Abstract
Objective
Individuals taking antipsychotic medications have increased risk of obesity-related early morbidity/mortality. This report presents weight maintenance results from a successful weight loss and behavioral lifestyle change program developed for people taking antipsychotic medications.
Design and Methods
STRIDE was a 2-arm, randomized controlled trial. Intervention participants attended weekly group meetings for 6 months, then monthly group meetings for 6 months. Assessments were completed at baseline, 6, 12, and 24 months.
Results
At 24-months, intervention participants lost 3.7% of baseline weight and control participants 2.1%, a non-significant difference. Fasting glucose results followed a similar pattern. There was a statistically significant difference, however, for fasting insulin—the intervention group’s levels decreased between the end of the intensive intervention (at 6 months) and 24 months (10.1 to 7.91μU/mL); control participants’ levels increased (11.66 to 12.92μU/mL) during this period. There were also fewer medical hospitalizations among intervention participants (5.7%) than controls (21.1%; Χ2=8.47, p=0.004) during the 12 to 24-month post-intervention maintenance period.
Conclusions
Weight-change differences between arms diminished following the intervention period, though fasting insulin levels continued to improve. Reduced hospitalizations suggest that weight loss, even with regain, may have long-term positive benefits for people taking antipsychotic medications and may reduce costs.
Introduction
Individuals with serious mental illnesses are at greatly increased risk of early morbidity and mortality (1-3). Serious mental illnesses are associated with obesity-related risk factors, irrespective of treatment (4-6), while psychiatric medications add to risks of weight gain, diabetes, and metabolic syndrome (7, 8).
Behavioral approaches to reducing cardiometabolic risk are considered the first line of treatment for overweight and obese adults in the general population (9). Recent evidence, including from the STRIDE project (10), suggests such interventions can be equally effective for adults with serious mental illnesses (11, 12) During STRIDE’s 6-month intensive intervention period (weekly sessions), intervention participants lost 4.4 kg more than control participants. Following an additional 6 months of monthly sessions (maintenance intervention), intervention participants were 2.6 kg lighter than controls. (10) Fasting glucose levels increased in controls at the 12-month measurement (from 106.0 mg/dL to 109.5 mg/dL), but decreased among intervention participants (from 106.3 mg/dL to 100.4 mg/dL). (10) Medical hospitalizations for the 12-month intervention period were significantly reduced in intervention compared to control participants, and at 12 months, 47% of intervention participants had lost at least 5% of baseline body weight, compared with 36% of controls. (10)
Despite short-term successes, however, long-term maintenance of weight loss and improved lifestyle have been difficult to achieve in general populations (13) and, as of this writing, we are aware of only one study that has examined maintenance of weight loss and lifestyle change among people with serious mental illnesses (14). Predictors of long-term weight-loss maintenance in general populations include: 1) reduced dietary fat, increased dietary fiber, increased exercise, and increased self-monitoring (15, 16); 2) among Caucasians, better mental and physical health (17); consumption of diets with higher protein and lower glycemic indices (18); and, among women, flexible cognitive restraint, lower levels of uncontrolled eating in response to stimuli, exercise self-efficacy, exercise intrinsic motivation, and body dissatisfaction (19). Among people with serious mental illnesses, the In SHAPE replication is the only study to date of long-term maintenance following a successful lifestyle intervention (14). In contrast to STRIDE’s group intervention model, In SHAPE (14) tested an individualized health coach plus fitness club membership intervention compared to fitness club membership alone. The intevention reduced weight and improved fitness, reducing cardiovascular risks. Most importantly, improvements (exercise levels, improved diet, weight loss, improved fitness) were sustained in the 6 months after the intervention was withdrawn, though the investigators did not report predictors of maintenance beyond sustained dietary change and exercise.
In this paper, we report maintenance outcomes of the STRIDE weight-loss and lifestyle change program for the period 12-24 months following the end of the intervention. We also report exploratory analyses of predictors of maintaining a weight loss of 5% or more of baseline weight one year after the intervention.
Methods
The STRIDE trial (10) tested an adaptation of the PREMIER lifestyle intervention with DASH eating plan (20). PREMIER/DASH successfully reduced weight and blood pressure among people in the general population (21), and the DASH eating plan has been shown to reduce other cardiometabolic risks, through increases in HDL cholesterol, lowered triglycerides, reduced fasting blood glucose levels, and improved insulin resistance (22-24). Details of the STRIDE study design, rationale, implementation process and 12-month outcomes have been described elsewhere (10, 25, 26). The intervention manual, participant workbook, and other materials are also available: http://www.kpchr.org/research/public/stride/stride.htm.
