Abstract
Objective
To assess the relationship between secondhand smoke (SHS) exposure and disease severity among children hospitalized with community-acquired pneumonia (CAP).
Study Design
Children hospitalized with clinical and radiographic CAP were enrolled from January 1, 2010 through June 30, 2012 at three hospitals in Tennessee and Utah as part of the Centers for Disease Control and Prevention Etiology of Pneumonia in the Community (EPIC) study. Household SHS exposure was defined as the number of smokers in the child’s home. Outcomes included hospital length of stay, intensive care unit admission, and mechanical ventilation. Proportional hazards and logistic regression models were used to assess associations between SHS exposure and outcomes. All models were adjusted for age, sex, race/ethnicity, household education level, government insurance, comorbidities, enrollment site, year, and season.
Results
Of the 2219 included children, SHS exposure was reported in 785 (35.4%), including 325 (14.8%) with ≥2 smokers. Children exposed to ≥2 smokers had longer length of stay (median 70.4 vs. 64.4 hours; adjusted hazard ratio 0.85, 95% CI 0.75–0.97) and were more likely to receive intensive care (25.2% vs. 20.9%; adjusted odds ratio 1.44, 95% CI 1.05–1.96) but not mechanical ventilation compared with non-exposed children. Outcomes for children exposed to only one household smoker were similar to those for non-exposed children.
Conclusions
Children hospitalized with CAP from households with ≥2 smokers had a longer length of stay and were more likely to require intensive care than children from households with no smokers, suggesting they experienced greater pneumonia severity.
Keywords: pneumonia, pediatrics, secondhand smoke, outcomes research, hospitalized child
There is no safe level of exposure to secondhand smoke (SHS). (1) Although smoking rates and involuntary exposure to secondhand smoke (SHS) are declining in the United States (U.S.), the burden and negative health effects of SHS exposure remain substantial. (2) The pediatric population is particularly vulnerable, and with approximately 40% of U.S. children 3–11 years regularly exposed to cigarette smoke, (3) much work remains.
Secondhand smoke exposure is an established risk factor for both upper and lower respiratory tract illness in children. (4–10) Chronic SHS exposure induces inflammatory and functional changes in the airway that increase the risk of acute and chronic respiratory illness. (6, 11–13) Exposure to SHS also is associated with increased illness severity among children with asthma, including higher frequency of acute exacerbations and poorer long-term lung function. (14–16) Similarly, SHS-exposed children who are hospitalized with bronchiolitis or influenza experience more severe illness compared with non-exposed children. (17–19) However, few studies have assessed effects of SHS exposure on the severity of all-cause childhood pneumonia.
Pneumonia is a leading causes of pediatric hospitalization in the U.S. (20) and identifying modifiable risk factors to reduce pneumonia severity is a priority. (21) The objective of our study was to examine the relationship between household SHS exposure and disease severity among children <18 years hospitalized with community-acquired pneumonia (CAP) at three US children’s hospitals, as measured by hospital length of stay, intensive care unit admission, and invasive mechanical ventilation.
METHODS
This study used data from children <18 years old enrolled in the Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study. (22) The EPIC study is a prospective, population-based surveillance study of childhood CAP hospitalizations at three U.S. children’s hospitals (Le Bonheur Children’s Hospital [Memphis, TN], Primary Children’s Medical Center [Salt Lake City, UT], and Monroe Carell Jr. Children’s Hospital at Vanderbilt [Nashville, TN]). Children were enrolled in the EPIC study from January 2010 to June 2012. Eligible children were hospitalized at a study hospital and resided within a defined study catchment area. Inclusion criteria were: (1) evidence of acute infection (eg, fever); (2) signs or symptoms of acute respiratory illness (eg, new cough); and (3) radiographic evidence of pneumonia. Exclusion criteria were any of the following: (1) recent hospitalization (30 days for immunocompetent or 90 days for immunocompromised); (2) cystic fibrosis; (3) significant immunosuppression; (4) tracheostomy; or (5) a clear alternative diagnosis (eg, pulmonary embolism).
