Abstract
Aims
Roux-en-Y gastric bypass surgery (GB) is characterized by accentuated, but short-lived postprandial elevations of blood glucose and insulin. This profile has been attributed to effects of relative hyperglycemia to directly stimulate β-cells and an augmented incretin effect. We hypothesized additional glucose-independent stimulation of insulin secretion in GB subjects.
Methods
Fifteen subjects with prior GB, and six matched obese non-surgical controls, and seven lean individuals were recruited. Islet hormones were measured before and after meal ingestion during hyperinsulinemic hypoglycemic clamps to minimize the direct effects of glycemia and glucose-dependent gastrointestinal hormones on insulin secretion.
Results
The GB subjects had less suppression of fasting β-cell secretion during the insulin clamp compared to controls. In addition, meal-induced insulin secretion increased in the GB subjects but not controls during fixed sub-basal glycemia. In contrast the glucagon responses to hypoglycemia and meal ingestion were lower in the GB subjects than controls.
Conclusions
Among subjects with GB the response of insulin and glucagon secretion to decreasing blood glucose is blunted, but meal-induced insulin secretion is stimulated even at fixed systemic sub-basal glycemia. These findings indicate that following GB islet hormone secretion is altered as a result of factors beyond circulatory glucose levels.
Keywords: gastric bypass surgery, meal-induced insulin secretion, glucose-independent, GLP-1, pancreatic polypeptide, glucagon
Introduction
The past decade has seen a dramatic increase in the use of bariatric surgery for weight loss and mitigation of the co-morbidities of obesity(1). Gastric bypass (GB) is one of the most common and effective procedures, involving the creation of a small gastric pouch connected to the jejunum with diversion of meal contents to the mid-gut. In addition to reducing food intake and causing weight loss, GB has dramatic effects on the regulation of blood glucose(2, 3). Because individuals with GB have rapid passage of ingested nutrients into the intestine, their blood glucose levels rise rapidly after meals, achieving earlier and higher glycemic peaks followed by lower nadirs(4, 5). These changes in postprandial glucose are associated with increased insulin and GLP-1 responses(6). The commonly held explanation for the meal-induced hyperinsulinemia typical of GB is that stimulation of β-cells by glucose and incretins is enhanced (6, 7). However, we recently observed that subjects with GB have increased insulin secretion rates in the latter phases of meal absorption when blood glucose and GLP-1 levels have declined to near basal levels (8). This finding suggests that factors beyond direct glucose stimulation and glucose-potentiation of incretins act on the β-cell in persons with GB.
In the present study we measured the islet cell response to meal ingestion during a period of fixed, mild hypoglycemia (3-3.5 mmol/l) using a hyperinsulinemic (80 mU.m-2.min-1) glucose clamp (9, 10) in GB subjects compared to non-surgical controls. This approach eliminates the direct effects of increasing blood glucose to stimulate the β-cells and neutralizes the insulinotropic actions of the incretins, which are dependent on hyperglycemia (11, 12, 13). We hypothesized that subjects with GB would have increased meal-stimulated β-cell responses during sub-basal glycemic levels.
Methods
Subjects
Fifteen subjects with previous GB were recruited from the Endocrinology clinics at the University of Cincinnati as well as by general advertisement. Six subjects without prior surgery, matched for BMI and age of the GB subjects, were recruited as controls for the GB subjects (Obese-Controls, O-CON), as well as a group of 7 lean young subjects (L-CON) to approximate the normative range independent of BMI and age in non-surgical individuals. The GB subjects did not have a prior history of diabetes, had an average of 54 ± 5 kg (19-80 kg) of weight loss in a mean of 5.9 ± 0.5 y (3-8 y) since surgery, and had been weight stable for at least 6 months prior to the studies. The control subjects had no personal or family history of diabetes and had normal oral glucose tolerance tests before enrollment. All subjects were free of active gastrointestinal disease, renal dysfunction, or liver disorders and none took any medications that interfere with glucose metabolism or blood pressure for at least one week prior to the studies. The institutional review board of the University of Cincinnati approved the protocol, and all participants provided written informed consent before participating in any experiments.
Experimental protocols
All studies were performed at the Clinical and Translational Research Center at Cincinnati Children’s Hospital in the morning after an overnight fast. Participants were instructed to maintain normal carbohydrate ingestion for 3 d before each visit, and not to engage in excessive physical activity. On the first day of study, body composition was assessed using dual-energy X-ray absorptiometry only in GB and O-CON subjects. For glucose clamps, intravenous catheters were placed in each forearm for the withdrawal of blood and the infusion of insulin and glucose; the arm used for blood sampling was continuously warmed with a heating pad to maintain consistent blood flow.
Hyperinsulinemic clamp/MTT
The insulin infusate consisted of recombinant human insulin (Humulin 100 U/ml) diluted in isotonic saline / 25% human serum albumin. After withdrawal of 3 fasting blood samples, a 10-min priming infusion of insulin was followed by constant administration of 80 mU/m2 surface area per min for the duration of the study (14). Blood was sampled at 5-min intervals and a variable infusion of 20% dextrose was infused to clamp blood glucose at a target of 3-3.5 mmol/l. At 120 min, a 140-ml liquid mixed meal containing 3 kcal/kg distributed as 40% protein, 40% fat, and 20% glucose was consumed within 10 min. Blood samples were removed at timed intervals and stored on ice, plasma was separated within 60 min, and these were stored at -80° C until assay. Each subject’s heart rate was monitored throughout the studies using the GE Dash 3000 monitoring system (GE Healthcare, Milwaukee, WI) with values averaged over every 5-min period throughout the study.