The weight-loss intervention used in STRIDE provided 6 months of weekly group meetings including 20-30 minutes of exercise, followed by 6 months of monthly maintenance meetings, also with exercise. No intervention activities were conducted between 12 and 24 months. This paper presents 24-month outcomes.
STRIDE was implemented using a multi-site, parallel, two-arm (balanced 1:1) randomized controlled trial design. A full description of the protocol is available elsewhere (37) The focus of the intervention was on moderate caloric restriction, increased moderate exercise, and improved dietary practices (DASH Eating Plan: increased fruit, vegetable, and low-fat dairy consumption; reduced fat consumption). The intervention goal was weight loss of 4.5-6.8 kg (10-15 pounds) over 6 months, the same goal as the PREMIER intervention. The trial was registered on ClinicalTrials.gov (NCT00790517) Clinical Trials.gov, NCT00790517; http://clinicaltrials.gov/ct2/show/NCT00790517?term=STRIDE&rank=1.
Settings
This study was conducted in Kaiser Permanente Northwest (KPNW), a not-for-profit integrated health system, and CMHCs in the Portland, Oregon metropolitan area (Cascadia Behavioral Healthcare [Cascadia] and LifeWorks Northwest [LifeWorks]). Most individuals served by these CMHCs are low-income; KPNW’s membership is insured and demographically representative of the surrounding metropolitan area. All settings provide mental health and addiction treatment; KPNW also provides medical treatment.
Participants
Participants were 200 adults (56 men, 144 women) age ≥18 years (mean age = 47.2, SD = 10.6). All had been taking antipsychotic agents for at least 30 days before enrollment, and had a body mass index (BMI) of 27 or more. Using a stratified randomized block design, 96 were randomized to the usual care control and 104 to intervention. Individuals were excluded if they were pregnant, breastfeeding, or planning a pregnancy during the study period; had an inpatient psychiatric hospitalization within 30 days (although we allowed deferred participation); had a history of, or plans for, bariatric surgery; had a history of a cancer diagnosis in the 2 years prior to enrollment; had experienced a heart attack or stroke within 6 months; or had cognitive impairment that could interfere with informed consent.
We collected data between July 2009 and October 2013. Detailed recruitment and participant demographic information are available elsewhere. (25) Blinded staff collected data at all study periods, including scale-measured weights. Follow-up rates were 90.5% of participants at 6 months (n = 181), 85% at 1 year (n = 170), and 81.5% (n = 164) at 2 years (83.2% if 3 deaths are removed). We found no significant differences in attrition between study arms at any assessment point. Participants from all study arms who had elevated blood pressure or laboratory results were referred to primary or urgent care after each assessment. The KPNW Institutional Review Board reviewed, approved, and monitored all study sites and study procedures.
Measures
Our pre-specified outcome measures were weight change from baseline, BMI change from baseline, fasting glucose, fasting insulin, the HOMA-IR index of insulin resistance (27); the Framingham Diabetes Risk Score (28), HDL and LDL cholesterol, triglycerides, and diastolic and systolic blood pressure. In addition, we collected data on a variety of other measures for exploratory analyses including: the SF-36v2 General Health subscale (29); sleep quality, measured with items from the Berlin sleep apnea scale (30); and the BASIS-24 measure of mental health (31). Other measures included the Patient Activation Measure of health-related self-efficacy; positive and negative social support for lifestyle changes; the B-WISE body image scale; the Wisconsin Quality of Life Index General Quality of Life Subscale; dietary self-efficacy; weight outcome expectancies; past 7-day exercise; and dietary content from Block Screeners (see Yarborough et al. (25) for details and citations for these secondary measures). We also assessed adverse events occurring in the periods between assessment visits, including all medical and psychiatric hospitalizations.
