Abstract
Objective
Pernicious anemia (PA) is an autoimmune disease that causes achlorhydria or profound hypochlorhydria. We conducted a population-based study to determine whether individuals with PA are at increased risk for community-acquired pneumonia (CAP).
Methods
We performed a retrospective cohort study using The Health Improvement Network (THIN) from the United Kingdom (1993 to 2009). The eligible study cohort included individuals 18 years of age or older and with at least 1 year of THIN follow-up. The exposed group consisted of individuals with a diagnosis code for PA. The unexposed group consisted of individuals without a diagnosis of PA and was frequency matched with the exposed group with respect to age, sex, and practice site. Cox regression analysis was used to determine the hazard ratio (HR) with 95% confidence interval (CI) for CAP associated with PA, accounting for a comprehensive list of potential confounders.
Results
The study included 13,605 individuals with PA and 50,586 non-PA subjects. The crude incidence rate of CAP was 9.4 per 1000 person-years for those with PA, versus 6.4 per 1000 person-years for those without PA. The multivariable adjusted HR for CAP associated with PA was 1.18, 95% CI 1.08 – 1.29.
Conclusions
In this large population-based cohort study, individuals with PA and presumed chronic achlorhydria were at increased risk for CAP.
Keywords: pernicious anemia, achlorhydria, pneumonia, epidemiology
Introduction
Pernicious anemia (PA) is an autoimmune disease characterized by auto-antibodies directed against gastric parietal cells.[1] This loss of gastric parietal cells leads to atrophic gastritis and subsequent achlorhydria. It has been postulated that the increased gastric pH facilitates bacterial colonization of the stomach, which then potentially leads to colonization of the upper aerodigesitve tract with pathogens and subsequently increased risk of pneumonia.[2, 3] To our knowledge, the relationship between PA and the risk of pneumonia has not been previously studied.
An important parallel group of patients with chronic gastric acid suppression are those who take proton-pump inhibitors (PPIs). However, existing epidemiological studies that investigated the association between PPI therapy and the risk of community-acquired pneumonia (CAP) have yielded conflicting results.[4, 5, 6, 7, 8, 9, 10, 11]
If a true causal relationship exists between suppression of gastric acid production and CAP, then this effect should be manifested to its fullest extent in patients with PA and achlorhydria. Thus, the aim of this population-based retrospective cohort study was to determine whether patients with PA are at increased risk for developing CAP.
Methods
Study Design
We performed a retrospective cohort study using The Health Improvement Network (THIN) from the United Kingdom (U.K.). Our study was approved by the University of Pennsylvania Institutional Review Board and the THIN Scientific Review Committee.
Data Source
THIN is a computerized medical record database system for general practitioners in the U.K.[12] It covers patients in over 500 general practices that are representative of the U.K. population by age, gender, medical conditions, and death rates adjusted for demographics and social deprivation.[13, 14] THIN contains data that are publicly available, de-identified, and without any patient identifiers. Data collected includes demographic information, diagnoses, symptoms, prescriptions issued, tests and results, as well as dates of entry in and out of the database.[13] Medical diagnoses are recorded into THIN using the Read Clinical Classification version 2. Medications prescribed to patients are logged into THIN using a drug dictionary. All practices contributing data to THIN are instructed to follow a standardized protocol of entering information, and data quality is monitored through routine analysis of the entered data. Numerous prior studies have validated the high quality of the diagnoses, prescription records, and documentation in THIN.[15, 16, 17, 18, 19, 20, 21]
Study Cohort
THIN data from 1993 to 2009 included approximately 9.5 million patients. Our study cohort consisted of all individuals who were 18 years of age or older at the start of THIN follow-up and who had at least 1 year of THIN follow-up. Individuals with a history of CAP prior to the start of THIN follow-up were excluded from the study cohort.
Exposed Group
The exposed group comprised all individuals from the study cohort with a diagnosis code for PA in THIN. Start of follow-up for the exposed group was defined as the latest of the following: date PA diagnosis was made, Vision date, or six months following THIN registration date. Registration date is defined as the date when patients were first registered with a practice in THIN, and Vision date is the date when a practice began using in-practice Vision software that collects information for the THIN database. The end of the follow-up period was defined by either a CAP diagnosis or patient exit from THIN follow-up due to migration or death.