Assays
Blood samples were collected in heparin for measurement of insulin and glucose and in aprotinin/heparin/EDTA for assay of glucagon, pancreatic polypeptide (PP), C-peptide, and GLP-1(7, 8, 15). Blood glucose concentrations were determined at the bedside using an automated glucose analyzer (YSI). C-peptide and glucagon were measured by commercial radioimmunoassay (Millipore, Billerica, MA), and insulin (ALPCO Diagnostics, Salem, NH), GLP-1 and PP (Millipore, Billerica, MS) using commercial ELISA, according to the manufacturers’ specifications.
Calculations and analysis
Fasting values of blood glucose and hormones were computed as the average of the 3 samples drawn before the clamp, and the pre-meal values as the average of the samples drawn in the 20 min before the test meal (100-120 min). The effects of the sub-basal hyperinsulinemic clamp on islet hormone release and heart rates were computed as: [(fasting values - premeal values) / fasting values] × 100. Islet hormone responses to the test meal were summarized as the incremental areas under the curve (AUC) over premeal values using the trapezoidal rule. Postprandial changes in heart rates were calculated as: [(postprandial values - premeal values) / premeal values] × 100. Insulin sensitivity was computed as the average of the glucose infusion rate from 100-120 min divided by mean plasma insulin levels over the same period (16). Fasting clearance rates of insulin were calculated from the insulin infusion rate divided by the steady-state insulin concentrations at 100-120 minutes corrected for endogenous insulin secretion (16). Systemic appearance of ingested glucose (RaOral) was computed as the integrated reduction of glucose infused after meal ingestion (9, 10).
Statistical analysis
Data are presented as mean ± SEM. The parameters of interest at baseline and during the hyperinsulinemic clamp were compared using ANOVA based on pre-specified comparisons between GB and O-CON as well as GB and L-CON. Statistical analyses were performed using SPSS 22 (SPSS Inc., Chicago, IL).
Results
Subject characteristics (Table 1)
Table 1.
Baseline characteristics, glucose, and hormonal profile of study participants
| GB (15) | O-CON (6) | L-CON (7) | GB vs. O-CON (p value) | GB vs. L-CON (p value) | |
|---|---|---|---|---|---|
| Gender (M/F) | 2/13 | 1/6 | 3/4 | 0.66 | 0.05 |
| Age (yr) | 50.9±2.7 | 48.7±1.9 | 20.7±0.3 | 0.58 | <0.001 |
| Current BMI (kg/m2) | 32.0±1.4 | 30.9±2.7 | 23.1±0.8 | 0.74 | <0.001 |
| Total fat mass (kg)# | 38±4 | 33±4 | - | 0.41 | - |
| Total lean mass (kg)# | 56±2 | 56±3 | - | 0.95 | - |
| HbA1C (%) | 5.2±0.1 | 5.4±0.2 | 4.8±0.2 | 0.51 | 0.08 |
| Blood glucose (mmol/l)* | 4.5±0.1 | 4.8±0.1 | 4.8±0.1 | 0.10 | 0.10 |
| Insulin (pmol/l)* | 12±3 | 69±17 | 13±5 | 0.04 | 0.75 |
| C-peptide (nmol/l)* | 3.6±0.3 | 6.0±0.9 | 3.3±0.6 | 0.42 | 0.26 |
| GLP-1 (pmol/l)* | 3.6±0.3 | 3.1±0.7 | 4.0±0.7 | 0.59 | 0.56 |
| Glucagon (pg/ml)* | 33.5±1.8 | 36.1±1.6 | 39.6±2.6 | 0.42 | 0.06 |
| PP (pg/ml)* | 287±153 | 108±56 | 34±9 | 0.42 | 0.26 |
| Heart rate (bpm)* | 68±6 | 68±6 | 57±2 | 0.42 | 0.13 |
| Insulin sensitivity (M/I)** | 7.3±0.9 | 4.2±0.9 | 6.1±0.5 | 0.05 | 0.30 |
| Insulin clearance (ml.m-2.min-1)** | 903±61 | 682±56 | 897±52 | 0.04 | 0.94 |
Data are presented as mean ± SEM unless specified otherwise. P values for ANOVA or X2 comparison between GB and O-CON and GB and L-CON are provided;
baseline values;
derived from clamp studies before meal ingestion;
data were obtained in 10 GB and 5 O-CON subjects.
The GB and the O-control subjects had similar BMI, fat and lean body mass, age, A1C, and female to male ratio. The L-CON subjects were younger and leaner than the GB subjects.
Glucose clamp and insulin sensitivity
Glucose levels at baseline were similar among the 3 groups (Table 1) and decreased rapidly to the target with infusion of insulin (GB: 3.3 ± 0.1, O-CON: 3.3 ± 0.1, and L-CON: 3.0 ± 0.1 mmol/l; Fig.1a). The mean coefficient of variation of blood glucose levels from 40-120 min was 5 ± 1 % for the three groups. Basal insulin levels were significantly lower in the GB subjects compared to the O-CON individuals but they did not differ between the GB and L-CON groups (Table1). A square wave of hyperinsulinemia was achieved in each of the three groups with mean steady state (100-120 min) levels of 669 ± 49, 909 ± 78, and 647 ± 42 pmol/L in the GB, O-CON, and L-CON subjects, respectively (Fig.1b); representing comparable increments over basal values in the groups.
