Abstract
Objective
The PI3K/Akt pathway is frequently dysregulated in endometrial cancer, the most common gynecologic malignancy. Emerging evidence identifies the ubiquitin ligase NEDD4 as a key regulator of the PI3K/Akt pathway via activation of insulin-like growth factor-1 receptor (IGF-1R). Our objective was to understand the role of NEDD4 in endometrial cancer.
Methods
NEDD4 expression was assessed by immunohistochemistry in a tissue microarray with 77 endometrial lesions ranging from normal benign endometrium to tumor specimens of varying stage and grade. Studies were extended to a panel of eight endometrial cancer cell lines phenotypically representing the most common endometrial patient tumors.
Results
Immunohistochemistry demonstrated robust staining of NEDD4 in endometrial tumor specimens, with greater NEDD4 expression in the most aggressive tumors. Expression of NEDD4 was detected in a majority of endometrial cancer cell lines surveyed. Exogenous overexpression of murine Nedd4 in endometrial cancer cell lines with modest endogenous NEDD4 expression resulted in a significant increase in the rate of proliferation. Nedd4 overexpression also promoted an increase in cell surface localization of IGF-1R and activation of Akt. Inhibition of PI3K/Akt signaling reversed the enhanced cell growth in Nedd4-overexpressing endometrial cancer cells. In addition, expression of NEDD4 in endometrial tumors positively correlated with the Akt downstream effector FoxM1.
Conclusions
This study identifies NEDD4 as a putative oncogene in endometrial cancer that may augment activation of the IGF-1R/PI3K/Akt signaling pathway.
Keywords: Nedd4, IGF-1R, endometrial cancer, PI3K
Introduction
Endometrial cancer, the most common gynecologic malignancy, is frequently associated with alternations in the phosphoinositide-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway [1, 2]. Activation of PI3K is a hallmark for aggressive endometrial tumors [3]. The mechanism of constitutive PI3K/Akt activation in endometrial tumors includes inactivating mutations in PTEN, amplification of the PI3K catalytic subunit (PIK3CA), and activating mutations in PIK3CA and the PI3K regulatory (PI3KR) subunits [4]. Mutations in the fibroblast growth factor receptor type II (FGFR2), which occur in ~10–12% of endometrial tumors [5, 6], may also result in sustained PI3K/Akt activity. In addition, a subset of endometrial tumors is addicted to signaling through the insulin-like growth factor receptor (IGF-1R) [7, 8], another growth factor receptor that produces PI3K/Akt activation.
IGF-1R activation and downstream signaling is tightly controlled through multiple mechanisms, including regulation of cell-surface expression and stability of adaptor proteins. For example, NEDD4, a prototypic member of the family of the HECT-type ubiquitin ligases, is required for embryonic growth by positively regulating IGF-1R signaling. Several mechanisms have been proposed, including regulation of IGF-1R cell-surface expression and stability as well as ubiquitination of insulin receptor substrate-2 (IRS-2), an IGF-1R substrate [9–12]. Genetic deletion of murine NEDD4 (Nedd4) results in a significant reduction in the cell surface expression of IGF-1R and a strong inhibition of the PI3K/Akt signaling [9]. These data implicate NEDD4 as a potentiator of IGF-1R survival signaling, which may have deleterious consequences in the setting of cancer. Consistent with this notion, NEDD4 overexpression has been documented in prostate, gastric, colorectal, and bladder cancers and non-small cell lung carcinoma [12, 13], though no studies to date have evaluated NEDD4 expression in endometrial cancer.
Building on our previous studies of murine Nedd4 in IGF-1R normal biology, our objective in this study was to understand the role of NEDD4 in endometrial cancer. Utilizing patient tumor specimens and a panel of endometrial cancer cell lines, our data demonstrate increased expression of NEDD4 in endometrial cancer, with levels increasing with the progression of endometrial cancer from an inflammatory or hyperplastic endometrium. Exogenous overexpression of Nedd4 in endometrial cancer cells resulted in accelerated growth, increased activation of Akt and cell-surface localization of IGF-1R. Levels of NEDD4 in endometrial tumors correlated with expression of FoxM1, a transcription factor downstream of Akt that regulates expression of numerous pro-growth genes including NEDD4 [14]. These data suggest that NEDD4 may be an oncogene in endometrial cancer.