Unexposed Group
All individuals in the study cohort without a diagnosis code of PA were initially considered as potentially eligible to be included in the unexposed group. We randomly selected non-PA individuals from this group to form the unexposed group by frequency matching this unexposed group with the exposed group at a 4:1 unexposed to exposed ratio, with respect to age at start of THIN follow-up (+/- 5 years), sex, and practice site. Start of THIN follow-up for the unexposed group was defined as the latest of the following: Vision date or six months following THIN registration date. The end of the follow-up period was defined by a CAP diagnosis or by patient exit from THIN follow-up.
Outcomes and Covariates
The primary outcome in our study was time to the first episode of CAP recorded during the follow-up period. The diagnosis of CAP was based on the presence of Read codes (Supplementary Table 1). We examined a comprehensive list of potential confounders including age, sex, smoking status, and comorbidities (i.e. alcoholism, chronic obstructive pulmonary disease, asthma, hypertension, coronary artery disease, congestive heart failure, chronic renal failure, diabetes mellitus, cancer other than basal cell carcinoma, stroke, dementia, history of organ transplant, connective tissue disorder, peripheral vascular disease, cirrhosis, inflammatory bowel disease, gastroesophageal reflux disease (GERD), peptic ulcer disease, and dysphagia).
In addition, medications that were included as covariates in the model were anxiolytics, antidepressants, anti-Parkinson drugs, antipsychotics, barbiturates, opiates, corticosteroids, antibiotics, non-steroidal anti-inflammatories, PPIs, and histamine-2-receptor (H2R) antagonists. Because the potential effect of these medications on CAP risk would likely occur following a short exposure period and over a short latency period, we treated these variables as time-varying by dividing the entire follow-up period of each individual into consecutive time blocks of two-month duration (with the last block being less than or equal to two months in duration). We then determined the exposure status of each individual for each medication in each of these 2-month blocks separately. We considered a medication as being actively used if the intended duration of the prescription for the medication was more than 50% of the duration of the entire block. Furthermore, a patient was considered to have a comorbid condition for a 2-month follow-up block if the earliest recorded date for that diagnosis in THIN wason or before the start date of the 2-month block.
Statistical Analyses
All statistical analyses were performed using Stata 13.1 (StataCorp LP, College Station, TX). A p-value < .05 was considered statistically significant in all analyses. Descriptive statistics were performed to determine the baseline characteristics of the exposed and unexposed groups. Student's t-test and the chi-square test were used to analyze continuous and categorical variables, respectively. The crude incidence of CAP in each group was estimated by dividing the number of incident CAP by the total person-years of follow-up.
We performed a multivariable Cox regression analysis to estimate hazard ratios (HR) and 95% confidence intervals (CI) for risk of CAP in patients with and without PA, with robust estimate of variance to account for clustering due to the inclusion of time-varying covariates. Potential confounders as listed above were included in the multivariable regression model.
Sensitivity Analyses
Given the potential for misclassification of PA status based on diagnostic code alone, we performed three sensitivity analyses to increase the validity of the PA exposure definition. First, we restricted the PA group to patients with a PA diagnosis code and documented vitamin B12 therapy. Second, given patients with true PA and achlorhydria would not be expected to be prescribed gastric acid suppressive medications, we performed another analysis limiting the PA group to individuals who had a PA diagnosis code, were on vitamin B12 therapy, and who had not been prescribed a PPI or H2R antagonist. Third, we further restricted the PA group to incident cases (i.e. patients diagnosed with PA at least 6 months after the start of THIN follow-up) who were also on vitamin B12 therapy and who had not been on acid-suppressive medications. Previous work in a similar U.K. database showed that the 6-month threshold was adequate for capturing incident diagnosis of chronic conditions.[22] For all sensitivity analyses, we performed multivariable Cox regression analysis as described above to calculate HRs and 95% CIs for risk of CAP.
