Abstract
Objective
The cerebral microvasculature is rendered more vulnerable to thrombus formation following a brief (5.0 min) period of focal ischemia. This study examined the contribution of interleukin-6 (IL-6), a neuroprotective and prothrombotic cytokine produced by the brain, to transient ischemia-induced thrombosis in cerebral arterioles.
Approach & results
The middle cerebral artery of C57BL/6J mice was occluded for 5 minutes, followed by 24 hrs of reperfusion (MCAo/R). Intravital fluorescence microscopy was used to monitor thrombus development in cerebral arterioles induced by light/dye photoactivation. Thrombosis was quantified as the time of onset of platelet aggregation on the vessel wall and the time for complete blood flow cessation. MCAo/R in wild type (WT) mice yielded an acceleration of thrombus formation that was accompanied by increased IL-6 levels in plasma and in post-ischemic brain tissue. The exaggerated thrombosis response to MCAo/R was blunted in WT mice receiving an IL-6 receptor-blocking antibody and in IL-6 deficient (IL-6−/−) mice. Bone marrow chimeras, produced by transplanting IL-6−/− marrow into WT recipients, did not exhibit protection against MCAo/R-induced thrombosis.
Conclusions
The increased vulnerability of the cerebral vasculature to thrombus development after MCAo/R is mediated by IL-6, which is likely derived from brain cells rather than circulating blood cells. These findings suggest that anti-IL-6 therapy may reduce the likelihood of cerebral thrombus development after a transient ischemic attack.
Keywords: Transient ischemic attack, thrombosis, interleukin-6, cerebral arterioles, ischemia-reperfusion
Introduction
The effects of ischemia, with or without reperfusion, on the brain have been extensively characterized. These studies have revealed the influence of a variety of factors on the brain injury response to an ischemic episode, with the magnitude and duration of insult representing major determinants of the injury response. Ischemic insults of long duration typically elicit severe tissue injury characterized by neuronal cell death, blood-brain barrier dysfunction and edema, which collectively result in brain infarction and a potentially debilitating stroke (Jung et al, 2010). Ischemic insults of shorter durations are better tolerated by the brain and often result in little or no lasting brain damage and neurological impairment. There is also a large body of evidence demonstrating that prior exposure of the brain to a transient ischemic insult of short duration confers a protective phenotype that allows brain tissue to tolerate a subsequent and more severe ischemic insult (Kirino, 2002; Gidday, 2006). The phenomenon of ischemic preconditioning (or ischemic tolerance) has been attributed to an increased expression of protective genes and the production of neuroprotective agents that render the brain more resistant to ischemic injury. While neurons are generally considered the primary cellular target of the ischemic preconditioning (IPC) response in brain (Gidday, 2006), there is evidence that the protection is also evidenced in different cellular components of the cerebral microvasculature, including endothelial cells, and is manifested as improved blood brain-barrier function, decreased endothelial adhesiveness to circulating leukocytes, and enhanced angiogenesis (Kirino, 2002; Gidday, 2006). A recently described deleterious consequence of a brief, transient ischemic insult on the cerebral vasculature is accelerated thrombus development, which is more pronounced in arterioles than in venules (Tang et al, 2014). The enhanced thrombogenesis occurs despite evidence for prolonged coagulation and bleeding times after IPC (Chen et al, 2005; Kim et al, 2008), and the thrombosis response is not altered by treatment with anti-platelet agents such as aspirin, clopidogrel and dypiridamole (Tang et al, 2014). The nature and origin of the prothrombogenic stimulus that mediates this deleterious response to transient cerebral ischemia remain unclear.
Interleukin-6, one of the major cytokines produced by the central nervous system (Erta et al, 2012; Gadient et al, 1994; Schobitz et al, 1993), exerts a variety of biological actions that impact on the injury and repair responses of the brain to an ischemic insult (Tuttolomondo et al, 2008). Potential beneficial effects of the cytokine include stimulation of neurogenesis and angiogenesis, inhibition of apoptosis, and blood-brain barrier stabilization (Jung et al, 2011; Erta et al, 2012). In contrast to these neuroprotective and neurotrophic effects, IL-6 has been reported to exert pro-inflammatory, pro-oxidative, and prothrombogenic actions (Spychalowicz et al, 2012; Senchenkova et al, 2013). A net protective effect of IL-6 in ischemic stroke is suggested by some animal studies that show an exaggerated brain injury response to mild transient ischemia in both IL-6−/− mice (Herrmann 2003; Gertz et al, 2012) and in obese (ob/ob) mice treated with an IL-6 neutralizing antibody (Terao et al, 2008). Ischemic stroke and transient ischemic attacks in humans are associated with significant increases in plasma IL-6 concentration, and the results of several clinical studies have revealed the cytokine to be a strong predictor of brain injury and clinical outcome (Hoshi et al, 2005; Orion et al, 2008). Similar increases in plasma, as well as brain tissue, IL-6 concentration (or mRNA) have been described in animal models of ischemic stroke (Chapman et al, 2009; Offner et al, 2009; Terao et al, 2008, Gertz, 2012).