Materials and Methods
Immunohistochemistry
Tissue microarray (TMA) was purchased from US Biomax (Cat. UT801, Rockville, MD), which contains tissue samples covering the spectrum of endometrial disease. Within the malignant endometrial adenocarcinoma (EA) samples, all were endometrioid adenocarcinomas except for two grade I serous adenocarcinomas and one grade II serous adenocarcinoma. Immunohistochemical staining of the TMAs was conducted with standard method using Vectastain ABC kits. Briefly, slides were de-paraffinized according to standard protocols and blocked in 5% normal goat serum for 1 hr before application of the primary antibody (NEDD4, Cell Signaling, Danvers MA; FoxM1, LifeSpan BioSciences, Seattle WA). The slides were counterstained with hematoxylin, dehydrated, and mounted using standard procedures. Tissues were first reviewed by a pathologist and then scored independently by two investigators blinded to the sample identity.
Cell culture and generation of Nedd4-overexpressing cells
Six endometrial cancer cell lines (AN3CA, RL95-2, Hec1A, SKUT1B, ECC-1, and KLE) were obtained from ATCC and grown according to the recommended guidelines. Two endometrial cancer cell lines, Ishikawa H and Hec50co (gifts from Dr. Erlio Gurpide, New York University), were grown in DMEM media supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin. To generate a Nedd4-overexpressing endometrial cancer cell line, FLAG-tagged mouse Nedd4 cDNA (a generous gift from Dr. Sharad Kumar, University of Adelaide, Australia) was subcloned into the FIV shuttle vector FIV3.2RSVmcsCMVmCherry (obtained from the Gene Transfer Core Facility, University of Iowa). The mouse Nedd4 is under the control of an RSV promoter while the expression of mCherry is under the control of a CMV promoter. Nedd4-expression FIV vector was generated at the Gene Transfer Core Facility at the University of Iowa and the final viral yield was 1 × 109 TU/ml. Three different viral titers were used for infection of Ishikawa cells: 3.3 × 106, 6.4 × 105, and 1.3 × 105 TU/ml. Briefly, 70% confluent Ishikawa cells were used for infection, in DMEM with 2% FBS, with indicated viral titer, at 37°C for 6 hrs, then replaced with DMEM with 10% FBS. Twenty-four to 48 hrs after infection, mCherry expression status was examined and clonal populations were generated from single mCherry-positive cells. Cells were maintained in DMEM supplemented with 10% FBS.
Cell growth measurement
An equal number of cells (300,000) were plated per well. After growth for 24, 48 and 72 hrs in normal standard growth media containing 10% FBS, cells were trypsinized, stained with trypan blue, and counted with a hemacytometer. Data are expressed as the fold increase in cell number relative to the initial number of cells plated.
Western blotting
Cells were solubilized in cold NP-40 cell lysis buffer (150 mM NaCl, 50 mM Tris/HCl, pH 7.4, 1% NP-40, and a protease and phosphatase inhibitor cocktail from Pierce). Lysates were analyzed by Western blotting with specific primary (NEDD4, (Cell Signaling, Danvers MA), Akt (#4691, Cell Signaling), Akt phospho-S473 (#4060, Cell Signaling), phospho-p44/42 MAPK (Erk1/2 Thr202/Tyr204, #4370, Cell Signaling), and β-actin (#A1978, Sigma Aldrich) and HRP-conjugated secondary antibodies. Where indicated, cells were serum-starved in DMEM with 1% FBS for 2 hrs prior to treatment with IGF-1 (Santa Cruz Biotechnology) for 10 min.
Cell viability assays
Ninety-six well plates were seeded with 10,000 cells in each well 24 hrs prior to drug treatment. Cells were treated with ZSTK474 or BEZ235 (LC Laboratory) or DMSO control for 3 days, followed by examination of cell viability using the WST-1 assay (Clontech) per the manufacturer’s instructions. Each experiment was carried out in triplicate and % cell viability calculated relative to parental Ishikawa cells treated with control DMSO. Data are reported as the average of three biological replicates.
