Abstract
Prolonged morphine treatment in neonatal pediatric populations is associated with a high incidence of opioid tolerance and dependence. Despite the clinical relevance of this problem, our knowledge of the long-term consequences is sparse. The main objective of this study was to investigate whether prolonged morphine administration in a neonatal rat is associated with long-term behavioral changes in adulthood. Newborn animals received either morphine (10mg/kg) or equal volume of saline subcutaneously twice daily for the first 2 weeks of life. Morphine treated animals underwent 10 days of morphine weaning to reduce the potential for observable physical signs of withdrawal. Animals were subjected to non-stressful testing (locomotor activity recording and a Novel-Object Recognition test) at a young age (PD27-31) or later in adulthood (PD55-56), as well as stressful testing (calibrated forceps test, Hot Plate test, and Forced Swim test) only in adulthood. Analysis revealed that prolonged neonatal morphine exposure resulted in decreased thermal, but not mechanical threshold. Importantly, no differences were found for total locomotor activity (proxy of drug reward/reinforcement behavior), individual Forced Swim test behaviors (proxy of affective processing), or Novel-Object Recognition test. Performance on the Novel-Object Recognition test was compromised in the morphine treated group at the young age, however the effect disappeared in adulthood. These novel results provide insight into the long-term consequences of opioid treatment during an early developmental period and suggest long-term neuroplastic differences in sensory processing related to thermal stimuli.
Keywords: Behavioral sensitization, Hot Plate, Locomotor activity, Novel-Object Recognition test, Opioid, Swim, Threshold
INTRODUCTION
Pain management of infants and children has greatly evolved over the past 25 years, from no treatment at all to an emphasis on pain management. This was based on advances in our understanding of the neurobiology of sensory processing during development. As early as the 24th week of gestation, painful stimuli are associated with physiologic, hormonal, and metabolic markers of the stress response (Anand & Hickey, 1987; Lee, Ralston, Drey, Partridge, & Rosen, 2005). In addition to feeling pain, infants and children may have decreased pain thresholds and increased physiological responses to both noxious and innocuous stimuli as compared to older children and adults (Craig, Whitfield, Grunau, Linton, & Hadjistavropoulos, 1993; Grunau, Whitfield, Petrie, & Fryer, 1994; Johnston, Stevens, Yang, & Horton, 1995; Johnston, Stevens, Yang, & Horton, 1996). More importantly, several studies demonstrated that untreated painful stimulation, especially in preterm infants leads to long-term effects that may involve permanent changes in pain processing and impaired brain development (Brummelte et al., 2012; Fitzgerald & Walker, 2009; Johnston & Stevens, 1996; Vinall et al., 2012) including increased pain sensitivity and maladaptive behavior later in life (Anand & Scalzo, 2000; Taddio & Katz, 2005). Hence, due to the known negative implications of untreated pain in infants and children (Fitzgerald, 1991; Fitzgerald, Millard, & McIntosh, 1989; Fitzgerald & Walker, 2009), there has been an increase in the use of analgesic agents in the pediatric population.
Opioids have been shown to relieve acute pain in infants and have become the ‘gold standard’ for pain treatment in procedural and perioperative settings (Anand, 2001). Furthermore, even in the absence of surgical pain, critically ill neonates and children receive prolonged opioids for sedation to reduce anxiety, agitation, stress responses and to facilitate ventilation (Anand, 2001; Berde & Sethna, 2002; Chambliss & Anand, 1997). Although the administration of opioids has been shown to improve behavioral measures of comfort in mechanically ventilated infants (Guinsburg et al., 1998; Saarenmaa, Huttunen, Leppaluoto, & Fellman, 1996), there is a gap in the literature regarding the effectiveness and hazards (benefit/risk ratio) of these agents. Moreover, such treatment is associated with markedly high incidence (35–57%) of analgesic tolerance and opioid dependence (Anand et al., 2010; Fonsmark, Rasmussen, & Carl, 1999; Katz, Kelly, & Hsi, 1994).
The impact of chronic maternal opioid use on in utero brain development has been widely studied and is known to be associated with neurocognitive and motor impairments later in life (Hunt, Tzioumi, Collins, & Jeffery, 2008; McGlone, Mactier, & Weaver, 2009; van Baar & de Graaff, 1994). The added complexity of human studies makes it difficult to identify factors that are due to opiate use alone or from confounding factors (e.g. the abuse of other drugs, poor prenatal care, poor nutrition, etc.) Furthermore, the evidence for long-term neurodevelopmental delay following postnatal opioid exposure is limited (Bellu, de Waal, & Zanini, 2008; Bellu, de Waal, & Zanini, 2010; Ferguson, Ward, Paule, Hall, & Anand, 2012). It is possible that prolonged postnatal opioid treatment associated with the development of opioid dependence significantly alters neural pathways. Given that administration of opioids in both newborn and infant periods in the absence of pain (e.g. for sedation) is considered standard clinical care, the objective of our study was to address possible long-term behavioral sequelae of prolonged postnatal morphine exposure in a rodent model in the absence of nociception as opposed to prenatal rat models when dams are treated. Both the maturation and function of pain pathways, as well as the mechanisms of prolonged opioid effects in a rat model are age dependent. Specifically, increased excitability of nociceptive circuits peaks at postnatal day (PD)6 and decreases to an adult-like level by PD21 (Fitzgerald & Jennings, 1999; Jennings & Fitzgerald, 1998). Furthermore, some of the mechanisms of opioid tolerance (Zhu & Barr, 2003) and dependence (Zhu & Barr, 2001a, 2001b) partially correspond to those of the adult rats at PD14 and are equivalent to an adult at the PD21. Therefore, we decided to expose developing rat pups to prolonged morphine administration during this early period of the first two weeks of life (PD1-14) when different mechanisms of pain perception and opioid treatment are in effect in comparison to adult rats. Although the exact equivalencies cannot be made to human developing age, there is a consensus that PD1-14 roughly extends from the last trimester of pregnancy up to the first few years of postnatal life in human (Clancy, Darlington, & Finlay, 2001; Clancy, Finlay, Darlington, & Anand, 2007; Huttenlocher & Dabholkar, 1997). Specifically, we hypothesized that prolonged morphine administration in a modified neonatal rat model of antinociceptive tolerance and dependence (Bajic, Berde, & Commons, 2012; Jones & Barr, 1995; Zhu & Barr, 2003), is associated with long-term behavioral changes in adulthood. To test our hypothesis, we investigated possible long-term influence on (1) sensory processing by measuring mechanical and thermal threshold; (2) sensitization by evaluating locomotor activation, a proxy of drug reward/reinforcement behavior; (3) stress/anxiety by using a Forced Swim test; and (4) short-term recognition memory using a Novel-Object Recognition test.
METHODS
Animal Care and Use
The Institutional Animal Care and Use Committee at Boston Children’s Hospital approved the experimental protocols for the use of vertebrate animals in this study. Experiments were conducted according to the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals, and the guide for the Care and Use of Laboratory Animals (NIH Publications No.80-23, revised 1996) prepared by the National Academy of Sciences’ Institute for Laboratory Animal Research. All efforts were made to minimize the number of animals used and their discomfort. Pregnant rat dams (Sprague Dawley, Sasco; Charles River Laboratories International, Inc., Wilmington, MA, USA) were received on day 18 of gestation and were handled for several days before delivery. Cages with pregnant dams were checked at 9AM and 5 PM daily and pups found at either times were termed 0 days of age. The progeny from 8 litters were used in this study. Each litter had between 9 and 12 pups that were randomly assigned to each of the pharmacological groups; this split-litter (within-litter) design represents all treatment groups within a single litter (Booze & Mactutus, 1985). Furthermore, we used balanced treatment distribution per litter (Festing, 2006). Pups from both sexes were included in the study. Animals were housed with their litters and were maintained on a 12-h light/dark cycle with food and water given ad libitum. Pups were weaned from dams at 3 weeks of age (postnatal day (PD)18).
Pharmacological Treatment
Barr’s group originally described a method for prolonged morphine administration in rat pups that is associated with morphine dependence (Jones & Barr, 1995) and development of analgesic tolerance (Zhu & Barr, 2003) in newborn rats; the latter was also confirmed in our lab (Bajic et al., 2012). We used subcutaneous (sc) injections to minimize nociceptive experience from intraperitoneal drug administration and, in this report we extended the period of administration from 6 ½ (1 week) to 13 ½ days (2 weeks). The first day of injection always occurred at PD1. Specifically, animals received twice-daily sc injections for 14 days (9 AM and 5 PM), from PD1 through PD14. All injections were done using either a 10 or 100μl Hamilton syringe (Hamilton Company, Reno, NV, USA). Morphine sulfate (10 mg/kg; Baxter Healthcare Corp., Deerfield, IL, USA) or equal volume of saline was administered in the sc area of the upper or lower back. Experimental groups included: (1) control group that received saline injections and (2) morphine group that received morphine injections.