Studies in other organ systems and different pathological conditions have revealed that IL-6 is also a potent prothrombogenic cytokine (van der Poll et al, 1994; Mutlu et al, 2007; Yan et al, 2014). Anti-IL-6 therapies have been reported to reduce thrombus development in animal models of deep vein thrombosis (Wojcik BM, 2011) and in the microvessel thrombosis associated with experimental colitis (Senchenkova, 2013). The cytokine is known to promote a variety of responses that favor coagulation/thrombosis, including thrombocytosis secondary to stimulation of thrombopoeisis (Zhang et al, 2013), induction of platelet-leukocyte aggregation (Yan et al, 2014), and platelet activation (Peng et al, 1994; Yan et al, 2014). The cytokine also exerts an influence on the coagulation system, as evidenced by its ability to enhance the expression of tissue factor, fibrinogen, factor VIII and von Willebrand factor, and to increase thrombin generation. IL-6 also reduces inhibitors of hemostasis such as anti-thrombin and protein S (Tuttolomondo et al, 2008). These prothrombotic actions of IL-6, coupled to the well-described elevation in plasma and tissue IL-6 concentration that occurs in response to brain ischemia (Hoshi et al, 2005; Orion et al, 2008), suggest that it is a viable candidate mediator of the prothrombogenic phenotype that is assumed by the cerebral microvasculature in response to transient cerebral ischemia. Hence, the overall goal of this study was to assess, using both immunologic and genetic approaches, the contribution of IL-6 to the accelerated thrombus formation elicited in cerebral arterioles following a brief ischemic episode. IL-6 deficient bone marrow chimeras were also used to identify the likely source of IL-6 in mediating the ischemia-induced thrombogenesis.
Materials and Methods
Animals
Male (23-33 g body weight) C57BL/6J and IL-6−/− (B6.129S6-IL6tml Kopf, C57BL/6J background) mice were purchased from Jackson Laboratory (Bar Harbor, ME). A total of 123 mice were used in the study. The distribution of mice in the different experimental groups is outlined in the figure legends. The experimental procedures were approved by the Institutional Animal Care and Use Committee at LSU Health Sciences Center and are in compliance with National Institutes of Health guidelines. All efforts were made to minimize animal distress and the number of animals used.
Induction of transient cerebral ischemia (MCAo/R)
Mice were anesthetized with 150 mg/kg ketamine and 7.5 mg/kg xylazine. Core body temperature was monitored and maintained at 36 ± 0.5°C using a heating blanket during surgery and until recovery from anesthesia. Transient focal cerebral ischemia was induced using the intraluminal filament method, as described previously (Tang et al, 2014). Briefly, a blunted 6-0 silicone-coated monofilament (Doccol Corporation, CA) was introduced into the common carotid artery through an arteriotomy below the carotid bifurcation and advanced along the internal carotid artery into the Circle of Willis until mild resistance was felt, indicating that the filament had entered the anterior cerebral artery and blocked the origin of middle cerebral artery (MCA). Following MCA occlusion for a period of 5 min, the filament was withdrawn, allowing for brain reperfusion for a period of 24 hrs. We have previously demonstrated that the 5 min ischemia/24 reperfusion (MCAo/R) protocol does not yield significant sustained neurological deficits nor does it produce detectable brain infarction (Tang et al, 2014). Sham mice were subjected to the same procedure without arteriotomy and monofilament insertion.
Intravital videomicroscopy
Following 24 hrs of reperfusion (after MCA occlusion), the mice were prepared for intravital microscopic observation of the brain, as previously described (Tang, 2014). Briefly, the mice were anesthetized, a craniectomy was performed and the animal was placed on the stage of an upright fluorescent microscope (BX51WI; Olympus, Japan) and allowed to equilibrate for 30 minutes. Visualization of individual cerebral microvessels and the induction of thrombus formation were achieved using a 40× water immersion objective lens (LUMPlan FI/IR 40×/0.80×; Olympus). A silicon-intensified target color video camera (VK-C150; Hitachi, Japan) or a charge-coupled device video camera (XC-77; Hamamatsu, Japan) projected the images onto a monitor (Trinitron PVM-2030; Sony, Japan), which was connected to a video timer (Time-Date Generator WJ-810; Panasonic, Japan) to record time and date. The images were recorded on a DVD recorder (SR-MV50; JVC, Wayne, NJ). The diameters of the selected microvessels were measured by video analysis software (Image J software version 1.37; NIH, US) on a personal computer (G4 Macintosh; Apple, CA).
