Abstract
Magnetic nanoparticles (MNPs) have been used as effective vehicles for targeted delivery of theranostic agents in the brain. The advantage of magnetic targeting lies in the ability to control the concentration and distribution of therapy to a desired target region using external driving magnets. In this work, we investigated the behavior and safety of MNP motion in brain tissue. We found that MNPs move and, form nanoparticle chains in the presence of a uniform magnetic field, and that this chaining is influenced by the applied magnetic field intensity and the concentration of MNPs in the tissue. Using electrophysiology recordings, imaging and immunohistochemistry, we assessed the functional health of neurons and neural circuits and found no adverse effects associated with MNP motion through brain tissue.
Keywords: Magnetic nanoparticles, transport, drug delivery, brain, safety
Graphical Abstract
We studied the mechanisms of magnetic field induced motion of magnetic nanoparticles in brain tissue. In addition, we determined the safety of moving magnetic nanoparticles in brain using electrophysiological recordings, calcium imaging and immunohistochemistry.
Background
Nanotechnology based solutions for the treatment of brain tumors have been used in recent years to address the challenges faced by conventional cancer therapeutics1 such as surgery2,3, chemotherapy4,5,6 and radiation therapy7,8. Drugs such as doxyrubicin9 and oxantrazole10 can be combined with appropriate nanocarriers to penetrate the blood brain barrier (BBB) and increase the intracellular concentration of drugs in tumor cells11,12,13. Magnetic nanoparticles (MNPs) have been investigated as effective nanocarriers for targeted drug delivery in the brain14,15,16,17. Such MNPs, with attached drugs, proteins, or genes, could be imaged using MRI technology and guided towards brain tumor locations using external magnets.
MNPs with an aminosilane coating have been investigated in human trials for targeting glioblastoma multiforme cells and have been shown not to cause any adverse effects in patients. In the presence of an alternating magnetic field, the MNPs were found to extend tumor necrosis with minor or no side effects in the patients17. Hassan and Gallo showed that after a systemic injection of magnetic chitosan microspheres coated with oxantrazole, while in the presence of a 0.6 T magnetic field, the guided microspheres accumulated in targeted rat brain tissue10. Thus MNPs have been shown to cross the BBB and reach targets in brain tissue without disrupting the barrier in rat models15,18.
Furthermore, endothelial progenitor cells (EPCs) from humans have been loaded with MNPs and guided to targets in mouse brains19. These EPCs loaded with MNPs have shown increase in secretion and migration of growth factors such as VEGF and FGF, in vitro, thereby promoting angiogenesis for neural regeneration. Various in vitro studies have shown that cancer cells can be made to internalize a higher level of nanoparticles with drugs by appropriate targeting of receptors20,21,22,23. The MNPs, therefore, can be used as a potential option to circumvent the challenges faced by conventional drug delivery techniques.
Most of the work mentioned above has focused on the motion of MNPs through blood vessels and the observation of MNP presence in living tissue15,24,25. The motion of MNPs in brain tissue surrounding the blood vessels is expected to differ from its motion in the vessels. Hence there is a need for a better understanding of the motion of MNPs in brain tissue after extravasating from blood vessels. The MNP motion in tissue should be safe. It must not be cytotoxic, nor should it affect the normal function of the intricate neural networks in the brain so as to eliminate the possibility of permanent side effects in the brain following the delivery of drugs using MNPs. Addressing these needs will result in better nanotherapeutic schemes to target tumors in brain tissue.
In the current work, we have studied the movement of magnetic nanoparticles (MNPs) in brain tissue under an applied magnetic field. The movement of MNPs throughout this work includes the interactive motion of MNPs towards each other caused by the influence of an external magnetic field. The mechanisms of this MNP motion and the primary factors that impact this motion have been explored. We found that the motion of MNPs did not cause any detrimental effects on the functional health of the neurons or the circuit function in the main olfactory bulb, a well studied region in the brain. We examined the functional safety aspects of MNP motion by using whole-cell patch recordings, imaging and immunohistochemistry in the main olfactory bulb.
Methods
Characterization of magnetic nanoparticles
The physical properties (mean hydrodynamic diameter, polydispersity index) of MNPs (nano-screenMag, Chemicell, listed as 300 nm diameter) used in our experiments were determined using dynamic light scattering. The MNPs were required to be monodispersed to avoid non-uniformity in their motion in the tissue caused by particle size variations. For the dynamic light scattering measurements, the stock concentration of MNPs (25 mg/mL in double distilled water) was diluted with de-ionized water to a concentration of 0.25 mg/mL. Three samples of 3 mL of the diluted solution were used for the measurement assays. The particle size distribution curve was plotted for these samples and used to calculate the polydispersity index (Figure 1A in Supplementary materials).
The magnetic properties of the nanoparticles including magnetic susceptibility and saturation magnetization were measured using a vibrating sample magnetometer (Lake Shore Cryotronics Inc.). Sample volumes of 60 μL of MNPs in DI water were pipetted into the sample holder (Kel-F) and the holder was placed in the vibrating sample magnetometer setup. The experiments were performed at room temperature (298 K). The samples were exposed to a cycle of different magnetic field values in the range of −1.5 to +1.5 Tesla and the corresponding net magnetization produced in the samples were recorded. The magnetic properties (susceptibility and saturation magnetization) of the samples were then calculated from the magnetization versus magnetic field (M vs H) plot obtained from the vibrating sample magnetometer (Figure 1B in Supplementary materials).