Figure 1.
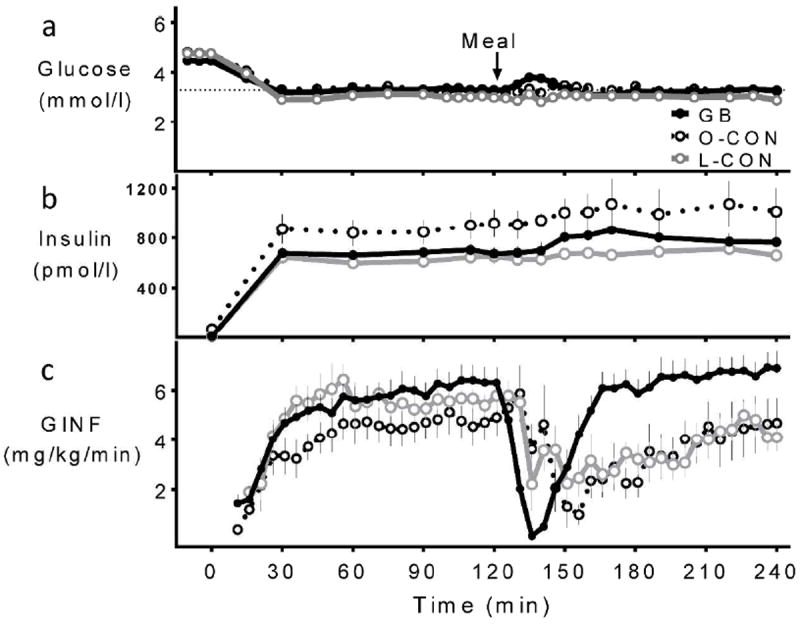
Blood glucose (a) and insulin (b) levels and glucose infusion rates (c) during hyperinsulinemic hypoglycemic clamp combined with MTT in GB subjects (solid black line, close circle), matched obese controls (dashed black line, open circle), and lean controls (grey line, open circle).
The glucose infusion rate needed to achieve the premeal glycemic target was similar in the GB subjects compared to the L-CON subjects but there was a trend for lower value in the O-CON subjects compared to GB individuals (p=0.13; Fig.1c). Accordingly, the GB subjects were more insulin sensitive and had greater insulin clearance compared to the O-CON subjects, while these parameters were similar among the GB subjects and L-CON individuals (Table1).
Fasting islet hormone responses to the hyperinsulinemic glucose clamp
Fasting C-peptide values were lower in the GB compared to the matched O-CON subjects (Fig.2b, Table 1). With gradual reduction of blood glucose from basal during the clamp, endogenous insulin secretion, reflected by C-peptide levels, declined. Despite a significant difference in fasting C-peptide levels among GB and O-CON individuals, the absolute C-peptide levels from 100-120 min were similar in both groups. Thus the relative change in C-peptide in response to the clamp was significantly lower in GB patients compared to the O-CON subjects (GB: 59 ± 4 and O-CON: 76 ± 1 %; p<0.01; Fig.2b). Fasting C-peptide levels were comparable in the GB and L-CON subjects (Fig.2b, Table 1). However, the absolute C-peptide levels from 100-120 min were significantly greater in the GB subjects, indicative of a smaller relative rate of suppression of C-peptide as a result of the glucose clamp in the GB subjects compared to L-CON individuals (GB: 59 ± 4 and L-CON: 80 ± 3%; p<0.01; Fig.2b).
Figure 2.
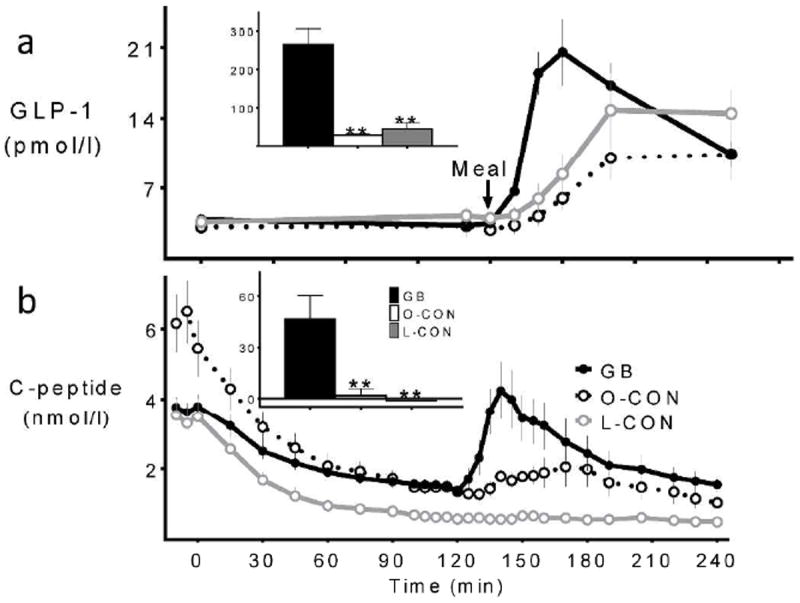
GLP-1 (a) and C-peptide (b) concentrations during the hyperinsulinemic hypoglycemic clamp combined with MTT in GB subjects (solid black line, black bar), matched obese controls (dashed black line, white bar), and lean controls (grey line, grey bar). Corresponding postprandial AUC(120-150min) shown in inset; ** (p<0.01) compared to the GB subjects
Despite similar fasting glucagon levels among the groups (Table 1), the relative increase in premeal glucagon in response to glucose reduction during the hyperinsulinemic clamp was significantly greater in the L-CON compared to the GB subjects (GB: -21 ± 4% vs. L-CON: 49 ± 17%; p<0.01; Fig.3b). Compared to the O-CON individuals, the GB subjects tended to reduce premeal glucagon with responses that were significantly lower than those of the matched non-surgical group (GB: -21 ± 4% vs. O-CON: 0 ± 6%; p=0.02; Fig.3b). Similarly, fasting PP levels did not differ among the 3 groups (Table 1), but followed a generally similar trajectory as glucagon. The L-CON subjects had the largest PP response to the premeal decrease in glycemia, with no significant differences between the GB and the O-CON groups (Fig.3a).