Data collection for the EPIC study included standardized interviews (collecting socio-demographic characteristics, medical history, and history of present illness) and medical record reviews (capturing hospitalization course and outcomes). Informed consent was obtained prior to enrollment. The study protocol was approved by the IRB of each study institution and CDC.
Household Second Hand Smoke Exposure
SHS exposure was determined by caregiver response to the question, “How many household members smoke (either indoors or outdoors)?” Children whose caregiver responded “none” were considered non-exposed (referent group). Those indicating the presence of household smokers (hereafter referred to as any household smoker) were further categorized as one household smoker and ≥2 household smokers. Children missing household exposure data were excluded. Children >9 years of age who identified themselves as current smokers and those missing personal smoking history data were also excluded.
Outcomes
The primary outcome was hospital length of stay, measured in hours. Secondary outcomes included intensive care unit admission and invasive mechanical ventilation. Although no single, validated measure to assess childhood pneumonia outcomes is available, our selected measures often are used to assess disease outcomes among children hospitalized with acute respiratory illness, including pneumonia. (18, 23) Children missing outcome data were excluded.
Statistical Analyses
Descriptive statistics included frequencies and percentages for categorical variables and median and interquartile range (IQR) values for continuous variables. Baseline characteristics according to reported home smoke exposure were compared using the Kruskall-Wallis and chi-squared tests for continuous and categorical variables, respectively. Multivariable Cox proportional hazard regression was used to model the association between SHS exposure and hospital length of stay (i.e. time to discharge). In Cox regression, a hazard ratio <1 indicates a lower probability of experiencing the outcome for the comparator group at any given point in time. Thus, in our study, a hazard ratio <1 indicated a lower probability of discharge for children exposed to SHS compared with non-exposed children. Visual inspection of log-log plots were used to confirm the proportional hazards assumption (data not shown). For categorical outcomes (intensive care unit admission and invasive mechanical ventilation), multivariable logistic regression was used. For each outcome, two separate models were constructed. The first model compared no reported home smoke exposure with any household smoker. The second model compared no reported home smoke exposure with one household smoker and also ≥2 household smokers. All models were adjusted for the following factors selected a priori based on hypothesized associations with SHS exposure and/or outcomes: age, sex, race/ethnicity, household education level, government insurance, individual high-risk comorbidities (persistent asthma [requiring inhaled corticosteroids], prematurity [if age < 24 months], neurologic disorder, cardiopulmonary disorder, and other [includes endocrine, renal, hepatic, hematologic, immunologic, chromosomal, and genetic/metabolic disorders]), enrollment site, season (winter, spring, summer, fall), and year. Adjusted hazard ratios (aHR) or odds ratios (aOR) and 95% confidence intervals (CIs) were reported.
For the primary outcome, two alternative models also were examined. First, the main analysis was repeated after including terms for microbiologic etiology (bacterial and viral detection from blood and/or respiratory specimens—methods previously described (22)). Second, the main analysis was repeated after stratifying by asthma history. However, results of both analyses were similar to the main analysis (data not shown). All analyses were conducted using Stata 13.1 (StataCorp, College Station, TX).
