that heat treatment leads to sperm PLCζ inactivation in our experiments. Interestingly, freeze thawing of sperm reduces the activity and distribution of PLCζ (Kashir et al., 2011). This is consistent with our finding that this step slightly reduces the number of Ca2+ oscillations after ICSI as well as the percentage of embryos developing to the blastocyst stage. This could be due to some PLCζ enzyme inactivation due to the freeze thaw cycle, or to loss of PLCζ from the sperm. Nevertheless, in our study, defining the exact cause of loss of Ca2+ oscillations is not critical since we have identified a simple way of mimicking the clinical observation in humans where sperm from subfertile males shows a reduced ability to cause Ca2+ oscillations in oocytes after ICSI.
One advantage of using 56°C heat treatment to deplete the ability of sperm to activate oocytes after ICSI is that it enables provision of different time points that creates a range of effects. Indeed we found that varying the duration of sperm heating from 2.5 to 30 min led to progressive loss of Ca2+ oscillation-inducing activity. Along with the loss of Ca2+ oscillations, there was a loss of oocyte activation and embryo development up to the blastocyst stage. We found that embryo development to the blastocyst stage fell from ∼50% down to 0% correlating directly with the reduced number of Ca2+ spikes caused by increasingly longer heat treatments. It should be noted that we used mouse oocytes from the MF1 strain which develop to blastocysts in vitro at a rate of ∼50%, which matches clinical scenarios with human embryos. These data suggests that an attenuation of the Ca2+ oscillations following ICSI leads to decreased rates of oocyte activation and development. This is a scenario that appears to occur in some cases of subfertility found by practitioners of ICSI (Vanden Meerschaut et al., 2013a; Nikiforaki et al., 2014).
In clinical cases where poor ICSI oocyte activation rates are observed, the current treatment option is the use of Ca2+ ionophores, which provide an artificial stimulus (Neri et al., 2014). It has been used in many cases of complete activation failure. It can give rise to live births in treatment cycles that would otherwise fail (Yanagida et al., 2008; Nasr-Esfahani et al., 2010), but the efficiency of this activation protocol compared with a physiological stimulus has not been critically examined. Ca2+ ionophores can only cause one or, in some protocols, two large Ca2+ increases, which fails to mimic the multiple Ca2+ oscillations that occur at fertilization (Vincent et al., 1992; Heytens et al., 2008). In our study, we tested the ability of ionomycin to overcome failed oocyte activation after ICSI with heat-treated sperm and found that it could restore activation. Ionomycin treatment also lead to development to the blastocyst stage, but it was less effective than either Sr2+ media or recombinant PLCζ at producing oocyte activations. PLCζ recombinant protein has been previously shown to overcome activation failure after ICSI in human oocytes (Yoon et al., 2012). This set a precedent for the use of PLCζ as a means of rescuing oocytes from activation failure. However, the efficiency of activation was not compared with other protocols. In our current experiments, the higher rates of Sr2+ and PLCζ-mediated oocyte activation versus ionophore-mediated oocyte activation also lead to better rates of embryo development to the blastocyst. Even though it may not be usable for human oocytes, we used Sr2+ media because it induces Ca2+ oscillations. Consequently our data indicated that it is because PLCζ causes Ca2+ oscillations that it is a more effective stimulus for rescuing oocyte activation after ICSI failure than Ca2+ ionophores. This is consistent with previous data showing that multiple Ca2+ transients are the most effective way to activate mouse or rabbit oocytes (Ducibella et al., 2002, 2006).
In our protocol, we have used a second injection to introduce PLCζ and induced Ca2+ oscillations in the mouse oocyte after ICSI. The use of a second injection has been used in at least one clinic where Ca2+ is injected after ICSI, and then ionophores are applied, as part of a protocol to rescue oocyte from ICSI failure (Vanden Meerschaut et al., 2013a, b). Human oocytes are more robust than mouse oocytes since mouse oocytes specifically require piezo devices, or electrical oscillation, for pipette insertion (Swann, 1990; Yoshida and Perry, 2007). Hence, a second injection of PLCζ protein should be possible in a clinical setting. We additionally anticipate that introducing PLCζ into an oocyte should be relatively safe for embryos. So far the treatment of oocytes with Ca2+ ionophores, which produces an unnaturally large rise in Ca2+, has been shown to have no discernible damaging effect on embryos or live young (Yanagida, 2004; Vanden Meerschaut et al., 2013a). PLCζ causes Ca2+ oscillations indistinguishable from those seen at fertilization and so should not lead to any effects upon development other than those arising naturally.