Figure 3.
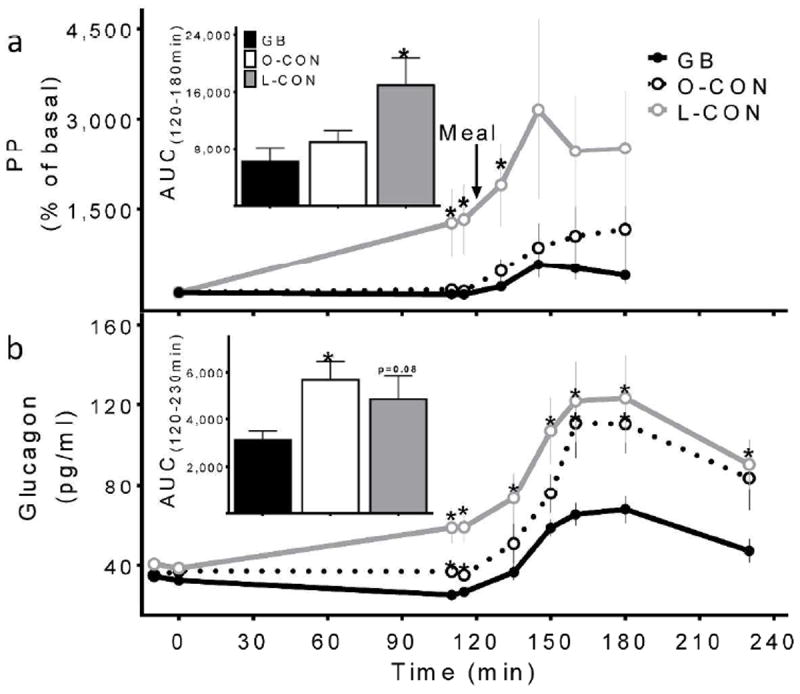
Pancreatic polypeptide (a) and glucagon (b) concentrations during the hyperinsulinemic hypoglycemic clamp combined with MTT in GB subjects (solid black line, black bar), matched obese controls (dashed black line, white bar), and lean controls (grey line, grey bar). Corresponding AUCs shown in insets; * (p<0.05) compared to the GB subjects
Fasting heart rates were similar among the surgical and nonsurgical groups (Table 1). Heart rates increased in response to the sub-basal glucose clamp in all subjects, with the largest enhancement in the L-CON individuals while the relative increase was similar in the BMI-matched controls and surgical groups (Fig.4a).
Figure 4.
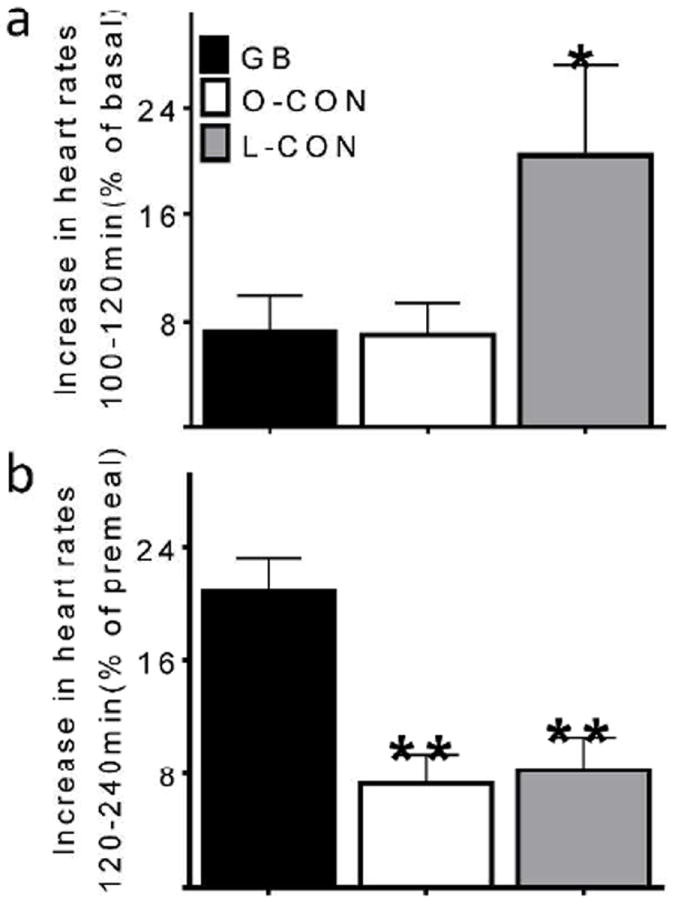
Heart rate response to hypoglycemia (a) and meal ingestion (b) during hyperinsulinemic hypoglycemic clamp in GB subjects (black bar), matched obese controls (white bar), and lean controls (grey bar). * (p<0.05) and ** (p<0.01) compared to the GB subjects
Systemic appearance of ingested glucose
Following meal ingestion the glucose infusion rates were sharply reduced to compensate for glucose influx from the gut, but then gradually increased at a different pace among surgical and nonsurgical subjects (Fig.1c). Blood glucose was maintained at the target in all groups except for 5 subjects in the GB group, whose levels rose slightly above the target for a short period, yet remained below fasting levels (absolute difference between glycemia from 125-150 min and 100-120 min: 0.25 ± 0.07, 0.03 ± 0.1, and -0.03 ± 0.09 mmol/l for GB, O-CON, and L-CON, respectively; Fig.1a). The average coefficient of variation for glucose concentration from 120-240 min was 9 ± 1, 7 ± 1, and 7 ± 1 % for GB, O-CON, L-CON respectively. The completion of oral glucose absorption, marked by the return of glucose infusion rates to steady state values, was achieved in surgical subjects over the 60 min following meal ingestion (Fig.1c). When the data are expressed as meal glucose appearance, it is apparent that postprandial glucose absorption occurred at a faster rate in the GB subjects (Fig.5).