Analyses
We examined distributions for all outcomes to determine whether transformations or different models were needed, and used generalized estimating equations (GEE) because they estimate population-averaged effects while controlling for the non-independence of observations inherent in repeated-measures data. (32) Time was dummy coded using the 24-month time point as the reference group because comparisons among the other time points have been reported. (10) We tested whether the joint effect of the interaction of time and study arm equaled zero using Wald tests. Each time-by-arm interaction term represents the difference in the change from baseline, 6 months, or 12 months, to the 24-month time point—all time points are included in figures and tables to provide a full picture of changes over time. We included age and study site as time-invariant covariates, and whether or not outcomes-related medications (e.g., for blood pressure, lipids, or diabetes; and medications producing weight gain or weight loss) were being taken at a given time, as time-varying covariates. We used GEE models with a normal distribution and identity link. The working covariance matrix was specified as exchangeable. We report covariate-adjusted results using robust estimates of standard errors.
We also conducted sensitivity analyses for each outcome using transformations that improved the normality of the outcome, a different family and link (e.g., negative binomial with log link) where appropriate, and unstructured working covariance matrices. For time-varying covariates, we also ran models that specified them as time-invariant (i.e., baseline only). There were no substantive differences among models in almost all cases.
We examined between-group differences at 24 months for percentage weight change, proportion of participants at or below baseline weight, and proportion of participants who had lost at least 5% or 10% of baseline weight. In contrast to other analyses, these were not intent-to-treat results because computation of change scores required complete data. We then tested differences in percentage weight change between intervention and control groups using oneway ANCOVAs, and used multiple logistic regression analyses to test whether the proportion of participants at or below baseline weight at 24 months differed between the 2 arms. Finally, we used multiple logistic regression analyses to explore factors associated with maintaining a weight loss of 5% or more from baseline weight, irrespective of study group, and we used chi-square analyses to examine differences in medical and psychiatric hospitalizations at 24 months.
Results
Outcomes at 24 Months
We found no significant differences in intent-to-treat analyses comparing weight at 24 months to weight at previous assessments: baseline (2.10, 95% CI [−0.96, 5.15]); 6 months (−2.45, 95% CI [−6.00,1.10]); or 12 months (−0.60, 95% CI [−3.09,1.90]) (the omnibus test for the interaction was significant because there was a difference in the weight change between groups during the intervention phase) (see Table 1).
Table 1.
Adjusted time-by-group coefficients and confidence intervals for intent-to-treat generalized estimating equation analyses for 2-year outcomes.
| Δ Baseline – 2 year | Δ 6 mo. – 2 year | Δ 12 mo. – 2 year | |||||
|---|---|---|---|---|---|---|---|
| Coef. a | 95% CI (lower, upper) |
Coef. | 95% CI (lower, upper) |
Coef. | 95% CI (lower, upper) |
Omnibus
p valueb |
|
| Weight, kg | 2.098 | −0.958, 5.154 | −2.449 | −5.995, 1.097 | −0.596 | −3.090, 1.899 | 0.007 |
| BMI, kg/m2 | 0.708 | −0.405, 1.822 | −0.907 | −2.179, 0.365 | −0.299 | −1.232, 0.635 | 0.007 |
| Systolic blood pressure, mmHg |
−0.470 | −4.157, 3.218 | −2.135 | −5.939, 1.670 | −0.370 | −4.123, 3.383 | 0.676 |
| Diastolic blood pressure, mmHg |
−0.984 | −3.494, 1.525 | −2.307 | −5.022, 0.407 | −1.354 | −3.915. 1.207 | 0.412 |
| Fasting Glucose, mg/dL |
0.047 | −0.017, 0.111 | 0.032 | −0.024, 0.089 | −0.042 | −0.101, 0.018 | 0.049 |
| Fasting insulin, μU/mL | 0.231 | −0.080, 0.543 | 0.347* | 0.054, 0.640 | 0.225 | −0.224, 0.675 | 0.118 |
| HOMA-IRc | 0.100 | −0.149, 0.349 | 0.180 | −0.089, 0.448 | −0.035 | −0.276, 0.207 | 0.352 |
| Diabetes riskd | −0.269 | −2.015, 1.478 | −0.099 | −1.917, 1.720 | −1.612 | −3.336, 0.111 | 0.225 |
| Fasting Triglycerides, mg/dL |
0.070 | −0.066, 0.205 | 0.056 | −0.086, 0.198 | 0.006 | −0.122, 0.134 | 0.653 |
| Fasting LDL, mg/dL | −6.313 | −15.703, 3.076 | −8.369 | −17.390, 0.651 | −6.188 | −15.011, 2.636 | 0.343 |
| Fasting HDL, mg/dL | −2.152 | −5.420, 1.116 | −0.676 | −3.838, 2.486 | 0.285 | −3.262, 3.831 | 0.193 |
p <.05 for the difference between the end of the intensive intervention at 6 months and the two-year follow-up assessment. No other comparisons were significant at the 2-year follow-up.
