Abstract
The invasive yellow-legged hornet Vespa velutina nigrithorax was accidentally introduced in Europe in the early 2000s. As is the case in colonies of other wasp and hornet species, V. velutina colonies are known to produce sexuals (males and new queens) at the end of the summer. We show that early-stage colonies in French populations frequently produce males well before the usual reproductive period. The vast majority of the males produced are diploid, which is consistent with the loss of genetic diversity previously reported in introduced populations in France. Since males do not participate in colony activities, the production of early diploid males at the expense of workers is expected to hamper colony growth and, ultimately, decrease the expansion of the species in its invasive range in Europe.
Introduction
In haplodiploid hymenopteran species, sex is typically determined by one polyallelic locus (single-locus complementary sex determination, or sl-CSD) [1–4]. Individuals heterozygous at the sex-determining locus develop into diploid females, while hemizygotes develop into haploid males. However, diploid homozygotes at the sex locus develop into diploid males. Diploid male production (DMP) results in direct fitness costs to parents [4–7]. In a number of species, diploid males experience sterility or reduced survival to adulthood [8]. When fertile and viable, they produce diploid sperm and can father sterile triploid female progeny (but see [9, 10] for examples of diploid males siring diploid female offspring). In social hymenopterans, DMP represents an additional cost because males are produced at the expense of female workers but do not contribute to colony productivity [11, 12]. This phenomenon has been shown to reduce colony growth in bumble bees [13] and to increase mortality during colony founding in ants [11]. Recent theoretical studies have also suggested that DMP can increase the risk of population extinction [14].
Diploid males are rare in large outbred populations because negative frequency-dependent selection maintains a large number of alleles at the sex locus [15]. However, inbreeding, limited gene flow, and genetic drift reduce sex allele diversity and are expected to increase the frequency of diploid males in hymenopteran populations with sl-CSD. In particular, invasive species are predicted to suffer from such reduced allelic diversity due to genetic bottlenecks that occur during founding events [16–18]. In line with this, reduced genetic diversity, the loss of sex alleles, and DMP have been observed in introduced populations of the fire ant Solenopsis invicta [19] and the European bumblebee Bombus terrestris [20].
Native to China, the invasive yellow-legged hornet, Vespa velutina nigrithorax, was accidentally introduced to southwestern France around 2004, most likely via imported ceramic pottery [21]. The species successfully expanded its range, which now spans over more than 70% of France, and is currently colonizing neighboring countries (Spain, Portugal, Belgium, and Italy) [22–25]. The negative impact of V. velutina invasion in France is twofold. First, the species preys on several insect and arthropod taxa, thus potentially affecting biodiversity. For example, in southwestern France, V. velutina is a predator of the domestic honeybee, Apis mellifera, and could be induced in colony losses [22–24]. Second, the species presents a risk to human health. Accidents have occurred, some fatal, when people have inadvertently approached the hornet’s nests [26]. Typically, new colonies of V. velutina are established in the spring by mated queens, after the overwintering period. Colonies first pass through an ergonomic stage by rearing an increasingly large number of workers to ensure colony growth and then produce sexuals at the end of the summer [24, 27]. Males reach the adult stage before the new queens (gynes) (protandry); males and new queens (gynes) emerge in late August/early September and early/mid September, respectively. They take part in reproductive flights; the females then disperse, and the males die.
Recent genetic analyses based on the combination of mitochondrial and nuclear markers support a single introduction event of the yellow-legged hornet in France with a strong founder effect [28]. All populations sampled experience a dramatic loss of genetic diversity. Moreover, the production of “early males” (i.e., males produced before the end of August) has been observed in a few V. velutina colonies in France [27]. Early male production has been documented in several social Hymenoptera, but its exact function remains unclear [4, 5, 29–30]. Mating between early males and workers has been seen in orphaned colonies of Polistes wasps and allows workers to lay both fertilized and unfertilized eggs [31, 32]. However, such behavior appears to be rare [33, 34] and is viewed as an alternative reproductive strategy that is adopted only when a colony has lost its queen [29, 35, 36]. Another explanation for the production of early males is that they develop from fertilized eggs that are homozygous at the sex locus.