Immunofluorescence
Cells were plated on glass coverslips and, 24 hrs later, serum starved for 2 hrs in DMEM with 1% FBS. After treatment with IGF-1 for 10 min, cells were fixed and immunostained with primary antibody against IGF-1R and Alexa-Fluor 488-conjugated secondary antibody. Nuclei were stained with DAPI and cells were visualized by confocal microscopy.
Statistical analysis
One-way or two-way analysis of variance (ANOVA) was used for comparison among multiple groups. A p value < 0.05 was considered statistically significant.
Results
NEDD4 expression is increased during tumorigenesis in the endometrium
We first examined expression of NEDD4 in endometrial tumor specimens and found that NEDD4 levels were markedly higher in malignant tissue as compared to benign endometrium (Figure 1A). Quantitation of NEDD4 immunostaining in normal, inflammatory, hyperplastic, and malignant endometrial tissues demonstrated a progressive increase in NEDD4 expression (Figure 1B). NEDD4 is overexpressed in different types of endometrial cancer as demonstrated by the increased expression of NEDD4 in endometrial adenocarcinomas of varying grades as well as in squamous cell carcinoma and metastatic lesions (Figure 1C). We detected a trend towards higher NEDD4 expression in endometrial adenocarcinomas relative to the other cell types, consistent with the frequent aberrations in PI3K/Akt signaling in this tumor subtype [4].
Figure 1. NEDD4 expression increases with progression from hyperplastic endometrium to endometrial cancer.
(A) Expression of NEDD4 by immunohistochemistry. Box in left panels indicates region presented in right panels. Scale bar = 200 μm (left panels); 100 μm (right panels) (B) Relative NEDD4 levels in indicated endometrial specimens as determined by immunohistochemistry. ‡ p<0.05 vs. normal endometrium (Normal); † p<0.05 vs. inflammatory (Inflam) endometrium; * p < 0.05 vs. endometrial hyperplasia (Hyper). Mal, malignant. (C) Relative NEDD4 levels in endometrial tumors of varying stage and grade. EA, endometrial adenocarcinoma; SCC, squamous cell carcinoma; MEA, metastatic endometrial adenocarcinoma. N values for B and C are denoted in the figures.
Nedd4 overexpression increases cell growth and Akt activation
We next surveyed NEDD4 protein expression in a panel of eight endometrial cancer cell lines. NEDD4 was detected at varying levels in all surveyed endometrial cancer cell lines (Figure 2A). In order to understand the role of NEDD4 in tumor aggressiveness, we overexpressed it in Ishikawa cells that have low baseline NEDD4 levels. Specifically, we introduced murine Nedd4 using a lentiviral vector that expresses mCherry under a separate promoter. We first established that an MOI of 10 was sufficient to achieve infection of >90% of Ishikawa cells (Figure 2B). Two stable Nedd4-overexpressing Ishikawa cell lines were generated, and Nedd4 levels were more than 10-fold higher in these cells as compared to parental Ishikawa cells (Figure 2C). The band present in control Ishikawa cells is human NEDD4; no commercially available antibodies can distinguish between mouse Nedd4 and human NEDD4. Nedd4 overexpression was associated with a significant increase in cell growth at 24, 48 and 72 hrs (Figure 2D).
Figure 2. Lentiviral-mediated overexpression of murine Nedd4 in Ishikawa endometrial cancer cells accelerates cell growth.
(A) Representative immunoblot of NEDD4 (115 kDa) as determined by Western blotting. β-actin, loading control. Ish, Ishikawa. (B) Infection efficiency of murine Nedd4 in Ishikawa cells (MOI=10) as determined by expression of mCherry. Left panel, bright field; middle panel, mCherry fluorescence; right panel, merged image. (C) Expression of Nedd4/NEDD4 in control Ishikawa cells (Ish) or Nedd4-overexpressing Ishikawa cells (IshNedd4) by Western blotting. β-actin, loading control. (D) Cell growth as determined by cell counting. Data were quantitated as the fold increase in cell number relative to the initial number of cells plated (T0, time 0). * p < 0.05 vs. control Ishikawa cells.