Uniform magnetic field using a two magnet setup
A system was created to apply a uniform magnetic field to magnetic nanoparticles inside brain tissue slices. A uniform magnetic field was desired so that all MNPs in the tissue would experience the same magnetic field irrespective of their location in the tissue. Two permanent magnets, appropriately sized and placed as shown in Figure 1A, were sufficient to create a uniform magnetic field. The uniformity of the field was verified by a 3-channel Gaussmeter (Lake Shore Inc.) mounted on a piezo positioning stage (VXM Motor Inc.). The Gaussmeter measured the spatial distribution of the magnetic field intensity between the two magnets and it was found that the deviation from the mean magnetic field intensity in the tissue sample volume was less than 1%. This data is displayed in Figure 2 in the Supplementary Materials.
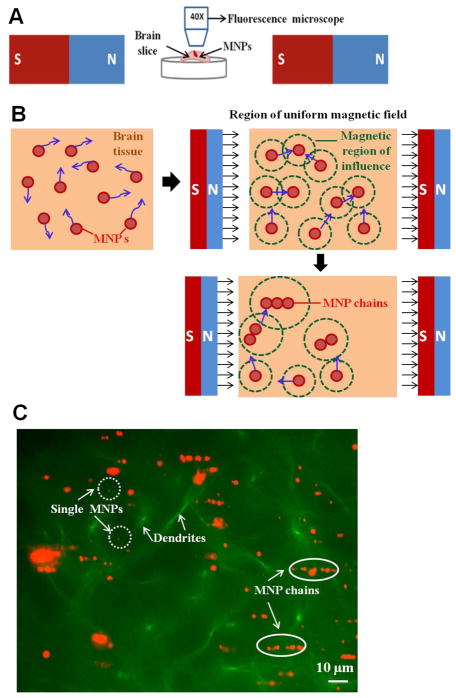
Figure 1.
(A) The diagram of two-magnet setup used to study movement of MNPs in brain tissue. The tissue loaded with MNPs was mounted and visualized under a fluorescence microscope after exposing it to the uniform magnetic field (B) An illustration of how MNPs behave in brain tissue with and without an applied uniform magnetic field. The MNPs diffuse in different directions (blue arrows) in the absence of a uniform magnetic field (left, top). After the introduction of the magnetic field, the MNPs move towards each other due to an overlap of induced magnetic fields of influence (green circles). As a result, the MNPs form chains as they move towards each other and longer chains have a larger field of influence which recruits additional particles to the chain (bottom). (C) Chaining of MNPs experimentally observed in mouse brain tissue (pre-frontal cortex region) in the presence of a uniform magnetic field. The MNP chains (orange) and the barely-visible single MNPs are marked by white ovals and white dotted circles respectively. The dendrites (green) in the tissue are indicated by white arrows.
Motion experiments of MNPs in the brain tissue
The motion of MNPs towards each other under the influence of an applied uniform magnetic field was studied in rat brain tissue using a total of 12 rats (Sprague Dawley). Each different motion experiment was repeated three times using tissue from different rats to ensure that the data was independent of animal to animal variability. The rat brains were dissected out and immediately stored at 4°C in 1X Phosphate Buffer Saline (PBS) solution to increase their viability. After 15 minutes, the brains were injected in the prefrontal cortex with 4 μL of the MNPs, using a 10 μL micro-syringe (Hamilton). Following this injection we obtained cortical slices using a razor blade. The slicing was facilitated by the low temperature storage of the brain samples. The slices containing the injected MNPs were then stabilized at room temperature in 1X PBS solution in a petri dish. The MNPs were visualized by fluorescence using a lipophilic dye coating (Texas Red, Chemicell) with excitation and emission wavelengths of 578 nm and 613 nm respectively. The petri dish containing the brain tissue, immersed in PBS, was placed in the uniform magnetic field region of the two magnet setup. The effect of the uniform magnetic field on the MNPs in the brain tissue was observed using a fluorescence microscope (Zeiss) with 40X magnification and recorded using a video camera (Hamamatsu). The videos were post-processed in MATLAB (Mathworks) to quantify the movement of the MNPs in the uniform magnetic field.
Functional health experiments
All animal studies were conducted in accordance with the policies and recommendations of the National Institute of Health Guide for the Care and Use of Laboratory Animals, and under approval from the Institutional Animal Care and Use Committee of the University of Maryland. The functional health experiments were performed in brain slices extracted from wild-type BL6/C57 mice (Jackson Labs), or 4–6-week-old transgenic mice expressing green fluorescent protein (GFP) and subjected to MNP motion. Specifically, we used the ChAT-Tau-GFP line, generously provided by Dr. Sukumar Vijayaraghavan 26. We performed these electrophysiology experiments in mice because of the feasibility of transgenic modification in a mouse model compared to a rat model. All the functional experiments involved whole-cell patching of neurons in an electrophysiology setup. The transgenic modification of mice enabled us to visualize the GFP expressing neurons in the presence of MNPs around them using a fluorescence microscope with multiple wavelength filters. Neurons from at least 5 different brain tissue slices were used for the studies. The animals were anaesthetized with isofluorane and decapitated. The whole brain was removed and immediately placed in ice-cold oxygenated artificial cerebrospinal fluid (ACSF). The ACSF used for the experiments contained the following composition (in mM) : 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3 KCl, 2 CaCl2, 1 MgCl2, 3 myo-inositol, 0.3 ascorbic acid, 2 Na-pyruvate, and 15 glucose. The solution was maintained at a constant pH of 7.4 and osmolarity of ~350 mOsm by continuous oxygenation (95% O2-5% CO2). A block of the extracted tissue, containing the olfactory bulb, was glued to a stage with cyanoacrylate and bathed in ice-cold low Ca2+, high Mg2+ ACSF. Sagittal brain sections (250–300 μm), containing the olfactory bulb were sliced using a vibratome slicer (Leica). The slices were held at 34 °C for 30 minutes and then at room temperature to recuperate.