Figure 5.
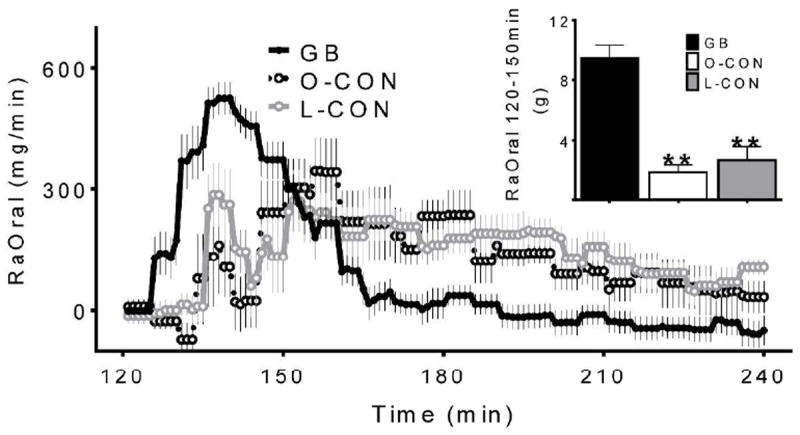
Systemic appearance of ingested glucose during hyperinsulinemic clamp in GB subjects (solid black line, black bar), matched obese controls (dashed black line, white bar), and lean controls (grey line, grey bar). Corresponding postprandial AUC(120-150min) shown in inset; ** (p<0.01) compared to the GB subjects
Islet hormone responses to meal ingestion during a sub-basal hyperinsulinemic glucose clamp
Following meal ingestion, the L-CON and O-CON subjects had a minimal release of C-peptide (Fig.2b). In contrast almost all of the surgical subjects had measurable C-peptide release in the first 30-60 min after eating (Fig.2b). There was no association of meal-induced β-cell output with the small changes in postprandial glucose levels (Supplementary Figure). Meal ingestion increased GLP-1 levels in both surgical and non-surgical groups, with the largest and earliest response occurring in the GB subjects (Fig.2a).
After meal ingestion, there was a 2-3-fold increase in glucagon concentrations in all groups (Fig.3b). However, meal-induced glucagon release was significantly lower in the surgical subjects compared to both O-CON and L-CON individuals (Fig.3b). Similar to the glucagon response, meal ingestion led to augmented PP values during the clamp in all subjects, with a larger response in the L-CON compared to the GB, and no differences between the surgical and O-CON subjects (Fig.3a).
Meal ingestion increased heart rates in all groups, with the largest enhancement in the surgical individuals compared to the non-surgical controls (Fig.4b).
Discussion
Postprandial hyperinsulinemia in subjects with GB has been attributed mainly to the rapid and substantial changes in glycemia combined with the enhanced incretin effect that is typical after surgery. In this study we assessed the GB effect on meal-induced β-cell secretion, independent of postprandial changes in systemic glucose levels. Our findings demonstrate that in contrast to persons without surgery, those with GB have meal-induced β-cell responses in the absence of stimulatory changes in blood glucose. Also, there is blunted suppression of β-cell secretion after GB in response to glucose lowering compared to non-surgical individuals. Taken together with the distinct glucagon responses observed in GB subjects, these findings suggest systematic differences in islet cell regulation after surgery, and for the first time dissociate these from changes in systemic blood glucose.
To address glucose-independent regulation of islet hormone secretion we refined a meal/hyperinsulinemic clamp protocol similar to one previously used to demonstrate abnormal insulin secretion in patients with insulinoma(17). We selected a glucose target of 3-3.5 mmol/l to prevent glycemic stimulation of insulin and to neutralize the effect of incretins, which are glucose-dependent in non-surgical cohorts (11, 12, 13). Subjects were given a mixed nutrient meal with relatively low carbohydrate content to minimize postprandial glucose excursions. As validated in previous studies (9, 10), we used the meal/clamp method to measure systemic meal glucose appearance, although this parameter may have been underestimated in the GB subjects given the differences in postprandial insulin and glucagon response in these individuals compared to the controls.