In this study, we examined the production patterns and ploidy of males generated by the invasive hornet V. velutina in France. We collected colonies during the wasp’s active season to analyze colony composition. We found that significant numbers of males are produced in the spring and early summer, i.e. before the normal reproductive period. Flow cytometric analyses show that the vast majority of these “early males” are diploid. While the production of early males at the expense of workers during the ergonomic stage is expected to hinder colony growth and, ultimately, the expansion of V. velutina in introduced populations, the species has spread throughout Europe during the last decade [23, 28]. We propose possible explanations to account for the success of this species in its invasive range.
Materials and Methods
Sample collection
Thirty-one colonies of Vespa velutina were collected between April and December from 2012 to 2014 in France, mainly in the Indre-et-Loire region (see Table 1 for geographic coordinates). They were brought to the laboratory and kept at -20°C for 48h to kill the hornets. Numbers of queens, workers, gynes, and males in each colony were counted (Table 1).
Table 1. Caste composition of 31 Vespa velutina colonies collected in France between April and December from 2012 to 2014.
Colonies typically produce only workers until the end of summer (end of August) and then produce males and new queens [24, 27]. In the populations sampled in this study, males were reared before the reproductive period. The numbers of workers, gynes (new reproductive queens), and males found in each colony are provided, as are the percentages of males (of all adults) and of diploid males (of all randomly sampled males). Q+: queenright colonies; Q-: queenless colonies.
| Geographic coordinates | Month | Colony/Queen | Males | Workers | Gynes | % Males | Ploidy level of males | % Diploid males | |
|---|---|---|---|---|---|---|---|---|---|
| n | 2n | ||||||||
| 47°24'09.0"N—0°39'50.0"E | April | C1 / Q+ | 3 | 3 | 0 | 42.86 | 0 | 3 | 100 |
| 44°48'29.9"N—0°32'47.0"W | May | C2 / Q+ | 2 | 0 | 0 | 66.66 | 1 | 1 | 50 |
| 44°48'29.9"N—0°32'47.0"W | May | C3 / Q+ | 5 | 5 | 0 | 45.45 | 0 | 4 | 100 |
| 43°45′25.0″N—0°41′06.0″W | June | C4 / Q+ | 0 | 1 | 0 | 0 | - | - | - |
| 43°45′25.0″N—0°41′06.0″W | June | C5 / Q+ | 2 | 3 | 0 | 33.33 | - | - | - |
| 43°45′25.0″N—0°41′06.0″W | June | C6 / Q+ | 3 | 3 | 0 | 42.86 | - | - | - |
| 47°08′57.0″N—0°10′58.0″E | June | C7 / Q+ | 1 | 0 | 0 | 50 | - | - | - |
| 47°15'02.9"N—0°52'40.0"E | July | C8 / Q- | 28 | 178 | 0 | 13.59 | 0 | 12 | 100 |
| 47°24'24.8"N—0°59'09.0"E | July | C9 / Q- | 0 | 61 | 0 | 0 | - | - | - |
| 47°20'17.2"N—0°42'50.0"E | July | C10 / Q+ | 7 | 19 | 0 | 25.93 | 0 | 7 | 100 |