Light/dye induced thrombosis
A 10 ml/kg body weight dose of 5% fluorescein isothiocyanate dextran (FITC; 150 000 molecular weight; Sigma-Aldrich, US) was injected into the femoral vein cannula and allowed to circulate for 10 minutes before photoactivation. Photoactivation of FITC (excitation, 495 nm; emission, 519 nm) was initiated by exposing 100 μm vessel (arteriole) length to epi-illumination with a 175-W xenon lamp (Lamda LS, Sutter, US) coupled with a fluorescein filter cube (HQ-FITC; Chroma Technology, US). The excitation power density was calibrated daily (ILT 1700 Radiometer, SED033 detector; International Light Technologies, US) and maintained within 1% of 0.17 W/cm2, as previously described (Tang et al, 2014). During continuous epi-illumination, thrombus formation was visualized and quantified in both venules and arterioles (diameters: 20–40μm) by determining the time of onset of platelet deposition/aggregation (onset time) and the time to complete blood flow cessation (cessation time). Epi-illumination of a vessel under study was discontinued once blood flow ceased. The results of both onset time and cessation time were averaged from 2–4 induced thrombi in each arterioles of the same mouse.
IL-6 in plasma and brain tissue
A cytometric bead array (Mouse soluble protein master buffer kit; BD Biosciences USA) was used to measure the concentration of IL-6. Briefly, plasma and supernatants of left and right hemisphere brain tissues were collected at 24 hrs after MCAo/R. For these experiments, mice were transcardially perfused (to flush the cerebral vasculature) with 1 × phosphate buffered saline (PBS). The samples were processed and analyzed with the cytometric beads as per the manufacturers instructions. The concentration of IL-6 was expressed as pg/ml in plasma or pg/g brain weight for the tissue samples.
Generation of IL-6−/− bone marrow chimeras
Bone marrow (BM) chimeras were produced by transplanting bone marrow from either WT or IL-6−/− donor mice into lethally irradiated WT recipient mice, as previously described (Stokes et al, 2006). Briefly, BM was isolated from the femurs and tibias of donor mice expressing CD45.2 leukocyte antigen and a minimum of 2×106 cells in 200μl PBS were administered via the femoral vein into recipient mice previously irradiated with two doses of 500-525 rad, 3 hr apart. The chimeric mice were kept in autoclaved cages, with 0.2% neomycin drinking water for 2 weeks, followed by normal water. Flow cytometry was used to verify chimera reconstitution at 6-8 weeks by staining for leukocyte CD45.2 expression using a FITC-conjugated antibody and CD45.1 expression using a PE-conjugated antibody. Mice exhibiting > 90% chimerization were used.
Some WT mice received an antibody that blocks the alpha subunit of the IL-6 receptor (rat anti-IL-6α (CD126) from Angio-Proteomic, Boston, MA, 4 mg/Kg) that was administered (i.v.) 24 hr before the experiment.
Laser Speckle Contrast Analysis (LASCA)
Blood flow in the brain region perfused by the MCA was monitored using a high resolution LASCA imaging system (PeriCam PSI, Perimed, Järfälla-Stockholm, Sweden), as previously described (Ayata et al, 2004). After the induction of anesthesia, a midline sagittal scalp incision was made and the periosteum removed to expose the surface of the skull. The skull surface was cleansed with saline and lightly swabbed with mineral oil to prevent drying. Speckle images (18mm × 20mm) were acquired at 6 images/s under 785 nm laser illumination. Both wild type and IL-6−/− mice were imaged before (baseline) and 5 minutes after filament insertion for MCAo. PIMSoft software was use to drive the system and capture images/data for offline analysis. Blood perfusion units were quantified in a 3.5 mm2 region of interest in the MCA branches region. Because the MCA branches region was not always visible on the ispilateral side, the 3.5 mm2 region of interest in this area was placed in the identical location as the baseline image.
Statistical analysis
Data are presented as means ± SEM unless otherwise indicated. One-way ANOVA was used with the Bonferroni or Fisher’s post hoc test. Differences between two data sets were determined using the two-tailed unpaired t-test. Statistical significance was set at p < 0.05.