The slices were then transferred to a petri dish and the MNPs were injected into the slices using a glass micro-pipette (≈5 μm diameter) attached to a micro injection system (Toohey spritzer). The MNPs in the brain slice were visualized using a fluorescence microscope and the two magnet setup was introduced for 5 minutes to produce MNP motion and chaining. Then the two magnet system was rotated by 90° to produce motion of MNPs in a perpendicular direction to ensure that the functional safety of neurons did not depend on the direction of MNP movement. The slices were then placed in the electrophysiology recording chamber mounted on the stage of an upright fluorescence microscope (Zeiss) and the region of the tissue containing MNPs was identified using fluorescence. Then neurons in that region were patched for electrophysiology recordings. The recordings were carried out in current-clamp and voltage-clamp mode using standard patch pipettes (3–7 MΩ resistance) pulled on a horizontal puller (Sutter). To further assess neuronal integrity and viability in slices loaded with MNPs, after the application of a magnetic field, we included the fluorescent dye Alexa-Fluor 488 (10 μM, Life Technologies) in the recording pipette solution. Data was acquired using a dual EPC10 amplifier (HEKA) and analyzed offline using the IgorPro software (Wavemetrics). We conducted control experiments in slices obtained from the same brain but not injected with MNPs or injected with the MNPs but not subjected to the magnetic field.
Calcium imaging
Following the post-slicing recuperation period, slices were transferred to a 30 mm Milicell culture dish insert (Millipore Corp, Billerica, Ma) containing 5 mL of normal oxygenated ACSF with 5 μM freshly prepared Fluo-4 AM Pluronic Acid F-127 20% solution in DMSO (Molecular Probes, Life Technologies). Slices were submerged in the dye for 20 minutes then transferred to a submerged recording chamber mounted on the stage of an Olympus BX51 microscope for acquisition.
We visualized labeled slices using epifluorescence illumination and a 40X water immersion objective. Illumination was achieved using an OPTOLED green LED (exciter 488 nm center wavelength, Chroma; Cairn Research LTD), emitted light was collected by an ORCA-Flash4.0 V2 sCMOS camera (Hamamatsu), and images were recorded using the HCimage software (Hamamatsu). Imaging analysis was performed offline using the ImageJ and IgorPro (Wavemetrics) softwares. (S)-1-Aminopropane-1,3-dicarboxylic acid (Glutamate) was prepared from a stock solution and added to the bathing solution. The calcium indicator, Fluo-4 AM (Molecular Probes, Life Technologies), was excited at a wavelength of ~490 nm and the resulting emission detected at ~520 nm. The optical recording data is shown as the ratio of the change in fluorescence caused by glutamate in cells after 60 seconds to the baseline fluorescence (Δf/f0) for the indicated regions of interest.
Immunohistochemistry
The ex-vivo brain tissue slices from ChAT-tau-GFP mice were analyzed using immunohistochemistry after magnetic field induced MNP motion. The nerve fibers in the slices were visualized using anti-GFP immunostaining to assess any damage caused due to MNP movement. The slices were extracted as above, injected with MNPs in the main olfactory bulb, and exposed to a uniform magnetic field in two different directions as described in the previous section. The slices were then fixed in 4% paraformaldehyde for 5 minutes, transferred to saline solution at 4° C, and then quickly washed with 1X PBS for 2 minutes. The slices were then incubated with the blocker (10% Donkey serum in PBS-T) for 1 hour, followed by incubation with the primary antibody in 2.5% Donkey serum in PBS-T overnight at room temperature. The slices were then washed once in PBS-T and then 7X for 5 minutes each in PBS-T and incubated in the secondary Alexa-488 antibody solution (1:750 concentration) for 2 hours at room temperature. The slices were washed 3X for 5 minutes in PBS-T, then further rinsed 3X for 5 minutes each in PBS. At this point, immunostained slices were visualized using confocal microscopy with appropriate fluorescence filters for the MNPs and the GFP-stained fibers.
Results
The MNPs were analyzed using dynamic light scattering to calculate the particle size distribution and the extent of polydispersity. The mean hydrodynamic diameter of the samples was measured to be 274.6 ± 40 nm (n = 3 samples) with a polydispersity index of 2%. The distribution of hydrodynamic diameter in the samples is shown in Supplementary Figure 1A. The magnetization of the particles was measured using the vibrating sample magnetometer for different field intensities and the hysteresis curve for the MNPs is shown in Supplementary Figure 1B. The saturation magnetization of the particles was calculated to be 0.06 emu at a saturating magnetic field of 0.5 T. The magnetic susceptibility of the nanoparticles was calculated from the M vs H plot and was found to be χm = 15.2. Based on these measurements, the MNPs exhibited superparamagnetic behavior and were confirmed to be monodispersed.