| 47°26'17.0"N—0°38'20.0"E | July | C11 / Q+ | 5 | 71 | 0 | 6.49 | 0 | 5 | 100 |
| 47°16′41.0″N—0°37′31.0″E | July | C12 / Q- | 0 | 72 | 0 | 0 | - | - | - |
| 47°19'14.2"N—0°55'02.0"E | July | C13 / Q+ | 0 | 61 | 0 | 0 | - | - | - |
| 47°15'42.1"N—0°27'58.0"E | July | C14 / Q+ | 8 | 9 | 0 | 44.44 | 0 | 8 | 100 |
| 47°33'47.2"N—1°12'53.0"E | August | C15 / Q- | 1 | 30 | 0 | 3.23 | - | - | - |
| 47°15'54.0"N—0°21'09.0"E | August | C16 / Q- | 21 | 57 | 0 | 26.92 | 0 | 12 | 100 |
| 47°21'22.0"N—0°54'34.0"E | August | C17 / Q+ | 0 | 98 | 0 | 0 | - | - | - |
| 47°25'18.1"N—0°50'52.0"E | August | C18 / Q- | 0 | 244 | 10 | 0 | - | - | - |
| 47°24'11.2"N—0°36'07.0"E | August | C19 / Q- | 0 | 62 | 0 | 0 | - | - | - |
| 47°35'19.0"N—1°19'39.0"E | August | C20 / Q- | 6 | 8 | 0 | 38.46 | 1 | 5 | 83.33 |
| 47°21'58.0"N—0°43'45.0"E | August | C21 / Q+ | 28 | 186 | 1 | 12.96 | 0 | 14 | 100 |
| 47°26'17.0"N—0°38'20.0"E | August | C22 / Q- | 14 | 9 | 0 | 60.87 | 0 | 13 | 100 |
| 47°24'09.0"N—0°39'50.0"E | September | C23 / Q+ | 1 | 195 | 0 | 0.51 | 1 | 0 | 0 |
| 47°24'24.8"N—0°59'09.0"E | September | C24 / Q- | 0 | 13 | 0 | 0 | - | - | - |
| 47°23'34.1"N—0°41'01.0"E | September | C25 / Q+ | 63 | 86 | 2 | 41.45 | 0 | 23 | 100 |
| 47°23'34.1"N—0°41'01.0"E | September | C26 / Q+ | 57 | 80 | 0 | 41.3 | 0 | 24 | 100 |
| 47°02'31.9"N—0°49'08.0"E | October | C27 / Q+ | 106 | 150 | 0 | 41.25 | - | - | - |
| 47°19'14.2"N—0°55'02.0"E | November | C28 / Q- | 162 | 96 | 50 | 52.6 | 5 | 5 | 50 |
| 47°54'14.0"N—1°54'26.0"E | November | C29 / Q- | 3 | 2 | 2 | 42.86 | 1 | 2 | 66.67 |
| 47°19'58.1"N—1°02'57.0"E | December | C30 / Q- | 10 | 8 | 10 | 35.71 | 10 | 0 | 0 |
| 47°14'12.8"N—0°07'40.0"E | December | C31 / Q- | 33 | 44 | 108 | 17.83 | 4 | 0 | 0 |
Our field study did not involve endangered or protected species. Therefore, no specific permissions were required to collect hornets in France. Rather, as V. velutina is an invasive species, the French government recommends the elimination of colonies (DGAL/SDSPA/N2013-8082, 10 May 2013).
Ploidy analysis
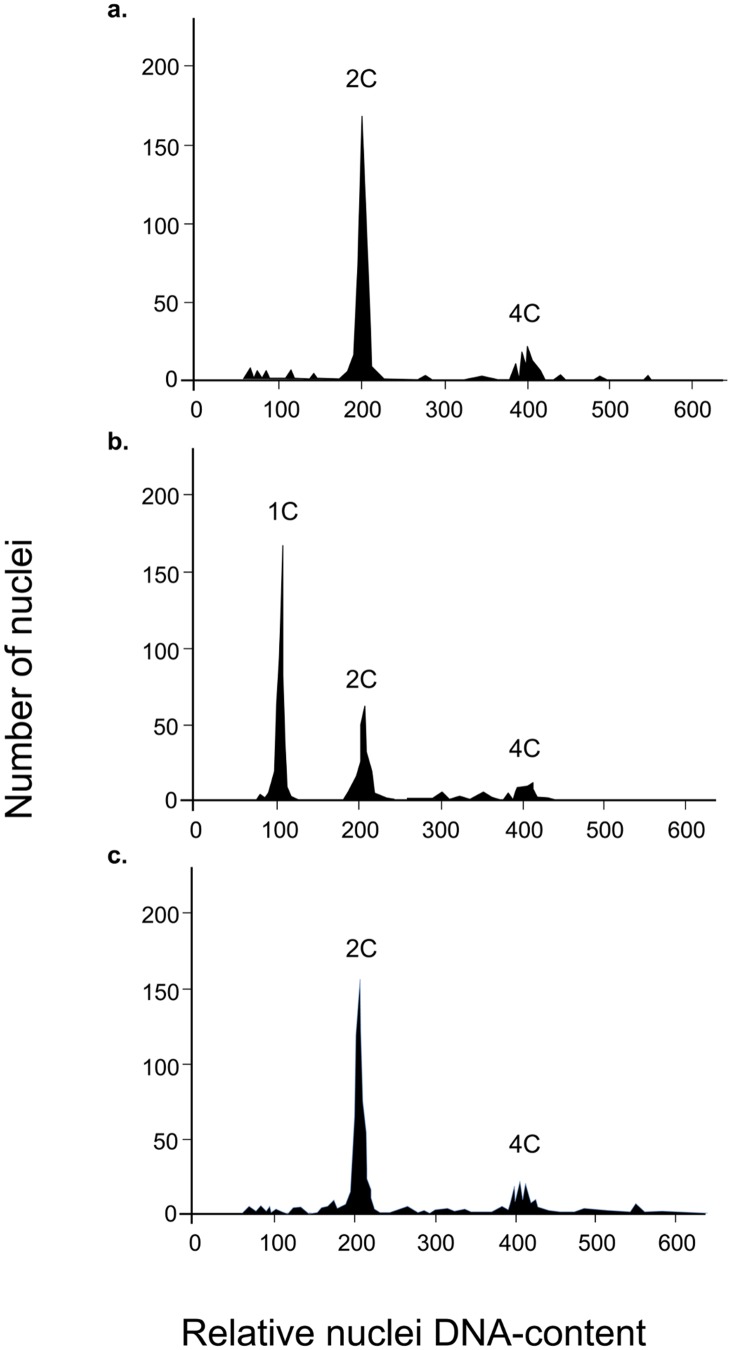
The ploidy level of the V. velutina males sampled was determined by flow cytometry [5, 37]. Colonies and individual males were randomly sampled. Samples were prepared by pulverizing the head of each individual hornet in a 1-ml 4′,6-diamino-2-phenylindole dihydrochloride (DAPI) solution (CyStain) with a pestle. The suspension was subsequently filtered using CellTrics (mesh size: 20 μm). Flow cytometric analyses were performed using a PA-I flow cytometer (PARTEC, Partec Gmbh, Münster, Germany) equipped with a UV-LED 365 nm light source and employing an optical configuration described elsewhere [38]. For each sample, the ploidy of 2,500 nuclei was analyzed. A threshold on FL2-A was used to exclude very small debris. We used flow cytometric DNA histograms of known haploid males and diploid females as references to determine the ploidy of unknown males (Fig 1).