Results
Estimates of cerebral blood flow derived from LASCA imaging (Supplementary Figure S1) revealed that MCAo yielded a significant reduction in brain tissue blood flow in the region (ipsilateral) perfused by the MCA, without accompanying changes in the same region of the contralateral hemisphere. MCA occlusion elicited a similar reduction in ipsilateral blood flow in both wild type and IL-6−/− mice, and the magnitude of the reduction observed in both groups is comparable to values previously reported using laser speckle imaging following MCAo (Ayata et al, 2004).
IL-6 concentration increases in plasma and brain tissue following transient brain ischemia
Following 5 min cerebral ischemia and 24 hrs reperfusion (MCAo/R), the concentration of IL-6 was measured in plasma and both brain (ipsilateral and contralateral) hemispheres. IL-6 concentration in plasma was significantly elevated (3.3-fold) following MCAo/R (Figure 1A). A significant (3.7-fold) increase in IL-6 concentration was detected in the ipsilateral hemisphere while a smaller (75%) increase was noted in the contralateral hemisphere (Figure 1 B).
Figure 1.

Changes in plasma and brain tissue interleukin-6 concentrations elicited by 5 min middle cerebral artery occlusion and 24 hrs reperfusion (MCAo/R) in control (n=10) and MCAo/R (n=8) mice. * indicates p<0.05 vs corresponding control group. † indicates p<0.05 vs MCAo ispilateral tissue.
IL-6 receptor (IL-6R) immunoblockade blunts the thrombosis response in cerebral arterioles subjected to MCAo/R
Thrombus formation in cerebral arterioles, assessed using the time of onset of platelet deposition/aggregation (onset time) as well as the time for complete blood flow cessation after light/dye-induced vessel injury, was quantified in control (untreated) WT, untreated WT mice subjected to MCAo/R, and WT-MCAo/R mice treated with an IL-6R blocking antibody (Figure 2). As previously reported (Tang et al, 2014), MCA0 (5 min) followed by 24 hr reperfusion significantly enhanced thrombus development induced by light/dye injury in cerebral arterioles of WT mice. Immunoblockade of IL-6R significantly blunted the accelerated thrombus development associated with MCAo/R, as reflected by increases in both onset and cessation times.
Figure 2.
Effects of interleukin-6 receptor (IL-6R) immunoblockade on light/dye-induced thrombus development in cerebral arterioles following MCAo/R. Control (n=10), MCAo/R (n=11), MCAo/R + IL-6R mAb (n=6) *denotes p<0.05 compared to corresponding control value, # indicates p<0.05 vs corresponding untreated MCAo/R values.
MCAo/R accelerated thrombosis is attenuated in IL-6 deficient mice
IL-6−/− mice were used to determine whether genetic deficiency of this cytokine imparts a protective effect similar to that observed with IL-6R immunoblockade. Figure 3 illustrates that while transient cerebral ischemia elicits a significant and profound acceleration of light/dye-induced thrombosis in cerebral arterioles of WT mice, this response was not observed in IL-6 deficient mice. The protective effect of IL-6 deficiency was manifested in both indices of thrombogenicity, i.e., onset time (panel A) and time to flow cessation (panel B).
Figure 3.

Comparison of light/dye-induced thrombosis responses following MCAo/R between wild type (WT) and interleukin-6 deficient (IL-6−/−) mice. WT control (n=10), WT MCAo/R (n=11), IL-6−/− control (n=5), IL-6−/− MCAo/R (n=8) * denotes p<0.05 compared to corresponding MCAo/R value, # indicates p<0.05 compared to WT-MCAo/R
Contribution of circulating blood cells to changes in IL-6 concentration in plasma and brain tissue following MCAo/R
Bone marrow (BM) chimeras were produced by transplanting BM from either wild type mice (WT→WT) or IL-6−/− mice (IL-6−/−→WT) into WT recipients. A comparison of the changes in brain tissue IL-6 concentration after MCAo/R in WT→WT and IL-6−/−→WT mice (Figure 4, panel A) revealed that the control (WT→WT) chimeras yielded an increased IL-6 concentration in ipsilateral (but no contralateral) brain tissue similar (3.2-fold) to that noted in WT mice (Figure 1). However, only an 80% increase in ipsilateral tissue IL-6 concentration was detected in bone marrow chimeras with circulating blood cells deficient in IL-6 (IL-6−/−→WT mice), suggesting that the IL-6 detected in postischemic brain tissue is mostly (67%) derived from circulating cells. WT→WT chimeras exhibited a significant (~80%) increase in plasma IL-6 concentration following MCAo/R (Figure 4 panel B), while no significant change in plasma IL-6 concentration was noted in IL-6−/−→WT chimeras.