The characterized MNPs were injected in rat brain tissue and exposed to a uniform magnetic field as shown in Figure 1A. These ex-vivo cortical slices were maintained at a low temperature in order to preserve structure and extend sample viability. Prior to applying a magnetic field, it was observed that the MNPs diffused in random directions in the tissue. However, when the uniform field was applied to the tissue using the two magnet system, each magnetized MNP produced a magnetic field of influence around it. An MNP falling in the field of influence of any neighboring MNP experiences an attractive magnetic force towards its neighbor27. This attractive force between particles causes the motion of MNPs towards their neighbors. The interactive motion of MNPs in the presence of a uniform magnetic field resulted in the formation of MNP chains in the prepared rat brain tissue. Figure 1C shows a representative image of this chaining of MNPs in a mouse brain tissue (GFP line) after the application of magnetic field. The MNP chains increased in size over time as new particles were recruited to the chain and as the corresponding region of the magnetic field of influence grew larger. The phenomena of movement and agglomeration of MNPs into chains in the brain tissue was observed in all the tissue slices from different rats (n=12).
The motion of MNPs in brain tissue was further evaluated after varying two key parameters in the above experiment, namely, magnetic field intensity and MNP volume concentration. The experiments were performed in brain tissue slices combining either high (0.1 T) or low (0.02 T) uniform magnetic field intensity with either high (0.5 mg/mL) or low (0.05 mg/mL) MNP concentration. Each of these four experiments was repeated over three slices from different rats. In 3 out of the 4 experiments, MNPs formed chains in the presence of a uniform magnetic field while in one case, at the combined low magnetic field and low magnetic concentration, the MNPs were too far apart and the magnetic field was too small to produce any chaining. Table 1 shows a comparison of the extent of chaining observed for each combination of parameters. The amount of chaining for each of the experiments was defined by the average MNP chain length observed in the tissue after 10 minutes of applying the uniform magnetic field. As anticipated, the largest MNP chaining was observed for a combination of high magnetic field and high magnetic concentration (12.51 ± 3.5 μm). In addition, the chain length observed in a high magnetic field and low MNP concentration (5.84 ± 1.1 μm ) was higher than observed for the case of a low magnetic field and a high MNP concentration (2.76 ± 0.8 μm). This indicated a dominant effect of magnetic field intensity over the MNP concentration in the process of MNP movement and chaining.
Table 1.
Average chain length after 10 minutes for different applied magnetic field intensity and MNP concentration combinations in rat brain tissue
| MNP Concentration | High Concentration (0.5 mg/mL) | Low Concentration (0.05 mg/mL) |
|---|---|---|
| Magnetic Field | ||
| High field (0.1 T) | 12.51 ± 3.5 μm | 5.84 ± 1.1 μm |
| Low field (0.02 T) | 2.76 ± 0.8 μm | No Chaining |
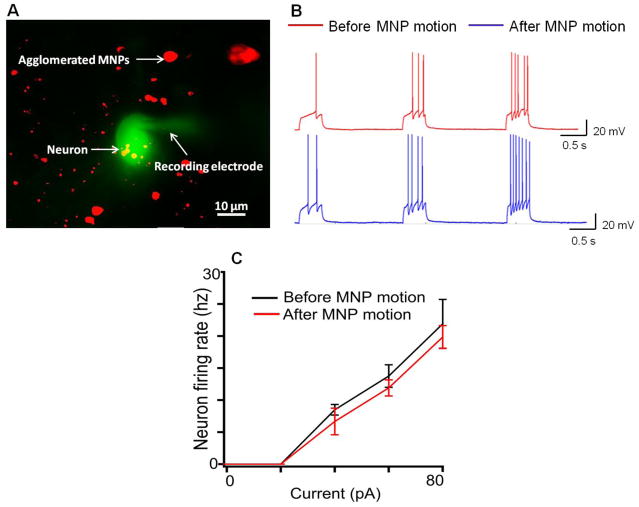
To determine the functionality of cells after moving MNPs through or near them, we performed standard electrophysiology recordings in the neurons of the olfactory bulb28,29. Mitral cells from the main olfactory bulb were targeted for whole-cell recordings, after moving MNPs through a region that contained those cells. In these experiments the recording pipette contained a fluorescent dye (see methods), which allowed us to visually verify the integrity of the recorded neuron. As shown in Figure 2B, following the movement of MNPs, mitral cells remain excitable as determined by current injections, indicating that basic processes such as influx and efflux of sodium and potassium ions30 respectively were unaffected by the motion of MNPs. The motion of MNPs did not alter the dependence of neuron firing frequency for different constant currents injected into the cells (Figure 2C). Additionally, we tested synaptic functionality by examining the occurrence of spontaneous inhibitory post-synaptic currents (sIPSCs) in mitral cells. Previously, it has been shown that noradrenaline, a neuromodulatory transmitter, enhances the release of gamma-Aminobutyric acid (GABA) from granule cells in the main olfactory bulb, and greatly enhances the frequency of spontaneous inhibitory post-synaptic currents in mitral cells31. As shown in Figure 3B, slices exposed to noradrenaline (NA, 10 uM, for 3 minutes) after MNP motion showed a significant increase in spontaneous inhibitory post-synaptic current frequency, suggesting that the synaptic connectivity between granule and mitral cells in the main olfactory bulb remained functional.
Figure 2.
Functional health of brain tissue after MNP motion. (A) Recording from a mitral cell in the olfactory bulb after the slices treated with MNPs were subjected to a magnetic field. The recording electrode contained the fluorescent dye Alexa-488 (green), which diffuses into the neuron during the recording. The MNPs contained a fluorophore Texas-Red (red). Note this is a total summed two wavelength images (B). Current-clamp recordings in mitral cells before (red) and after magnet induced MNP movement (blue). Increasing depolarizing current pulses (not shown) elicited action potentials in both control and treated neurons. (C) In the range of depolarizing current used, the frequency of neuronal firing increased linearly and it was comparable for different constant current stimuli before (black) and after MNP motion (red).