Coef=Coefficient for the time-by-group indicators estimated from the GEE models.
Omnibus Wald test assessing whether the joint effect of the time-by-group indicators = 0; all significant omnibus tests reflect significant differences during the intervention periods (intensive or maintenance).
The Homeostasis Model Assessment Index for Insulin Resistance (HOMA-IR) is calculated as follows: fasting glucose [mmol/L] x fasting insulin [μU/mL]/22.5. Lower scores indicate lower risk for developing insulin resistance. Coefficients represent the change in the natural log of the HOMA-IR index.
Based on the Framingham Diabetes Risk Scale. Lower scores represent decreased risk of developing diabetes.
In analyses of complete cases at 2 years, intervention participants had lost 3.7% of their baseline weight, and control participants had lost 2.1% of baseline weight, F(1,156)=1.2, p=0.29. Table 1 presents adjusted time-by-group coefficients and confidence intervals for intent-to-treat analyses. Figures 1 and 2 show estimated marginal means for all study outcomes (Table 2 provides the estimated marginal means for 12 and 24 months).
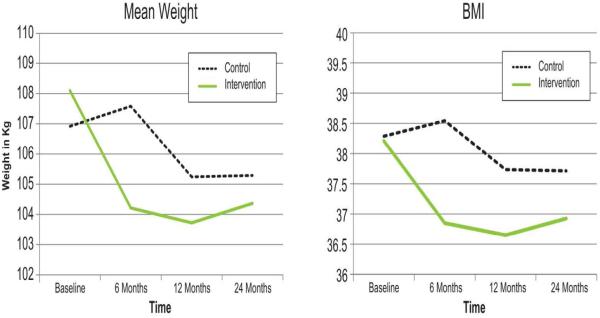
Figure 1.
Adjusted mean weigth and adjusted BMI of intervention and control groups from baseline to 24 months.
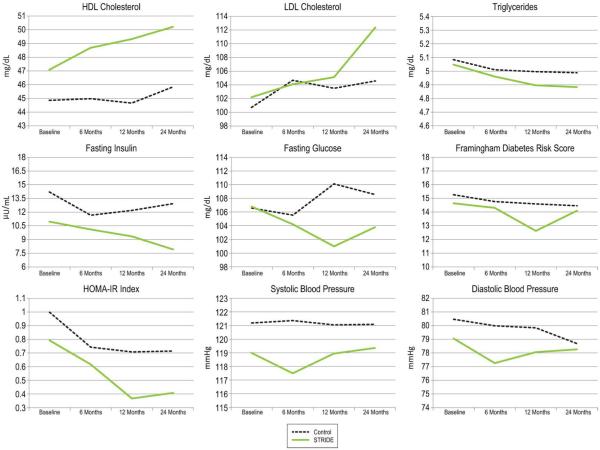
Figure 2.
Adjusted mean outcomes of intervention and control groups from baseline to 24 months.
Table 2.
Estimated Marginal Means at 12 and 24 Months for Control and Intervention Arms.
| Control | Intervention | |||
|---|---|---|---|---|
| 12 Months | 24 Months | 12 Months | 24 Months | |
| Weight, kg | 105.241 | 105.290 | 103.725 | 104.369 |
| BMI, kg/m2 | 37.735 | 37.713 | 36.649 | 36.927 |
| HOMA-IRa | .707 | .714 | .367 | .408 |
| Fasting insulin, μU/mL | 12.186 | 12.929 | 9.343 | 7.912 |
| Fasting HDL, mg/dL | 44.656 | 45.833 | 49.321 | 50.213 |
| Fasting LDL, mg/dL | 103.508 | 104.571 | 105.117 | 112.367 |
| Diastolic blood pressure, mmHg |
79.836 | 78.683 | 78.058 | 78.259 |
| Systolic blood pressure, mmHg |
121.046 | 121.084 | 118.942 | 119.351 |
| Fasting Triglycerides, mg/dLb | 4.996 | 4.988 | 4.896 | 4.883 |
| Diabetes riskc | 14.594 | 14.458 | 12.620 | 14.096 |
| Glucose | 110.112 | 108.557 | 100.974 | 103.776 |
The Homeostasis Model Assessment Index for Insulin Resistance (HOMA-IR) is calculated as follows: fasting glucose [mmol/L] x fasting insulin [μU/mL]/22.5. Lower scores indicate lower risk for developing insulin resistance. Means represent the change in the natural log of the HOMA-IR index.
b Means represent the change in the natural log of Fasting Triglycerides.