Previous studies have suggested that human sperm contain variable amounts of PLCζ and also have a variable ability to cause Ca2+ oscillations in oocytes (Grasa et al., 2008; Kashir et al., 2013). During ICSI, an embryologist is unable to assess the ability of a chosen sperm to cause Ca2+ oscillations. If PLCζ is injected into ICSI oocytes to overcome cases of suspected activation failure, then it is possible that these oocytes will receive some endogenous Ca2+ releasing activity from the sperm in addition to that from the injected PLCζ. We tested the developmental consequence of this by examining the scenario where control sperm was used for ICSI and then the same oocytes were subsequently injected with the single sperm equivalent dose of PLCζ used for rescuing complete activation failure. We also injected PLCζ into oocytes that had ICSI with frozen–thawed sperm and have only a slightly reduced number of Ca2+ oscillations. In both these cases of ICSI + PLCζ injection, we found that after oocyte activation, the development rates to the blastocyst stage were similar to that for control ICSI. This benign effect might seem surprising since previous studies have shown that PLCζ over-expression can lead to poor development to the blastocyst stage (Yu et al., 2008). However, the previous deleterious effects on development required over-expression of about 10-fold more PLCζ than is effective in causing oocyte activation. In the current study, we estimate that oocytes should have received little more than double the normal PLCζ level in one sperm. The lack of change in the frequency of Ca2+ oscillations is consistent with previous studies in mouse oocytes that have found only a marginal difference in Ca2+ oscillation frequency after fertilization by two versus one sperm in zona-free oocytes (Faure et al., 1999). These data suggest that PLCζ can be injected at a dose to successfully activate an oocyte that had not been activated by ICSI, and that this will not lead to detrimental effects on early embryo development.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/
Authors' roles
R.S. and Y.Y. carried out experiments on oocytes and embryos and analysed the data. M.N. purified and characterized the recombinant human PLCζ protein. K.S. and F.A.L. planned and supervised the project. All authors prepared the manuscript.
Funding
R.S. is supported by a research scholarship from the Libyan Ministry of Education. M.N. holds an EU-FP7 Marie Curie Intra-European Fellowship (628634). This work was also partly funded by a research grant from Cook Medical Technologies LLC. Funding to pay the Open Access publication charges for this article was provided by Cardiff University.
Conflict of interest
F.A.L. and K.S. hold patents with Cardiff University on PLCζ Sperm factor sequences. Other authors declare no conflict of interest.
Supplementary Material
Acknowledgements
We thank Junaid Kashir for helpful comments on the manuscript.
References
- Araki Y, Yoshizawa M, Abe H, Murase Y. Use of mouse oocytes to evaluate the ability of human sperm to activate oocytes after failure of activation by intracytoplasmic sperm injection. Zygote 2004;12:111–116. [DOI] [PubMed] [Google Scholar]
- Bukulmez O, Yarali H, Yucel A, Sari T, Gurgan T. Intracytoplasmic sperm injection versus in vitro fertilization for patients with a tubal factor as their sole cause of infertility: a prospective, randomized trial. Fertil Steril 2000;73:38–42. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol 2002;250:280–291. [PubMed] [Google Scholar]
- Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol 2006;17:324–332. [DOI] [PubMed] [Google Scholar]
- Faure JE, Myles DG, Primakoff P. The frequency of calcium oscillations in mouse eggs at fertilization is modulated by the number of fused sperm. Dev Biol 1999;213:370–377. [DOI] [PubMed] [Google Scholar]
- Grasa P, Coward K, Young C, Parrington J. The pattern of localization of the putative oocyte activation factor, phospholipase Cζ, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod 2008;23:2513–2522. [DOI] [PubMed] [Google Scholar]
- Heindryckx B, Van Der Elst J, De sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod 2005;20:2237–2241. [DOI] [PubMed] [Google Scholar]
- Heytens E, Soleimani R, Lierman S, De Meester S, Gerris J, Dhont M, Van der Elst J, De Sutter P.. Effect of ionomycin on oocyte activation and embryo development in mouse. Reprod Biomed Online 2008;17:764–771. [DOI] [PubMed] [Google Scholar]
- Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, Fissore RA, Hamer R, Deane CM, Ruas M et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCζ) in spermatozoa from infertile men. Hum Reprod 2009;24:2417–2428. [DOI] [PubMed] [Google Scholar]
- Ito J, Parrington J, Fissore RA. PLCζ and its role as a trigger of development in vertebrates. Mol Reprod Dev 2011;78:846–853. [DOI] [PubMed] [Google Scholar]
- Javed M, Esfandiari N, Casper RF. Failed fertilization after clinical intracytoplasmic sperm injection. Reprod Biomed Online 2010;20:56–67. [DOI] [PubMed] [Google Scholar]
- Johnson LNC, Sasson IE, Sammel MD, Dokras A. Does intracytoplasmic sperm injection improve the fertilization rate and decrease the total fertilization failure rate in couples with well-defined unexplained infertility? A systematic review and meta-analysis. Fertil Steril 2013;100:704–711. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Merriman JA, Whittingham DG, Kono T. Repetitive sperm-induced Ca2+ transients in mouse oocytes are cell cycle dependent. Development 1995;121:3259–3266. [DOI] [PubMed] [Google Scholar]
- Kashir J, Jones C, Lee HC, Rietdorf K, Nikiforaki D, Durrans C, Ruas M, Tee ST, Heindryckx B, Galione A et al. Loss of activity mutations in phospholipase C zeta (PLCζ) abolishes calcium oscillatory ability of human recombinant protein in mouse oocytes. Hum Reprod 2011;26:3372–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashir J, Konstantinidis M, Jones C, Lemmon B, Chang Lee H, Hamer R, Heindryckx B, Deane CM, De Sutter P, Fissore RA et al. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum Reprod 2012;27:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashir J, Jones C, Mounce G, Ramadan WM, Lemmon B, Heindryckx B, De Sutter P, Parrington J, Turner K, Child T et al. Variance in total levels of phospholipase C zeta (PLC-ζ) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertil Steril 2013;99:107–117.e3. [DOI] [PubMed] [Google Scholar]
- Kashir J, Nomikos M, Lai FA, Swann K. Sperm-induced Ca2+ release during egg activation in mammals. Biochem Biophys Res Commun 2014;450:1204–1211. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato KI, Fissore RA. Mammalian fertilization: from sperm factor to phospholipase Cζ. Biol Cell 2004;96:37–45. [DOI] [PubMed] [Google Scholar]
- Kyono K, Takisawa T, Nakajo Y, Doshida M, Toya M. Birth and follow-up of babies born following ICSI with oocyte activation using strontium chloride or calcium ionophore A23187. J Mamm Ova Res 2012;29:35–40. [Google Scholar]
- Liu J, Nagy Z, Joris H, Tournaye H, Smitz J, Camus M, Devroey P, Van Steirteghem A.. Analysis of 76 total fertilization failure cycles out of 2732 intracytoplasmic sperm injection cycles. Hum Reprod 1995;10:2630–2636. [PubMed] [Google Scholar]
- Loutradis D, Drakakis P, Vomvolaki E, Antsaklis A. Different ovarian stimulation protocols for women with diminished ovarian reserve. J Assist Reprod Genet 2007;24:597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. Calcium signalling during mammalian fertilization. Ciba Found Symp 1995;188:235–247; discussion 247. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Ito M. Calcium signals for egg activation in mammals. J Pharmacol Sci 2006;100:545–552. [DOI] [PubMed] [Google Scholar]
- Nasr-esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril 2010;94:520–526. [DOI] [PubMed] [Google Scholar]
- Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium 2014;55:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforaki D, Vanden Meerschaut F, De Gheselle S, Qian C, Van den Abbeel E, De vos WH, Deroo T, De Sutter P, Heindryckx B. Sperm involved in recurrent partial hydatidiform moles cannot induce the normal pattern of calcium oscillations. Fertil Steril 2014;102:581–588.e1. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Blayney LM, Larman MG, Campbell K, Rossbach A, Saunders CM, Swann K, Lai FA. Role of phospholipase C-ζ domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J Biol Chem 2005;280:31011–31018. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Kashir J, Swann K, Lai FA. Sperm PLCζ: From structure to Ca2+ oscillations, egg activation and therapeutic potential. FEBS Lett 2013a;587:3609–3616. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Yu Y, Elgmati K, Theodorido M, Campbell K, Vassilakopoulou V, Zikos C, Livaniou E, Amso N, Nounesis G et al. Phospholipase Cζ rescues failed oocyte activation in a prototype of male factor infertility. Fertil Steril 2013b;1:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos M, Sanders JR, Theodoridou M, Kashir J, Matthews E, Nounesis G, Lai FA, Swann K. Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes. Mol Hum Reprod 2014;20:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozil JP, Swann K. Stimulation of repetitive calcium transients in mouse eggs. J Physiol 1995;483:331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340:17–18. [DOI] [PubMed] [Google Scholar]