Fig 1. Flow cytometric DNA histogram of diploid female (a), haploid male (b) and diploid male (c).
Each histogram shows the nuclear frequency with regard to DNA content for the head of a single individual. The first peak corresponds to ploidy level, the second peak to nuclei with a double DNA content and the third peak to polyploid nuclei. In haploid males, the second peak (2C) corresponds to nuclei from mandibular muscles where cells are diploids.
Results
Several V. velutina colonies produced males throughout the entire season, not just at the end of the summer (Table 1). Fifteen of the 22 colonies (68%) collected from April to August (i.e., before the reproductive period) contained early males. These males accounted for between 3 and 67% of colony members. Flow cytometric analyses revealed that more than 97% (84/86) of the early males were diploid; only two males were haploid.
Of the 9 colonies collected during the reproductive period (September–December), 8 contained mature males. These males represented between 0.5 and 53% of colony members. Of the 75 males sampled, 54 (72%) were diploid. The remaining 21 were produced by arrhenotokous parthenogenesis and were haploid. Two colonies (C30 and C31; Table 1) produced only haploid males.
The very high proportion (>50%) of diploid males produced among offspring in colonies C2, C22 and C28 likely results from sampling bias, e.g. due to workers being not collected because they were foraging or some died before sampling.
Discussion
This study shows that colonies of the invasive yellow-legged hornet Vespa velutina nigrithorax in France (1) produce males throughout the species’ active season, even well before the reproductive period, and (2) that most of these males are diploid. The predominance of diploid males is consistent with the genetic bottleneck experienced by this species following its introduction into France [28]. Similarly high levels of diploid males have been observed in the fire ant, Solenopsis invicta, in its introduced range in North America [11]. DMP is attributable to a loss of allelic diversity at the sex-determining locus [39]. The number of sex alleles in V. velutina remains unknown. Estimates based on the frequency of diploid males in populations suggest that the effective number of alleles at the sex-determining locus varies greatly within Hymenoptera, with an average of 5 alleles in Halictus poeyi [40], 15 in Solenopsis invicta [39], 19 in Apis mellifera [41], 20 in Melipona compressipes fasciculate [42], 24 or more in Bombus terrestris [43], and 33 in Polistes chinensis antennalis [30].