We next examined potential mechanisms by which Nedd4 overexpression augments growth of Ishikawa endometrial cancer cells. Baseline phosphorylation of ERK and Akt was substantially higher in Nedd4-overexpressing cells as compared to control (Figure 3A). IGF-1 treatment produced the anticipated increase in Akt phosphorylation at Ser 473, and this effect was augmented by Nedd4 overexpression (Figure 3A). By contrast, IGF-1 stimulation had no effect on ERK phosphorylation in either control or Nedd4-overexpressing cells, indicating the potential for ERK activation through other non-IGF-1-dependent pathways. Control and Nedd4-overexpressing cells were also treated with increasing concentrations of BEZ235 and ZSTK474, inhibitors of PI3K/Akt signaling. As shown in Figure 3B and C, both inhibitors reversed the enhanced cell growth in Nedd4-overexpressing cells to levels observed in parental cells.
Figure 3. Overexpression of murine Nedd4 in endometrial cancer cells augments Akt activation.

(A) Representative immunoblots of phospho-ERK (Thr 202/204), phospho-Akt (Ser 473), total Akt, and β-actin in control (Ishikawa) or Nedd4-overexpressing Ishikawa cells (IshikawaNedd4) treated with the indicated concentrations of IGF-1 for 10 min. (B, C) Cell viability after exposure to increasing concentrations of BEZ235 (B) or ZSTK474 (C) for 72 hrs. The percent (%) cell viability was calculated relative to control Ishikawa cells treated with DMSO. Data are reported as the average of three biological replicates.
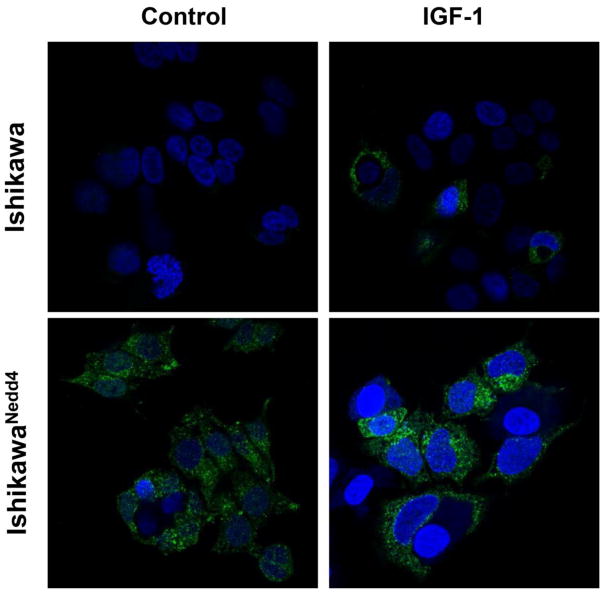
Nedd4 overexpression induces IGF-1R cell-surface expression
We previously reported that Nedd4 deficiency in mice abrogates PI3K/Akt signaling by sequestering IGF-1R in the cytoplasm [9]. We therefore assessed distribution of IGF-1R in Nedd4-overexpressing Ishikawa cells. In unstimulated control Ishikawa cells, minimal cell-surface expression of IGF-1R was detected by fluorescence microscopy (Figure 4). By contrast, cell-surface levels of IGF-1R were notably higher in Nedd4-overexpressing cells, which is consistent with the increased baseline activation of Akt in these cells. Treatment with IGF-1 for 5 min promoted redistribution of IGF-1R to the cell surface in both control and Nedd4-overexpressing Ishikawa cells (Figure 4).
Figure 4. Overexpression of murine Nedd4 in endometrial cancer cells promotes cell-surface expression of IGF-1R.
Representative images of immunostaining for IGF-1R (green) using an antibody that recognizes the extracellular epitope. Cells were starved for 2 hrs in 1% FBS, followed by treatment with or without IGF-1 (10 ng/ml) for 5 min. Nuclei were counterstained with DAPI (blue).
Correlation of NEDD4 with FoxM1 in endometrial tumors
Akt has been shown to relieve FoxO3a-mediated suppression of the transcription factor FoxM1 [15], which regulates expression of numerous genes including NEDD4 [14]. We therefore assessed the relationship between NEDD4 and FoxM1 expression in the benign endometrium and endometrial tumors. We observed a robust positive correlation in expression of NEDD4 and FoxM1 (Figure 5). These data, taken with previously published findings, suggest a positive feedback loop whereby elevated levels of NEDD4 in endometrial cancer drive IGF-1R signaling through PI3K/Akt, which activates the downstream transcription factor FoxM1 and further augments NEDD4 expression (Figure 6).