To determine the lowest absolute C-peptide levels reached during the clamp, GB subjects were also compared with lean non-operated individuals, whose fasting C-peptide levels were expected to match those of the GB subjects (18). Despite differences in fasting C-peptide between the lean and obese control groups, exogenous insulin infusion suppressed plasma C-peptide to the same extent in both groups as described by previous investigators (16); certainly the sub-basal glucose target used here contributed to this effect. However, the GB subjects had less suppression of β-cell output before meal consumption compared to both lean and obese controls, suggesting abnormal glucose sensing with declining glycemia in these individuals. This observation is consistent with our previous findings that GB subjects with hyperinsulinemic hypoglycemia maintain higher rates of insulin secretion in the latter phases of a MTT when glucose concentrations approach basal and sub-basal levels (8). Previous studies have identified pancreatic denervation as causing a similar lack of β-cell response to relative hypoglycemia (19, 20), and altered neural control of insulin secretion is plausible in GB subjects. However, despite the attenuated response to declining glycemia, there was a 2.5-fold increase in postprandial C-peptide in the GB cohorts at fixed, sub-basal glycemia, an insulin response that was not associated with the extent of glycemic deviation from the clamp target. In contrast, the non-surgical controls had virtually no β-cell secretion after eating. This finding supports factors other than glycemia to promote the elevated rates of postprandial insulin secretion typical of individuals with GB. Potential mechanisms include differential β-cell stimulation by non-glucose nutrients (21, 22) or neural signals as a result of enhanced pace of nutrient flux, increased sensitivity to the effects of the incretins, or a combination of these factors. Any of these mechanisms, and others as well, are best accounted for by chronic effects of a reconfigured GI anatomy and an altered metabolic milieu. The important implication of the results presented here is that the effect of GB on β-cell function is not wholly explained by acute changes in systemic glycemia.
It is notable that although we maintained blood glucose 20-30% below fasting levels, we did not see the robust glucagon response typical of hypoglycemic counter-regulation among the surgical and obese non-surgical controls. Moreover, fasting heart rates did not increase by > 10% of basal in the majority of GB and O-CON subjects whereas heart rates in lean controls rose by ~ 25%. In contrast, the L-CON individuals had marked increases in glucagon and PP secretion as well as heart rate. The lean subjects were significantly younger than the GB and O-CON subjects, which may contribute to this difference, but given that the study population as a whole consisted of young and middle-aged adults, these findings suggest blunted activation of the autonomic nervous system by reductions in blood glucose in obese subjects, with and without GB.
The role of autonomic nervous system activation of the β-cell has been extensively studied during the preabsorptive phase of insulin secretion(23, 24), and as an anticipatory response to food intake or to oral nutrient sensory stimulation(25). However, beyond premeal insulin secretion, parasympathetic nervous system (PNS) activation has been found to make an important contribution to the β-cell response to food intake (26, 27). Circulating pancreatic polypeptide (PP) concentrations have been generally taken to be a marker of PNS input to the islet (28). PP secretion from islet F-cells is under cholinergic control and the PP levels tend to increase reliably during hypoglycemia (29, 30). In our cohorts, plasma PP measures do not provide clear insights into islet neural signaling. The PP responses to hypoglycemia and meal ingestion in the L-CON subjects were compatible with previous studies, increasing significantly in response to both stimuli (29, 30, 31). However, the O-CON and GB subjects did not have a rise in PP during glucose lowering with the first 2 h of the glucose clamp. Overall the pattern of PP secretion followed very closely the glucagon profiles in these studies, with blunted postprandial responses in the GB groups. Therefore, plasma PP does not support increased parasympathetic stimulation of the β-cell response to the meal in the GB subjects. On the other hand cholinergic inputs are not the only neural stimuli to insulin secretion (24), and recent work has demonstrated that prandial insulin and PP secretion can be dissociated (32). Therefore, the profile of altered PP responses in the GB subjects does not exclude neural stimulation in the response to meal ingestion at sub-basal glucose.
It is conceivable that our findings could be explained by effects of meal-induced GLP-1 given the relatively large and early secretion of the peptide after eating in the GB subjects. The glucose dependency of gut hormones at normal glucose levels has been established in meal studies with fixed basal, glucose levels (11) or intravenous GLP-1 infusion studies in non-operated individuals (12, 13). Therefore, these findings may not apply to GB where large amounts of GLP-1 are secreted into the portal vein after eating, causing an exaggerated discrepancy between portal and peripheral levels (33), and potentially activating portal GLP-1 sensors (34). These possibilities do not exclude, and even support, an eventual neural contribution to meal-induced insulin secretion.
It is surprising that following meal ingestion the GB subjects had relatively smaller glucagon responses than the non-surgical controls. Several studies have reported that the glucagon response to meal ingestion is enhanced after GB(5, 35), and is similar in post-surgical subjects with and without hypoglycemia(8, 36). While the mechanism(s) underlying distinct glucagon responses of GB subjects has not been explained, several possibilities could be considered to explain our findings. A diminished glucagon response to hypoglycemia could be due to antecedent hypoglycemia (37, 38), the short but extreme glycemic excursion with lower nadir glucose levels, in GB subjects might affect subsequent α-cell responses. In addition, it has been demonstrated previously that carbohydrate ingestion during hyperinsulinemic clamps reduces the glucagon response to hypoglycemia in healthy subjects (29), likely via neurally-mediated process initiated by nutrient sensing in the GI tract. While this mechanism does not explain the different responses in our surgical and nonsurgical subjects, it is possible that rapid nutrient flux from the gut in the GB individuals exaggerates this neurally-mediated response. Finally, inhibition of the α-cell due to paracrine regulation by factors released from the β-cell has been well established (39, 40). Thus, less meal induced glucagon release could be attributed to greater suppression by β-cell products. Based on our current findings it appears that α-cell responses in GB subjects are glucose dependent, with hypersecretion during meals or other conditions with elevated blood glucose (7, 8), but muted in the setting described here where glucose levels are held fixed and low. The present study adds to the case that α-cell, as well as β-cell, function is significantly changed by GB.