- Pelinck MJ, Hoek A, Simons AHM, Heineman MJ. Efficacy of natural cycle IVF: a review of the literature. Hum Reprod Update 2002;8:129–139. [DOI] [PubMed] [Google Scholar]
- Perry ACF, Wakayama T, Yanagimachi R. A novel trans-complementation assay suggests full mammalian oocyte activation is coordinately initiated by multiple, submembrane sperm components. Biol Reprod 1999;60:747–755. [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Massobrio M, Pepperell JR, Keefe DL, Buradgunta S. Dissociation between intracellular calcium elevation and development of human oocytes treated with calcium ionophore. Fertil Steril 1997;68:1086–1092. [DOI] [PubMed] [Google Scholar]
- Rogers NT, Hobson E, Pickering S, Lai FA, Braude P, Swann K. Phospholipase Cζ causes Ca2+ oscillations and parthenogenetic activation of human oocytes. Reproduction 2004;128:697–702. [DOI] [PubMed] [Google Scholar]
- Sato MS, Yoshitomo M, Mohri T, Miyazaki S. Spatiotemporal analysis of [Ca2+]i rises in mouse eggs after intracytoplasmic sperm injection (ICSI). Cell Calcium 1999;26:49–58. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLCζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 2002;129:3533–3544. [DOI] [PubMed] [Google Scholar]
- Summers MC, Bhatnagar PR, Lawitts JA, Biggers JD. Fertilization in vitro of mouse ova from inbred and outbred strains: complete preimplantation embryo development in glucose-supplemented KSOM. Biol Reprod 1995;53:431–437. [DOI] [PubMed] [Google Scholar]
- Sunkara SK, Rittenberg V, Raine-fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod 2011;26:1768–1774. [DOI] [PubMed] [Google Scholar]
- Swann K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 1990;110:1295–1302. [DOI] [PubMed] [Google Scholar]
- Swann K, Lai FA. PLCζ and the initiation of Ca2+ oscillations in fertilizing mammalian eggs. Cell Calcium 2013;53:55–62. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev 1999;79:1089–1125. [DOI] [PubMed] [Google Scholar]
- Vanden Meerschaut F, Leybaert L, Nikiforaki D, Qian C, Heindryckx B, De Sutter P.. Diagnostic and prognostic value of calcium oscillatory pattern analysis for patients with ICSI fertilization failure. Hum Reprod 2013a;28:87–98. [DOI] [PubMed] [Google Scholar]
- Vanden Meerschaut F, Nikiforaki D, De Roo C, Lierman S, Qian C, Schmitt-john T, De sutter P, Heindryckx B. Comparison of pre-and post-implantation development following the application of three artificial activating stimuli in a mouse model with round-headed sperm cells deficient for oocyte activation. Hum Reprod 2013b;28:1190–1198. [DOI] [PubMed] [Google Scholar]
- Vincent C, Cheek TR, Johnson MH. Cell cycle progression of parthenogenetically activated mouse oocytes to interphase is dependent on the level of internal calcium. J Cell Sci 1992;103:389–396. [DOI] [PubMed] [Google Scholar]
- Yanagida K. Complete fertilization failure in ICSI. Hum Cell 2004;17:187–193. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Fujikura Y, Katayose H. The present status of artificial oocyte activation in assisted reproductive technology. Reprod Med Biol 2008;7:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SY, Jellerette T, Salicioni AM, Hoi CL, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest 2008;118:3671–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SY, Eum JH, Lee JE, Lee HC, Kim YS, Han JE, Won HJ, Park SH, Shim SH, Lee WS et al. Recombinant human phospholipase C zeta 1 induces intracellular calcium oscillations and oocyte activation in mouse and human oocytes. Hum Reprod 2012;27:1768–1780. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Perry ACF. Piezo-actuated mouse intracytoplasmic sperm injection (ICSI). Nat Protoc 2007;2:296–304. [DOI] [PubMed] [Google Scholar]
- Yu Y, Saunders CM, Lai FA, Swann K. Preimplantation development of mouse oocytes activated by different levels of human phospholipase C zeta. Hum Reprod 2008;23:365–373. [DOI] [PubMed] [Google Scholar]
- Yuzpe AA, Liu Z, Fluker MR. Rescue intracytoplasmic sperm injection (ICSI)—salvaging in vitro fertilization (IVF) cycles after total or near-total fertilization failure. Fertil Steril 2000;73:1115–1119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.