Figure 4.
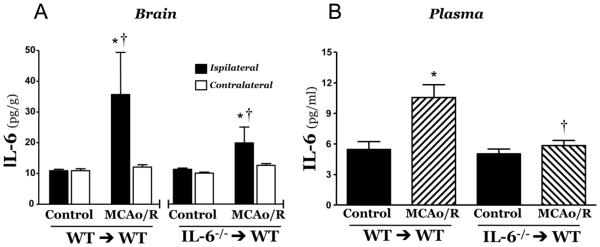
Changes in plasma and brain tissue interleukin-6 concentrations elicited by 5 min middle cerebral artery occlusion and 24 hrs reperfusion (MCAo/R) in WT→WT and IL-6−/− →WT bone marrow chimeras. WT→WT control (n=9), WT→WT MCAo/R (n=8), IL-6−/−→WT (n=9), IL-6−/−→WT MCAo/R (n=8) * indicates p<0.05 vs corresponding control group. † indicates p<0.05 vs corresponding MCAo contralateral tissue.
Blood cell-derived IL-6 does not contribute to the accelerated thrombus development elicited by MCAo/R
Figure 5 summarizes the results of thrombosis experiments in bone marrow chimeras (WT→WT and IL-6−/− →WT) following exposure to transient cerebral ischemia. In both WT→WT and IL-6−/− →WT chimeras, MCAo/R resulted in an acceleration of the time of onset (panel A) and time to flow cessation (panel B). The lack of protection noted in the IL-6−/− →WT chimeras suggest that blood cell derived IL-6 does not contribute to the enhanced light/dye induced thrombosis elicited by MCAo/R.
Figure 5.

Light/dye-induced thrombosis responses following MCAo/R in WT (WT→WT) and IL-6−/− (IL-6−/− →WT) bone marrow chimeras. A comparable acceleration in onset time and time to flow cessation was noted following MCAo/R in the WT and IL-6 chimeras. WT→WT control (n=6), WT→WT MCAo/R (n=8), IL-6−/−→WT (n=8), IL-6−/−→WT MCAo/R (n=9) *indicates p<0.05 compared to corresponding control values
Discussion
Transient ischemic attacks (TIA), traditionally defined as an ischemic stroke of short duration that produces focal neurological dysfunction without objective evidence of brain infarction (E et al, 2009; Sacco et al, 2013), are known to render the brain more vulnerable to a subsequent, more severe ischemic episode, with approximately 30% of TIA patients later experiencing a serious stroke (Dennis M, 1990). Antiplatelet drugs, which represent the first line of therapeutic intervention after TIA, show significant clinical efficacy in the secondary prevention of strokes (Johnston, 2005). However, the risk of stroke is reduced by only one-third even in TIA patients placed on a combination of antiplatelet drugs, such as aspirin and dipyridamole (Diener et al, 1996; CAPRIE Steering Committee, 1996; Field et al, 2013), suggesting the need for alternate therapeutic targets. In a recent animal study from our laboratory (Tang et al, 2014), it was revealed that a brief ischemic insult (2.5, 5.0, or 10.0 min) renders the cerebral vasculature hyper-responsive to thrombus formation in response to vessel wall injury. The transient ischemia-induced enhancement of thrombus development in murine cerebral arterioles was evident as long as 28 days following the ischemic insult, and the thrombosis was unresponsive to treatment with antiplatelet and antithrombotic agents. The results of the present study implicates IL-6, a prothrombotic cytokine that accumulates in plasma and brain tissue after a TIA or stroke (Hoshi et al, 2005; Orion et al, 2008), as a major mediator of the accelerated thrombosis response elicited in cerebral arterioles following a brief episode of focal brain ischemia. This novel finding raises the possibility of targeting IL-6 for the secondary prevention of stroke following a TIA.