RESULTS
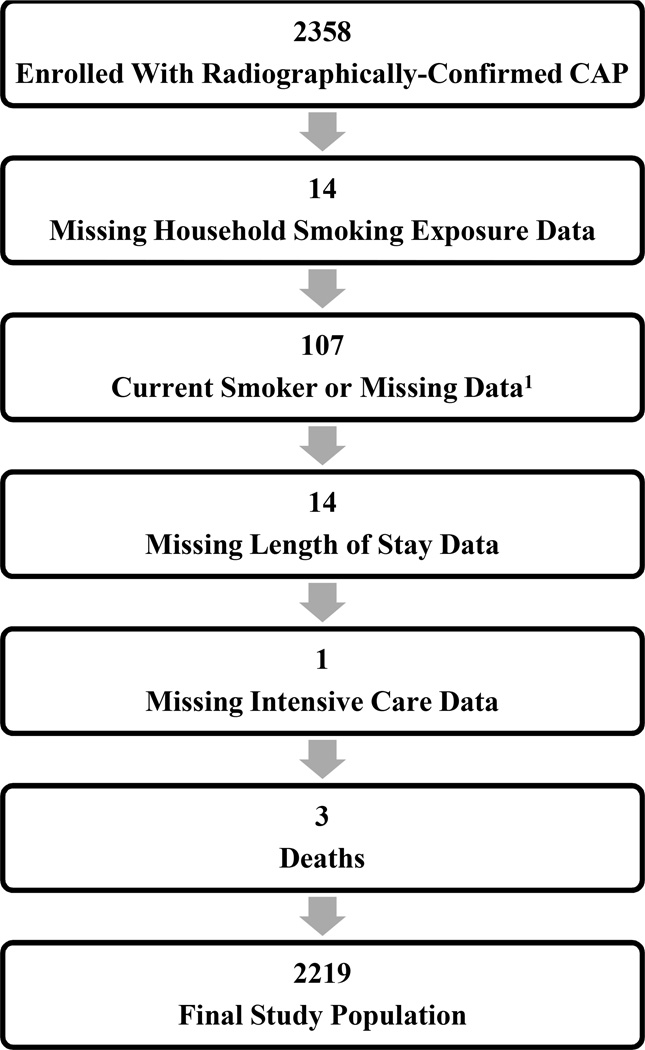
Overall, 2358 children with CAP were enrolled in the EPIC study. Of these, 2219 children (94.1%) with complete data constituted the final study population (Figure 1; available at www.jpeds.com). Among these children, 1434 (64.6%) were non-exposed to SHS, 460 (20.7%) were exposed to one household smoker and 325 (14.7%) were exposed to ≥2 household smokers (Table I). The median age was 26 months (interquartile range [IQR] 12, 63); 55.0% were male; 39.2%, 33.1%, and 19.5% were non-Hispanic white, non-Hispanic black, and Hispanic, respectively. Overall, 36.7% of children had one or more high-risk comorbidities, including 11.8% with persistent asthma. Asthma occurred with similar frequency among SHS exposed children (12.6% and 12.3% in households with one and ≥2 smokers, respectively) and non-exposed children (11.4%, p=0.76). Overall, median length of stay was 65.7 hours (IQR 42.3, 110.5); 470 children (21.2%) were admitted to intensive care and 159 (7.2%) required invasive mechanical ventilation.
Figure 1. Study Population.
Abbreviations: CAP, community-acquired pneumonia; 1restricted to children >9 years of age and includes 4 self-reported current smokers and 103 children with missing data
Table 1.