Figure 3.
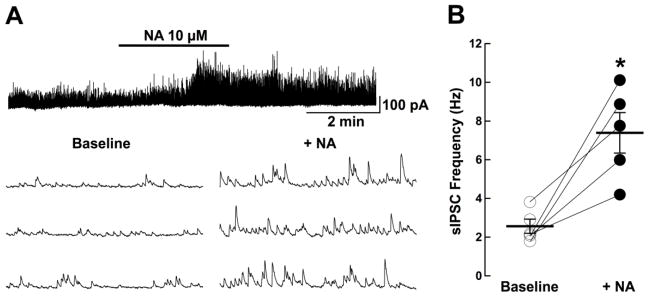
Synaptic connectivity in the olfactory bulb after MNP motion (A) Recording from a mitral cell showing the spontaneous occurrence of GABA IPSCs after MNP motion in brain slices. Top, application of noradrenaline (NA, 10 μM, 3 min) produced a long lasting increase in sIPSC frequency in this cell. Bottom, select traces from above, in an expanded time scale, showing sIPSC before (left) and after NA (right). (B) NA significantly increased the sIPSC frequency; baseline, 2.56 ± 0.82 Hz, NA, 7.39 ± 2.34 Hz (*, p<0.003; n = 5). The observed increase in sIPSC frequency caused by NA after MNP motion is similar to the trend observed previously by Zimnik et al. 31
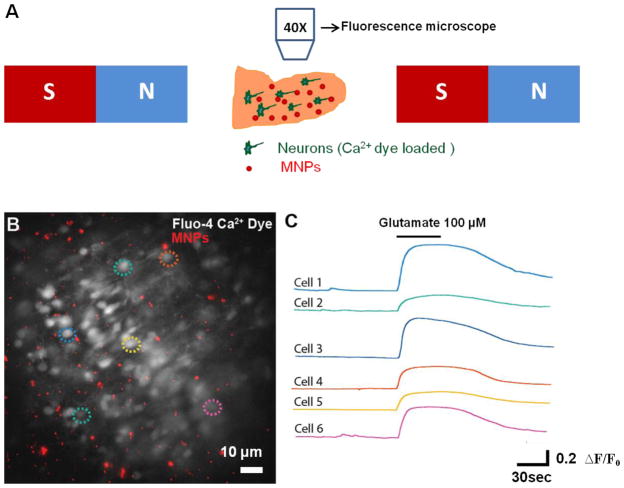
Next, we assessed whether the magnetically induced movement of MNPs disrupted the neural circuit function in the olfactory bulb. The olfactory bulb has a well-characterized neural circuit in which sensory inputs excite principal neurons, specifically the mitral/tufted cells32. Activation of mitral cells then excites the surrounding granule cells at dendrodendritic synapses. Thus, by monitoring the granule cells after MNP movement, we studied the effect of MNP motion on the excitatory synapses in the olfactory bulb 32. To investigate olfactory bulb neural circuit function, we loaded olfactory bulb slices with a Ca2+ sensing dye (Fluo-4 AM dye, 5 μM, see Methods) to visualize and monitor the neural activity of the circuit, in particular granule cells (the most abundant neuron in the olfactory bulb) (Fig 4B). Fluo-4 dye AM is a cell permeable dye that exhibits an increase in fluorescence upon binding to Ca2+ (indicating neural activation), and allows for the monitoring of a large number of neurons simultaneously. MNPs were applied to the slice 30 minutes before the acquisition of images began and they were moved by exposure to a uniform magnetic field. We then assessed the responsiveness of granule cells to activation by the excitatory neurotransmitter glutamate after MNP movement in the region (Supplementary video 1). As shown in Fig 4B, following the movement of MNPs in the slice, granule cells show normal fluorescence labeling suggesting that the overall morphology is maintained. In these slices, application of glutamate (100 μM) resulted in a robust increase in intracellular Ca2+ as evidenced by the changes in ΔF/F0 (45.25± 8.2%, n= 6 cells). Hence the responses to excitatory stimuli in granule cells were not affected by the MNP movement in the region.
Figure 4.
Calcium imaging recording in brain slices after MNP motion. (A) Experimental setup used for the calcium imaging experiments. After loading the calcium dye, MNPs are placed on the slice and subjected to a magnetic field. (B) Fluorescence image showing a network of functionally active neurons in a brain slice loaded with the calcium dye Fluo-4 AM (white) and MNPs (red), after exposing the slice loaded with MNPs to a uniform magnetic field. Dotted colored circles represent the neurons used for quantification of fluorescence changes shown on the right. (C) Optical fluorescence recordings of the selected cells shown in B. Images were taken at a rate of 1 Hz FPS. Application of the excitatory neurotransmitter, glutamate (100 μM, 45 seconds) resulted in a large, and reversible, increase in intracellular calcium levels. The color of each plot corresponds to cells indicated by the colored dotted circles in (B).
To further determine whether the movement of MNPs disrupted neural connections, we used transgenic mice (ChAT Tau-GFP) that expressed GFP under the promoter of choline acetyl transferase (ChAT), an enzyme involved in the synthesis of acetylcholine. Since the main olfactory bulb receives a rich cholinergic projection from the basal forebrain33, we visualized the effect of MNPs on the fibers in this particular region. The slices used in these experiments were divided into three main categories: treated, untreated, and control. The treated slices were injected with MNPs and were subjected to the applied uniform magnetic field for 5 minutes, followed by a rotation of the field for 5 minutes as explained in the Methods section. The untreated slices were just injected with MNPs and no magnetic field was applied. The control slices contained no MNPs and no magnetic field was applied. As shown in Fig 5, immunostaining of GFP in control mice samples (Fig 5, left) revealed abundant fibers throughout the different cellular layers of the main olfactory bulb. The untreated (Fig 5, middle) and treated slices (Fig 5, right) showed no difference in the pattern of distribution of GFP-positive fibers. Hence the motion or presence of MNPs did not disrupt the neural connections in the brain independent of the direction of MNP motion.