In social Hymenoptera, DMP may severely hamper colony growth because a large percentage of diploid eggs yield males instead of females [11, 14]. First, a colony may lose out if queens produce diploid males instead of workers during the ergonomic stage because males do not benefit the colony, in contrast to workers (e.g., building the nest and feeding larvae). Of the offspring produced by queens who mate with a single male carrying the same sex allele, 50% will be diploid males. Field studies in France have found that V. velutina nests may be abandoned early on, which suggests that colony founding has failed [44]. Second, diploid males impose particularly high fitness costs on the colony since they are usually sterile. Even when they are not, they produce diploid sperm and father sterile, triploid female progeny (reviewed in [8]). The production of triploid offspring, both females and males, has indeed been observed in various hymenopteran species, including Athalia rosae [45], the parasitoid wasp Cotesia vestalis [46], the bumblebee Bombus terrestris [47] or the ant Tapinoma erraticum [5]. A triploid male was also found in V. velutina (unpublished data). These findings suggest that diploid males produce diploid sperm and father triploid offspring.
In introduced species, founder effects and genetic drift can reduce the genetic diversity as populations are becoming established. They may result in inbreeding depression, a main contributor to population extinction [48–50]. In haplodiploids, the production of nonviable or sterile diploid males due to inbreeding is expected to reduce population growth rates and effective sizes, potentially creating a rapid extinction vortex [18, 51]. Remarkably, while DMP is predicted to affect the expansion range of introduced V. velutina populations, the species has spread throughout Europe during the last decade [24,28]. This indicates that the yellow-legged hornet can establish successful populations, even from a limited number of foundresses with low genetic variability [28].
Several biological and environmental factors may have contributed to the success of this invasive species in Europe, including favorable climatic conditions [52], the abundance of preys (honeybees), multiple mating by queens [28] and occasional production of early haploid males. If DMP has a large negative impact on colony foundation and survival, there may be a selective advantage for queens to mate with multiple partners to reduce the likelihood of producing diploid males [53, 54]. Recently, genetic analyses of French populations of V. velutina have revealed that queens mate with an average of 4.6 males (SD = 2.3; [28]). Interestingly, this value is higher than that observed in other Vespa species [28, 55]. To date, queen mating frequency and diploid male production in native populations of V. velutina nigrithorax remain unknown. Whether multiple mating has been selected for in invasive populations to reduce the costs associated with DMP and/or the probability of mating with diploid males remains to be studied. Our data also show that V. velutina colonies rear a small number of haploid males before the reproductive season. The function of these early males is enigmatic. One hypothesis is that early males could mate with virgin reproductive females (gynes) that survive the winter. Another, non-exclusive hypothesis is that early males mate with workers from orphaned colonies; mated workers could then become new queens and leave their natal nests to found new colonies. Both these scenarios illustrate alternative reproductive strategies adopted by gynes and workers [29, 33, 56–59] and may have contributed to the rapid expansion of V. velutina in Europe.
Clearly, future research should explore (1) the reproductive function of early males and their potential role in alternative female reproductive strategies, (2) the inbreeding coefficient resulting from nonrandom mating within invasive populations, (3) whether diploid males father triploid fertile females and, more generally, (4) the ecological consequences of DMP for populations of the invasive hornet V. velutina in Europe.
Acknowledgments
The authors thank C. Poirier (CP) and E. Roumaillac (ER) for their help in collecting colonies in the field, and M. Coquet (MC) for her help to manage samples. This work was funded by the local government (Région Centre, France) (ED) and several grants from Belgium’s National Fund for Scientific Research (SA).
Data Availability
All relevant data are within the paper.
Funding Statement
This work was funded by the local government (Région Centre, France) (ED) and several grants from Belgium’s National Fund for Scientific Research (SA).