Morphine Weaning and Quantification of Physical Signs of Morphine Withdrawal
Physical dependence is manifested indirectly as a myriad of physiological disturbances and physical symptoms of withdrawal that result from abrupt discontinuation of dosage reduction (spontaneous withdrawal). After the period of pharmacological treatment (PD1-14), pups injected with morphine underwent morphine weaning treatment for 10 days (PD15-25) to reduce the potential for observable physical signs of withdrawal. Specifically, pups received incrementally decreasing morphine dosages: 3 days of twice-daily 5mg/kg, 3 days of twice-daily 2.5mg/kg, 2 days of twice daily 1.25mg/kg and 2 days of once daily 1.25mg/kg. To ensure that the weaning protocol was appropriate in preventing development of observable physical signs of withdrawal, we quantified the pups’ daily behavior for 10 days of morphine weaning. We quantified the physical signs of withdrawal in progeny from the first two litters analyzed: N=8 (4 female and 4 male) for the saline group and N=10 (8 female and 2 male) for the morphine group. Specifically, a 10 min video was recorded daily before morning injections. We selected this time point since it reflects behavior following the longest time-period since the last injection (14–16 hr). An observer blind to the treatment group manually scored the individual behaviors of the animals every 15 seconds. The scoring rubric was adapted from a previous study (Gellert & Holtzman, 1978). Briefly, 11 scored withdrawal behaviors corresponded to 2 different types of physical signs: ‘checked signs’ and ‘graded signs’ with 5 and 6 behaviors in each category, respectively. Checked signs are behaviors for which only the absence or presence of the behavior is evaluated and include diarrhea, facial fasciculation/teeth chattering, swallowing movements, profuse salivation, ptosis and abnormal posture. Graded signs are scored based on the frequency (number of events) and include escape attempts, abdominal contractions, wet dog shakes, rearing and grooming. Modifications of Gellert-Holtzmann method (Gellert & Holtzman, 1978) included the elimination of weight loss (as no animals lost weight during morphine weaning; Fig. 1B) and the addition of two graded behaviors (rearing and grooming) not previously analyzed. Withdrawal scores were expressed as individual sign means ± SD (not shown) as well as the mean of the sum of all behavioral scores (total global score mean ± SD; Fig. 1C) for the 10 min of observed behavior.
Figure 1. Prolonged morphine administration in newborn rats.
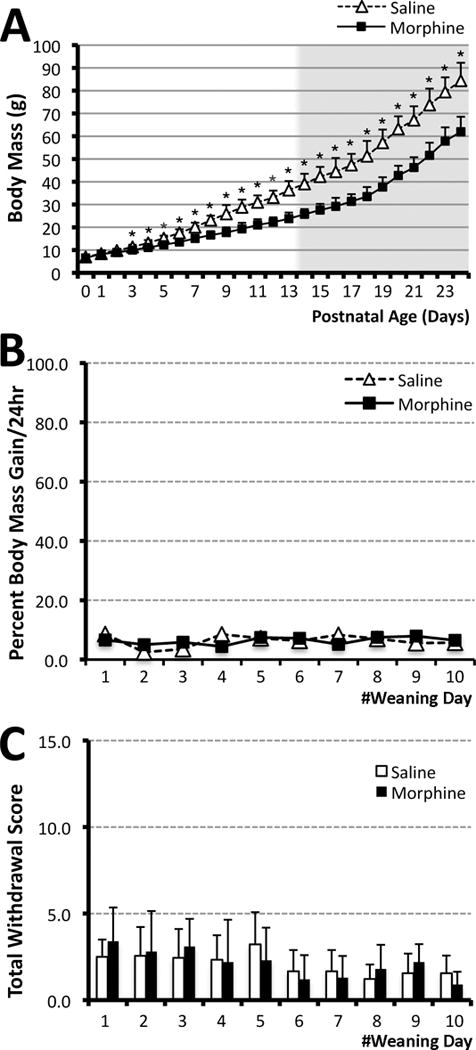
Graph A illustrates the effect of repeated morphine treatment on the body mass of newborn rats (white background) in comparison to saline treated controls. Day of birth was considered day 0 of life. Morphine sulfate (10mg/kg sc twice-daily) or equivalent volume of saline was injected for 14 days starting on postnatal day (PD)1. Afterwards, animals underwent a 10-day weaning period (PD15-24; gray background; see Methods section for details). Progeny from 4 litters was used to analyze the weight. Systemic morphine exposure (N=21; 15 female and 6 male) significantly decreased body mass (± SD) in newborn rats in comparison to saline control group (N=18; 8 female and 10 male) from PD3. Panels B and C show analysis of physical signs of withdrawal that were analyzed in progeny of the first two litters: saline (N=8) and morphine (N=10). Graph B illustrates percent body mass gain/24 hr (± SD) during the weaning period of 10 days. Consistent percent body mass gain was maintained through the period of morphine weaning and no significant differences were observed between the morphine and saline treated animals at any of the days analyzed. Graph C shows analysis of total withdrawal score (± SD) for each day of the weaning period (PD15-24). There were no differences in any individual (not shown) or total daily withdrawal scores between morphine and saline treated animals. Two-tailed t-test was used to compare mean values/day (± SD; A–C): *p<0.05.
Behavioral Analyses
Following prolonged pharmacological treatment after birth (PD1-14) and morphine weaning treatment (PD15-24), animals underwent stressful testing only one time in adulthood (PD55-56) for nociceptive and thermal threshold, as well as the Forced swim test (saline group N=25, 12 female and 13 male rats from 8 litters; and morphine group N=24, 15 female and 9 male from 6 litters). Two non-stressful tests, locomotor activity recording and Novel-Object Recognition test were performed at two different time points: at younger age (PD30; saline group N=10, 5 female and 5 male from 4 litters; morphine group N=7, 4 female and 3 male from 4 litters), and in adulthood (PD55-56; saline group N=17, 8 female and 9 male from 4 litters; morphine group N=21, 15 female and 6 male from 4 litters). None of these animals underwent testing twice for the same test. When tested in adulthood, tests were performed from the least to the most stimulating manipulations and proceeded as follows: (1) Novel-Object Recognition test, (2) locomotor recording, (3) calibrated forceps, (4) Hot-Plate test, and (5) Forced Swim test. An individual blinded to the pharmacological treatment performed all the tests.
Calibrated Forceps Testing
Mechanical threshold response was evaluated by using calibrated forceps (Rodent pincher-analgesia meter; Bioseb In Vivo Research Instruments, Vitrolles, France). This algometer allows calibrated forceps to induce quantifiable mechanical stimulation in the animal on a linear scale. As previously described by Luis-Delgado et al. (2006), we recorded the effects of 3 repetitive measurements on each hindpaw (total 6 measurements per individual rat) to provide a sensitive and reliable way of testing mechanical threshold. The force applied to the foot was increased incrementally at approximately 200 g every 3 seconds until the paw was withdrawn. A 5 min period was allowed between repeated testing. The maximum force applied to the paw at the time of withdrawal was recorded as displayed by the dynamometer in grams (g). Withdrawal latency was expressed as mean ± SD per pharmacological group.
Hot-Plate Test
The hot-plate test was carried out to measure thermal withdrawal latency as an index of nociceptive threshold using previously described methods (Bannon & Malmberg, 2007; Eddy & Leimbach, 1953; Shi, Qi, Gao, Wang, & Luo, 2010). Briefly, rats were placed in a clear plexiglas box standing on a hot plate (Cold Hot Plate Analgesia Meter; Bioseb In Vivo Research Instruments, Vitrolles, France) heated to 52.5°C (Allen & Yaksh, 2004). The latency time was defined as the period between the animal’s initial placement on the hot plate surface and the time when the animal licked its paws, jumped to avoid thermal nociceptive stimulus, and/or urinated. To minimize tissue damage, a cut-off time of 40 sec was adopted. We recorded the effects of 3 repetitive measures per individual rat performed at a minimum of 2 min apart. Thermal withdrawal time (sec) was expressed as mean ± SD per pharmacological group.
Forced Swim test
Adult animals underwent modified Forced Swim testing on PD55-56. The Forced Swim test, as originally reported by Porsolt et al. (Porsolt, Le Pichon, & Jalfre, 1977), measures coping strategy to an acute stress, and has become a widely used model for assessing depressant/anti-depressant-like activity in rats. We used a protocol that was identical to those previously described by others (Borsini, Lecci, Sessarego, Frassine, & Meli, 1989; Borsini & Meli, 1988; Detke, Rickels, & Lucki, 1995; Porsolt, Bertin, & Jalfre, 1977) except that we increased a water depth from 15 cm to 30 cm. Briefly, rats were placed in a cylindrical glass tank (46 cm high × 20 cm diameter) filled with water (25 ± 1°C) to a depth of 30 cm for 15 min. The 30 cm depth allowed rats to swim or float without their tails touching the bottom of the tank. Test sessions were video recorded (Flip Video UltraHD Video Camera, Cisco Systems, Inc., San Jose, CA) for later scoring. Immediately after the Forced Swim test, rats were removed from the tank, towel dried, and put in a warming cage (37°C) that contained a heating pad covered with towels for 15 min. Rats were then returned to their home cage. Videotaped sessions were reviewed and scored using a time-sampling technique wherein the predominant behavior over each 5-second period of the test is recorded. This is in contrast to the original swim test, which used a cumulative timing measure, where the total amount of time is recorded for each behavior in seconds. Three types of predominant behavior (swimming, climbing, immobile) (Slattery & Cryan, 2012) were recorded at the end of every 5 sec period during the 15 min testing session. Floating was defined as rats keeping their heads above the water’s surface with minimal body movements. Climbing was defined as vigorous movements of all four limbs, with the front paws breaking through the surface of the water against the wall of the tank. In contrast, during swimming, rats created coordinated and sustained movements with all four limbs, usually traveling around the interior of the cylinder but without breaking the surface of the water with their forelimbs. During diving, rats would submerge entirely beneath the water. Diving was counted as a swimming activity (Slattery & Cryan, 2012). An individual blinded to the treatment group scored video-recorded tests. Frequency score measure for individual behaviors (swimming, climbing, immobility) was expressed as mean ± SD per pharmacological group.
Locomotor Activity Assay
Animals underwent locomotor testing either at a younger age (PD27), or in adulthood (PD55-56). Locomotor activity was recorded in clear plexiglass cages (10″ ×19″ ×8″h; Photobeam Activity System, San Diego Instruments, San Diego, CA) over twelve 5-min intervals for 60 min. Considering that we used an unpaired paradigm (animals tested in an environment that differed (unpaired) from that used for injection), recorded locomotor activity is considered context-independent sensitization (Vezina, Giovino, Wise, & Stewart, 1989). Total locomotor activity data (ambulatory, fine, and rearing movements) recorded over all 12 intervals (period of 1 hr) are expressed as mean ± SD per pharmacological group.