Figure 5.
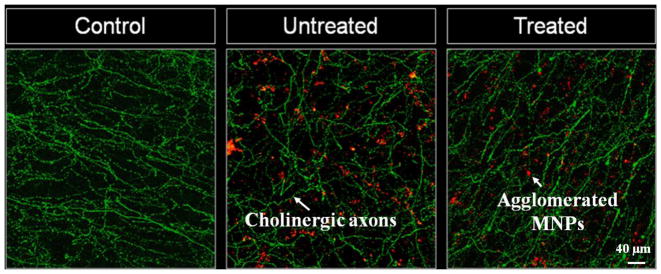
Confocal microscopy images of the granular cell layer in the main olfactory bulb from ChAT-Tau-GFP mice, after immunostaining for GFP. In control conditions (left) the slices show abundant distribution of GFP labelled fibers, corresponding to the axonal processes of cholinergic neurons. The pattern of distribution of axonal fibers was not affected in slices treated with MNPs without application of the magnetic field (middle) or after the MNPs exhibited motion into chains under an applied uniform magnetic field (right).
Discussion
In previous works, MNPs of various sizes, shapes, and coatings have been successfully utilized in drug delivery, gene transfection, tumor imaging, and regenerative medicine 16,18,34,35,36,. In principle, such MNPs can be controlled in the human body using external magnet systems to direct drugs and other biological factors to specific targets. Here we examined the motion of MNPs in brain tissue, to investigate both the character of MNP motion in the brain and its safety. We showed that monodispersed starch-coated MNPs are able to move towards each other in brain tissue when exposed to a uniform magnetic field and, importantly, that this movement produced no apparent disruption of the neural circuit function in the main olfactory bulb.
We observed that the MNPs agglomerated into chain like structures as they moved in the brain tissue under the influence of a uniform magnetic field. Such an agglomeration of MNPs in a uniform magnetic field has been previously studied in various media such as in water, bovine serum albumin and sodium dodecyl sulphate 37,38,27. The dynamics of chain formation and the distribution of chain length have been modeled and compared with experiments38,39,40. Based on these prior studies, the mechanism of chain formation can be classified into two main cases: diffusion dominated and magnetic drift dominated agglomeration. In diffusion dominated agglomeration, the MNPs undergo diffusion in the media until they are close enough so that they bring each other together by the magnetic forces between them41,42,43. In the drift dominated agglomeration, the magnetic force has a sufficiently long range that it drives the motion of MNPs together from the start 44,45,46. In our experiments in brain tissue, the average chain length of MNPs was higher in a high magnetic field and low MNP concentration than in a low magnetic field and high concentration condition. This indicates that a high magnetic field intensity can bring even sparsely distributed nanoparticles together. Thus for our experimental conditions, the MNPs exhibit a magnetic drift dominated mechanism of agglomeration as they moved in brain tissue.
The MNPs used in this work have been shown to not produce cytotoxicity in various cell types and in-vivo studies13,47,48. However, it is equally important to study and ascertain that the motion of these nanoparticles in brain tissue does not affect the normal function of neurons or their connectivity. By taking electrophysiological recordings of neurons before and after MNP movement, we have shown that the MNP motion and chaining did not affect neural functionality. Current injections produced a robust depolarization in the neurons, and they exhibited a stimulus-dependent increase in firing when a constant current stimulus was provided to the cell. Importantly, the change in neural firing rate elicited by incremental current stimuli was not affected by the MNP motion. Therefore, we conclude that MNP presence, motion, or chaining did not affect the physiological properties of the neurons.
In addition, we showed that the movement of MNPs did not affect the inhibitory neural circuit in the olfactory bulb; a critical component of olfactory processing in the bulb. The frequency and amplitude of the GABA sIPSCs after movement of the MNPs was similar to the previously reported values 31. Further, since the sIPSCs recorded in the mitral cells are produced by the summation of multiple synapses from several interneuron types, these results suggest that circuit level basal release from interneurons and post synaptic mitral cells activation were not affected following MNP motion. Furthermore, noradrenaline caused a robust increase in the spontaneous inhibitory post-synaptic current frequency in mitral cells, suggesting that the overall functionality of interneurons was also not affected by the MNPs movement (see also 31). This conclusion was further supported by the analysis of excitatory glutamatergic responses in a population of granule cells using a calcium indicator. In these optical recordings we found that a wide field of granule cells showed an increase in fluorescence after exposure to glutamate despite MNP motion in the same region (Supplementary video 1). The increase in fluorescence corresponds to an increase in intracellular calcium ions in the granule cells, in response to the glutamate-induced excitation. Together, these results provide evidence that excitatory and inhibitory responses of the olfactory bulb neural network were not affected by the MNP movement.
Apart from the functional health of the neurons, the immunohistochemistry experiments suggested that the MNPs did not disrupt the fibers as they moved and chained in the tissue. The slices containing MNPs (both with and without an applied uniform magnetic field) did not exhibit any noticeable difference in the density of cholinergic fibers in the granule cell layer, as compared with the control slices with no MNPs and no applied magnetic field. These experiments ruled out the possibility that the passive diffusion or magnetically induced movement of MNPs disrupted neural connections.