Clinical studies have revealed that IL-6 levels are elevated in plasma of patients suffering from TIA or ischemic stroke, and these studies suggest that plasma IL-6 concentration is predictive of the severity of the stroke as well as clinical outcome (Lambertsen et al, 2012, Shaafi et al, 2014). Animal experiments have also demonstrated elevated plasma IL-6 concentrations in the reperfusion period following 45-60 min of MCA occlusion (Chapman et al, 2009; Offner et al, 2009; Terao et al, 2008). The results of our study reveal that plasma IL-6 concentration is significantly elevated even following a very brief (5.0 min) period of ischemia and 24 hr of reperfusion, which is consistent with reports describing increased plasma IL-6 levels in TIA patients (Hoshi et al, 2005). The increased systemic (plasma) IL-6 concentration following ischemic stroke is accompanied by a significant increase in brain tissue IL-6 concentration, which typically achieves a peak tissue level several hours following the early (1-3 hrs after reperfusion) elevation in plasma IL-6 concentration (Chapman et al, 2009). Immune cells (either circulating or residing in the spleen) and several resident cell populations in the brain (e.g, neurons, astrocytes, microglia, endothelial cells) are known to synthesize IL-6 (Woodroofe et al., 1991; Schobitz et al., 1993; Reyes et al., 1999; Van Wagoner et al., 1999). Consequently, the elevated brain tissue IL-6 concentration noted following ischemic stroke may reflect either locally and/or systemically generated IL-6. In an effort to determine the relative contributions of local vs systemic sources of IL-6 to the elevated cytokine level detected in ipsilateral brain tissue after a brief episode of MCA occlusion, we measured tissue IL-6 concentration in plasma and in brain tissue of bone marrow (BM) chimeric mice wherein the circulating cells are incapable of producing IL-6 (IL-6−/−→WT chimeras). The results of these experiments suggest that circulating cells account for all of the rise in plasma IL-6 concentration, while approximately two-thirds of the IL-6 in brain tissue after MCAo/R is derived from circulating cells, with the remaining one-third generated by resident brain cells. This observation is inconsistent with the results of a previous study, wherein WT→IL-6−/− BM chimeras revealed that brain cells are the major source of IL-6 in a different mouse model of MCAo/R that involved a 30 min occlusion period, followed by 48 hrs reperfusion (Gertz et al, 2012). This inconsistency could result from a waning influence of circulating immune cells on IL-6 production in postischemic brain at later reperfusion periods or may reflect a differential response of IL-6 producing brain cells to a more severe ischemic insult.
The results of this study implicate IL-6 in the hyper-responsiveness of cerebral arterioles to thrombus development following exposure of the brain to a brief period of focal ischemia. Using both immunologic (IL-6R blocking antibody) and genetic (IL-6 deficient mice) approaches, we demonstrated that ablation of IL-6 function largely prevented the accelerated thrombus formation elicited by MCAo/R. Our observation that IL-6 ablation protects that cerebral vasculature from thrombosis is consistent with several reports that describe the ability of this cytokine to promote coagulation and alter platelet function (van der Poll et al, 1994; Peng et al, 1994). Furthermore, our results are in line with findings derived from other experimental models of human disease, including sepsis (van der Poll et al, 1994), deep vein thrombosis (Wojcik et al, 2011), and inflammatory bowel disease (Senchenkova et al, 2013), that implicate IL-6 as a critical player in the thrombogenesis that accompanies these conditions. In a recent study (Yan et al, 2014), it was demonstrated that chronic (7-day) infusion of IL-6 via an Alzet pump into WT mice significantly enhanced light/dye-induced thrombus development in cremaster muscle arterioles, as reflected by shorter times of thrombus onset and flow cessation. The plasma IL-6 concentration achieved with this infusion protocol (33 pg/ml) is comparable to the IL-6 level detected in our MCAo/R model (Figure 1), which supports the view that the elevated IL-6 levels elicited by a brief period of brain ischemia is sufficient to promote thrombus development in arterioles of the brain and other organs. Given the pleiotropic actions of IL-6, which include effects on endothelial cells, platelets and the coagulation system, it remains unclear how IL-6 exerts its prothrombotic effects in the postischemic brain and whether this IL-6 dependent reaction reflects the responses on a single or multiple cellular and/or chemical targets.
Another issue addressed in this study is the source of IL-6 that ultimately induces the prothrombotic phenotype in the postischemic cerebral microvasculature. A comparison the cerebral arteriolar thrombosis responses between WT →WT and IL-6−/−→WT BM chimeras revealed similar thrombosis responses to light/dye injury, suggesting that eliminating the IL-6 derived from circulating blood cells did not afford the protection observed in IL-6−/− mice. This observation, coupled to our IL-6 measurements in plasma and brain tissue of the BM chimeras (Figure 4), indicates that the IL-6 derived from brain cells, rather than plasma, is largely responsible for inducing the prothrombogenic phenotype in the postischemic cerebral vasculature. While our findings indicate that brain cells account for only one-third of the increased tissue IL-6 concentration detected in ipsilateral brain tissue after MCAo/R, it would appear that either this concentration is sufficient to elicit the thrombosis response or, more likely, that the cells generating the IL-6 lie within (e.g., endothelial cells) and/or in close proximity (e.g., neurons, astrocytes) to cerebral arterioles. For example, astrocytes, which extend their foot processes around cerebral arterioles in mouse brain (Yata et al, 2014), may generate locally high levels of IL-6 that account for the critical role of this cytokine in mediating the accelerated arteriolar thrombosis in the postischemic brain. Additional work is needed to definitively identify the cellular source(s) of the IL-6 that underlies the MCAo/R induced thrombogenesis.