Novel-Object Recognition Test
Animals underwent Novel-Object Recognition testing either at a younger age (PD31), or in adulthood (PD55-56). We used a protocol identical to those previously described (Bevins & Besheer, 2006). This test takes advantage of animals’ tendency to approach and explore novelty (Berlyne, Koenig, & Hirota, 1966), does not require preliminary training, and has a high-throughput potential being that it is conducted in one session. All testing was conducted in a non-transparent plastic chamber (10″×10″×10″ for pups; 13″×13″×13″ for adults). Briefly, to acclimate to the new environment, all animals were pre-exposed to the testing environment for 5 minutes the day before testing. Novel-Object Recognition testing consisted of two sessions (training, and 1hr testing), which were all videotaped (Flip Video UltraHD Video Camera, Cisco Systems, Inc., San Jose, CA). In the first 10 min session (‘training session’), animals interacted with two identical (sample/familiar) objects that were placed in the back left and right corners of the box. Animals were always placed in the center of the box, facing the wall opposite the testing objects. This orientation prevented any unintentional bias towards one object/side of the box. The experimenters left the procedure area so as not to serve as a cue for the rat. After this initial session, rats were returned to their cage for a period of 1 hour (‘training-to-testing interval’). Afterwards, one of the sample objects was replaced with an object novel to the animal. The animals were then placed in the testing chamber again for 4 min (‘1hr testing session). An experimenter blinded to the group treatment scored video-taped behaviors. The first 3 min of each video were analyzed. Two stopwatches were used to monitor the time an animal spent in ‘directed contact’ with each object. This included contact of the mouth, nose and/or paw with the object. It did not include accidental touches, like bumping or backing into the object. Also, standing or leaning on the object was not included in this definition of contact (Bevins & Besheer, 2006). In addition to mean interaction time (sec) ± SD spent with objects per pharmacological group, we also used the discrimination ratio as a measure of novel object recognition. The discrimination ratio is the time spent with the novel object divided by the total interaction time (time spent with both objects). Using this measure, a value of 0.5 indicates the same amount of time spent with both objects, whereas novel object recognition is determined by a discrimination ratio greater than 0.5.
Statistical Analyses
Considering that we used split-litter (within-litter) design when individual pups within a single litter were subjected to different treatments, the analysis unit (‘N’) is based on the number of individual pups in a treatment group (Festing, 2006). A statistical power analysis was performed for sample size estimation. The standardized effect size in this study was 1 (large) (Hulley et al., 2001). With an alpha = 0.05 and power = 0.80, the projected sample size needed with this effect size is approximately N=17. Thus, our adult group sizes (17–25/group) were more than adequate for detecting for meaningful differences between the pharmacological groups. Being that sample sizes for younger animals were smaller, the likelihood of type 2 error is higher. No differences were found in any of the behavioral analyses between male and female rats (N=12 female and 13 male saline treated; N=15 female and 9 male morphine treated; data not shown). Since all tested behaviors were sex independent, data for each treatment group were collapsed for clarity. A two-tailed independent Student’s t-test was used to determine mean differences between pharmacological groups for each day of the morphine weaning period (PD15-25), mechanical and thermal thresholds, individual Forced Swim test behaviors (swimming, climbing, immobility). Furthermore, we used one-way ANOVA with Tukey HSD test to identify: (1) mean differences with age and pharmacological treatment for non-repeated measures locomotor activity data, as well as (2) mean differences with pharmacological treatment and type of objects for interaction time and discrimination ratio of the Novel-Object Recognition test. P-values <0.05 were considered significant. All statistical analyses were done with VassarStats, a website for statistical computation.
RESULTS
Body Mass and Withdrawal Symptoms Evaluation in Newborn Period
We used a rat model of prolonged postnatal morphine exposure (PD1-14). Since we are interested in understanding long-term behavioral effects of this prolonged and early morphine administration, it was important to avoid development of withdrawal symptoms with weight loss being a cardinal symptom. Similar to our previous work (Bajic, Commons, & Soriano, 2013), prolonged morphine administration in neonatal rats (twice daily 10 mg/kg) leads to slower body mass gain (Fig. 1A; N=18 for control; N=21 for morphine group). This could be explained, in part, by the fact that morphine treated animals were asleep after the injections giving a nursing advantage to the saline treated pups. However, morphine treated animals did not show any loss of weight (based on day to day measurements) either during the period of treatment (PD1-14), or during the period of morphine weaning (PD15-25). In fact, percent body mass gain was maintained through the period of morphine weaning and was not different from the saline group (Fig. 1B). Animals were separated from mothers on PD18 (day 4 of morphine weaning), which was not associated with change in growth. In addition, none of the individual physical signs of withdrawal (not shown), or total daily withdrawal scores were different between morphine (N=10) and saline (N=8) treated animals (Fig. 1C) confirming that animals did not exhibit any of the physical signs of withdrawal during the weaning period. In adulthood (PD55-56), male rats weighed more than female rats but did not differ between treatment groups (data not shown; N=12 female and 13 male saline treated; N=15 female and 9 male morphine treated), suggesting that during development animals overcame the initial body mass differences.
Long-Term Mechanical and Thermal Nociception
To examine the influence of prolonged postnatal morphine exposure on nociceptive pathways, both mechanical and thermal tests were performed when animals reached adulthood (PD55-56; N=25 saline; N=24 morphine group). Average hindpaw-withdrawal mechanical threshold (g ± SD) measured with calibrated forceps was not significantly different between pharmacological treatment groups (Fig. 2A). In other words, prolonged postnatal morphine treatment did not change the mean mechanical threshold (573.09g ± 149.44) when compared to the saline treated animals (568.4g ± 154.75; t(47)= −0.11, p=0.91) in adulthood. This was in contrast to the average thermal threshold (sec ± SD) that was measured using the Hot-Plate test (Fig. 2B). Prolonged postnatal morphine exposure is associated with long-term thermal hypersensitivity in adulthood (9.26 ± 2.91) in comparison to the saline treated group (11.32 ± 2.92; t(47)= 2.48, p=0.02).
Figure 2. Mechanical and Thermal Threshold.
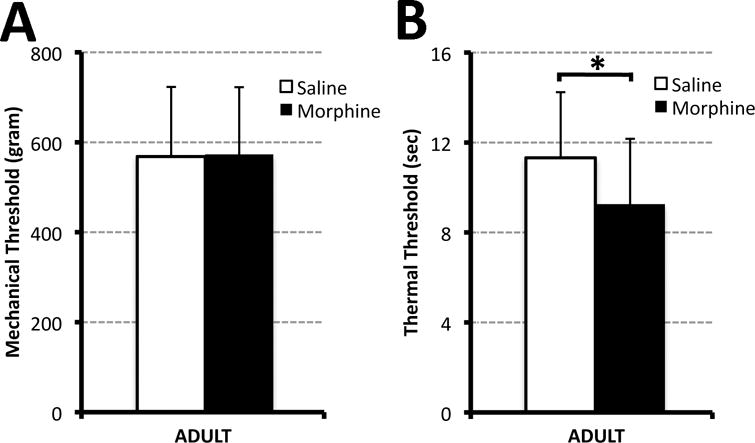
Graphs illustrate average mechanical (Panel A) and thermal (Panel B) threshold (± SD) in adult rats (PD55-56) that were exposed to different pharmacological treatment in the first two weeks of life. Morphine sulfate (10mg/kg sc twice-daily; N=24) or equivalent volume of saline (N=25 saline) was injected for 14 days starting on postnatal day 1. Hindpaw-withdrawal mechanical threshold (gram ± SD) was measured using calibrated forceps. Postnatal morphine treatment did not change the mean mechanical threshold in comparison to controls in adulthood (A). Panel B illustrates average thermal thresholds (sec ± SD), which were measured using the Hot-Plate test (52.5°C). Prolonged postnatal morphine exposure is associated with long-term thermal hypersensitivity in the adulthood in comparison to saline treated group. Two-tailed t-test: *, p<0.05.
Long-Term Forced Swim Test
The Forced Swim test is based on the assumption that animals try to escape from an aversive stimulus and is used to measure coping strategy to an acute stress (Borsini et al., 1989; Borsini & Meli, 1988; Detke et al., 1995; Porsolt, Anton, Blavet, & Jalfre, 1978; Porsolt, Bertin, et al., 1977). In this experiment we quantified individual swim test behaviors (swimming, climbing, immobility) using a frequency score measure/15 min (± SD) in adult animals (PD55-56) following either prolonged postnatal morphine (N=24) or saline treatment (N=25). The pattern of individual swim behaviors was not different between treatment groups (Fig. 3). Specifically, no statistical differences between morphine and saline treated groups were found for swimming (t(47)=−1.91, p=0.06), climbing (t(47)=0.16, p=0.87) or immobility behavior (t(47)= −0.33, p=0.74).
Figure 3. Forced Swim test.