Based on the Framingham Diabetes Risk Scale. Lower scores represent decreased risk of developing diabetes.
There was no evidence that the intervention group was more likely to be at or below their baseline weight at 24 months compared to the control group (odds ratio = 1.33; 95% CI [0.69, 2.58], p=0.40). Similarly, there was no indication at 24 months that the intervention group had a greater proportion of participants who lost 5% or more (odds ratio = 1.22; 95% CI [0.63, 2.36], p=0.55) or 10% or more (odds ratio = 1.37; 95% CI [0.60, 3.15], p=0.46) of baseline weight compared to the control group.
Because the distributions for fasting glucose, insulin, and HOMA-IR index were positively skewed, we fitted log transformations for these outcomes using a Gaussian-based GEE model, and a GEE model using the negative binomial distribution and log link (except for HOMA). For fasting glucose and insulin, we report results of the negative binomial GEE. There were no significant time-by-group interactions for fasting insulin, Framingham Diabetes Risk Score, or HOMA-IR. For fasting glucose, there were no significant differences in the change of the log of the incidence rate ratio at 24 months compared to baseline (.047, 95% CI [−.017, .111]). For fasting insulin, the time-by-group interaction was not significant (p=0.12), although the coefficient representing the difference in change from 6 to 24 months was significant (log of the incidence rate ratio = .347, 95% CI[.054,.640]). On average, fasting insulin in the intervention group decreased from 10.1 to 7.91 μU/mL and the control group’s level increased from 11.66 to 12.92 μU/mL.
In addition, there were significantly fewer medical hospitalizations in the intervention group (4.8%) compared to the control group at 24 months (16.7%; Χ2[1, 200] = 7.47, p=0.006; risk ratio = 0.29), while psychiatric hospitalizations did not differ. Given that the intervention was designed to improve physical health outcomes but not psychiatric outcomes, these results are consistent with intervention goals. Moreover, this was a randomized trial, so hospitalizations should have been equal at baseline. There was also no evidence for differences in self-reported health or functioning between intervention and control groups at baseline.
Changes in systolic and diastolic blood pressure were not significant, though because average values were within normal ranges at baseline, no significant change was expected. Time-by-group interactions were not significant for triglycerides, LDL, or HDL cholesterol; average LDL cholesterol was also within normal range at baseline.
Exploratory Analyses
We also conducted exploratory analyses of factors associated with maintenance of weight loss of 5% or more of baseline weight, irrespective of study arm. Individuals who reported better 24-month body image were more likely to have lost 5% or more of their baseline weight at 24 months (odds ratio = 1.22; 95% CI [1.07, 1.41], p=0.004), adjusting for all other predictors in the model. Their 24-month outcomes expectancies also differed from those who were unable to achieve and maintain this level of weight loss. Those who felt more strongly that they would achieve their goal weight (odds ratio = 2.11; 95% CI [1.23, 3.63], p=0.007) and reported a smaller difference between their actual weight and goal weight (odds ratio = 0.93; 95% CI [0.87, 0.99], p=0.026) were more likely to have maintained a 5% or greater weight loss. Factors not found to be associated with weight loss maintenance in this sample were general or mental health, sleep quality, confidence in ability to change eating habits, patient activation/health-related self-efficacy, general quality of life, positive or negative social support for lifestyle changes, marital status, 7-day exercise frequency, or dietary content.
