Abstract
Latent variables may exist in experimental designs and may interfere with reproducibility of findings. The present study reveals one such variable, the individual differences in affective response to chronic injection stress, by using the novelty-seeking phenotype as a model of differential emotional reactivity. The phenotype is identified by exposing a population of experimentally-naïve outbred rats to the mild stress of a novel environment and classifying them as high responders (HR; upper 1/3rd) and low responders (LR; lower 1/3rd) based on their locomotor reactivity. Research has shown that HR/LR animals differ in their basal levels of anxiety- and depressive-like behavior, as well as in their response to environmental and pharmacological challenges; suggesting validity of this model in studying individual differences in stress reactivity. The present data showed that 14 daily, intraperitoneal saline injections did not alter the phenotypic differences in social behavior observed basally in HR/LR rats. However, injections significantly increased time spent immobile in the forced swim test in LRs, while the identical regimen significantly decreased the same measure in HRs, compared to handled-controls. These data indicate that individual differences in stress reactivity can have a significant impact on the depressive-like responses to repeated intraperitoneal injections in rats. Given that such underlying emotional variability exists within standard, outbred rat populations, this study highlights the importance of accounting for such variability in any study investigating the effects of repeated drug administration on depressive-like behavior for reliability and replicability of findings. Thus, we recommend including an uninjected control group in all studies.
Keywords: Replication of findings, stress reactivity, depressive-like behavior, repeated injection stress, individual differences
Introduction
Replication and corroboration of results is crucial to the process of scientific research. However, latent variables may exist in experimental designs and it is important to uncover them for reproducibility of findings. For example, in a 2014 study Sorge et al. showed that exposure of mice or rats to male but not female experimenters produces pain inhibition, suggesting that gender of the experimenter can be a latent factor influencing baseline behavioral responses and possibly interfere with replicability of findings. One other latent variable in experimental designs can be the stress of repeated injections. Experimental design of studies investigating the effects of chronic, peripheral injections of drugs includes vehicle-injected groups to reveal the effects of the administered drug by controlling for the procedural and pharmacological effects of vehicle injections. However, chronic vehicle injections can be stressful in their own right and this stress is rarely considered in experimental designs. Moreover, similar to humans, animals vary in their stress perception and reactivity hence, the response to the stress of chronic injections may differ within experimental groups.
Individual differences in stress perception and reactivity has been modeled in animals using a rat model of the novelty-seeking phenotype where, experimentally-naïve outbred rats are exposed to the mild stress of novel environment and classified as high responders (HR; upper 1/3 of the population) and low responders (LR; lower 1/3 of the population) based on their locomotor reactivity. Previous research has shown that compared to LRs, HRs appear less anxious in the light–dark box and elevated plus maze tests (Kabbaj et al., 2000; Aydin et al., 2011) and, surprisingly, exhibit prolonged stress-induced secretion of corticosterone (Piazza and Le Moal, 1996), suggesting differences in behavioral and physiological responses to environmental stimuli between these two phenotypes. Moreover, these differences correlate with differential expression of stress molecules in the brain: Compared to LRs, HRs express lower levels of basal corticotrophin-releasing hormone (CRH) mRNA in the central nucleus of the amygdala (which may contribute to lower anxiety behavior) and higher levels of CRH mRNA in the paraventricular nucleus of the hypothalamus coupled with lower levels of glucocorticoid receptor mRNA in the hippocampus (which may contribute to their hyper responsive HPA; Kabbaj et al., 2000). Together, these data demonstrate basal, as well stress-induced differences between LRs and HRs, confirming the validity of the novelty-seeking phenotype as a model to study the behavioral and molecular basis of individual differences in stress reactivity.
In a recent, unpublished study, investigating the effects of the administration of a drug intraperitoneally for 14 days on depressive-like behavior, we realized that the baseline differences that we have previously observed in HR/LR rats in the forced swim test (FST) were altered in the vehicle injected animals. This observation prompted us to ask: Does the chronic mild stress, associated with repeated injections impact outbred rats differently? This is an important question to address in any behavioral study that investigates the effects of a given drug, as any underlying individual differences in stress reactivity may confound the effects of the actual experimental drug being tested. Thus, the present study determined whether there are individual differences in the affective response to chronic intraperitoneal (i.p) saline injections within an outbred rat population by using the HR/LR phenotype as a model of differential emotional reactivity.