Figure 5. Increased NEDD4 in endometrial tumors correlates with elevated FoxM1.
(A) Representative immunohistochemical images of FoxM1 expression in normal and malignant endometrial tumor specimens. (B) Correlation of NEDD4 and FoxM1 expression in normal and malignant endometrial tumor specimens.
Figure 6. Schematic depicting the proposed role for NEDD4 in endometrial cancer via regulation of IGF-1R signaling.
Increased growth with Nedd4 overexpression is associated with increased cell-surface expression of IGF-1R as well as enhanced Akt activation in response to IGF-1. The positive correlation of Nedd4 and FoxM1 overexpression in endometrial cancer suggests a positive feedback loop in which increased Nedd4 results in Akt activation, relief of FoxM1 suppression, and FoxM1-mediated transcription of Nedd4.
Discussion
NEDD4 is established as a key regulator of IGF-1R function. Since endometrial cancer is typified by alterations in PI3K/Akt signaling, our objective was to determine whether NEDD4 possesses oncogenic function in this tumor type. We first established that NEDD4 levels increase with endometrial tumor development. While we did not detect appreciable differences in NEDD4 in different endometrial tumor subtypes, there was a trend towards higher NEDD4 in endometrial adenocarcinomas. The tissue array contained primarily endometrioid adenocarcinoma samples, which have frequent aberrations in PI3K/Akt signaling [4]. Overexpression of murine Nedd4 in endometrial cancer cells with a low level of endogenous NEDD4 resulted in increased cell-surface localization of IGF-1R, IGF-1-mediated Akt activation, and cell growth. We also provide evidence that inhibition of the PI3K/Akt pathway using two different inhibitors reverses the enhanced growth associated with Nedd4 overexpression. In addition, NEDD4 levels in endometrial tumors positively correlated with expression of the transcription factor FoxM1, suggesting activation of PI3K/Akt signaling through IGF-1R. Taken together, our data suggest that NEDD4 may be a master regulator of the PI3K/Akt pathway in endometrial cancer by controlling cell-surface expression of IGF-1R.
The first evidence for NEDD4 in tumorigenesis came from studies of murine prostate tumors and human bladder cancer specimens [16]. More recently, expression of NEDD4 has been documented in more than 80% of colorectal carcinomas, 75% of gastric carcinomas, 80% of non small-cell lung carcinomas, and tumors of neurologic origin [14, 17, 18]. Few studies have provided mechanistic insight into the effects of elevated NEDD4 in cancer. Most of the studies that have examined NEDD4 in cancer have demonstrated an inverse relationship between NEDD4 and PTEN [12]. More recently, NEDD4 has been identified as the E3 ligase for proteins other than PTEN such as IRS-2 [11] and the prostate-specific androgen-inducible gene PMEPA1, which negatively regulates signaling through androgen receptor [19]. These studies provide additional mechanisms for the putative oncogenic role of NEDD4.
Our previous studies using Nedd4-deficient mouse embryonic fibroblasts revealed that Nedd4 also can affect IGF-1R localization and thereby PI3K signaling independent of its effects on PTEN expression [9]. Our data herein demonstrate that Nedd4 overexpression in endometrial cancer cells augments re-distribution of IGF-1R to the cell surface and increased Akt activation. These effects were observed at baseline and were enhanced by stimulation with IGF-1. Nedd4-overexpressing cells also displayed elevated cell growth at 24, 48 and 72 hrs after plating, and treatment with inhibitors of the PI3K/Akt signaling pathway reverted cell viability to levels observed in parental cells. It is important to recognize that the endometrial cancer cell line Ishikawa used for Nedd4 overexpression studies lacks functional PTEN, which allowed us to examine other effects of Nedd4 independent of its role in PTEN regulation. Thus, our data provide an additional mechanism by which increased levels of NEDD4 provide a growth advantage in cancer. Our findings are congruent with a previous study in colorectal cancer cells that implicated NEDD4 in cell growth-independent of actions on PTEN [20].