References
- 1. Cook JM. Sex determination in the Hymenoptera: a review of models and evidence. Heredity. 1993;71:421–435. [Google Scholar]
- 2. Cook JM, Crozier RH. Sex determination and population biology in hymenoptera. Trends in Ecol Evol. 1995;10(7):281–286. [DOI] [PubMed] [Google Scholar]
- 3. Beye M, Hasselmann M, Fondrk MK, Page RE Jr, Omholt SW. The gene csd ls the primary signal for sexual development in the Honeybee and encodes an SR-type protein. Cell. 2003;114(4):419–429. [DOI] [PubMed] [Google Scholar]
- 4. van Wilgenburg E, Driessen G, Beukeboom LW. Single locus sex determination in Hymenoptera: an ‘unintelligent’ design? Front Zool. 2006;3:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cournault L, Aron S. Diploid males, diploid sperm production, and triploid females in the ant Tapinoma erraticum . Naturwissenschaften. 2009;96(12):1393–1400. 10.1007/s00114-009-0590-1 [DOI] [PubMed] [Google Scholar]
- 6. Darvill B, Lepais O, Woodall LC, Goulson D. Triploid bumblebees indicate a direct cost of inbreeding in fragmented populations. Mol Ecol. 2012;21(16):3988–3995. 10.1111/j.1365-294X.2012.05679.x [DOI] [PubMed] [Google Scholar]
- 7. Harpur BA, Sobhani M, Zayed A. A review of the consequences of complementary sex determination and diploid male production on mating failures in the Hymenoptera. Ent Exp Appl. 2013;146(1):156–164. [Google Scholar]
- 8. Heimpel GE, de Boer JG. Sex determination in the Hymenoptera. Annu Rev Entomol. 2008;53:209–230. [DOI] [PubMed] [Google Scholar]
- 9. Cowan DP, Stahlhut JK. Functionally reproductive diploid and haploid males in an inbreeding hymenopteran with complementary sex determination. Proc Natl Acad Sci USA. 2004;101(28):10374–10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elias J, Mazzi D, Dorn S. No need to discriminate? Reproductive diploid males in a parasitoid with complementary sex determination. PLoS ONE 2009;4(6):e6024 10.1371/journal.pone.0006024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ross KG, Fletcher DJC. Diploid male production—a significant colony mortality factor in the fire ant Solenopsis invicta (Hymenoptera: Formicidae). Behav Ecol Sociobiol. 1986;19(4):283–291. [Google Scholar]
- 12. Whitehorn PR, Tinsley MC, Brown MJF, Darvill B, Goulson D. Impact of inbreeding on bumblebee colony fitness under field conditions. BMC Evol Biol. 2009;9:152 10.1186/1471-2148-9-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plowright RC, Pallett MJ. Worker-male conflict and inbreeding in bumble bees (Hymenoptera: Apidae). Can Entomol. 1979;111(3):289–294. [Google Scholar]
- 14. Zayed A, Packer L. Complementary sex determination substantially increases extinction proneness of haplodiploid populations. Proc Natl Acad Sci USA. 2005;102(30):10742–10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page RE, Metcalf RA. Multiple mating, sperm utilization, and social evolution. Am Nat. 1982;119(2): 263–281. [Google Scholar]
- 16. Packer L, Owen RE. Population genetic aspects of pollinator decline. Cons Ecol. 2001;5:1 [Google Scholar]
- 17. Tsutsui ND, Suarez AV, Holway DA, Case TJ. Reduced genetic variation and the success of an invasive species. Proc Natl Acad Sci USA. 2000;97(11): 5948–5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dlugosch KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol. 2008;17: 431–449. [DOI] [PubMed] [Google Scholar]
- 19. Ross KG, Vargo EL, Keller L, Trager JC. Effect of founder event on variation in the genetic sex-determining system of the fire ant Solenopsis invicta . Genetics. 1993;135, 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmid-Hempel P, Schmid-Hempel R, Brunner PC, Seeman OD, Allen GR. Invasion success of the bumblebee, Bombus terrestris, despite a drastic genetic bottleneck. Heredity. 2007;99:414–422. [DOI] [PubMed] [Google Scholar]