Materials and methods
Animal housing and the HR/LR phenotype screening
Animals were treated in accordance with the National Institute of Health guidelines on laboratory animal use and care. All efforts were made to minimize animal suffering and to reduce the number of animals used. A grand total of 42 male Sprague-Dawley rats (Charles River) arrived at postnatal day (PN) 50 and were kept on a 12 h light/dark cycle (lights on at 7:00 A.M.). Rats were pair-housed throughout the entire experiment and food and water were available ad libitum. Animals were allowed to habituate to the housing conditions for 7 days. On PN 57, all rats were screened for locomotor reactivity to the mild stress of a novel environment for 1 hr using Plexiglas locomotion chambers with stainless steel grid flooring, made in-house. Activity was monitored by means of photocells equally spaced along the sides of the box. At the end of the screening session, total locomotor activity (i.e., rearing and lateral movements) was pooled and the rats were ranked as HRs (i.e., rats that exhibited locomotor scores in the highest third of the sample; n=14) or LRs (i.e., rats that exhibited locomotor scores in the lowest third of the sample n=14). The intermediary responders (IRs) were only used as stimulus rats in the social interaction test described below.
Chronic saline injections
Following phenotype screening, cages were reorganized in a way that each rat was housed with a cage mate of the same phenotype. Each cage was assigned to injection or control handling groups (n=6–8). Five days after phenotype-screening, animals in the chronic injections groups received i.p saline injections (1ml/kg) once a day for 2 weeks. Control handled rats underwent the identical handling procedure as the saline injected animals daily, for 2 weeks but did not receive the injections. The day after the final saline injection or handling session all rats underwent pretesting for the FST. One day after the pretest session, rats were tested on the social interaction and forced swim tests as described below.
Social interaction test (SIT)
Testing took place in an open topped, rectangular, transparent social interaction box. The stimulus rat was a conspecific of similar age and weight and had no previous contact with the experimental rat. Rats were placed simultaneously in the box and the amount of time the experimental rat spent initiating social interaction (i.e., grooming, sniffing, following, and crawling over or under) with the stimulus rat was scored for 5 min.
Forced swim test (FST)
Testing was performed according to a published protocol (Porsolt et al., 1977) with some modifications. A 15-min pretest was conducted the day before testing. On the day of testing, rats were placed in a cylinder filled with water at room temperature. Each rat was tested individually and the cylinder was cleaned and filled with fresh water following each animal. Time rats spent immobile (lack of movement except necessary movements to keep the head above water), as well as swimming and climbing were scored for 5 min.
Statistical analysis
Locomotor reactivity in response to a novel environment was analyzed by a one-way ANOVA. Data pertaining to SIT and FST were analyzed by two-way ANOVAs: Phenotype (LR, HR) and Exposure (INJ, HANDL). Significant main effects and interactions were followed by post-hoc comparisons using the Bonferroni-Dunn corrections.