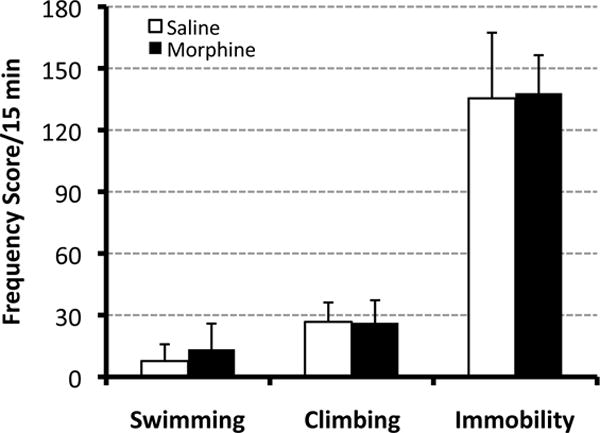
Graph illustrates mean frequency scores (± SD) /15 min for individual behaviors of the Forced Swim test: swimming, climbing, and immobility. Adult animals (PD55-56) were tested following either prolonged postnatal morphine exposure (N=24) or saline control (N=25). Specifically, morphine sulfate (10mg/kg sc twice-daily) or equivalent volume of saline was injected from PD1-14. We report no significant differences between two different pharmacological groups for any of the individual Forced Swim test behaviors: swimming, climbing, or immobility behavior. Two-tailed t-test.
Long-Term Locomotor Activity
Increased locomotor activity has been proposed to reflect neuroadaptations caused by the drug of abuse, such as heightened drug reward/reinforcement behavior, and is a long-lasting phenomenon. Total locomotor activity data was recorded over twelve 5 min intervals (total period of 1 hr; Fig. 4A). Animals did not receive any morphine pretreatment before the measurement of spontaneous locomotor behavior. We report no significant differences following prolonged postnatal morphine administration (PD1-14) in comparison to saline control with respect to average individual (ambulatory, fine, and rearing movements; not shown) or total locomotor activity (± SD) at a young age (PD27; N=10 for saline group, N=7 for morphine group) or long-term, in adulthood (PD55-56; N=17 for saline group, N=21 for morphine group). Specifically, total average locomotor activity/animal/hr (± SD) either at a young age (1 week after treatment; 3 days after last injection during weaning) or in adulthood (8 weeks after treatment) did not differ with either treatment or age (F(3,44)=2.51, p=0.07; Fig. 4B).
Figure 4. Locomotor Activity.
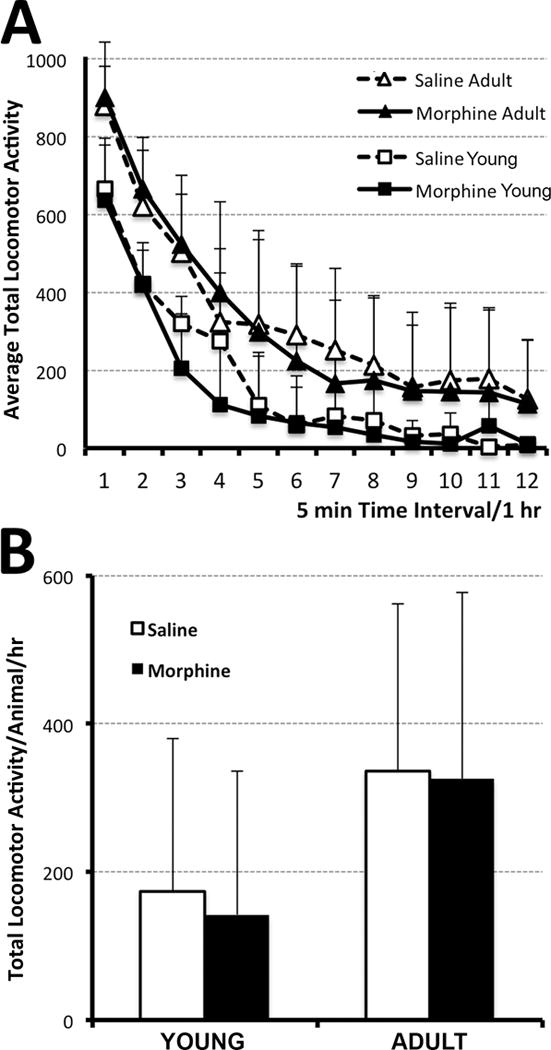
Graphs illustrate average total locomotor activity (which include ambulatory, fine, and rearing movements) ± SD at two different ages: either at young age (one-week after the pharmacological treatment; PD27) or in adulthood (8 weeks after treatment; PD55-56). Morphine sulfate (10mg/kg sc twice-daily) or equivalent volume of saline was injected for 14 days starting on PD1. (A) Average Total Locomotor Activity over a period of 1 hr (twelve 5 min intervals). Although the mean values for locomotor activity are slightly lower for younger animals (N=10 for saline group; N=7 for morphine group) in comparison to adult (N=17 for saline group; N=21 for morphine group), there were no significant differences at any of the time points. Panel B summarized total average locomotor activity/animal/hr. It did not differ with either pharmacological treatment or age. One-way ANOVA with Tukey HSD test.
Long-Term Novel-Object Recognition Test
Rodents’ tendency to interact more with novel objects than previously explored (familiar) objects (Berlyne et al., 1966) has been used to study memory in a form of a Novel-Object Recognition test (Bevins & Besheer, 2006). In the first phase of the experiment (Fig. 5A and B – TRAINING), animals were exposed to two identical objects (familiar objects). There were no differences in interaction time between either the left or the right familiar objects at a young age (PD31; N=10 for saline group; N=7 for morphine group) or later in adulthood (PD55-56; N=17 for saline group; N=21 for morphine group). Analysis also showed that during training session, morphine treated young animals spent more time with one of the objects in comparison to saline group (F(3,30)=3.34, p=0.03), but not during adulhood (F(3,72)=1.9, p=0.14). Equal time spend with both objects is also represented as a discrimination ratio (the time spent with one of the objects divided by the total interaction time) of 0.5 (Fig. 5A′ and B′). In the second phase of the experiment, when a novel object was introduced instead of a familiar one (Fig. 5A and B – 1hr TESTING), animals spent more time interacting with the novel object. This significant increase in interaction time (± SD) was demonstrated for young (F(3,30)=15.38, p<0.001) and adult animals (F(3,72)=7.86, p<0.001). Specifically, at the young age, both saline (p<0.01) and morphine (p<0.05) groups interacted significantly longer with a novel object (Fig. 5A). In adulthood, althought both saline and morphine groups interacted longer with a novel object, only morphine group reached significance (p<0.01; Fig. 5B). However, we report a significant increase in the discrimination ratio at 1 hour testing in comparison to training session for both young (Fig. 5A′; F(3,30)=27.72, p<0.01) and adult rats (Fig. 5B′; F(3,72)=13.98, p<0.01). Discrimination ratio greater than 0.5 indicates that animals spend more time with the novel object. At a young age, the calculated discrimination ratio at 1 hour testing was significantly higher in saline than morphine treated animals (Fig. 5A′; p<0.05). This difference disappeared in adulthood (Fig. 5B′).
Figure 5. Novel-Object Recognition Test.
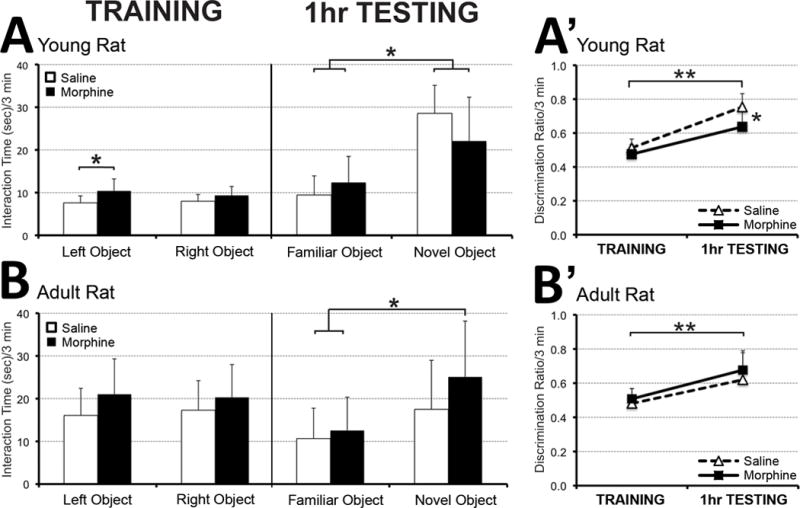
Graphs illustrate interaction time (sec) during two phases of the Novel-Object Recognition test performed at a younger age (PD31; Panel A) and later in adulthood (PD55-56; Panel B). Animals were initially treated with morphine sulfate (10mg/kg sc twice-daily) or equivalent volume of saline from PD1-14. During the ‘training session,’ animals interacted with each of the two identical objects (familiar objects) for equal amount of time both at the young age (PD31; N=10 for saline group; N=7 for morphine group; Panel A) and in adulthood (PD55-56; N=17 for saline group; N=21 for morphine group; Panel B). In a second phase (‘testing session after 1hr’), one of the familiar objects was replaced by a novel object. At a young age, both saline and morphine-treated animals spent significantly more time interacting with the novel object. In adulthood, althought both saline and morphine groups interacted longer with a novel object, only morphine group reached significance. Panels A′ and B′ illustrate discrimination ratio (the time spent with the novel object divided by total interaction time). During the training session, a discrimination ratio of 0.5 indicates that animals spent equal amounts of time with both objects, whereas values greater than 0.5 during the testing session after 1 hr indicate that animals interacted more with the novel object. We report significantly increased discrimination ratio for both young (Fig. A′) and adult animals (Fig. B′). Although morphine treated animals discriminated between the familiar and the novel object at both ages, calculated discrimination ratio was significantly higher in saline treated young animals (Fig. A′). This difference disappeared in adulthood (Fig. B′). One-way ANOVA with Tukey HSD test; *, p<0.05; **, p<0.001.
DISCUSSION
Our novel findings show that prolonged morphine exposure in neonatal rats altered long-term nociceptive processing, as manifested by thermal hyperalgesia, and not mechanical allodynia later in adulthood. Equally important, prolonged morphine exposure in neonatal rats is not associated with any long-term changes in locomotor sensitization (proxy of drug reward/ reinforcement behavior in rats), or the Forced Swim test (proxy of coping behavior to an acute stress). Initially compromised Novel-Object Recognition test (short term recognition memory) at a younger age was not different from control in adulthood.