Discussion
The weight loss intervention tested in this trial produced significant weight loss during the 1-year intervention period, but the difference in weight loss between the trial arms diminished after the intervention period ended and was no longer statistically significant. Similar relapse following the end of interventions is typical in weight loss studies conducted with the general population of overweight and obese adults. Our results suggest, however, that some health improvements were sustained over two years (see Figures 1 and 2), and that some had lasting clinical significance, as evidenced by continued reduced hospitalization rates among intervention participants during the second year of follow-up. These reductions are consistent with recent findings showing reduced hospitalizations among lifestyle intervention participants with diabetes (33) and findings of sustained cardiovascular risk reduction among people with serious mental illnesses who received health coaching and a fitness club membership. (14) In a cost-effectiveness analysis conducted for this study, we estimated that reduced hospitalization costs would save approximately $137,500/year in each of the two years studied. (34) Furthermore, diabetes prevention studies have consistently shown that lifestyle interventions reduce diabetes risk long after the trials have ended. (35, 36) The steady decreases in fasting insulin levels found in this trial suggest that improved insulin sensitivity may help explain the long-term benefits of such lifestyle modification. Interestingly, improvements seen among control participants in the trial likely result from a combination of factors that could inform future interventions. These include: the importance of being ready to change; the value of routine measurement and feedback about weight and other outcomes provided as part of participation; and the importance of routine monitoring and referral to treatment for at-risk lab or other values.
Analyses of qualitative interviews with participants indicated that the support, common mental health and medication-related experiences, and the accountability provided by group members were extremely important to participants, and that they had a much harder time maintaining lifestyle changes after the intervention ended. (37) Together, these results are consistent with recent evidence suggesting that adopting a chronic disease perspective, with corresponding extended care for weight maintenance and other lifestyle changes, is likely to be more effective than shorter-term interventions. (38) These results are also consistent with evidence that extended care can be beneficial even when it is of low intensity. (39)
Given the special needs and risks experienced by individuals with serious mental illnesses taking antipsychotic medications, interventions that produce long-term changes in weight and cardiometabolic risks are needed to reduce this population’s rates of early cardiometabolic morbidity and mortality. In this context, it is noteworthy that participants reported that the end of intervention contacts at 1 year made their continued efforts to improve their lifestyles more difficult. Recent evidence in the general population suggests that extended care provided in group formats (as opposed to individual contacts with interventionists) is more effective for producing long-term weight maintenance and sustained lifestyle improvements. (38) In contrast, however, In SHAPE’s weekly personalized one-on-one health coaching produced longer-term benefits without continued intervention, though the In SHAPE 6-month no-intervention maintenance period was not as long as STRIDE’s 12-month period, and outcomes may have changed after an additional 6 months. (14) Nevertheless, it is possible that individuals with serious mental illnesses benefit more from individualized attention than other populations. Because such interventions are likely to be more costly to deliver than group interventions, future research should compare effects of longer-term follow-up in group contexts vs. shorter one-on-one interventions. Cost-effectiveness analyses of these different approaches could be useful to policy makers and program managers.
Our findings that better body image was associated with sustained weight loss, and that outcomes expectancies were more closely associated with actual weight loss among individuals who successfully maintained a 5% reduction in weight, have important implications beyond weight and health. First, there may be significant psychological benefits to weight loss resulting from improved body image. In this already stigmatized population, known for reduced social integration, both better feelings about oneself, and improved perceptions of others, (40) could improve quality of life and facilitate mental health recovery. Second, specifically targeting outcomes expectancies as part of interventions to help participants develop more realistic expectations may help people feel more successful in their efforts and enhance their ability to sustain behavior changes.
Limitations
Participants in the intervention were not as representative of the target population as we had planned. Their average age was about 47 years (with well-established chronic illnesses), we had fewer male participants (28%) than expected, and we had few participants from racial or ethnic minorities (14%). While this pattern is typical of lifestyle change programs generally, it may limit the generalizability of these results to men and members of minority groups in this patient population. In addition, we report results for significant changes in fasting glucose thought 6-24 months when the omnibus test was marginal. For this reason, these results should be interpreted with caution and examined in future research.
Conclusions
People with serious mental illnesses taking antipsychotic medications can lose weight and improve diabetes and other cardiometabolic risks factors when offered a group-based lifestyle intervention. Few changes are likely to be maintained without extended care, though significant clinical benefits may be sustained, as indicated by reduced hospitalizations beyond the intervention period.
What is already known about this subject?
Little is known about weight loss and lifestyle change maintenance among people with serious mental illnesses.
Little is known about long-term cardiometabolic effects of weight loss or lifestyle change among people taking antipsychotic medications.