Results
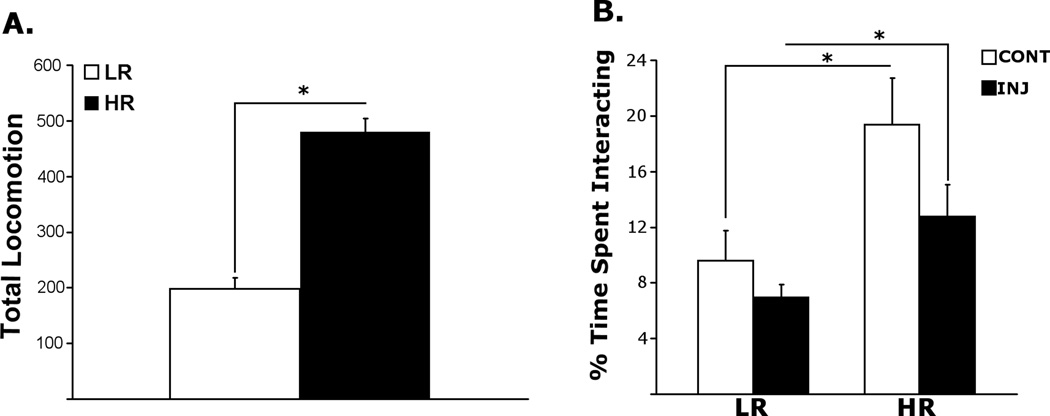
Figure 1 shows the baseline differences in novelty-induced locomotion in LRs versus HRs (A), as well as the effects of chronic saline injections on social interaction in LRHR rats by means of percent time the experimental rats spent interacting with the stimulus rats in SIT (B). A one-way ANOVA revealed a significant main effect of Phenotype [LR, HR; F(1, 25) = 86.11, p < 0.0001] in measures of novelty-induced locomotion, where HRs had higher locomotion scores compared to LRs [p < 0.0001]. Analysis of the SIT data by a two-way ANOVA revealed significant main effects of Phenotype [LR, HR; F(1, 23) = 11.24, p = 0.003], but not Exposure [INJ, HANDL; F(1, 23) = 3.83, p = 0.063], or interactions between Phenotype (LR, HR) and Exposure [INJ, HANDL; F(1, 23) = 0.86, p = 0.363]. Specific post-hoc comparisons showed that in agreement with the literature (Aydin et al., 2011), control handled HRs spent more time interacting with the stimulus rat compared to handled LRs [p = 0.044], and this effect remained preserved between chronically-injected LRs and HRs [p = 0.044].
Figure 1.
Baseline locomotor response to a novel environment (A), and percent time spent interacting with the stimulus rat in the SIT following 14, daily saline (1ml/kg; i.p) injections (B). All values are group means± SEMs.
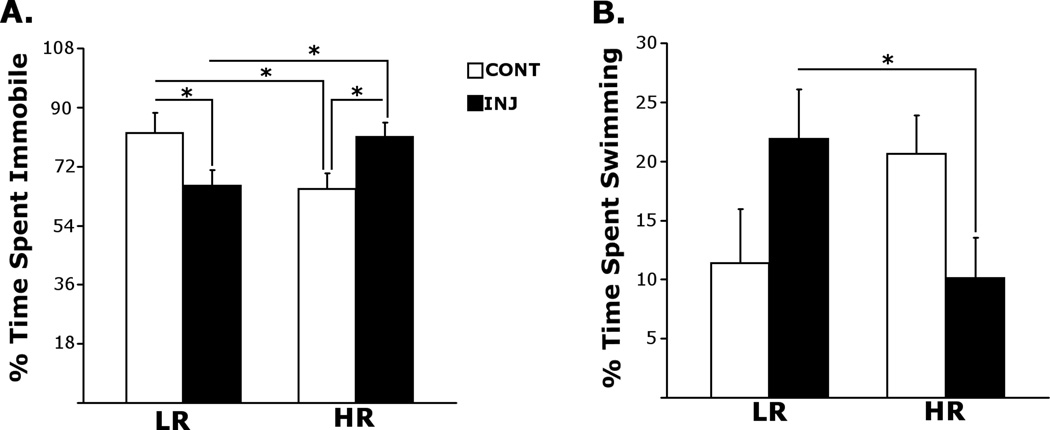
Figure 2 demonstrates the effects of chronic saline injections on depressive-like behavior in LRHR rats by means of percent time spent immobile (A), and swimming (B) in FST. Analysis of the percent time spent immobile in the FST by a two-way ANOVA showed that there were no significant main effects of Phenotype [LR, HR; F(1, 23) =0.001 , p = 0.973] or Exposure [INJ, HANDL; F(1, 23) =0.005 , p = 0.819], but interactions between Phenotype (LR, HR) and Exposure [INJ, HANDL; F(1, 23) =12.71, p = 0.002] were significant. Similarly, in swimming behavior, there were no significant main effects of Phenotype [LR, HR; F(1, 23) =0.19, p = 0.669] or Exposure [INJ, HANDL; F(1, 23) = 0.01, p = 0.907], but interactions between Phenotype (LR, HR) and Exposure [INJ, HANDL; F(1, 23) =8.176, p = 0.009] were significant. Specific post-hoc comparisons showed that control handled HRs spent less time immobile in FST compared to handled LRs [p = 0.043]. Interestingly, chronic saline injections resulted in decreased time spent immobile in LRs [p = 0.029], but an increase in the same measure in HRs [p = 0.027] compared to handled controls, which resulted in more time spent immobile by chronically-injected HRs compared to chronically-injected LRs [p = 0.016]. A similar effect was observed in time spent swimming; in that, chronic saline injections resulted in more time spent swimming in LRs compared to chronically-injected HRs [p = 0.031].