In summary, we have shown that MNPs can move towards each other in brain tissue under an applied uniform magnetic field. This motion of MNPs results in the formation of chain like agglomerates in the tissue and for our experimental conditions this chaining was determined to be drift dominated (as opposed to diffusion dominated) behavior. We found that the chained MNP agglomerates did not affect the normal functioning of neurons in the main olfactory bulb. The MNP agglomerates also did not disrupt the dense connections between the neurons in this region. Since it is known that MNP chaining, and the resulting ability for magnetic fields to effective move MNP through tissue49,50,51 depends on particle properties (size, shape, concentration), in the future the studies above could be expanded to select optimal MNP properties to enable effective but safe MNP motion in the brain. Enabling safe and effective manipulation of MNPs in the brain would aid drug and gene delivery and other tissue engineering applications in the brain.
Supplementary Material
Acknowledgments
Funding sources: Funding from the National Institutes of Health (NIH, grant numbers 1R21CA140068-01, 1R41DC013534-01A1), the National Science Foundation (NSF, grant number NSF 1261938) and National Institute on Deafness and Other Communication Disorders (NIDCD, grant number DCR01-DC-009817) is gratefully acknowledged
Footnotes
Conflict of interest: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woodworth GF, Dunn GP, Nance EA, Hanes J, Brem H. Emerging Insights into Barriers to Effective Brain Tumor Therapeutics. Front Oncol. 2014;4 doi: 10.3389/fonc.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia: Preliminary report. J Am Med Assoc. 1928;90:823–825. [Google Scholar]
- 3.Matsukado Y, Maccarty CS, Kernohan JW. The growth of glioblastoma multiforme (astrocytomas, grades 3 and 4) in neurosurgical practice. J Neurosurg. 1961;18:636–644. doi: 10.3171/jns.1961.18.5.0636. [DOI] [PubMed] [Google Scholar]
- 4.Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1 A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1:55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser MG, et al. Tissue distribution and antitumor activity of topotecan delivered by intracerebral clysis in a rat glioma model. Neurosurgery. 2000;47:1391–1398. discussion 1398–1399. [PubMed] [Google Scholar]
- 6.Brem H, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 8.Chinot OL, et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 9.Arruebo M, et al. Sustained release of doxorubicin from zeolite–magnetite nanocomposites prepared by mechanical activation. Nanotechnology. 2006;17:4057. doi: 10.1088/0957-4484/17/16/011. [DOI] [PubMed] [Google Scholar]
- 10.Hassan EE, Gallo JM. Targeting Anticancer Drugs to the Brain. I: Enhanced Brain Delivery of Oxantrazole following Administration in Magnetic Cationic Microspheres. J Drug Target. 1993;1:7–14. doi: 10.3109/10611869308998759. [DOI] [PubMed] [Google Scholar]
- 11.Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle Technology for Drug Delivery Across the Blood-Brain Barrier. Drug Dev Ind Pharm. 2002;28:1–13. doi: 10.1081/ddc-120001481. [DOI] [PubMed] [Google Scholar]
- 12.Pardridge WM. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Burgo LS, Hernández RM, Orive G, Pedraz JL. Nanotherapeutic approaches for brain cancer management. Nanomedicine Nanotechnol Biol Med. 2014;10:905–919. doi: 10.1016/j.nano.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Maier-Hauff K, et al. Intracranial Thermotherapy using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J Neurooncol. 2007;81:53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 15.Kong SD, et al. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J Control Release Off J Control Release Soc. 2012;164:49–57. doi: 10.1016/j.jconrel.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wankhede M, Bouras A, Kaluzova M, Hadjipanayis CG. Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy. Expert Rev Clin Pharmacol. 2012;5:173–186. doi: 10.1586/ecp.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Landeghem FKH, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30:52–57. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 18.Sensenig R, Sapir Y, MacDonald C, Cohen S, Polyak B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomed. 2012;7:1425–1442. doi: 10.2217/nnm.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carenza E, et al. In vitro angiogenic performance and in vivo brain targeting of magnetized endothelial progenitor cells for neurorepair therapies. Nanomedicine Nanotechnol Biol Med. 2014;10:225–234. doi: 10.1016/j.nano.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhan C, et al. Micelle-Based Brain-Targeted Drug Delivery Enabled by a Nicotine Acetylcholine Receptor Ligand. Angew Chem Int Ed. 2011;50:5482–5485. doi: 10.1002/anie.201100875. [DOI] [PubMed] [Google Scholar]
- 21.Feng B, et al. Delivery of sodium borocaptate to glioma cells using immunoliposome conjugated with anti-EGFR antibodies by ZZ-His. Biomaterials. 2009;30:1746–1755. doi: 10.1016/j.biomaterials.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Wu XX, et al. Doxorubicin enhances TRAIL-induced apoptosis in prostate cancer. Int J Oncol. 2002;20:949–954. [PubMed] [Google Scholar]