In conclusion, the findings of this study support a role for interleukin-6 in mediating the enhanced thrombus development that occurs in the cerebral microvasculature following a brief episode of focal brain ischemia. Our results also suggest that brain cell-derived IL-6 is largely responsible for this microvascular response. The relevance of these findings to thrombotic strokes of intracranial origin that involve thrombus development in larger arteries remains unclear. However, this work suggests that consideration should be given to IL-6 as a therapeutic target for secondary stroke prevention following a transient ischemic attack.
Supplementary Material
Highlights.
Transient brain ischemia accelerated thrombus formation in cerebral arterioles.
The enhanced thrombosis response was associated with elevated brain & plasma IL-6.
Genetic or immunologic blockade of IL-6 blunted the ischemia-enhanced thrombosis.
The IL-6 mediating this response is derived from brain cells rather than blood cells.
Acknowledgments
This work was supported by a grant from the National Heart Lung and Blood Institute (HL26441-32).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayata C, Dunn AK, Gursoy-OZdemir Y, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–755. doi: 10.1097/01.WCB.0000122745.72175.D5. [DOI] [PubMed] [Google Scholar]
- CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996 Nov 16;348:1329–39. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- Chapman KZ, Dale VQ, Dénes A, Bennett G, Rothwell NJ, Allan SM, McColl BW. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cereb Blood Flow Metab. 2009;29:1764–8. doi: 10.1038/jcbfm.2009.113. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J. Cereb. Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischemic attacks in the Oxfordshire Community Stroke Project. Stroke. 1990;21(6):848–53. doi: 10.1161/01.str.21.6.848. [DOI] [PubMed] [Google Scholar]
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143(1-2):1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276–93. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8(9):1254–66. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field TS, Nakajima M, Benavente OR. Combination aspirin and clopidogrel for secondary prevention of ischemic stroke. Curr Treat Options Cardiovasc Med. 2013;15(3):348–59. doi: 10.1007/s11936-013-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadient RA, Otten U. Identification of interleukin-6 (IL-6)-expressing neurons in the cerebellum and hippocampus of normal adult rats. Neurosci Lett. 1994;182:243–6. doi: 10.1016/0304-3940(94)90807-9. [DOI] [PubMed] [Google Scholar]
- Gertz K, Kronenberg G, Kälin RE, Baldinger T, Werner C, Balkaya M, Eom GD, Hellmann-Regen J, Kröber J, Miller KR, Lindauer U, Laufs U, Dirnagl U, Heppner FL, Endres M. Essential role of interleukin-6 in post-stroke angiogenesis. Brain. 2012;135:1964–80. doi: 10.1093/brain/aws075. Pt 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci. 2006;7(6):437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Herrmann O, Tarabin V, Suzuki S, Attigah N, Coserea I, Schneider A, Vogel J, Prinz S, Schwab S, Monyer H, Brombacher F, Schwaninger M. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:406–15. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M. Relations of serum high-sensitivity C-reactive protein and interleukin-6 levels with silent brain infarction. Stroke. 2005;36(4):768–72. doi: 10.1161/01.STR.0000158915.28329.51. [DOI] [PubMed] [Google Scholar]
- Johnston SC. Transient ischemic attack: a dangerous harbinger and an opportunity to intervene. Semin Neurol. 2005;25(4):362–370. doi: 10.1055/s-2005-923530. [DOI] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41(2-3):172–9. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Chan PH. Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke. 2011;42:3574–9. doi: 10.1161/STROKEAHA.111.626648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, Sawada N, Soydan G, Lee HS, Zhou Z, Hwang SK, et al. Additive effects of statin and dipyridamole on cerebral blood flow and stroke protection. J. Cereb blood Flow Metab. 2008;28(7):1285–1293. doi: 10.1038/jcbfm.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J. Cereb. Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32(9):1677–98. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu GM, Green D, Bellmeyer A, Baker CM, Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ, Chandel NS, Dean DA, Sznajder JI, Budinger GR. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117:2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158(3):1098–111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orion D, Schwammenthal Y, Reshef T, Schwartz R, Tsabari R, Merzeliak O, Chapman J, Mekori YA, Tanne D. Interleukin-6 and soluble intercellular adhesion molecule-1 in acute brain ischaemia. Eur J Neurol. 2008;15(4):323–8. doi: 10.1111/j.1468-1331.2008.02066.x. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Fabry Z, Coe CL. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain Res. 1999;851(1-2):215–20. doi: 10.1016/s0006-8993(99)02189-7. [DOI] [PubMed] [Google Scholar]