Characteristics of Children Hospitalized with Community-Acquired Pneumonia, by Reported Second Hand Smoker Exposure in the Home
| Home Smoke Exposure | ||||
|---|---|---|---|---|
| Characteristic | Non- Exposed, n=1434 |
1 Smoker, n=460 |
≥2 Smokers, n=325 |
p- value |
| Median age in months (IQR) | 27 (12–68) | 27 (13–63) | 20 (9–47) | <.01 |
| Sex, n (%) | .73 | |||
| Male | 793 (55.3) | 256 (55.7) | 171 (52.6) | |
| Race/Ethnicity | <.01 | |||
| Non-Hispanic White | 560 (39.1) | 157 (34.1) | 153 (47.1) | |
| Non-Hispanic Black | 426 (29.7) | 204 (44.4) | 105 (32.3) | |
| Hispanic | 337 (23.5) | 57 (12.4) | 38 (11.7) | |
| Other | 111 (7.7) | 42 (9.1) | 29 (8.9) | |
| Enrollment site | <.01 | |||
| Memphis, TN | 447 (31.2) | 213 (46.3) | 135 (41.5) | |
| Nashville, TN | 402 (28.0) | 136 (29.6) | 107 (32.9) | |
| Salt Lake City, UT | 585 (40.8) | 111 (24.1) | 83 (25.5) | |
| Enrollment year | .02 | |||
| 2010 | 426 (29.7) | 172 (37.4) | 108 (33.2) | |
| 2011 | 640 (44.6) | 188 (40.9) | 153 (47.1) | |
| 2012 | 368 (25.7) | 100 (21.7) | 64 (19.7) | |
| Enrollment season | .44 | |||
| Winter | 509 (35.5) | 155 (33.7) | 120 36.9) | |
| Spring | 461 (32.2) | 138 (30.0) | 95 (29.2) | |
| Fall | 251 (17.5) | 103 (22.4) | 65 (20.0) | |
| Summer | 213 (14.9) | 64 (13.9) | 45 (13.9) | |
| Insurance type | <.01 | |||
| Public | 781 (54.5) | 354 (77.0) | 260 (80.0) | |
| High-risk comorbidities, % | ||||
| Persistent Asthma1 | 164 (11.4) | 402 (87.4) | 40 (12.3) | .78 |
| Prematurity (less than 36 weeks)2 | 137 (9.6) | 43 (9.4) | 35 (10.8) | .82 |
| Neurologic | 111 (7.7) | 30 (6.5) | 25 (7.7) | .38 |
| Cardiopulmonary | 146 (10.2) | 38 (8.3) | 30 (9.2) | .27 |
| Other high-risk comorbidity3 | 169 (11.8) | 44 (9.6) | 27 (8.3) | .08 |
| Highest education in household | <.01 | |||
| <High School | 160 (11.2) | 61 (13.3) | 51 (15.7) | |
| High School Grad/Some College | 655 (45.7) | 300 (65.2) | 216 (66.5) | |
| College Grad/Advanced Degree | 519 (36.2) | 80 (17.4) | 43 (13.2) | |
Values displayed as median (IQR) or number (%);
defined as use of inhaled corticosteroids;
limited to children <24 months; 3 includes endocrine, renal, hepatic, hematologic, immunologic, chromosomal, and genetic/metabolic disorders
Hospital Length of Stay
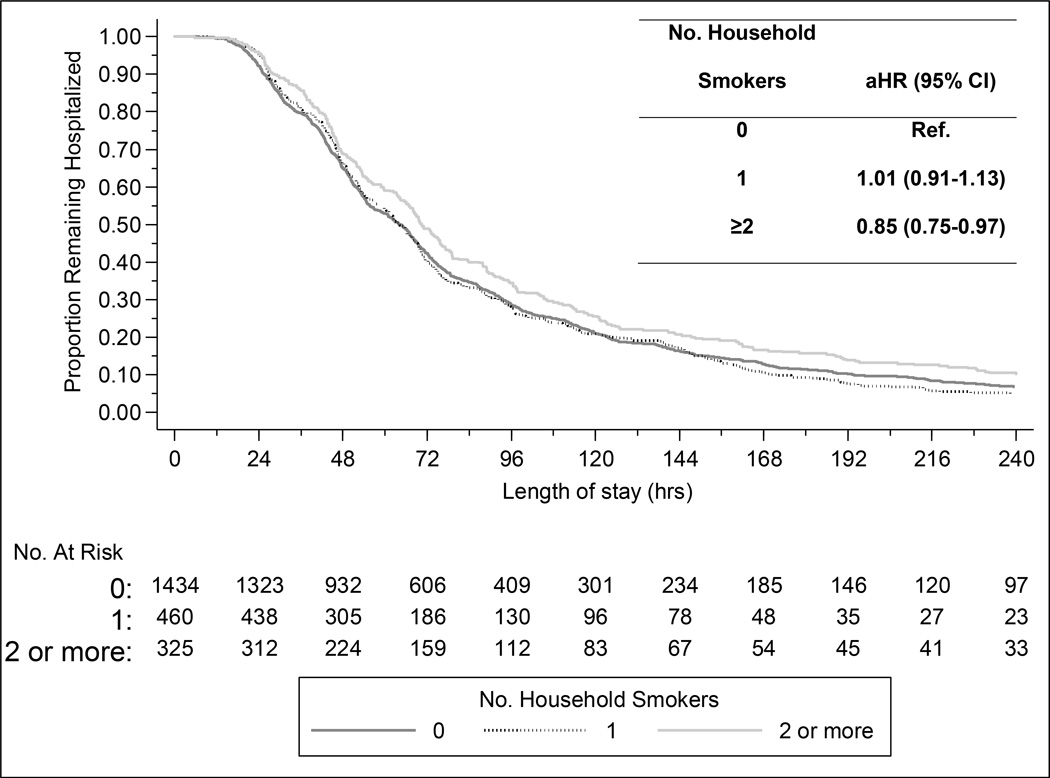
Overall, children from households with any household smoker had a slightly longer length of stay compared with non-exposed children, although this association did not reach statistical significance (median 67.4 vs. 64.4 hours; adjusted hazard ratio [aHR] 0.94, 95% CI 0.86–1.03). However, exposure to ≥2 household smokers was associated with a significantly longer length of stay compared with households without smokers (median 70.4 vs. 64.4 hours; aHR 0.85, 95% CI 0.75–0.97). Children from homes with only one household smoker had similar length of stay compared with non-exposed children (median 64.2 vs. 64.4 hours; aHR 1.01, 95% CI 0.91–1.13) (Figure 2).