Figure 2.
Percent time spent immobile (A) and swimming (B) in the forced swim test following 14, daily saline (1ml/kg; i.p) injections. All values are group means± SEMs.
Discussion
The present study uncovers the latent variable of individual differences in responsivity to the stress of repeated injections in studies investigating the effects of chronic drug administration on depressive-like behavior in outbred rats. Our data show that while chronic, intraperitoneal saline injections do not alter phenotypic differences in social behavior, they yield completely opposite effects on depressive-like behavior in the FST in two groups of animals with differential stress reactivity, i.e., LRs versus HRs. Thus, daily, i.p injections of saline decrease time spent immobile and increase time spent swimming in FST in LRs, suggesting a decrease in depressive-like behavior, while the same injection procedure results in longer time spent immobile and shorter time spent swimming in the HRs, indicating increased depressive-like behavior.
Individual variability in response to a neutral stimulus such as administration of an inert substance, or of a sham physical treatment is a phenomenon that has been observed in humans. For example, clinical studies report that personality traits related to stress resiliency and genetic factors significantly influence development of placebo effects (Jaksic et al., 2013; Pecina and Zubieta, 2014). Similarly, the present study shows that variability exists in affective response triggered by repeated i.p injections of saline within outbred rat populations. In that, chronic i.p saline injections result in reduction in the measures of depressive-like behavior in rats with low novelty-induced locomotion, LRs; but an escalation in the same measures in HRs, the rats with high novelty-induced locomotion. Moreover, the contrasting effects of the repeated injection stress on depressive-like behavior in these two groups of rats result in a phenotype-switch, such that HRs display greater depressive-like behavior compared to LRs that are basally more depressed-like compared to HRs (Calvo et al., 2011). It should be noted that according to a previously published study, a phenotype-switch is not observed in HR/LR rats following three injections of the vehicle over a 24-hr period prior to the test session of the FST, although this regimen blunts the phenotypic differences observed basally (Jama et al., 2008), suggesting that a chronic injection regimen is necessary for phenotype-reversal to occur. Moreover, this dramatic switch in depressed-like responses following repeated vehicle injections observed in the present study indicates that individual rats can vary in the way they react to environmental challenges. Indeed, previous studies using the HR/LR model have found that HRs and LRs are often differentially responsive to stressful or environmental stimuli. For example, social defeat stress induces a depressive-like phenotype in HRs, while leaving LRs unaffected (Calvo et al., 2011). Similarly, chronic exposure to environmental and social stimuli results in decreased time spent immobile in HRs, while it is ineffective in LRs in the FST (Oztan et al., 2011a). On the other hand, a chronic variable physical stress paradigm results in increased depressive-like behavior in LRs, but a decrease in the same measure in HRs; although the same paradigm affects social interactions uniformly (Oztan et al., 2011b). Notably, similar effects have been observed between performances on the FST and SIT in the present study, where phenotype differences in social behavior are preserved while a phenotype-switch is observed in the FST, possibly due to differences in the nature of these two tests and the behaviors they measure. Taken together, these findings indicate that the precise effects that any given environmental stress has on animals with differential stress reactivity may depend critically on the exact type (i.e. social or physical), duration, or severity of stress, as well as the emotional test employed. Indeed, the present data show that an experience as mild as repeated, i.p saline injections impact both HRs and LRs, but in completely opposite ways; increasing depressive-like behavior in HRs, while reducing it in LRs.