Results
Study Population
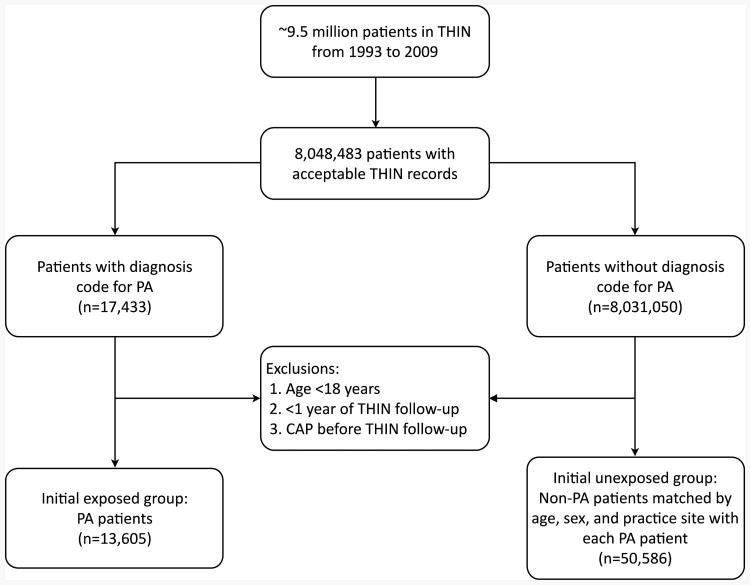
We identified approximately 9.5 million patients enrolled in THIN from 1993 to 2009, of whom approximately 8.0 million had acceptable records in THIN (Figure 1). Among this group,17,433 had a diagnosis code for PA. We excluded 3,828 PA patients since they were either less than 18 years old at start of THIN follow-up, had less than one year of follow-up in THIN, or had a history of CAP prior to the start of THIN follow-up. Therefore, 13,605 individuals comprised our PA exposure group. A total of 50,586 non-PA patients without a CAP diagnosis before THIN follow-up were selected to form the unexposed group which was frequency matched with the PA group with respect to sex, age at start of follow-up and practice site.
Figure 1.
Flow diagram of patient selection. CAP = community-acquired pneumonia; PA = pernicious anemia; THIN = The Health Improvement Network.
Table 1 depicts the characteristics of the study population at the start of THIN follow-up. Patients with PA were more likely to have potentially confounding medical diagnoses versus the control group. No differences were seen in prevalence of GERD between the two groups. PA patients were also more likely to be taking potentially confounding medications versus non-PA patients. The mean follow-up time for the non-PA subjects was slightly longer compared to the PA patients (5.5 vs. 5.2 years, p < .001).
Table 1.
Patient characteristics at start of follow-up.
| Variable | Non-PA patients (n = 50,586) | PA patients (n = 13,605) | p-value |
|---|---|---|---|
|
| |||
| Age at start of follow-up, year (SD) | 66.1 (16.4) | 67.7 (16.8) | < .001† |
|
| |||
| Male, % (n) | 31.1 (15,713) | 31.5 (4290) | .29‡ |
|
| |||
| Smoking status, % (n) | < .001‡ | ||
| Never smoker | 52.5 (26,572) | 51.2 (6968) | |
| Current smoker | 15.6 (7907) | 17.9 (2436) | |
| Ex-smoker | 18.9 (9581) | 20.0 (2725) | |
| Unknown | 12.9 (6526) | 10.8 (1476) | |
|
| |||
| Medical comorbidities, % (n): | |||
| Alcoholism | 0.8 (420) | 1.6 (212) | < .001‡ |
| COPD or asthma | 11.6 (5849) | 14.4 (1956) | < .001‡ |
| Hypertension | 31.4 (15,878) | 33.8 (4594) | < .001‡ |
| Coronary artery disease | 10.4 (5274) | 14.3 (1946) | < .001‡ |
| Congestive heart failure | 2.2 (1095) | 3.2 (436) | < .001‡ |
| Chronic renal failure | 0.7 (360) | 1.3 (174) | < .001‡ |
| Diabetes mellitus | 6.7 (3402) | 12.7 (1733) | < .001‡ |
| Any cancer other than BCC | 5.6 (2837) | 6.7 (909) | < .001‡ |
| Stroke | 5.9 (3005) | 8.6 (1168) | < .001‡ |
| Dementia | 1.3 (649) | 3.3 (451) | < .001‡ |
| History of organ transplant | 0.1 (42) | 0.1 (19) | .06‡ |
| Connective tissue disorder | 2.9 (1448) | 4.6 (624) | < .001‡ |
| Peripheral vascular disease | 1.5 (770) | 2.2 (300) | < .001‡ |
| Cirrhosis | 0.1 (74) | 0.3 (41) | < .001‡ |