Long-Term Effects on Nociceptive Thresholds
Prolonged postnatal morphine exposure did not produce mechanical allodynia in adulthood. However, it was associated with long-term thermal hyperalgesia (Fig. 2B). These findings are in contrast to recently published work by Zhang and Sweitzer (2008) who reported that neither mechanical nor thermal nociception was significantly lower than control in adulthood. Several factors might explain the discrepancy with our study, including differences in: (1) pharmacological treatment (3mg/kg once daily from PD1-9; vs. 10 mg/kg twice daily from PD1-14 that was used in our study); (2) differences in frequency of behavioral testing (total 8 testing sessions from PD11-48; vs. only one in adulthood at PD55-56 in our study); (3) methodological differences (heat exposure of the single hindpaw with Ugo Basile Plantar testing apparatus vs. exposure of all four limbs during Hot Plate test in our study); as well as (4) justification of the absence of the withdrawal symptoms as shown in our report. Unlike the Zhang and Sweitzer’s (2008) model, we avoided repeated nociceptive testing because it was reported to be associated with behavioral tolerance (Lane & Morgan, 2005). Despite differences in methodology between our study and that of Zhang and Sweitzer (2008), thermal response latencies were within a similar range. It is possible that the higher morphine dose administered in our study might have a long-term effect on thermal nociceptive processing. Future studies should investigate dose-dependent morphine effects on potential long-term behavioral sequelae on thermal threshold. Postnatal maturation that occurs during the first 3 postnatal weeks in rat involves dramatic changes in: (1) opioid receptor expression, binding, and locations in the brain (Georges, Normand, Bloch, & Le Moine, 1998; Kent, Pert, & Herkenham, 1981; Kivell, Day, McDonald, & Miller, 2004; Petrillo, Tavani, Verotta, Robson, & Kosterlitz, 1987; Tong et al., 2000; Winzer-Serhan, Chen, & Leslie, 2003), and the spinal cord (Beland & Fitzgerald, 2001; Nandi et al., 2004; Nandi & Fitzgerald, 2005; Rahman, Dashwood, Fitzgerald, Aynsley-Green, & Dickenson, 1998; Rahman & Dickenson, 1999); (2) distribution changes of afferent fibers (C, Aδ, and Aβ) in different laminae of the spinal cord dorsal horn (Fitzgerald, Butcher, & Shortland, 1994; Fitzgerald & Jennings, 1999); as well as (3) maturation of cortical (Colonnese, Phillips, Constantine-Paton, Kaila, & Jasanoff, 2008) and descending inhibitory mechanisms originating from the brainstem (Barr & Wang, 2013; Fitzgerald & Koltzenburg, 1986; Hathway, Koch, Low, & Fitzgerald, 2009; van Praag & Frenk, 1991). Therefore, additional studies should look into mechanistic elucidation of neuronal plasticity of both mechanical and thermal nociceptive circuitry at different levels of the neuroaxis.
Long-Term Effect on Forced Swim Test
The behavioral pattern elicited by the swim stress test measures animals’ coping strategy to an acute stress (Borsini et al., 1989; Borsini & Meli, 1988; Detke et al., 1995; Porsolt et al., 1978; Porsolt, Bertin, et al., 1977). Our novel findings show that prolonged postnatal morphine exposure (from PD1-14) has no long-term influence on the Forced Swim test behavior later in adulthood (Fig. 3). Similar studies addressing the potential behavioral changes using the Forced Swim test following postnatal morphine exposure unfortunately are lacking. In contrast, a study that looked into perinatal morphine exposure (whole gestation and lactation period) reported enhanced depressive-like changes (as indicated by increased percentage of time spent in immobile/floating posture) both immediately following cessation of the treatment in young age (PD25), as well as long-term in adulthood (PD56) (Klausz et al., 2011). Furthermore, our preliminary data at the young age (PD16) show no difference in immobility following 2 weeks of treatment (PD1-14) with either saline or morphine (Craig and Bajic, unpublished observations). It is possible that timing and/or the total opioid dose exposure might influence the possible neuronal adaptations that relate to increased risk of depressive-like behavior later in life. The serotonin system originating from the midbrain’s dorsal raphe nucleus that is implicated in mood and affective disorders is dramatically affected by swim stress. It is generally believed that the neuronal circuits underlying behavioral responses of swim stress engage inputs to GABAergic neurons in the dorsolateral sub-region of dorsal raphe nucleus, which in turn inhibit serotonergic neuronal activity and serotonin release in certain forebrain regions (Roche, Commons, Peoples, & Valentino, 2003), such as the lateral septum and amygdala (Kirby, Allen, & Lucki, 1995; Kirby, Chou-Green, Davis, & Lucki, 1997; Kirby & Lucki, 1997). Enhancement of serotonin neurotransmission may mediate swimming, whereas enhancement of another neurotransmitter, norepinephrine, has been implicated to mediate climbing in the Forced Swim test (Detke et al., 1995). Given this evidence, our results suggest that prolonged postnatal morphine exposure does not cause long-term changes of neurochemical substrates involved in control of serotonin, and possibly norepinephrine.
Long-Term Effects on Locomotor Activity
Behavioral sensitization process in rodent models is important for the incentive-motivational components of drug reward (see reviews (Robinson & Berridge, 1993; Wise & Bozarth, 1987)). It is defined by the augmented motor-stimulant response that occurs with repeated, intermittent exposure to a specific drug, and is a long-lasting phenomenon. Behavioral sensitization to the locomotor activating effects of morphine can be demonstrated without the period of withdrawal (Norwood, Al-Chaer, & Fantegrossi, 2014), which we have also shown in our previous work in adult rats (Bajic, Soiza-Reilly, Spalding, Berde, & Commons, 2015). Interestingly, our behavioral findings do not support the hypothesis that the period of the first two weeks of life (PD1-14) is crucial to long-term behavioral sensitization to prolonged morphine exposure 1 week after completion of treatment (3 days after the last injection during weaning; Fig. 4). This finding is consistent with reports that repeated psychostimulant treatment does not produce long-term sensitization in young rats (Fujiwara, Kazahaya, Nakashima, Sato, & Otsuki, 1987; Kolta, Scalzo, Ali, & Holson, 1990; McDougall, Duke, Bolanos, & Crawford, 1994; Ujike, Tsuchida, Akiyama, Fujiwara, & Kuroda, 1995) suggesting that the critical period for behavioral sensitization may be a late-developing effect, one that occurs after the 3rd week of rat postnatal life and corresponds to the period of presynaptic dopamine autoreceptor formation in the rat brain (Hedner & Lundborg, 1985; Murrin, Gibbens, & Ferrer, 1985). This finding in rodent models is consistent with the generally held belief that prolonged psychostimulant administration does not increase the likelihood of current or future drug abuse among children and adolescents. (Klein, 1995; Levin & Kleber, 1995; Spencer et al., 1996; St Dennis & Synoground, 1996). In contrast, a study by McDougall et al. (McDougall, Collins, Karper, Watson, & Crawford, 1999) demonstrated that young rats are capable of exhibiting locomotor sensitization after an abstinence period from repeated administration of comparatively high doses of a potent dopamine re-uptake blocker. We also show that the locomotor activation 8 weeks following the treatment was not evident in spontaneous activity (Fig. 4). Furthermore, our preliminary data show that when exposed to repeated morphine administration in adulthood, animals show no difference in locomotor sensitization whether they were exposed to morphine at an early age (as per protocol described in this report) or not (Craig and Bajic, unpublished observations). Taken together, these conflicting data indicate that the occurrence and persistence of behavioral sensitization in young animals may depend both on the amount and the type of psychostimulant. Indeed, dopaminergic neurons of the ventral tegmental area (Bozarth & Wise, 1981; Joyce & Iversen, 1979; Kalivas & Stewart, 1991; Spanagel & Shippenberg, 1993; Vezina & Stewart, 1984) as well as their terminal field in the nucleus accumbens are critical for locomotor sensitization by morphine (see Reviews: (Koob & Le Moal, 2001; Steketee & Kalivas, 2011)). Future studies should aim to examine critical time periods in ontogeny of the mesocorticolimbic dopaminergic system known to be involved in reward and addiction of morphine (Bozarth & Wise, 1981).
Long-Term Effect on Novel-Object Recognition Testing
Modulation of learning and memory processes by morphine and other opioids was previously demonstrated in adult animals (Bodnar & Klein, 2005; Canli, Cook, & Miczek, 1990). We used a Novel-Object Recognition test as a useful tool for assessing the behavioral and neural processes mediating storage and subsequent recall of the features that compromise the familiar object (Berlyne et al., 1966) under normal, low stress environmental conditions (Ennaceur & Delacour, 1988). Calculated discrimination ratio following Novel Object Recognition testing revealed that although compromised in the young age (Fig. 5A′), novel object recognition showed no differences in adulthood between morphine treated and control animals (Fig. 5B′). It was reported that chronic exposure to opioids in adult rats result in a residual long-term performance impairment on a memory task (Sala et al., 1994; Spain & Newsom, 1991). Furthermore, since spontaneous morphine withdrawal gives rise to memory impairment, the test has been reported to elucidate memory deficits in morphine withdrawal settings in the adult rodents (Mesripour, Hajhashemi, & Rabbani, 2008; Rabbani, Hajhashemi, & Mesripour, 2009; Vaseghi, Rabbani, & Hajhashemi, 2013). Our results are novel in assessing the long-term effects of prolonged postnatal morphine exposure in the absence of physical signs of withdrawal. These results suggest that possible neuronal maladaptations related to a novel object recognition at an early age are normalized by adulthood. This lack of discrimination at the younger age is unlikely to be explained by factors such as differences in locomotor efficiency (Fig. 4; locomotor activity test). However, future studies should include analysis of parametrically varying the retention interval being that a long retention interval may increase the subjective similarity between the stimuli (King, Jones, Pearlman, Tishman, & Felix, 2002). This should be evaluated along the stress reactivity (e.g. forced swim test) considering we evaluated physical and not affective signs of withdrawal during our weaning period. Finally, future behavioral studies (e.g. radial arm maze; Morris water maze; fear conditioning, etc.) should be done to strengthen presented results that are limited to non-spatial working memory.