Using Nedd4 knockout mice, we have previously reported that Nedd4-dependent IGF-1R signaling is mainly through PI3K/Akt and minimally through the ERK pathway [9]. Consistent with these data, in this study we did not observe appreciable activation of ERK upon stimulation of Ishikawa cells with IGF-1. Overexpression of Nedd4 in Ishikawa cells resulted in a higher baseline phosphorylation, but, as with the parental cells, IGF-1 did not induce ERK activation. These data suggest that overexpression of Nedd4 regulates a compensatory pathway that results in ERK activation. Future studies are necessary to determine the precise mechanism.
Analysis of endometrial tumor specimens revealed a positive correlation between NEDD4 levels and FoxM1 levels. FoxM1 is a transcription factor downstream of Akt that is recognized as a potent oncogene in many cancers [15]. FoxM1 controls expression of many genes that drive a malignant phenotype, including cell cycle checkpoint controllers such as polo-like kinase 1 (PLK1), pro-angiogenic ligand vascular endothelial growth factor (VEGF), and NEDD4 [14, 15]. FoxM1 transcriptional activity is normally suppressed by FoxO3a, which itself is negatively regulated by Akt. Thus, hyperactivation of Akt in tumors relieves FoxO3a-mediated repression of FoxM1 and propagates a tumorigenic phenotype. This is especially relevant in endometrial cancer in which mutations in the PI3K/Akt pathway predominate. The positive correlation between NEDD4 and FoxM1 in endometrial cancer suggests a positive feedback loop in which NEDD4-mediated activation of IGF-1R/PI3K/Akt releases inhibition of FoxM1 by FoxO3a, thus allowing transcription of FoxM1 target genes, including NEDD4. Interestingly, recent studies have shown that NEDD4 regulates Akt nuclear trafficking in response to IGF-1 signaling [21]. Thus, NEDD4 may “hyperactivate” Akt through both receptor-mediated Akt activation and enhanced nuclear transport.
In summary, we identify NEDD4 as a putative oncogene in endometrial cancer. Our mechanistic data suggest that, in addition to the established mechanisms for sustained PI3K/Akt signaling in endometrial tumors, elevated levels of NEDD4 may provide an additional mechanism to drive tumorigenesis. Future clinical efforts that target the PI3K/Akt pathway should take into consideration baseline levels of NEDD4, which may impact the response to treatment.
Research Highlights.
NEDD4 is overexpressed in malignant compared to benign endometrium
NEDD4 expression in endometrial tumors positively correlates with levels the oncogenic transcription factor FoxM1
Overexpression of NEDD4 in endometrial cancer cells promotes IGF-1R cell surface localization, Akt activation and cell growth
Acknowledgments
This work was supported by NIH R01CA99908 and R01CA184101 (KKL), American Cancer Society Institutional Research Grant (SY) and the Department of Obstetrics and Gynecology Research Development Fund (KKL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Authors’ contributions
Y.Z., R.G., K.W.T., K.L.L, and B.Y. conceptualized the hypotheses and designed the research strategy; R.G., Y.Z., Y.L., S.Y., C.J.W., and B.Y. performed the experiments; B.Y. performed the statistical analysis; R.G., Y.L., S.Y., K.W.T. K.K.L., and B.Y. analyzed data; and S.Y., K.K.L., and B.Y. wrote the paper. All authors read and approved the final manuscript.
Conflict of interest
K.W.T and B.Y. are co-owners of Immortagen, L.L.C. All other authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yuping Zhang, Email: Yuping-zhang@uiowa.edu.
Renee Goodfellow, Email: renee-goodfellow@uiowa.edu.
Yujun Li, Email: Yujun-li@uiowa.edu.
Shujie Yang, Email: Shujie-yang@uiowa.edu.
Christopher J. Winters, Email: Christopher-winters@uiowa.edu.
Kristina W. Thiel, Email: Kristina-thiel@uiowa.edu.
Kimberly K. Leslie, Email: Kimberly-leslie@uiowa.edu.
Baoli Yang, Email: Baoli-yang@uiowa.edu.