Figure 2. Length of stay for Children Hospitalized with Community-Acquired Pneumonia, According to Reported SHS Exposure.
Abbreviations: aHR, adjusted hazards ratio; CI, confidence interval;; proportional hazards regression was used to model the association between home smoke exposure and hospital length of stay; model was adjusted for the following potential confounding factors selected a priori: age, sex, race/ethnicity, household education level, government insurance, individual high-risk comorbidities (persistent asthma, prematurity, neurologic disorder, cardiopulmonary disorder, and other), enrollment site, year, and season.
Secondary Outcomes
The presence of any household smoker was not associated with increased admission to an intensive care unit (21.7% vs. 20.9%, aOR 1.22, 95% CI 0.97–1.55) or invasive mechanical ventilation (7.1% vs. 7.1%, aOR 0.94, 95% CI 0.65–1.36) compared with non-exposed children. Similar to the findings for length of stay, children exposed to ≥2 household smokers were more likely to be admitted to intensive care compared with non-exposed children (25.2% vs. 20.9%, aOR 1.44, 95% CI 1.05–1.96) (Table II). Invasive mechanical ventilation also was more common among children exposed to ≥2 household smokers compared with non-exposed children, but this association was not statistically significant (10.2% vs. 7.1%, aOR 1.28, 95% CI 0.81–2.02). Outcomes for children exposed to one household smoker were similar to non-exposed children.
Table 2.
Adjusted Odds Ratios for Severe Outcomes among Children Hospitalized with Community-Acquired Pneumonia, According to Reported Home Smoke Exposure
| Home Smoke Exposure | |||
|---|---|---|---|
| Outcomes | Non-Exposed, n=1434 |
1 Smoker, n=460 | ≥2 Smokers, n=325 |
| Intensive care unit admission | |||
| No. (%) | 300 (20.9) | 88 (19.1) | 82 (25.2) |
| aOR (95% CI) | Ref | 1.09 (0.82–1.45) | 1.44 (1.05–1.96) |
| Invasive mechanical ventilation | |||
| No. (%) | 100 (7.1) | 23 (5.2) | 33 (10.2) |
| aOR (95% CI) | Ref | 0.70 (0.43–1.13) | 1.28 (0.81–2.02) |
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; all models were adjusted for the following potential confounding factors selected a priori age, sex, race/ethnicity, household education level, government insurance, individual high-risk comorbidities (persistent asthma, prematurity, neurologic disorder, cardiopulmonary disorder, and other), enrollment site, year, and season.