- Peng J, Friese P, George JN, Dale GL, Burstein SA. Alteration of platelet function in dogs mediated by interleukin-6. Blood. 1994;83:398–403. [PubMed] [Google Scholar]
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV. American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–89. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöbitz B, de Kloet ER, Sutanto W, Holsboer F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Eur J Neurosci. 1993;5(11):1426–35. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Senchenkova EY, Komoto S, Russell J, Almeida-Paula LD, Yan LS, Zhang S, Granger DN. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am J Pathol. 2013;183:173–81. doi: 10.1016/j.ajpath.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaafi S, Sharifipour E, Rahmanifar R, Hejazi S, Andalib S, Nikanfar M, Baradarn B, Mehdizadeh R. Interleukin-6, a reliable prognostic factor for ischemic stroke. Iran J Neurol. 2014;13(2):70–6. [PMC free article] [PubMed] [Google Scholar]
- Spychalowicz A, Wilk G, Sliwa T, Ludew D, Guzik TJ. Novel therapeutic approaches in limiting oxidative stress and inflammation. Curr Pharm Biotechnol. 2012;13:2456–66. [PubMed] [Google Scholar]
- Stokes KY, Calahan L, Russell JM, Gurwara S, Granger DN. Role of platelets in hypercholesterolemia-induced leukocyte recruitment and arteriolar dysfunction. Microcirculation. 2006;13:377–388. doi: 10.1080/10739680600745877. [DOI] [PubMed] [Google Scholar]
- Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke. 2008;39(3):943–50. doi: 10.1161/STROKEAHA.107.494542. [DOI] [PubMed] [Google Scholar]
- Tang YH, Vital S, Russell J, Seifert H, Senchenkova E, Granger DN. Transient ischemia elicits a sustained enhancement of thrombus development in the cerebral microvasculature: effects of anti-thrombotic therapy. Exp Neurol. 2014;261:417–23. doi: 10.1016/j.expneurol.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des. 2008;14(33):3574–89. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Levi M, Hack CE, ten Cate H, van Deventer SJ, Eerenberg AJ, de Groot ER, Jansen J, Gallati H, Buller HR, ten Cate JW, Aarden LA. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179:1253–1259. doi: 10.1084/jem.179.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagoner NJ, Benveniste EN. Interleukin-6 expression and regulation in astrocytes. J Neuroimmunol. 1999;100(1-2):124–39. doi: 10.1016/s0165-5728(99)00187-3. [DOI] [PubMed] [Google Scholar]
- Wojcik BM, Wrobleski SK, Hawley AE, Wakefield TW, Myers DD, Jr, Diaz JA. Interleukin-6: a potential target for post-thrombotic syndrome. Ann Vasc Surg. 2011;25:229–39. doi: 10.1016/j.avsg.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Woodroofe MN, Sarna GS, Wadhwa M, Hayes GM, Loughlin AJ, Tinker A, Cuzner ML. Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J Neuroimmunol. 1991;33:227–36. doi: 10.1016/0165-5728(91)90110-s. [DOI] [PubMed] [Google Scholar]
- Yan SL, Russell J, Granger DN. Platelet activation and platelet-leukocyte aggregation elicited in experimental colitis are mediated by interleukin-6. Inflamm Bowel Dis. 2014;20(2):353–62. doi: 10.1097/01.MIB.0000440614.83703.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yata K, Nishimura Y, Unekawa M, Tomita Y, Suzuki N, Tanaka T, Mizoguchi A, Tomimoto H. In vivo imaging of the mouse neurovascular unit under chronic cerebral hypoperfusion. Stroke. 2014;45(12):3698–703. doi: 10.1161/STROKEAHA.114.005891. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lukowski R, Gaertner F, Lorenz M, Legate KR, Domes K, Angermeier E, Hofmann F, Massberg S. Thrombocytosis as a response to high interleukin-6 levels in cGMP-dependent protein kinase I mutant mice. Arterioscler Thromb Vasc Biol. 2013;33(8):1820–8. doi: 10.1161/ATVBAHA.113.301507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