References
- 1.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011;8:261–71. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 2.Llaurado M, Ruiz A, Majem B, Ertekin T, Colas E, Pedrola N, et al. Molecular bases of endometrial cancer: new roles for new actors in the diagnosis and the therapy of the disease. Mol Cell Endocrinol. 2012;358:244–55. doi: 10.1016/j.mce.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, Stefansson IM, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci U S A. 2009;106:4834–9. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollock PM, Gartside MG, Dejeza LC, Powell MA, Mallon MA, Davies H, et al. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene. 2007;26:7158–62. doi: 10.1038/sj.onc.1210529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C, et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci U S A. 2008;105:8713–7. doi: 10.1073/pnas.0803379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavelic J, Radakovic B, Pavelic K. Insulin-like growth factor 2 and its receptors (IGF 1R and IGF 2R/mannose 6-phosphate) in endometrial adenocarcinoma. Gynecol Oncol. 2007;105:727–35. doi: 10.1016/j.ygyno.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Bruchim I, Werner H. Targeting IGF-1 signaling pathways in gynecologic malignancies. Expert Opin Ther Targets. 2013;17:307–20. doi: 10.1517/14728222.2013.749863. [DOI] [PubMed] [Google Scholar]
- 9.Cao XR, Lill NL, Boase N, Shi PP, Croucher DR, Shan H, et al. Nedd4 controls animal growth by regulating IGF-1 signaling. Sci Signal. 2008;1:ra5. doi: 10.1126/scisignal.1160940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vecchione A, Marchese A, Henry P, Rotin D, Morrione A. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol Cell Biol. 2003;23:3363–72. doi: 10.1128/MCB.23.9.3363-3372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukushima T, Yoshihara H, Furuta H, Kamei H, Hakuno F, Luan J, et al. Nedd4-induced monoubiquitination of IRS-2 enhances IGF signalling and mitogenic activity. Nat Commun. 2015;6:6780. doi: 10.1038/ncomms7780. [DOI] [PubMed] [Google Scholar]
- 12.Boase NA, Kumar S. NEDD4: The founding member of a family of ubiquitin-protein ligases. Gene. 2015;557:113–22. doi: 10.1016/j.gene.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Matesic LE. The Nedd4-like family of E3 ubiquitin ligases and cancer. Cancer Metastasis Rev. 2007;26:587–604. doi: 10.1007/s10555-007-9091-x. [DOI] [PubMed] [Google Scholar]
- 14.Dai B, Pieper RO, Li D, Wei P, Liu M, Woo SY, et al. FoxM1B regulates NEDD4-1 expression, leading to cellular transformation and full malignant phenotype in immortalized human astrocytes. Cancer Res. 2010;70:2951–61. doi: 10.1158/0008-5472.CAN-09-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo CY, Muir KW, Lam EW. FOXM1: From cancer initiation to progression and treatment. Biochim Biophys Acta. 2012;1819:28–37. doi: 10.1016/j.bbagrm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–39. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SS, Yoo NJ, Jeong EG, Kim MS, Lee SH. Expression of NEDD4-1, a PTEN regulator, in gastric and colorectal carcinomas. APMIS. 2008;116:779–84. doi: 10.1111/j.1600-0463.2008.00999.x. [DOI] [PubMed] [Google Scholar]
- 18.Amodio N, Scrima M, Palaia L, Salman AN, Quintiero A, Franco R, et al. Oncogenic role of the E3 ubiquitin ligase NEDD4-1, a PTEN negative regulator, in non-small-cell lung carcinomas. Am J Pathol. 2010;177:2622–34. doi: 10.2353/ajpath.2010.091075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Xu LL, Masuda K, Raymundo E, McLeod DG, Dobi A, et al. A feedback loop between the androgen receptor and a NEDD4-binding protein, PMEPA1, in prostate cancer cells. J Biol Chem. 2008;283:28988–95. doi: 10.1074/jbc.M710528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eide PW, Cekaite L, Danielsen SA, Eilertsen IA, Kjenseth A, Fykerud TA, et al. NEDD4 is overexpressed in colorectal cancer and promotes colonic cell growth independently of the PI3K/PTEN/AKT pathway. Cell Signal. 2013;25:12–8. doi: 10.1016/j.cellsig.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Fan CD, Lum MA, Xu C, Black JD, Wang X. Ubiquitin-dependent regulation of phospho-AKT dynamics by the ubiquitin E3 ligase, NEDD4-1, in the insulin-like growth factor-1 response. J Biol Chem. 2013;288:1674–84. doi: 10.1074/jbc.M112.416339. [DOI] [PMC free article] [PubMed] [Google Scholar]