| Inflammatory bowel disease | 0.8 (414) | 3.0 (411) | < .001‡ |
| GERD | 6.5 (3266) | 6.9 (940) | .06‡ |
| Peptic ulcer disease | 5.1 (2577) | 6.6 (897) | < .001‡ |
| Dysphagia | 2.0 (991) | 2.8 (377) | < .001‡ |
|
| |||
| Medications, % (n): | |||
| Anxiolytics | 5.0 (2533) | 8.1 (1097) | < .001‡ |
| Antidepressants | 7.1 (3610) | 11.5 (1569) | < .001‡ |
| Anti-Parkinson drugs | 0.8 (387) | 1.1 (150) | < .001‡ |
| Antipsychotics | 1.2 (624) | 2.0 (269) | < .001‡ |
| Barbiturates | 0.03 (14) | 0.04 (5) | |
| Opiates | 2.3 (1152) | 4.4 (604) | .59‡ |
| Immunosuppressants | 2.9 (1455) | 5.3 (723) | < .001‡ |
| Antibiotics | 3.0 (1535) | 4.8 (652) | < .001‡ |
| NSAIDS | 33.8 (17,084) | 46.5 (6333) | < .001‡ |
| Proton-pump inhibitors | 7.8 (3954) | 11.2 (1520) | < .001‡ |
| H2R antagonists | 2.2 (1136) | 2.7 (361) | .005‡ |
|
| |||
BCC = basal cell carcinoma; COPD = chronic obstructive pulmonary disease; GERD = gastroesophageal reflux disease; H2R = histamine-2-receptor; NSAIDs = non-steroidal anti-inflammatory drugs; PA = pernicious anemia; SD = standard deviation.
Student t-test
Chi-squared test
Primary Analyses
Overall, the crude incidence rate of CAP was higher among patients with a diagnosis of PA (9.4 cases per 1000 person-years) compared to matched controls (6.4 cases per 1000 person-years). In unadjusted Cox regression analysis, PA patients had an increased risk of CAP compared to controls (HR 1.49; 95% CI 1.37 – 1.63) (Table 2). After adjusting for confounders, the HR for CAP associated with PA attenuated to 1.18, 95% CI 1.08 – 1.29.
Table 2.
Community-acquired pneumonia HRs according to PA status.
| Variable | Non-PA patients | PA patients |
|---|---|---|
| Total patients | 50,586 | 13,605 |
| Community-acquired pneumonia | 1755 | 671 |
| Total person-years | 276,763 | 71,337 |
| CAP unadjusted HR (95% CI) | reference | 1.49 (1.37 – 1.63) |
| CAP adjusted HR (95% CI)† | reference | 1.18 (1.08 – 1.29) |
CAP community-acquired pneumonia; CI = confidence interval; HR, = hazard ratio; PA = pernicious anemia.
Adjusted for age, sex, smoking status, and all medical comorbidities and medications listed in Table 1.
Sensitivity Analyses
Results from sensitivity analyses can be found in Table 3. The analysis restricting PA patients to those with documented vitamin B12 supplementation yielded nearly identical results as the primary analysis (adjusted HR 1.17, 95% CI1.06 – 1.28). Further exclusion of PA patients who received any acid-suppressants led to minimal change in the point estimate (adjusted HR 1.25, 95% CI 1.10 – 1.41). Lastly, we limited the exposure group to patients with incident diagnosis of PA and who were on vitamin B12 therapy but not on any acid-suppressive medications. Again, results were largely unchanged versus the primary analysis (adjusted HR 1.20, 95% CI 0.99 – 1.44).