- 21. Haxaire J, Bouguet J-P, Tamisier J-P. Vespa velutina Lepeletier, 1836, une redoutable nouveauté pour la faune de France (Hym., Vespidae). Bull Soc Entomol Fr. 2006;111:194. [Google Scholar]
- 22. Darrouzet E, Gévar J. La France peu à peu envahit par le frelon asiatique. Espèces. 2012;6:30–35. [Google Scholar]
- 23. Darrouzet E, Gévar J. Le frelon asiatique à la conquête de l’Europe. La Santé de l’Abeille, 2014;259:49–59. [Google Scholar]
- 24. Monceau K, Bonnard O, Thiéry D. Vespa velutina: a new invasive predator of honeybees in Europe. J Pest Sci. 2013; 10.1007/s10340-013-0537-3 [DOI] [Google Scholar]
- 25. Goldarazena A, de Heredia IP, Romon P, Iturrondobeitia JC, Gonzalez M, Lopez S. Spread of the yellow-legged hornet Vespa velutina nigrithorax du Buysson (Hymenoptera: Vespidae) across Northern Spain. EPPO Bulletin. 2015;45(1):1–6. [Google Scholar]
- 26. de Haro L, Labadie M, Chanseau P, Cabot C, Blanc-Brisset I, Penouil F. Medical consequences of the Asian black hornet (Vespa velutina) invasion in South Western France. Toxicon 2010;55:650–652 10.1016/j.toxicon.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 27. Rome Q, Muller FJ, Touret-Alby A, Darrouzet E, Perrard A, Villemant C. Caste differentiation and seasonal changes in Vespa velutina (Hym.: Vespidae) colonies in its introduced range. J Appl Entomol. 2015;(in press) [Google Scholar]
- 28. Arca M, Mougel F, Guillemaud T, Dupas S, Rome Q, Perrard A, et al. Reconstructing the invasion and the demographic history of the yellow-legged hornet, Vespa velutina, in Europe. Biol Invasions. 2015; 10.1007/s10530-015-0880-9 [DOI] [Google Scholar]
- 29. Yamasaki K, Takahashi J-I, Ono M, Tsuchida K. Reproductivity of early males of the temperate paper wasp Polistes rothneyi iwatai. Entomological Science. 2011;14(4):383–386. [Google Scholar]
- 30. Tsuchida K, Nagata N, Kojima J. Diploid males and sex determination in a paper wasp, Polistes chinensis antennalis (Hymenoptera, Vespidae). Ins Soc. 2002;49(2):120–124. [Google Scholar]
- 31. Tsuchida K, Saigo T, Tsujita S, Tabeuchi K. Early male production is not linked to a reproductive strategy in the Japanese paper wasp, Polistes chinensis antennalis (Hymenoptera: Vespidae). J Ethol. 2004;22(1):119–121. [Google Scholar]
- 32. Hagiwara Y, Kojima J. Reproductive options for first brood “workers” emerging in orphan nests of Polistes nipponensis (Hymenoptera, Vespidae). Ins Soc. 2002;49(3):191–195. [Google Scholar]
- 33. Page RE, Post DC, Metcalf RA Satellite nests, early males, and plasticity of reproductive behavior in a paper wasp. Am Nat 1989;134:731–748 [Google Scholar]
- 34. Kasuya E. Social behavior of early emerging males of a Japanese paper wasp, Polistes chinensis antennalis (Hymenoptera: Vespidae). Res Popul Ecol. 1983;25(1):143–149. [Google Scholar]
- 35. Suzuki T. Paradox of worker reproduction and worker mating in temperate paper wasps, Polistes chinensis and P. snelleni (Hymenoptera Vespidae). Ethol Ecol Evol. 1998;10(4):347–359. [Google Scholar]
- 36. Liebert AE, Johnson RN, Switz GT, Starks PT. Triploid females and diploid males: underreported phenomena in Polistes wasps? Ins Soc. 2004;51(3):205–211. [Google Scholar]
- 37. Aron S, de Menten L, Van Bockstaele DR, Blank SM, Roisin Y. When hymenopteran males reinvented diploidy. Current Biology. 2005;15(9):824–827. [DOI] [PubMed] [Google Scholar]
- 38. Cournault L, Aron S. Rapid determination of sperm number in ant queens by flow cytometry. Ins Soc. 2008;55:283–287. [Google Scholar]
- 39. Ross KG. The breeding system of the fire ant Solenopsis invicta: effects on colony genetic structure. Am Nat. 1993;141(4):554–576. 10.1086/285491 [DOI] [PubMed] [Google Scholar]