One study (In SHAPE) has reported successful maintenance of health behavior change and lifestyle and fitness outcomes among people with serious mental illnesses, though this study tested a one-on-one coaching intervention with a fitness club membership. Thus, the intervention differed in important ways from the STRIDE intervention studied here.
What does this study add?
First study of weight maintenance following a successful group-based weight loss and lifestyle intervention among people taking antipsychotic medications
First study to report cardiometabolic outcomes maintenance following a group-based lifestyle intervention for people taking antipsychotic medications.
First study to report reduced medical hospitalizations following the end of a group lifestyle intervention for people taking antipsychotic medications.
Acknowledgements
Funding for this study was provided by the National Institute of Diabetes and Digestive and Kidney Diseases, Grant R18DK076775, entitled “Reducing Weight and Diabetes Risk in an Underserved Population.” The authors would like to thank our scientific and community collaborators and the clinical and project management staff that supported this trial. Without their efforts, this project would not have been possible. We would also like to thank STRIDE participants, who gave their precious time and effort.
Funding: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
All authors have received funding from the National Institutes of Health. Drs. Green, Yarborough, and Perrin have received funding from the Agency for Healthcare Research and Quality.
Footnotes
Conflict of Interest Statement: None of the authors have other competing financial interests in relation to this work.
References
- 1.Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ. 2013;346:f2539. doi: 10.1136/bmj.f2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–31. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 3.Druss BG, Zhao L, Von ES, Morrato EH, Marcus SC. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599–604. doi: 10.1097/MLR.0b013e31820bf86e. [DOI] [PubMed] [Google Scholar]
- 4.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry. 2004;65(5):634–51. doi: 10.4088/jcp.v65n0507. [Review] [237 refs] quiz 730. [DOI] [PubMed] [Google Scholar]
- 5.Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry. 2003;160(2):284–9. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre RS, Konarski JZ, Misener VL, Kennedy SH. Bipolar disorder and diabetes mellitus: Epidemiology, etiology, and treatment implications. Ann Clin Psychiatry. 2005;17(2):83–93. doi: 10.1080/10401230590932380. [DOI] [PubMed] [Google Scholar]
- 7.Dent R, Blackmore A, Peterson J, Habib R, Kay GP, Gervais A, et al. Changes in body weight and psychotropic drugs: a systematic synthesis of the literature. PLoS One. 2012;7(6):e36889. doi: 10.1371/journal.pone.0036889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105(1-3):175–87. doi: 10.1016/j.schres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34(4):841–59. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green CA, Yarborough BJ, Leo MC, Yarborough MT, Stumbo SP, Janoff SL, et al. The STRIDE Weight Loss and Lifestyle Intervention for Individuals taking Antipsychotic Medications: A Randomized Trial. Am J Psychiatry. 2015;172(1):71–81. doi: 10.1176/appi.ajp.2014.14020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartels SJ, Pratt SI, Aschbrenner KA, Barre LK, Jue K, Wolfe RS, et al. Clinically significant improved fitness and weight loss among overweight persons with serious mental illness. Psychiatr Serv. 2013;64(8):729–36. doi: 10.1176/appi.ps.003622012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daumit GL, Dickerson FB, Wang NY, Dalcin A, Jerome GJ, Anderson CA, et al. A Behavioral Weight-Loss Intervention in Persons with Serious Mental Illness. NEJM. 2013;368(17):1594–602. doi: 10.1056/NEJMoa1214530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barte JC, ter Bogt NC, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev. 2010;11(12):899–906. doi: 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- 14.Bartels SJ, Pratt SI, Aschbrenner KA, Barre LK, Naslund JA, Wolfe R, et al. Pragmatic Replication Trial of Health Promotion Coaching for Obesity in Serious Mental Illness and Maintenance of Outcomes. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.14030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramage S, Farmer A, Apps EK, McCargar L. Healthy strategies for successful weight loss and weight maintenance: a systematic review. Applied Physiology, Nutrition, and Metabolism. 2014;39(1):1–20. doi: 10.1139/apnm-2013-0026. [DOI] [PubMed] [Google Scholar]