DISCUSSION
Among more than 2200 children hospitalized with pneumonia in this study, children from homes with ≥2 smokers had a longer hospital stay and were more likely to require intensive care compared with children not exposed to household smokers, suggesting that heavier SHS exposure is associated with higher pneumonia severity.
Our findings add to the increasing body of evidence supporting the association between SHS exposure and increased severity of pediatric respiratory infections. Several prior studies assessed the impact of SHS on respiratory disease severity among hospitalized children. Bradley et al. demonstrated lower oxygen saturations but similar length of stay among 206 infants with bronchiolitis whose mothers smoked compared with infants of non-smoking mothers. (17) A similar study among 378 infants hospitalized with bronchiolitis found that household tobacco smoking was associated with both an increased need for oxygen supplementation (aOR 2.45) and mechanical ventilation (aOR 5.49). (19) Among 117 children with laboratory-confirmed influenza, Wilson et al demonstrated a nearly 5-fold increase in the need for intensive care as well as a 70% longer length of stay among SHS exposed children. (18) None of these studies focused specifically on pneumonia.
In our study, children from homes with ≥2 household smokers, but not those from homes with only one household smoker, had a modest increase in length of stay and risk for intensive care admission compared with children from non-smoking households. Household SHS exposure was based on caregiver report, and there is potential for differential misclassification with some children from smoking households classified as non-exposed to SHS. Such underreporting of household smoking would result in a bias toward the null. Consequently, associations between SHS exposure and pneumonia disease severity are likely underestimated. In addition, families with only one household smoker may be more likely to implement home smoking bans compared with families with ≥2 household smokers, which may contribute to the lack of association observed with children from homes with one household smokers compared with those with ≥2 household smokers. Previous studies also demonstrate 200% higher air nicotine levels and >150% higher hair and blood cotinine levels among children residing in homes with ≥2 household smokers compared with homes with only one household smoker. (24, 25) Although no level of SHS exposure is considered safe, (1) our findings suggest an association between home SHS exposure intensity and illness severity for CAP. This is consistent with previous studies suggesting an association between the intensity of SHS exposure and disease severity for a variety of acute respiratory illnesses in children, such as RSV (26) and asthma (27).
In our study, 35.4% of children hospitalized with pneumonia were exposed to household smokers, an exposure prevalence comparable with other studies conducted among hospitalized children. (17, 18) Acute hospitalization for any reason, and particularly for respiratory illness, serves as an excellent “teachable moment” to reinforce the importance of smoke-free environments for children through caregiver counseling and active intervention. (28–30) However, SHS exposed children are not consistently identified as exposed based on admission documentation. (28) Improvements in SHS exposure screening in the inpatient setting are needed.
These findings also can inform policies aimed at further reducing the burden of SHS exposure in children. The overall prevalence of serum cotinine concentrations 0.05 ng/mL among nonsmoking Americans decreased by half from 53% in 1999–2000 to 25% in 2011–12. (3) This progress is attributable in part to the growing number of bans on indoor smoking in workplaces and public venues, such as hospitals, restaurants, bars and offices, as well as changing attitudes about SHS and voluntary restrictions on smoking around nonsmokers. (31) Still, millions of U.S. children remain exposed, and policy efforts on local and national levels should continue in order to minimize the impact of SHS exposure on child health.