- 40. Zayed A, Packer L. High levels of diploid male production in a primitively eusocial bee (Hymenoptera: Halictidae). Heredity 2001;87:631–636. [DOI] [PubMed] [Google Scholar]
- 41. Adams J, Rothman ED, Kerr WE, Paulino ZL. Estimation of the number of sex alleles and queen matings from diploid male frequencies in a population of Apis mellidera . Genetics. 1977;86(3):583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kerr WE. Sex determination in bees XXI. Number of xo-heteroalleles in a natural population of Melipona compressipes fasciculata (Apidae). Ins Soc. 1987;34(4):274–279. [Google Scholar]
- 43. Duchateau MJ, Mariën J. Sexual biology of haploid and diploid males in the bumble bee Bombus terrestris . Ins Soc. 1994;42:255–266. [Google Scholar]
- 44. Darrouzet E, Gévar J, Dupont S. A scientific note about an endoparasitoid that could help limit the spread of the honeybee killer Vespa velutina nigrithorax in Europe. Apidologie, 2015;46(1):130–132. [Google Scholar]
- 45. Naito T, Suzuki H. Sex determination in the Sawfly, Athalia rosae ruficornis (Hymenoptera): occurrence of triploid males. J Hered 1991;82(2):101–104. [Google Scholar]
- 46. de Boer JG, Ode PJ, Vet LEM, Whitfield JB, Heimpel GE. Diploid males sire triploid daughters and sons in the parasitoid wasp Cotesia vestalis . Heredity. 2007;99:288–294. [DOI] [PubMed] [Google Scholar]
- 47. Ayabe T, Hoshiba H, Ono M. Cytological evidence for triploid males and females in the bumblebee, Bombus terrestris . Chrom Res. 2004;12:215–223. [DOI] [PubMed] [Google Scholar]
- 48. Frankham R. Genetics and extinction. Biol Cons. 2005;126(2):131–140. [Google Scholar]
- 49. Wright LI, Tregenza T, Hosken DJ. Inbreeding, inbreeding depression and extinction. Cons Genet. 2008;9:833–843. [Google Scholar]
- 50. Charlesworth D, Willis JH. The genetics of inbreeding depression. Nat Rev Genet. 2009;10:783–796. 10.1038/nrg2664 [DOI] [PubMed] [Google Scholar]
- 51. Zayed A, Constantin SA, Packer L. Successful biological invasion despite a severe genetic load. PLoS ONE. 2007; 2: e868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Villemant C, Barbet-Massin M, Perrard A, Muller F, Gargominy O, Jiguet F, Rome Q. Predicting the invasion risk by the alien bee-hawking Yellow-legged hornet Vespa velutina nigrithorax across Europe and other continents with niche models. Biol Cons. 2011; 144: 2142–2150. [Google Scholar]
- 53. Crozier RH, Page RE. On being the right size: male contributions and multiple mating in social Hymenoptera. Behav Ecol Sociobiol. 1985;18:105–115. [Google Scholar]
- 54. Pamilo P, Sundström L, Fortelius W, Rosengren R. Diploid males and colony-level selection in Formica ants. Ethol Ecol Evol. 1994;6(2):221–235. [Google Scholar]
- 55. Hughes WOH, Ratnieks FLW, Oldroyd BP. Multiple paternity or multiple queens: two routes to greater intracolonial genetic diversity in the eusocial Hymenoptera. J Evol Biol. 2008;21(4):1090–5. 10.1111/j.1420-9101.2008.01532.x [DOI] [PubMed] [Google Scholar]
- 56. Kasuya E. Nest foundation by a single worker of the Japanese paper wasp Polistes chinensis antennalis (Hymenoptera: Vespidae). Ins Soc. 1981;28:341–342 [Google Scholar]
- 57. Strassmann JE. Evolutionary implications of early male and satellite nest production in Polistes exclamans colony cycles. Behav Ecol Sociobiol 1981;8:55–64 [Google Scholar]
- 58. Suzuki T. Mating and laying of female-producing eggs by orphaned workers of a paper wasp, Polistes snelleni (Hymenoptera: Vespidae). Ann Entomol Soc Am. 1985;78:736–739 [Google Scholar]
- 59. Suzuki T. Worker mating in queen-right colonies of a temperate paper wasp. Naturwissenshcaften. 1997;84:304–305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.