- 16.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125(9):1157–70. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brantley PJ, Stewart DW, Myers VH, Matthews-Ewald MR, Ard JD, Coughlin JW, et al. Psychosocial predictors of weight regain in the weight loss maintenance trial. J Behav Med. 2014 doi: 10.1007/s10865-014-9565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen TM, Dalskov SM, van BM, Jebb SA, Papadaki A, Pfeiffer AF, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. NEJM. 2010;363(22):2102–13. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teixeira PJ, Silva MN, Coutinho SR, Palmeira AL, Mata J, Vieira PN, et al. Mediators of weight loss and weight loss maintenance in middle-aged women. Obesity (Silver Spring) 2010;18(4):725–35. doi: 10.1038/oby.2009.281. [DOI] [PubMed] [Google Scholar]
- 20.Funk KL, Elmer PJ, Stevens VJ, Harsha DW, Craddick SR, Lin PH, et al. PREMIER--A trial of lifestyle interventions for blood pressure control: Intervention design and rationale. Health Promotion Practice. 2006;9(3):271–80. doi: 10.1177/1524839906289035. [DOI] [PubMed] [Google Scholar]
- 21.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083–93. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 22.Ard JD, Grambow SC, Liu D, Slentz CA, Kraus WE, Svetkey LP. The effect of the PREMIER interventions on insulin sensitivity. Diabetes Care. 2004;27(2):340–7. doi: 10.2337/diacare.27.2.340. [DOI] [PubMed] [Google Scholar]
- 23.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial effects of a dietary approaches to stop hypertension eating plan on features of the metabolic syndrome. Diabetes Care. 2005;28(12):2823–31. doi: 10.2337/diacare.28.12.2823. [DOI] [PubMed] [Google Scholar]
- 24.Obarzanek E, Sacks FM, Vollmer WM, Bray GA, Miller ER, III, Lin PH, et al. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. The American Journal of Clinical Nutrition. 2001;74(1):80–9. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- 25.Yarborough BJ, Leo MC, Stumbo S, Perrin NA, Green CA. STRIDE: a randomized trial of a lifestyle intervention to promote weight loss among individuals taking antipsychotic medications. BMC Psychiatry. 2013;13(1):238. doi: 10.1186/1471-244X-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yarborough BJ, Janoff SL, Stevens VJ, Kohler D, Green CA. Delivering a lifestyle and weight loss intervention to individuals in real-world mental health settings: Lessons and opportunities. Transl Behav Med. 2011;1(3):406–15. doi: 10.1007/s13142-011-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Wilson PW, Meighs J, Sullivan L, Fox C, Nathan DM, D'Agostino RB. Prediction of incident diabetes mellitus in middle aged adults. Arch Intern Med. 2007;167:1068–74. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr., Kosinski M, Turner-Bowker DM, Gandek B. How to score Version 2 of the SF-12 Health Survey (With a supplement documenting Version 1) QualityMetric Incorporated; Lincoln, R.I.: 2002. [Google Scholar]
- 30.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 31.Eisen SV, Gerena M, Ranganathan G, Esch D, Idiculla T. Reliability and validity of the BASIS-24 Mental Health Survey for Whites, African-Americans, and Latinos. J Behav Health Serv Res. 2006;33(3):304–23. doi: 10.1007/s11414-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 32.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 33.Espeland MA, Glick HA, Bertoni A, Brancati FL, Bray GA, Clark JM, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37(9):2548–56. doi: 10.2337/dc14-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meenan RT, Stumbo SP, Yarborough MT, Leo MC, Yarborough BH, Green CA. An economic evaluation of a weight loss intervention program for people with serious mental illnesses taking antipsychotic medications. Adm Policy Ment Health. Submitted. [DOI] [PMC free article] [PubMed]
- 35.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673–9. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 37.Yarborough BJ, Stumbo SP, Yarborough MT, Young TJ, Green CA. Improving lifestyle interventions for people with serious mental illnesses: Qualitative results from the STRIDE study. Psychiatr Rehab J. doi: 10.1037/prj0000151. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long-term maintenance of weight loss: a systematic review and meta-analysis. Obes Rev. 2012;13(6):509–17. doi: 10.1111/j.1467-789X.2011.00972.x. [DOI] [PubMed] [Google Scholar]
- 39.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 40.Carr D, Friedman MA. Is obesity stigmatizing? Body weight, perceived discrimination, and psychological well-being in the United States. J Health Soc Behav. 2005;46(3):244–59. doi: 10.1177/002214650504600303. [DOI] [PubMed] [Google Scholar]