This study has several limitations. As with all observational studies, there is potential for confounding. To address this concern, our modeling strategy considered a variety of variables hypothesized to be associated with SHS exposure or our selected outcomes. Nevertheless, residual confounding due to unmeasured or incompletely ascertained factors (e.g. socioeconomic status) is possible. Objective data on SHS exposure were not collected and subjective assessment of home smoke exposure could underestimate the proportion of SHS exposed children, although consistent findings are reported across studies that utilize either subjective reporting or objective measures of SHS exposure. (16) We did not gather data on the number of cigarettes smoked per day by household smokers or if smoking was allowed inside the home or in personal vehicles, so more detailed quantification of exposure was not possible. Finally, although SHS exposure among children occurs primarily in the home environment, (32, 33) we did not capture SHS exposure outside of the home nor did we assess in-home exposure from non-household members. Our findings underscore the importance of ongoing policy efforts and interventions to reduce and eliminate SHS exposure among children in order to improve child health.
ACKNOWLEDGMENTS
The authors wish to thank the children and families who graciously consented to participate in the EPIC study. We also wish to acknowledge all members of the EPIC team at CDC and the 3 study sites.
Supported by the National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIH; K23AI104779 [to D.W.]). The EPIC study was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases/Centers for Disease Control and Prevention (CDC) through cooperative agreements with each study site and was based on a competitive research funding opportunity. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.United States Public Health Service. xvii. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General; 2006. Office of the Surgeon General. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General; p. 709. [Google Scholar]
- 2.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environmental health perspectives. 2006;114(6):853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, et al. Vital signs: disparities in nonsmokers' exposure to secondhand smoke - United States, 1999–2012. MMWR Morbidity and mortality weekly report. 2015;64(4):103–108. [PMC free article] [PubMed] [Google Scholar]
- 4.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and metaanalysis. Pediatrics. 2012;129(4):735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 5.Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53(3):204–212. doi: 10.1136/thx.53.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook DG, Strachan DP. Health effects of passive smoking-10: Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54(4):357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics. 1998;101(2):E8. doi: 10.1542/peds.101.2.e8. [DOI] [PubMed] [Google Scholar]
- 8.Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2001;155(1):36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- 9.Jones LL, Hassanien A, Cook DG, Britton J, Leonardi-Bee J. Parental smoking and the risk of middle ear disease in children: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(1):18–27. doi: 10.1001/archpediatrics.2011.158. [DOI] [PubMed] [Google Scholar]
- 10.Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res. 2011;12:5. doi: 10.1186/1465-9921-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young S, Le Souef PN, Geelhoed GC, Stick SM, Turner KJ, Landau LI. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med. 1991;324(17):1168–1173. doi: 10.1056/NEJM199104253241704. [DOI] [PubMed] [Google Scholar]
- 12.Martinez FD, Antognoni G, Macri F, Bonci E, Midulla F, De Castro G, et al. Parental smoking enhances bronchial responsiveness in nine-year-old children. The American review of respiratory disease. 1988;138(3):518–523. doi: 10.1164/ajrccm/138.3.518. [DOI] [PubMed] [Google Scholar]