In summary, these findings extend previous studies demonstrating distinct patterns of islet hormone secretion in persons with GB. In particular, this study indicates that the insulin and glucagon responses to declining blood glucose are blunted, and that a component of meal-induced hyperinsulinemia after GB is attributable to factors beyond glucose. Taken together these factors support distinct regulation of the islet following GB. While there are many possible explanations for these observations, they implicate chronic effects of surgery, in addition to acute shifts in circulating regulatory factors, on islet function.
Supplementary Material
Known and unknown about this subject
Rapid passage of ingested glucose after gastric bypass (GB) is associated with earlier and larger insulin response to meal ingestion.
Meal-induced hyperinsulinemia after GB has been attributed to increased glucose and GLP-1 levels.
The role of factors beyond altered systemic glycemia on postprandial islet function remained to be understood.
What is added to the knowledge by this study
The islet cell secretory response before and after meal ingestion during hyperinsulinemic hypoglycemic clamp were measured and for the first time we have shown that α- and β-cell response to hypoglycemia is altered after GB.
Postprandial hyperinsulinemia after GB is partly attributable to factors beyond acute changes in systemic glycemia.
Acknowledgments
We thank Leslie Baum, Brianne Reedy, and Radhakrishna Krishna from Department of Medicine of University of Cincinnati for their technical support and nursing staff from Clinical Research Center of Cincinnati Children’s Hospital for their expert technical assistance. We owe a great debt to our research participants.
This work was supported by grants from the National Institutes of Health (DK083554 to M.S. and DK57900 to D.A.D.) and in part by National Center for Advancing Translational Sciences, National Institutes of Health grant 8 UL1 TR000077 as well as the Medical Research Service of the Department of Veterans Affairs.
Abbreviations
- GB
Roux-en-Y gastric bypass surgery
- GI
gastrointestinal
- GLP-1
glucagon-like peptide 1
- L-CON
non-operated lean controls
- MTT
meal tolerance test
- O-CON
non-operated BMI- and age-matched controls
- PNS
parasympathetic nervous system
- PP
pancreatic polypeptide
- RaOral
systemic meal-derived glucose appearance
Footnotes
MS designed and supervised the study and, obtained the data, analyzed and interpreted the data, and wrote the manuscript; DAD and SCW contributed to interpretation of data and review/editing of the manuscript. MS as the guarantor takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. The manuscript was revised and approved by all authors.
Disclosure summary: The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg. 2011;213:261–266. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 4.Camastra S, Muscelli E, Gastaldelli A, Holst JJ, Astiarraga B, Baldi S, et al. Long-term effects of bariatric surgery on meal disposal and beta-cell function in diabetic and nondiabetic patients. Diabetes. 2013;62:3709–3717. doi: 10.2337/db13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorgensen NB, Jacobsen SH, Dirksen C, Bojsen-Moller KN, Naver L, Hvolris L, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122–131. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 6.Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like Peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60:2308–2314. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99:2008–2017. doi: 10.1210/jc.2013-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludvik B, Nolan JJ, Roberts A, Baloga J, Joyce M, Bell JM, et al. A noninvasive method to measure splanchnic glucose uptake after oral glucose administration. J Clin Invest. 1995;95:2232–2238. doi: 10.1172/JCI117913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludvik B, Nolan JJ, Roberts A, Baloga J, Joyce M, Bell JM, et al. Evidence for decreased splanchnic glucose uptake after oral glucose administration in non-insulin-dependent diabetes mellitus. J Clin Invest. 1997;100:2354–2361. doi: 10.1172/JCI119775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen DK, Elahi D, Brown JC, Tobin JD, Andres R. Oral glucose augmentation of insulin secretion. Interactions of gastric inhibitory polypeptide with ambient glucose and insulin levels. J Clin Invest. 1978;62:152–161. doi: 10.1172/JCI109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355. [DOI] [PubMed] [Google Scholar]