Translational Aspects and Significance
The association of rat and human developmental stages depends upon several endpoints such as the number of brain cells, degree of myelination, brain growth rate, synaptogenesis, as well as measures related to more contemporary neuroinformatics (Clancy et al., 2001; Clancy et al., 2007). In rodents, this critical period of neuronal differentiation and synaptic development is limited to a time window up to a fourth postnatal week (PD1-28) (De Felipe, Marco, Fairen, & Jones, 1997; Micheva & Beaulieu, 1996, 1997). In humans, described brain growth spurt characterized with synaptogenesis and accompanied by dendritic and axonal growth, as well as myelination of the subcortical white matter extends from the last trimester of pregnancy up to the first few years of postnatal life (Huttenlocher & Dabholkar, 1997). In fact, the newborn rat model at PD7 and PD14 has been extensively used in relation to early (premature, neonatal, and infant) and childhood development in humans, respectively (McCann, Bellinger, Davidson, & Soriano, 2009; Zhang & Sweitzer, 2008).
Consensus statements on the analgesic treatment of neonatal pain and discomfort suggested the use of prolonged opioid treatment for preterm and term neonates undergoing ventilatory support (Anand et al., 2006). However, our understanding of such treatment in the developing brain on the possible long-term sequelae is limited. Results from the recent Neurological Outcomes and Preemptive Analgesia in Neonate (NEOPAN) trial (Ferguson et al., 2012) are strongly suggestive of long-lasting negative effects. Specifically, body weight (similar to our current animal study) and head circumference were still decreased in the morphine treated group at 5–7 years of age. Morphine-treated children had more social problems and exhibited increased latencies to choice responses in the short-term memory task. Future studies are needed to translate significance of a Novel-Object Recognition test used in a rat model (Fig. 5) to findings in NEOPAN study cohort of former preterm infants. Unfortunately, no clinical studies are available to provide insight into mechanical/thermal pain perception, addictive potential, or stress coping in the older age.
CONCLUSIONS
Administration of opioids for treatment of acute pain, as well as prolonged sedation of newborns and infants is considered standard clinical care. Since the treatment is often associated with a high incidence of opioid tolerance and dependence (Anand et al., 2010), it is necessary to investigate the possible long-term influence of prolonged morphine administration during this early developmental period. Clinical observations of possible long-term effects of prolonged morphine treatment in infants are informed by the use of animal models that permit analysis of the potentially neurotoxic effects, the importance of age, and the underlying mechanisms, while controlling for handling and maternal separation. Using a rat model of prolonged postnatal morphine exposure, our novel behavioral results demonstrate selective long-term neuroplastic differences in thermal but not mechanical sensory processing. Importantly, our results also suggest a lack of long-term alterations on drug reward/reinforcement behavior, affective processing, and novel object recognition. Future studies are needed to address the potential neuroplastic alterations at the molecular, cellular and/or brain networks levels, as well as the translational significance of the animal model findings to clinical sequelae in humans.
Acknowledgments
Authors would like to acknowledge Drs. Kathryn G. Commons and Nick Andrews for providing the equipment and instrumentation to complete the study. This work was supported by the NIH K08 DA035972-01 and Trailblazer Award from Department of Anesthesiology, Perioperative and Pain Medicine, Boston Children’s Hospital (D.B.). The content of the article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- g
gram
- PD
postnatal day
- sc
subcutaneous
Footnotes
Neither of the authors have any conflict of interest, including specific financial interests, relationships or affiliations relevant to the manuscript.
References
- Allen JW, Yaksh TL. Assessment of acute thermal nociception in rats. In: Luo DZ, editor. Pain Research: Methods and Protocols. Vol. 99. Totowa, New Jersey: Humana Press; 2004. pp. 11–24. (Methods in Molecular Medicine). [DOI] [PubMed] [Google Scholar]
- Anand KJ. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155(2):173–180. doi: 10.1001/archpedi.155.2.173. doi: poa00293 [pii] [DOI] [PubMed] [Google Scholar]
- Anand KJ, Aranda JV, Berde CB, Buckman S, Capparelli EV, Carlo W, Walco GA. Summary proceedings from the neonatal pain-control group. Pediatrics. 2006;117(3 Pt 2):S9–S22. doi: 10.1542/peds.2005-0620C. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317(21):1321–1329. doi: 10.1056/NEJM198711193172105. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate. 2000;77(2):69–82. doi: 10.1159/000014197. doi: 14197 [pii] [DOI] [PubMed] [Google Scholar]
- Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J, Nicholson C. Tolerance and Withdrawal From Prolonged Opioid Use in Critically Ill Children. Pediatrics. 2010;125:1208–1225. doi: 10.1542/peds.2009-0489. doi: peds.2009-0489 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajic D, Berde CB, Commons KG. Periaqueductal gray neuroplasticity following chronic morphine varies with age: role of oxidative stress. Neuroscience. 2012;226:165–177. doi: 10.1016/j.neuroscience.2012.09.028. doi: 10.1016/j.neuroscience.2012.09.028 S0306-4522(12)00936-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajic D, Commons KG, Soriano SG. Morphine-enhanced apoptosis in selective brain regions of neonatal rats. Int J Dev Neurosci. 2013;31(4):258–266. doi: 10.1016/j.ijdevneu.2013.02.009. doi: 10.1016/j.ijdevneu.2013.02.009 S0736-5748(13)00036-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajic D, Soiza-Reilly M, Spalding AL, Berde CB, Commons KG. Endogenous cholinergic neurotransmission contributes to behavioral sensitization to morphine. PloS One. 2015;10(2):e0117601. doi: 10.1371/journal.pone.0117601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci, Chapter. 2007 doi: 10.1002/0471142301.ns0809s41. Chapter 8, Unit 8 9. [DOI] [PubMed] [Google Scholar]
- Barr GA, Wang S. Analgesia induced by localized injection of opiate peptides into the brain of infant rats. Eur J Pain. 2013;17(5):676–691. doi: 10.1002/j.1532-2149.2012.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beland B, Fitzgerald M. Mu- and delta-opioid receptors are downregulated in the largest diameter primary sensory neurons during postnatal development in rats. Pain. 2001;90(1–2):143–150. doi: 10.1016/s0304-3959(00)00397-3. doi: S0304-3959(00)00397-3 [pii] [DOI] [PubMed] [Google Scholar]
- Bellu R, de Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database Syst Rev. 2008;(1):CD004212. doi: 10.1002/14651858.CD004212.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellu R, de Waal K, Zanini R. Opioids for neonates receiving mechanical ventilation: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2010;95(4):F241–251. doi: 10.1136/adc.2008.150318. doi: 10.1136/adc.2008.150318 adc.2008.150318 [pii] [DOI] [PubMed] [Google Scholar]
- Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N Engl J Med. 2002;347(14):1094–1103. doi: 10.1056/NEJMra012626. doi: 10.1056/NEJMra012626 347/14/1094 [pii] [DOI] [PubMed] [Google Scholar]
- Berlyne DE, Koenig ID, Hirota T. Novelty, arousal, and the reinforcement of diversive exploration in the rat. J Comp Physiol Psychol. 1966;62(2):222–226. doi: 10.1037/h0023681. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1(3):1306–1311. doi: 10.1038/nprot.2006.205. doi: nprot.2006.205 [pii] [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Klein GE. Endogenous opiates and behavior: 2004. Peptides. 2005;26(12):2629–2711. doi: 10.1016/j.peptides.2005.06.010. doi: S0196-9781(05)00305-0 [pii] [DOI] [PubMed] [Google Scholar]
- Booze RM, Mactutus CF. Experimental design considerations: a determinant of acute neonatal toxicity. Teratology. 1985;31(2):187–191. doi: 10.1002/tera.1420310203. [DOI] [PubMed] [Google Scholar]
- Borsini F, Lecci A, Sessarego A, Frassine R, Meli A. Discovery of antidepressant activity by forced swimming test may depend on pre-exposure of rats to a stressful situation. Psychopharmacology (Berl) 1989;97(2):183–188. doi: 10.1007/BF00442247. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 1988;94(2):147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28(5):551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Miller SP. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71(3):385–396. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Cook RG, Miczek KA. Opiate antagonists enhance the working memory of rats in the radial maze. Pharmacol Biochem Behav. 1990;36(3):521–525. doi: 10.1016/0091-3057(90)90250-l. doi: 0091-3057(90)90250-L [pii] [DOI] [PubMed] [Google Scholar]