In conclusion, the present study provides evidence for the notion that perception of a stimulus as stressful, neutral or enriching presents individual variability. Importantly, the phenotype switch following chronic injections of saline observed in the measures of depressive-like behavior in HR/LR rats indicates that this effect may be confounding studies where the vehicle injected animals are regarded no different than baseline animals, and interfere with replication of findings. Hence, the current data suggest that studies investigating the effects of repeated drug administration on depressive-like behavior in rats should account for the confound of differences in affective response to chronic i.p injections in their experimental design and interpretation of findings. This can be achieved by including a group of animals that do not receive any injections in the experimental design. In addition, we recommend characterization of rats prior to any experimental manipulation by exposing them to a novel environment and assessing their locomotor response. This is a simple and noninvasive way of determining the rats’ baseline stress reactivity and predicting their response to the stress of repeated injections, as demonstrated in the present study, which can then allow representation of each phenotype in the experimental groups, including the uninjected control groups, and ensure replication of findings.
Acknowledgements
Authors thank Mr. James Stewart for his technical assistance, and Dr. Pamela Maras for her valuable comments in preparation of the manuscript. This study was supported by the National Institute of Mental Health Grant R01-MH-104261, Office of Naval Research Grants N00014-09-1-0598 and N00014-12-1-0366, and Hope for Depression Research Foundation Grant RGA# 13-001 awarded to Dr. H. Akil. Dr. C. Aydin was supported by the NIDA Biology of Drug Abuse Training Grant T32 DA007268.
Abbreviations
- CRH
corticotropin-releasing hormone
- FST
Forced swim test
- HPA
hypothalamic-pituitary-adrenal axis
- HR
high responder
- i.p
Intraperitoneal
- IR
Intermediary responder
- LR
low responder
- PN
postnatal day
- SIT
Social interaction test
References
- 1.Aydin C, Oztan O, Isgor C. Effects of a selective Y2R antagonist, JNJ-31020028, on nicotine abstinence-related social anxiety-like behavior, neuropeptide Y and corticotropin releasing factor mRNA levels in the novelty-seeking phenotype. Behav Brain Res. 2011;222(2):332–341. doi: 10.1016/j.bbr.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvo N, Cecchi M, Kabbaj M, Watson SJ, Akil H. Differential effects of social defeat in rats with high and low locomotor response to novelty. Neuroscience. 2011;183:81–89. doi: 10.1016/j.neuroscience.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaksic N, Aukst-Margetic B, Jakovljevic M. Does personality play a relevant role in the placebo effect? Psychiatr Danub. 2013;25:17–23. [PubMed] [Google Scholar]
- 4.Jama A, Cecchi M, Calvo N, Watson SJ, Akil H. Inter-individual differences in novelty-seeking behavior in rats predict differential responses to desipramine in the forced swim test. Psychopharmacology (Berl) 2008;198(3):333–340. doi: 10.1007/s00213-008-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20(18):6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oztan O, Aydin C, Isgor C. Stressful environmental and social stimulation in adolescence causes antidepressant-like effects associated with epigenetic induction of the hippocampal BDNF and mossy fibre sprouting in the novelty-seeking phenotype. Neurosci Lett. 2011a;501(2):107–111. doi: 10.1016/j.neulet.2011.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oztan O, Aydin C, Isgor C. Chronic variable physical stress during the peripubertal-juvenile period causes differential depressive and anxiogenic effects in the novelty-seeking phenotype: functional implications for hippocampal and amygdalar brain-derived neurotrophic factor and the mossy fibre plasticity. Neuroscience. 2011b;192:334–344. doi: 10.1016/j.neuroscience.2011.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peciña M, Zubieta JK. Molecular mechanisms of placebo responses in humans. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36(1996):359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- 10.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men causes stress and related analgesia in rodents. Nat Methods. 2014;11(6):629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]