- 13.Health effects of exposure to environmental tobacco smoke. California Environmental Protection Agency. Tobacco control. 1997;6(4):346–353. doi: 10.1136/tc.6.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans D, Levison MJ, Feldman CH, Clark NM, Wasilewski Y, Levin B, et al. The impact of passive smoking on emergency room visits of urban children with asthma. The American review of respiratory disease. 1987;135(3):567–572. doi: 10.1164/arrd.1987.135.3.567. [DOI] [PubMed] [Google Scholar]
- 15.LeSon S, Gershwin ME. Risk factors for asthmatic patients requiring intubation. I. Observations in children. J Asthma. 1995;32(4):285–294. doi: 10.3109/02770909509044836. [DOI] [PubMed] [Google Scholar]
- 16.Chilmonczyk BA, Salmun LM, Megathlin KN, Neveux LM, Palomaki GE, Knight GJ, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N Engl J Med. 1993;328(23):1665–1669. doi: 10.1056/NEJM199306103282303. [DOI] [PubMed] [Google Scholar]
- 17.Bradley JP, Bacharier LB, Bonfiglio J, Schechtman KB, Strunk R, Storch G, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115(1):e7–e14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]
- 18.Wilson KM, Pier JC, Wesgate SC, Cohen JM, Blumkin AK. Secondhand tobacco smoke exposure and severity of influenza in hospitalized children. J Pediatr. 2013;162(1):16–21. doi: 10.1016/j.jpeds.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 19.Semple MG, Taylor-Robinson DC, Lane S, Smyth RL. Household tobacco smoke and admission weight predict severe bronchiolitis in infants independent of deprivation: prospective cohort study. PLoS One. 2011;6(7):e22425. doi: 10.1371/journal.pone.0022425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keren R, Luan X, Localio R, Hall M, McLeod L, Dai D, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164. doi: 10.1001/archpediatrics.2012.1266. [DOI] [PubMed] [Google Scholar]
- 21.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of america. Clin Infect Dis. 2011;53(7):e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClain L, Hall M, Shah SS, Tieder JS, Myers AL, Auger K, et al. Admission chest radiographs predict illness severity for children hospitalized with pneumonia. J Hosp Med. 2014;9(9):559–564. doi: 10.1002/jhm.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275(16):1233–1240. [PubMed] [Google Scholar]
- 25.Wipfli H, Avila-Tang E, Navas-Acien A, Kim S, Onicescu G, Yuan J, et al. Secondhand smoke exposure among women and children: evidence from 31 countries. Am J Public Health. 2008;98(4):672–679. doi: 10.2105/AJPH.2007.126631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carbonell X, Fullarton JR, Gooch KL, Figueras-Aloy J. The evolution of risk factors for respiratory syncytial virus-related hospitalisation in infants born at 32–35 weeks' gestational age: time-based analysis using data from the FLIP-2 study. Journal of perinatal medicine. 2012 doi: 10.1515/jpm-2011-0248. [DOI] [PubMed] [Google Scholar]
- 27.Morkjaroenpong V, Rand CS, Butz AM, Huss K, Eggleston P, Malveaux FJ, et al. Environmental tobacco smoke exposure and nocturnal symptoms among inner-city children with asthma. J Allergy Clin Immunol. 2002;110(1):147–153. doi: 10.1067/mai.2002.125832. [DOI] [PubMed] [Google Scholar]
- 28.Wilson KM, Wesgate SC, Best D, Blumkin AK, Klein JD. Admission screening for secondhand tobacco smoke exposure. Hosp Pediatr. 2012;2(1):26–33. doi: 10.1542/hpeds.2011-0005. [DOI] [PubMed] [Google Scholar]
- 29.Winickoff JP, Hillis VJ, Palfrey JS, Perrin JM, Rigotti NA. A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140–145. doi: 10.1542/peds.111.1.140. [DOI] [PubMed] [Google Scholar]
- 30.Winickoff JP, Hibberd PL, Case B, Sinha P, Rigotti NA. Child hospitalization: an opportunity for parental smoking intervention. Am J Prev Med. 2001;21(3):218–220. doi: 10.1016/s0749-3797(01)00355-5. [DOI] [PubMed] [Google Scholar]
- 31.United States Public Health Service. xxii. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General; 2014. Office of the Surgeon General. The health consequences of smoking--50 years of progress: a report of the Surgeon General; p. 943. [Google Scholar]
- 32.Gergen PJ. Environmental tobacco smoke as a risk factor for respiratory disease in children. Respiration physiology. 2001;128(1):39–46. doi: 10.1016/s0034-5687(01)00263-8. [DOI] [PubMed] [Google Scholar]
- 33.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of exposure analysis and environmental epidemiology. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]