- 13.Qualmann C, Nauck MA, Holst JJ, Orskov C, Creutzfeldt W. Insulinotropic actions of intravenous glucagon-like peptide-1 (GLP-1) [7-36 amide] in the fasting state in healthy subjects. Acta Diabetol. 1995;32:13–16. doi: 10.1007/BF00581038. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 15.Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146:669–680. e662. doi: 10.1053/j.gastro.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elahi D, Nagulesparan M, Hershcopf RJ, Muller DC, Tobin JD, Blix PM, et al. Feedback inhibition of insulin secretion by insulin: relation to the hyperinsulinemia of obesity. N Engl J Med. 1982;306:1196–1202. doi: 10.1056/NEJM198205203062002. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, Park JY, Shong YK, Hong SK, Kim GS, Lee KU. Suppression of endogenous insulin secretion by exogenous insulin in patients with insulinoma. Clin Endocrinol (Oxf) 2000;52:87–92. doi: 10.1046/j.1365-2265.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- 18.Immonen H, Hannukainen JC, Iozzo P, Soinio M, Salminen P, Saunavaara V, et al. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients. J Hepatol. 2014;60:377–383. doi: 10.1016/j.jhep.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Luzi L, Battezzati A, Perseghin G, Bianchi E, Vergani S, Secchi A, et al. Lack of feedback inhibition of insulin secretion in denervated human pancreas. Diabetes. 1992;41:1632–1639. doi: 10.2337/diab.41.12.1632. [DOI] [PubMed] [Google Scholar]
- 20.Boden G, Chen X, DeSantis R, Kolaczynski J, Morris M. Evidence that suppression of insulin secretion by insulin itself is neurally mediated. Metabolism. 1993;42:786–789. doi: 10.1016/0026-0495(93)90250-r. [DOI] [PubMed] [Google Scholar]
- 21.Lindgren O, Carr RD, Deacon CF, Holst JJ, Pacini G, Mari A, et al. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J Clin Endocrinol Metab. 2011;96:2519–2524. doi: 10.1210/jc.2011-0266. [DOI] [PubMed] [Google Scholar]
- 22.Claessens M, Calame W, Siemensma AD, van Baak MA, Saris WH. The effect of different protein hydrolysate/carbohydrate mixtures on postprandial glucagon and insulin responses in healthy subjects. Eur J Clin Nutr. 2009;63:48–56. doi: 10.1038/sj.ejcn.1602896. [DOI] [PubMed] [Google Scholar]
- 23.Teff KL, Townsend RR. Early phase insulin infusion and muscarinic blockade in obese and lean subjects. Am J Physiol. 1999;277:R198–208. doi: 10.1152/ajpregu.1999.277.1.R198. [DOI] [PubMed] [Google Scholar]
- 24.Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 25.Teff KL, Levin BE, Engelman K. Oral sensory stimulation in men: effects on insulin, C-peptide, and catecholamines. Am J Physiol. 1993;265:R1223–1230. doi: 10.1152/ajpregu.1993.265.6.R1223. [DOI] [PubMed] [Google Scholar]
- 26.D’Alessio DA, Kieffer TJ, Taborsky GJ, Jr, Havel PJ. Activation of the parasympathetic nervous system is necessary for normal meal-induced insulin secretion in rhesus macaques. J Clin Endocrinol Metab. 2001;86:1253–1259. doi: 10.1210/jcem.86.3.7367. [DOI] [PubMed] [Google Scholar]
- 27.Ahren B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50:1030–1038. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz TW, Holst JJ, Fahrenkrug J, Jensen SL, Nielsen OV, Rehfeld JF, et al. Vagal, cholinergic regulation of pancreatic polypeptide secretion. J Clin Invest. 1978;61:781–789. doi: 10.1172/JCI108992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ertl AC, Mann S, Richardson A, Briscoe VJ, Blair HB, Tate DB, et al. Effects of oral carbohydrate on autonomic nervous system counterregulatory responses during hyperinsulinemic hypoglycemia and euglycemia. Am J Physiol Endocrinol Metab. 2008;295:E618–625. doi: 10.1152/ajpendo.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossetti P, Porcellati F, Busciantella Ricci N, Candeloro P, Cioli P, Nair KS, et al. Effect of oral amino acids on counterregulatory responses and cognitive function during insulin-induced hypoglycemia in nondiabetic and type 1 diabetic people. Diabetes. 2008;57:1905–1917. doi: 10.2337/db08-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adrian TE, Bloom SR, Bryant MG, Polak JM, Heitz P. Proceedings: Radioimmunoassay of a new gut hormone-human pancreatic polypeptide. Gut. 1976;17:393–394. [PubMed] [Google Scholar]
- 32.Vozarova de Courten B, Weyer C, Stefan N, Horton M, DelParigi A, Havel P, et al. Parasympathetic blockade attenuates augmented pancreatic polypeptide but not insulin secretion in Pima Indians. Diabetes. 2004;53:663–671. doi: 10.2337/diabetes.53.3.663. [DOI] [PubMed] [Google Scholar]
- 33.Ionut V, Liberty IF, Hucking K, Lottati M, Stefanovski D, Zheng D, et al. Exogenously imposed postprandial-like rises in systemic glucose and GLP-1 do not produce an incretin effect, suggesting an indirect mechanism of GLP-1 action. Am J Physiol Endocrinol Metab. 2006;291:E779–785. doi: 10.1152/ajpendo.00106.2005. [DOI] [PubMed] [Google Scholar]
- 34.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 35.Falken Y, Hellstrom PM, Holst JJ, Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 36.Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 37.Davis SN, Galassetti P, Wasserman DH, Tate D. Effects of antecedent hypoglycemia on subsequent counterregulatory responses to exercise. Diabetes. 2000;49:73–81. doi: 10.2337/diabetes.49.1.73. [DOI] [PubMed] [Google Scholar]
- 38.Diedrich L, Sandoval D, Davis SN. Hypoglycemia associated autonomic failure. Clin Auton Res. 2002;12:358–365. doi: 10.1007/s10286-002-0035-9. [DOI] [PubMed] [Google Scholar]
- 39.Greenbaum CJ, Havel PJ, Taborsky GJ, Jr, Klaff LJ. Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J Clin Invest. 1991;88:767–773. doi: 10.1172/JCI115375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes. 2002;51:958–965. doi: 10.2337/diabetes.51.4.958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