- 23.Yang C, et al. Nanoimmunoliposome delivery of superparamagnetic iron oxide markedly enhances targeting and uptake in human cancer cells in vitro and in vivo. Nanomedicine Nanotechnol Biol Med. 2008;4:318–329. doi: 10.1016/j.nano.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nacev A, Beni C, Bruno O, Shapiro B. Magnetic nanoparticle transport within flowing blood and into surrounding tissue. Nanomed. 2010;5:1459–1466. doi: 10.2217/nnm.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huynh NT, et al. Treatment of 9L gliosarcoma in rats by ferrociphenol-loaded lipid nanocapsules based on a passive targeting strategy via the EPR effect. Pharm Res. 2011;28:3189–3198. doi: 10.1007/s11095-011-0501-y. [DOI] [PubMed] [Google Scholar]
- 26.Salcedo E, et al. Activity-dependent changes in cholinergic innervation of the mouse olfactory bulb. PloS One. 2011;6:e25441. doi: 10.1371/journal.pone.0025441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erb RM. Magnetic Manipulation and Assembly of Multi-component Particle Suspensions. Duke University; 2009. [Google Scholar]
- 28.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflüg Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 29.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 30.Sakmann B, Neher E. Single-channel recording. Springer; New York: 2009. [Google Scholar]
- 31.Zimnik NC, Treadway T, Smith RS, Araneda RC. α(1A)-Adrenergic regulation of inhibition in the olfactory bulb. J Physiol. 2013;591:1631–1643. doi: 10.1113/jphysiol.2012.248591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol. 1996;30:123–176. doi: 10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 33.Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: A study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13:627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 34.Richter H, et al. Magnetorelaxometric quantification of magnetic nanoparticles in an artery model after ex vivo magnetic drug targeting. Phys Med Biol. 2009;54:N417. doi: 10.1088/0031-9155/54/18/N03. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, et al. Smart multifunctional core–shell nanospheres with drug and gene co-loaded for enhancing the therapeutic effect in a rat intracranial tumor model. Nanoscale. 2012;4:6501. doi: 10.1039/c2nr31263h. [DOI] [PubMed] [Google Scholar]
- 36.Ang D, et al. Insights into the mechanism of magnetic particle assisted gene delivery. Acta Biomater. 2011;7:1319–1326. doi: 10.1016/j.actbio.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Jones GA, Niedoba H. IMP-Field induced agglomeration in thin films of aqueous based magnetic fluids. J Magn Magn Mater. 1988;73:33–38. [Google Scholar]
- 38.Domínguez-García P, Melle S, Pastor JM, Rubio MA. Scaling in the aggregation dynamics of a magnetorheological fluid. Phys Rev E. 2007;76:051403. doi: 10.1103/PhysRevE.76.051403. [DOI] [PubMed] [Google Scholar]
- 39.Helgesen G, Skjeltorp AT, Mors PM, Botet R, Jullien R. Aggregation of Magnetic Microspheres: Experiments and Simulations. Phys Rev Lett. 1988;61:1736–1739. doi: 10.1103/PhysRevLett.61.1736. [DOI] [PubMed] [Google Scholar]
- 40.Černák J, Helgesen G, Skjeltorp AT. Aggregation dynamics of nonmagnetic particles in a ferrofluid. Phys Rev E. 2004;70:031504. doi: 10.1103/PhysRevE.70.031504. [DOI] [PubMed] [Google Scholar]
- 41.Vicsek T, Family F. Dynamic Scaling for Aggregation of Clusters. Phys Rev Lett. 1984;52:1669–1672. [Google Scholar]
- 42.Witten TA, Sander LM. Diffusion-Limited Aggregation, a Kinetic Critical Phenomenon. Phys Rev Lett. 1981;47:1400–1403. [Google Scholar]
- 43.Miyazima S, Meakin P, Family F. Aggregation of oriented anisotropic particles. Phys Rev A. 1987;36:1421–1427. doi: 10.1103/physreva.36.1421. [DOI] [PubMed] [Google Scholar]
- 44.Erb RM, Sebba DS, Lazarides AA, Yellen BB. Magnetic field induced concentration gradients in magnetic nanoparticle suspensions: Theory and experiment. J Appl Phys. 2008;103:063916–063916–5. [Google Scholar]
- 45.Vartholomeos P, Mavroidis C. In Silico Studies of Magnetic Microparticle Aggregations in Fluid Environments for MRI-Guided Drug Delivery. IEEE Trans Biomed Eng. 2012;59:3028–3038. doi: 10.1109/TBME.2012.2213340. [DOI] [PubMed] [Google Scholar]
- 46.Socoliuc V, Bica D, Vekas L. Estimation of magnetic particle clustering in magnetic fluids from static magnetization experiments. J Colloid Interface Sci. 2003;264:141–147. doi: 10.1016/s0021-9797(03)00426-0. [DOI] [PubMed] [Google Scholar]
- 47.Kim JS, et al. Toxicity and Tissue Distribution of Magnetic Nanoparticles in Mice. Toxicol Sci. 2006;89:338–347. doi: 10.1093/toxsci/kfj027. [DOI] [PubMed] [Google Scholar]
- 48.Talelli M, et al. Intrinsically active nanobody-modified polymeric micelles for tumor-targeted combination therapy. Biomaterials. 2013;34:1255–1260. doi: 10.1016/j.biomaterials.2012.09.064. [DOI] [PubMed] [Google Scholar]
- 49.Alexiou C, et al. Locoregional Cancer Treatment with Magnetic Drug Targeting. Cancer Res. 2000;60:6641–6648. [PubMed] [Google Scholar]
- 50.Nacev A, et al. Towards Control of Magnetic Fluids in Patients: Directing Therapeutic Nanoparticles to Disease Locations. IEEE Control Syst. 2012;32:32–74. [Google Scholar]
- 51.Probst R, et al. Planar Steering of a Single Ferrofluid Drop by Optimal Minimum Power Dynamic Feedback Control of Four Electromagnets at a Distance. J Magn Magn Mater. 2011;323:885–896. doi: 10.1016/j.jmmm.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.