Table 3.
Community-acquired pneumonia HRs from sensitivity analyses.
| PA exposure definition | CAP adjusted HR (95% CI)† |
|---|---|
| PA diagnosis code‡ | 1.18 (1.08 – 1.29) |
| PA diagnosis code and on vitamin B12 therapy‡ | 1.17 (1.06 – 1.28) |
| PA diagnosis code and on vitamin B12 therapy and not on acid-suppressive medications§ | 1.25 (1.10 – 1.41) |
| First PA diagnosis > 6 months after start of follow-up, on vitamin B12 therapy, and not on acid-suppressive medications§ | 1.20 (0.99 – 1.44) |
CAP community-acquired pneumonia; CI = confidence interval; HR, = hazard ratio; PA = pernicious anemia.
Adjusted for age, sex, smoking status, and all medical comorbidities and medications listed in Table 1.
Reference group is non-PA individuals.
Reference group is non-PA individuals who had not been prescribed acid-suppressive medications.
Discussion
In this population-based retrospective cohort study, individuals with PA and presumed chronic achlorhydria had a modestly increased risk for CAP compared to those without PA, even after adjusting for confounding medications and comorbidities. This association was robust to sensitivity analyses with a more stringent definition of PA and restriction to incident PA diagnosis.
Our findings suggest that PA should be considered an independent risk factor for CAP. This increased risk is likely due in part to the PA-induced achlorhydria which prevents formation of the “gastric bactericidal barrier.” Loss of this barrier has been theorized to facilitate bacterial colonization of the stomach thereby leading to possible colonization of the upper aerodigesitve tract with pathogens and CAP.[23, 24] Supporting this theory is the finding that when 109 viable bacteria was placed into fasting stomachs of PA patients (gastric pH > 6.8), no reduction in bacterial number was seen after one hour.[2] Among individuals with normal stomachs (pH < 3.0), no recoverable bacteria was noted after one hour.[2] Other investigators have also found that individuals with PA had gastric and duodenal juices that grew more nitrate-reducing bacteria compared to that of controls.[3]
An important parallel group of patients with chronic gastric acid suppression are patients who take PPIs. A meta-analysis performed by Johnstone and colleagues noted that PPI users had 36% higher odds for developing CAP versus non-users.[25] However, the risk increase was limited to short duration of PPI use, while longer-term PPI use did not increase CAP risk.[25] Our finding of a modest increase in the risk of CAP associated with PA is consistent with the lack of association between chronic PPI therapy and the risk of CAP. Chronic PPI use, even at high doses, generally does not achieve the same level of gastric acid suppression experienced by those with PA. The mean intragastric pH found with PPIs across different dosages ranged from 1.4 to 6.4,[26] compared to 6.8 to 8.3 for individuals with PA.[2, 27] Moreover, while PA leads to chronic and persistent achlorhydria, PPIs on the other hand do not result in 24-hour gastric acid suppression. Prior studies have found that the intragastric pH was < 4 for 10 to 14 hours per day among those taking PPIs once daily.[28] Even in individuals taking PPIs twice daily, nocturnal acid breakthrough has been demonstrated.[29] Thus, even if the “gastric bactericidal barrier” theory does have clinical relevance, the modest association we found between PA and CAP suggests that PPIs may not increase the risk for CAP, as they cannot achieve the same level of gastric acid suppression seen with PA.