- Chambliss CR, Anand KJ. Pain management in the pediatric intensive care unit. Curr Opin Pediatr. 1997;9(3):246–253. doi: 10.1097/00008480-199706000-00011. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105(1):7–17. doi: 10.1016/s0306-4522(01)00171-3. doi: S0306-4522(01)00171-3 [pii] [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28(5):931–937. doi: 10.1016/j.neuro.2007.01.014. doi: S0161-813X(07)00033-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Phillips MA, Constantine-Paton M, Kaila K, Jasanoff A. Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat Neurosci. 2008;11(1):72–79. doi: 10.1038/nn2017. doi: nn2017 [pii] [DOI] [PubMed] [Google Scholar]
- Craig KD, Whitfield MF, Grunau RV, Linton J, Hadjistavropoulos HD. Pain in the preterm neonate: behavioural and physiological indices. Pain. 1993;52(3):287–299. doi: 10.1016/0304-3959(93)90162-I. [DOI] [PubMed] [Google Scholar]
- De Felipe J, Marco P, Fairen A, Jones EG. Inhibitory synaptogenesis in mouse somatosensory cortex. Cereb Cortex. 1997;7(7):619–634. doi: 10.1093/cercor/7.7.619. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121(1):66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. doi: 0166-4328(88)90157-X [pii] [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Ward WL, Paule MG, Hall RW, Anand KJ. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol Teratol. 2012;34(1):47–55. doi: 10.1016/j.ntt.2011.10.008. doi: 10.1016/j.ntt.2011.10.008 S0892-0362(11)00207-8 [pii] [DOI] [PubMed] [Google Scholar]
- Festing MF. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47(1):5–14. doi: 10.1093/ilar.47.1.5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The developmental neurobiology of pain. In: Bond MR, Charlton JE, Woolf CJ, editors. Proceedings of teh 6th World Congress on Pain, Pain Research and Clinical Management. Amsterdam: Elsevier; 1991. pp. 253–262. [Google Scholar]
- Fitzgerald M, Butcher T, Shortland P. Developmental changes in the laminar termination of A fibre cutaneous sensory afferents in the rat spinal cord dorsal horn. J Comp Neurol. 1994;348(2):225–233. doi: 10.1002/cne.903480205. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proc Natl Acad Sci U S A. 1999;96(14):7719–7722. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Res. 1986;389(1–2):261–270. doi: 10.1016/0165-3806(86)90194-x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain. 1989;39(1):31–36. doi: 10.1016/0304-3959(89)90172-3. doi: 0304-3959(89)90172-3 [pii] [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5(1):35–50. doi: 10.1038/ncpneuro0984. doi: 10.1038/ncpneuro0984 ncpneuro0984 [pii] [DOI] [PubMed] [Google Scholar]
- Fonsmark L, Rasmussen YH, Carl P. Occurrence of withdrawal in critically ill sedated children. Crit Care Med. 1999;27(1):196–199. doi: 10.1097/00003246-199901000-00052. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Kazahaya Y, Nakashima M, Sato M, Otsuki S. Behavioral sensitization to methamphetamine in the rat: an ontogenic study. Psychopharmacology (Berl) 1987;91(3):316–319. doi: 10.1007/BF00518183. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205(3):536–546. [PubMed] [Google Scholar]
- Georges F, Normand E, Bloch B, Le Moine C. Opioid receptor gene expression in the rat brain during ontogeny, with special reference to the mesostriatal system: an in situ hybridization study. Brain Res Dev Brain Res. 1998;109(2):187–199. doi: 10.1016/s0165-3806(98)00082-0. [DOI] [PubMed] [Google Scholar]
- Grunau RV, Whitfield MF, Petrie JH, Fryer EL. Early pain experience, child and family factors, as precursors of somatization: a prospective study of extremely premature and fullterm children. Pain. 1994;56(3):353–359. doi: 10.1016/0304-3959(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Guinsburg R, Kopelman BI, Anand KJ, de Almeida MF, Peres Cde A, Miyoshi MH. Physiological, hormonal, and behavioral responses to a single fentanyl dose in intubated and ventilated preterm neonates. J Pediatr. 1998;132(6):954–959. doi: 10.1016/s0022-3476(98)70390-7. doi: S0022347698002571 [pii] [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem-spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol. 2009;587(Pt 12):2927–2935. doi: 10.1113/jphysiol.2008.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner T, Lundborg P. Development of dopamine autoreceptors in the postnatal rat brain. J Neural Transm. 1985;62(1–2):53–63. doi: 10.1007/BF01260415. [DOI] [PubMed] [Google Scholar]
- Hulley SB, Cummings SR, Browner WS, Grady D, Hearst N, Newman TB. Designing Clinical Research. 2nd. Lippincott Williams & Wilkins; 2001. Estimating Sample Size and Power; p. 85. [Google Scholar]
- Hunt RW, Tzioumi D, Collins E, Jeffery HE. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev. 2008;84(1):29–35. doi: 10.1016/j.earlhumdev.2007.01.013. doi: S0378-3782(07)00027-8 [pii] [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. doi: 10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z [pii] [DOI] [PubMed] [Google Scholar]
- Jennings E, Fitzgerald M. Postnatal changes in responses of rat dorsal horn cells to afferent stimulation: a fibre-induced sensitization. J Physiol. 1998;509(Pt 3):859–868. doi: 10.1111/j.1469-7793.1998.859bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics. 1996;98(5):925–930. [PubMed] [Google Scholar]
- Johnston CC, Stevens BJ, Yang F, Horton L. Differential response to pain by very premature neonates. Pain. 1995;61(3):471–479. doi: 10.1016/0304-3959(94)00213-X. doi: 030439599400213X [pii] [DOI] [PubMed] [Google Scholar]
- Johnston CC, Stevens B, Yang F, Horton L. Developmental changes in response to heelstick in preterm infants: a prospective cohort study. Dev Med Child Neurol. 1996;38(5):438–445. doi: 10.1111/j.1469-8749.1996.tb15101.x. [DOI] [PubMed] [Google Scholar]
- Jones KL, Barr GA. Ontogeny of morphine withdrawal in the rat. Behav Neurosci. 1995;109(6):1189–1198. doi: 10.1037//0735-7044.109.6.1189. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Iversen SD. The effect of morphine applied locally to mesencephalic dopamine cell bodies on spontaneous motor activity in the rat. Neurosci Lett. 1979;14(2–3):207–212. doi: 10.1016/0304-3940(79)96149-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16(3):223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit Care Med. 1994;22(5):763–767. doi: 10.1097/00003246-199405000-00009. [DOI] [PubMed] [Google Scholar]
- Kent JL, Pert CB, Herkenham M. Ontogeny of opiate receptors in rat forebrain: visualization by in vitro autoradiography. Brain Res. 1981;254(4):487–504. doi: 10.1016/0165-3806(81)90018-3. [DOI] [PubMed] [Google Scholar]
- King DL, Jones FL, Pearlman RC, Tishman A, Felix CA. The length of the retention interval, forgetting, and subjective similarity. J Exp Psychol Learn Mem Cogn. 2002;28(4):660–671. [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1995;682(1–2):189–196. doi: 10.1016/0006-8993(95)00349-u. doi: 0006-8993(95)00349-U [pii] [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760(1–2):218–230. doi: 10.1016/s0006-8993(97)00287-4. doi: S0006-8993(97)00287-4 [pii] [DOI] [PubMed] [Google Scholar]
- Kirby LG, Lucki I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J Pharmacol Exp Ther. 1997;282(2):967–976. [PubMed] [Google Scholar]
- Kivell BM, Day DJ, McDonald FJ, Miller JH. Developmental expression of mu and delta opioid receptors in the rat brainstem: evidence for a postnatal switch in mu isoform expression. Brain Res Dev Brain Res. 2004;148(2):185–196. doi: 10.1016/j.devbrainres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Klausz B, Pinter O, Sobor M, Gyarmati Z, Furst Z, Timar J, Zelena D. Changes in adaptability following perinatal morphine exposure in juvenile and adult rats. Eur J Pharmacol. 2011;654(2):166–172. doi: 10.1016/j.ejphar.2010.11.025. doi: 10.1016/j.ejphar.2010.11.025 S0014-2999(10)01195-7 [pii] [DOI] [PubMed] [Google Scholar]
- Klein RG. The role of methylphenidate in psychiatry. Arch Gen Psychiatry. 1995;52(6):429–433. [PubMed] [Google Scholar]
- Kolta MG, Scalzo FM, Ali SF, Holson RR. Ontogeny of the enhanced behavioral response to amphetamine in amphetamine-pretreated rats. Psychopharmacology (Berl) 1990;100(3):377–382. doi: 10.1007/BF02244610. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lane DA, Morgan MM. Antinociceptive tolerance to morphine from repeated nociceptive testing in the rat. Brain Res. 2005;1047(1):65–71. doi: 10.1016/j.brainres.2005.04.001. doi: S0006-8993(05)00517-2 [pii] [DOI] [PubMed] [Google Scholar]
- Lee SJ, Ralston HJ, Drey EA, Partridge JC, Rosen MA. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294(8):947–954. doi: 10.1001/jama.294.8.947. doi: 294/8/947 [pii] [DOI] [PubMed] [Google Scholar]
- Levin FR, Kleber HD. Attention-deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harv Rev Psychiatry. 1995;2(5):246–258. doi: 10.3109/10673229509017144. [DOI] [PubMed] [Google Scholar]
- Luis-Delgado OE, Barrot M, Rodeau JL, Schott G, Benbouzid M, Poisbeau P, Lasbennes F. Calibrated forceps: a sensitive and reliable tool for pain and analgesia studies. J Pain. 2006;7(1):32–39. doi: 10.1016/j.jpain.2005.07.011. doi: S1526-5900(05)00827-8 [pii] [DOI] [PubMed] [Google Scholar]
- McCann ME, Bellinger DC, Davidson AJ, Soriano SG. Clinical research approaches to studying pediatric anesthetic neurotoxicity. Neurotoxicology. 2009;30(5):766–771. doi: 10.1016/j.neuro.2009.02.013. doi: S0161-813X(09)00051-5 [pii] [DOI] [PubMed] [Google Scholar]
- McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA. Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Exp Clin Psychopharmacol. 1999;7(3):208–218. doi: 10.1037//1064-1297.7.3.208. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Duke MA, Bolanos CA, Crawford CA. Ontogeny of behavioral sensitization in the rat: effects of direct and indirect dopamine agonists. Psychopharmacology (Berl) 1994;116(4):483–490. doi: 10.1007/BF02247482. [DOI] [PubMed] [Google Scholar]
- McGlone L, Mactier H, Weaver LT. Drug misuse in pregnancy: losing sight of the baby? Arch Dis Child. 2009;94(9):708–712. doi: 10.1136/adc.2008.156851. doi: adc.2008.156851 [pii] [DOI] [PubMed] [Google Scholar]
- Mesripour A, Hajhashemi V, Rabbani M. Metyrapone and mifepristone reverse recognition memory loss induced by spontaneous morphine withdrawal in mice. Basic Clin Pharmacol Toxicol. 2008;102(4):377–381. doi: 10.1111/j.1742-7843.2007.00183.x. doi: 10.1111/j.1742-7843.2007.00183.x PTO183 [pii] [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J Comp Neurol. 1996;373(3):340–354. doi: 10.1002/(SICI)1096-9861(19960923)373:3<340::AID-CNE3>3.0.CO;2-2. doi: 10.1002/(SICI)1096-9861(19960923)373:3<340::AID-CNE3>3.0.CO;2-2 [pii] [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Development and plasticity of the inhibitory neocortical circuitry with an emphasis on the rodent barrel field cortex: a review. Can J Physiol Pharmacol. 1997;75(5):470–478. [PubMed] [Google Scholar]
- Murrin LC, Gibbens DL, Ferrer JR. Ontogeny of dopamine, serotonin and spirodecanone receptors in rat forebrain–an autoradiographic study. Brain Res. 1985;355(1):91–109. doi: 10.1016/0165-3806(85)90009-4. [DOI] [PubMed] [Google Scholar]
- Nandi R, Beacham D, Middleton J, Koltzenburg M, Howard RF, Fitzgerald M. The functional expression of mu opioid receptors on sensory neurons is developmentally regulated; morphine analgesia is less selective in the neonate. Pain. 2004;111(1–2):38–50. doi: 10.1016/j.pain.2004.05.025. doi: 10.1016/j.pain.2004.05.025 S0304-3959(04)00272-6 [pii] [DOI] [PubMed] [Google Scholar]
- Nandi R, Fitzgerald M. Opioid analgesia in the newborn. Eur J Pain. 2005;9(2):105–108. doi: 10.1016/j.ejpain.2004.05.005. doi: S1090-3801(04)00087-4 [pii] [DOI] [PubMed] [Google Scholar]
- Norwood AP, Al-Chaer ED, Fantegrossi WE. Predisposing effects of neonatal visceral pain on abuse-related effects of morphine in adult male Sprague Dawley rats. Psychopharmacology (Berl) 2014;231(22):4281–4289. doi: 10.1007/s00213-014-3574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo P, Tavani A, Verotta D, Robson LE, Kosterlitz HW. Differential postnatal development of mu-, delta- and kappa-opioid binding sites in rat brain. Brain Res. 1987;428(1):53–58. doi: 10.1016/0165-3806(87)90082-4. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47(4):379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rabbani M, Hajhashemi V, Mesripour A. Increase in brain corticosterone concentration and recognition memory impairment following morphine withdrawal in mice. Stress. 2009;12(5):451–456. doi: 10.1080/10253890802659612. doi: 10.1080/10253890802659612 908610523 [pii] [DOI] [PubMed] [Google Scholar]
- Rahman W, Dashwood MR, Fitzgerald M, Aynsley-Green A, Dickenson AH. Postnatal development of multiple opioid receptors in the spinal cord and development of spinal morphine analgesia. Brain Res Dev Brain Res. 1998;108(1–2):239–254. doi: 10.1016/s0165-3806(98)00054-6. [DOI] [PubMed] [Google Scholar]
- Rahman W, Dickenson AH. Development of spinal opioid systems. Reg Anesth Pain Med. 1999;24(5):383–385. doi: 10.1016/s1098-7339(99)90001-9. doi: S1098-7339(99)90001-9 [pii] [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23(3):970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. doi: 23/3/970 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarenmaa E, Huttunen P, Leppaluoto J, Fellman V. Alfentanil as procedural pain relief in newborn infants. Arch Dis Child Fetal Neonatal Ed. 1996;75(2):F103–107. doi: 10.1136/fn.75.2.f103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Leone MP, Calcaterra P, Frattola D, Gori E. Chronic morphine affects working memory during treatment and withdrawal in rats: possible residual long-term impairment. Behav Pharmacol. 1994;5(6):570–580. doi: 10.1097/00008877-199410000-00002. [DOI] [PubMed] [Google Scholar]
- Shi M, Qi WJ, Gao G, Wang JY, Luo F. Increased thermal and mechanical nociceptive thresholds in rats with depressive-like behaviors. Brain Res. 2010;1353:225–233. doi: 10.1016/j.brainres.2010.07.023. doi: 10.1016/j.brainres.2010.07.023 S0006-8993(10)01585-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7(6):1009–1014. doi: 10.1038/nprot.2012.044. doi: 10.1038/nprot.2012.044 nprot.2012.044 [pii] [DOI] [PubMed] [Google Scholar]
- Spain JW, Newsom GC. Chronic opioids impair acquisition of both radial maze and Y-maze choice escape. Psychopharmacology (Berl) 1991;105(1):101–106. doi: 10.1007/BF02316870. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Shippenberg TS. Modulation of morphine-induced sensitization by endogenous kappa opioid systems in the rat. Neurosci Lett. 1993;153(2):232–236. doi: 10.1016/0304-3940(93)90329-j. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409–432. doi: 10.1097/00004583-199604000-00008. doi: S0890-8567(09)63512-7 [pii] [DOI] [PubMed] [Google Scholar]
- St Dennis C, Synoground G. Methylphenidate. J Sch Nurs. 1996;12(1):5–8. 10–11. [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63(2):348–365. doi: 10.1124/pr.109.001933. doi: 10.1124/pr.109.001933 pr.109.001933 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddio A, Katz J. The effects of early pain experience in neonates on pain responses in infancy and childhood. Paediatr Drugs. 2005;7(4):245–257. doi: 10.2165/00148581-200507040-00004. doi: 744 [pii] [DOI] [PubMed] [Google Scholar]
- Tong Y, Chabot JG, Shen SH, O’Dowd BF, George SR, Quirion R. Ontogenic profile of the expression of the mu opioid receptor gene in the rat telencephalon and diencephalon: an in situ hybridization study. J Chem Neuroanat. 2000;18(4):209–222. doi: 10.1016/s0891-0618(00)00043-0. [DOI] [PubMed] [Google Scholar]
- Ujike H, Tsuchida K, Akiyama K, Fujiwara Y, Kuroda S. Ontogeny of behavioral sensitization to cocaine. Pharmacol Biochem Behav. 1995;50(4):613–617. doi: 10.1016/0091-3057(94)00352-1. doi: 0091305794003521 [pii] [DOI] [PubMed] [Google Scholar]
- van Baar A, de Graaff BM. Cognitive development at preschool-age of infants of drug-dependent mothers. Dev Med Child Neurol. 1994;36(12):1063–1075. doi: 10.1111/j.1469-8749.1994.tb11809.x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Frenk H. The development of stimulation-produced analgesia (SPA) in the rat. Brain Res Dev Brain Res. 1991;64(1–2):71–76. doi: 10.1016/0165-3806(91)90210-a. [DOI] [PubMed] [Google Scholar]
- Vaseghi G, Rabbani M, Hajhashemi V. The effect of AM281, a cannabinoid antagonist, on memory performance during spontaneous morphine withdrawal in mice. Res Pharm Sci. 2013;8(1):59–64. [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Giovino AA, Wise RA, Stewart J. Environment-specific cross-sensitization between the locomotor activating effects of morphine and amphetamine. Pharmacol Biochem Behav. 1989;32(2):581–584. doi: 10.1016/0091-3057(89)90201-3. doi: 0091-3057(89)90201-3 [pii] [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Conditioning and place-specific sensitization of increases in activity induced by morphine in the VTA. Pharmacol Biochem Behav. 1984;20(6):925–934. doi: 10.1016/0091-3057(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Vinall J, Miller SP, Chau V, Brummelte S, Synnes AR, Grunau RE. Neonatal pain in relation to postnatal growth in infants born very preterm. Pain. 2012;153(7):1374–1381. doi: 10.1016/j.pain.2012.02.007. doi: 10.1016/j.pain.2012.02.007 S0304-3959(12)00082-6 [pii] [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Chen Y, Leslie FM. Expression of opioid peptides and receptors in striatum and substantia nigra during rat brain development. J Chem Neuroanat. 2003;26(1):17–36. doi: 10.1016/s0891-0618(03)00031-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Zhang GH, Sweitzer SM. Neonatal morphine enhances nociception and decreases analgesia in young rats. Brain Res. 2008;1199:82–90. doi: 10.1016/j.brainres.2007.12.043. doi: 10.1016/j.brainres.2007.12.043 S0006-8993(07)03072-7 [pii] [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. Inhibition of morphine withdrawal by the NMDA receptor antagonist MK-801 in rat is age-dependent. Synapse. 2001a;40(4):282–293. doi: 10.1002/syn.1051. doi: 10.1002/syn.1051 [pii] [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. Opiate withdrawal during development: are NMDA receptors indispensable? Trends Pharmacol Sci. 2001b;22(8):404–408. doi: 10.1016/s0165-6147(00)01792-2. doi: S0165-6147(00)01792-2 [pii] [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. Ontogeny of NMDA receptor-mediated morphine tolerance in the postnatal rat. Pain. 2003;104(3):437–447. doi: 10.1016/S0304-3959(03)00051-4. doi: S0304395903000514 [pii] [DOI] [PubMed] [Google Scholar]