This study has limitations. First, we were not able to definitively confirm the PA diagnosis with results from Schilling tests or intrinsic factor antibody results, as such data could not be reliably obtained. However, 95% of the patients with a PA diagnosis had vitamin B12 supplementation documented in THIN, and our results were virtually unchanged when we restricted the PA group to those with a PA diagnosis and documented vitamin B12 use. Results from our analysis that additionally excluded individuals prescribed PPIs or H2R antagonists, medicines unlikely to have been prescribed to those with true PA, were also largely unchanged compared to our primary analysis. Also, limiting the exposed group to incident cases of PA did not significantly change the results as well. Data from these sensitivity analyses suggest potential misclassification of PA status did not have a significant impact on our study. Moreover, any misclassification of PA status would likely be non-differential, which would tend to bias the results towards the null. Thus, our calculated adjusted HR may be an underestimate of the true risk. Second, we did not collect information regarding concomitant immunodeficiencies. Limited reports have linked PA to common variable immunodeficiency, low serum immunoglobulin concentrations, and selective IgA deficiency.[30, 31, 32, 33] While these reports are not definitive, it is possible that some PA patients in our study had a concomitant immunodeficiency disorder, leading to an increased risk for CAP that was independent of the loss of the “gastric bactericidal barrier.” Lastly, we could not confirm the CAP diagnosis with microbiology or radiographic data, as this information was unavailable for most individuals with a CAP diagnosis code. However, CAP diagnosis codes in the General Practice Research Database (GPRD), another U.K. computerized medical record database similar to THIN in terms of data collection and recording,[18] were found to be valid for identification of CAP.[34] In fact, a previous GPRD study used the same definition of CAP as ours and found it to capture the effect of PPI-induced acid suppression similarly to a more stringent definition of hospitalized CAP.[10]
Conclusion
In this large population-representative cohort study, we observed a modest increased risk of CAP among PA patients with presumed chronic achlorhydria compared to those without PA, even after adjusting for confounding comorbidities and medications. This suggests that PA should be considered an independent risk factor for CAP.
Supplementary Material
Supplementary Table 1, Microsoft Word document.
Acknowledgments
None
Grant Support: Dr. Almario was supported by a National Institutes of Health T32 training grant (NIH T32DK07180-40) during his gastroenterology and health services research training at UCLA.
Footnotes
Conflicts of Interest: None declared
References
- 1.Epstein FH, Toh BH, van Driel IR, et al. Pernicious anemia. N Engl J Med. 1997;337:1441–48. doi: 10.1056/NEJM199711133372007. [DOI] [PubMed] [Google Scholar]
- 2.Giannella R, Broitman S, Zamcheck N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut. 1972;13:251–56. doi: 10.1136/gut.13.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockbruegger RW, Cotton PB, Menon GG, et al. Pernicious anaemia, intragastric bacterial overgrowth, and possible consequences. Scand J Gastroenterol. 1984;19:355–64. [PubMed] [Google Scholar]
- 4.Gulmez SE, Holm A, Frederiksen H, et al. Use of proton pump inhibitors and the risk of community-acquired pneumonia: A population-based case-control study. Arch Intern Med. 2007;167:950–55. doi: 10.1001/archinte.167.9.950. [DOI] [PubMed] [Google Scholar]
- 5.Laheij RJ, Sturkenboom MC, Hassing RJ, et al. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955–60. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 6.Myles PR, Hubbard RB, McKeever TM, et al. Risk of community-acquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2009;18:269–75. doi: 10.1002/pds.1715. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez LA, Ruigomez A, Wallander MA, et al. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology. 2009;20:800–06. doi: 10.1097/EDE.0b013e3181b5f27d. [DOI] [PubMed] [Google Scholar]
- 8.Eurich DT, Sadowski CA, Simpson SH, et al. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med. 2010;123:47–53. doi: 10.1016/j.amjmed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Dublin S, Walker RL, Jackson ML, et al. Use of proton pump inhibitors and H2 blockers and risk of pneumonia in older adults: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2010;19:792–802. doi: 10.1002/pds.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149:391–98. doi: 10.7326/0003-4819-149-6-200809160-00005. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Rev Clin Pharmacol. 2012;5:337–44. doi: 10.1586/ecp.12.20. [DOI] [PubMed] [Google Scholar]
- 12.Bourke A, Dattani H, Robinson M. Feasibility study and methodology to create a quality-evaluated database of primary care data. Inform Prim Care. 2004;12:171–7. doi: 10.14236/jhi.v12i3.124. [DOI] [PubMed] [Google Scholar]
- 13.CSD Medical Research. Our data. 2014 http://www.epic-uk.org/our-data/our-data.shtml.
- 14.University College London. THIN Database. 2013 http://www.ucl.ac.uk/pcph/research-groups-themes/thin-pub/database.
- 15.Denburg MR, Haynes K, Shults J, et al. Validation of The Health Improvement Network (THIN) database for epidemiologic studies of chronic kidney disease. Pharmacoepidemiol Drug Saf. 2011;20:1138–49. doi: 10.1002/pds.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaist D, Wallander MA, Gonzalez-Perez A, et al. Incidence of hemorrhagic stroke in the general population: validation of data from The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2013;22:176–82. doi: 10.1002/pds.3391. [DOI] [PubMed] [Google Scholar]
- 17.Langley TE, Szatkowski LC, Wythe S, et al. Can primary care data be used to monitor regional smoking prevalence? An analysis of The Health Improvement Network primary care data. BMC Public Health. 2011;11:773. doi: 10.1186/1471-2458-11-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis JD, Schinnar R, Bilker WB, et al. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 19.Meropol SB, Metlay JP. Accuracy of pneumonia hospital admissions in a primary care electronic medical record database. Pharmacoepidemiol Drug Saf. 2012;21:659–65. doi: 10.1002/pds.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogdie A, Alehashemi S, Love TJ, et al. Validity of psoriatic arthritis and capture of disease modifying antirheumatic drugs in the health improvement network. Pharmacoepidemiol Drug Saf. 2014;23:918–22. doi: 10.1002/pds.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seminara NM, Abuabara K, Shin DB, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol. 2011;164:602–9. doi: 10.1111/j.1365-2133.2010.10134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis JD, Bilker WB, Weinstein RB, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14:443–51. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 23.Hermos JA, Young MM, Fonda JR, et al. Risk of community-acquired pneumonia in veteran patients to whom proton pump inhibitors were dispensed. Clin Infect Dis. 2012;54:33–42. doi: 10.1093/cid/cir767. [DOI] [PubMed] [Google Scholar]
- 24.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139:1115–27. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther. 2010;31:1165–77. doi: 10.1111/j.1365-2036.2010.04284.x. [DOI] [PubMed] [Google Scholar]
- 26.Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors—comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19–31. doi: 10.1007/s00228-008-0576-5. [DOI] [PubMed] [Google Scholar]
- 27.Gray J, Shiner M. Influence of gastric pH on gastric and jejunal flora. Gut. 1967;8:574–81. doi: 10.1136/gut.8.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miner P, Katz PO, Chen Y, et al. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98:2616–20. doi: 10.1111/j.1572-0241.2003.08783.x. [DOI] [PubMed] [Google Scholar]
- 29.Peghini PL, Katz PO, Bracy NA, et al. Nocturnal recovery of gastric acid secretion with twice-daily dosing of proton pump inhibitors. Am J Gastroenterol. 1998;93:763–67. doi: 10.1111/j.1572-0241.1998.221_a.x. [DOI] [PubMed] [Google Scholar]
- 30.Hermans PE, Diaz-Buxo JA, Stobo JD. Idiopathic late-onset immunoglobulin deficiency: clinical observations in 50 patients. Am J Med. 1976;61:221–37. doi: 10.1016/0002-9343(76)90173-x. [DOI] [PubMed] [Google Scholar]
- 31.Ginsberg A, Mullinax F. Pernicious anemia and monoclonal gammopathy in a patient with IgA deficiency. Am J Med. 1970;48:787–91. doi: 10.1016/s0002-9343(70)80015-8. [DOI] [PubMed] [Google Scholar]
- 32.Ammann AJ, Hong R. Selective IgA deficiency: presentation of 30 cases and a review of the literature. Medicine (Baltimore) 1971;50:223. [PubMed] [Google Scholar]
- 33.Twomey J, Jordan P, Jarrold T, et al. The syndrome of immunoglobulin deficiency and pernicious anemia: A study of ten cases. Am J Med. 1969;47:340–50. doi: 10.1016/0002-9343(69)90218-6. [DOI] [PubMed] [Google Scholar]
- 34.Hansell A, Hollowell J, Nichols T, et al. Use of the General Practice Research Database (GPRD) for respiratory epidemiology: a comparison with the 4th Morbidity Survey in General Practice (MSGP4) Thorax. 1999;54:413–9. doi: 10.1136/thx.54.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1, Microsoft Word document.