Abstract
Vocalizations coordinate social interactions in many species and often are important for behaviors such as mate attraction or territorial defense. Although the neural circuitry underlying vocal communication is well-known for some species, such as songbirds, the motivational processes that regulate vocal signals are not as clearly understood. Neurotensin (NT) is a neuropeptide implicated in motivation that can modulate the activity of dopaminergic neurons. Dopaminergic projections from the ventral tegmental area (VTA) are key to mediating highly motivated, goal-directed behaviors, including sexually-motivated birdsong. However, the role of NT in modifying vocal communication or other social behaviors has not been well-studied. Here in European starlings (Sturnus vulgaris) we analyzed relationships between sexually-motivated song and NT and NT1 receptor (NTSR1) expression in VTA. Additionally, we examined NT and NTSR1 expression in four regions that receive dopaminergic projections from VTA and are involved in courtship song: the medial preoptic nucleus (POM), the lateral septum (LS), Area X, and HVC. Relationships between NT and NTSR1 expression and non-vocal courtship and agonistic behaviors were also examined. NT expression in Area X positively related to sexuallymotivated song production. NT expression in POM positively correlated with non-vocal courtship behavior and agonistic behavior. NT expression in POM was greatest in males owning nesting sites, and the opposite pattern was observed for NTSR1 expression in LS. These results are the first to implicate NT in Area X in birdsong, and further highlight NT as a potential neuromodulator for the control of vocal communication and other social behaviors.
Keywords: neurotensin, neuropeptide, vocal communication, motivation, songbird, social behavior
1. Introduction
Vocalizations coordinate social interactions in many species and often are important for behaviors such as mate attraction or territorial defense [1]. The neural circuitry underlying vocal communication is well-known for some animal groups, such as songbirds [2], but the motivational processes that regulate vocal signals are not as clearly understood. Neurotensin (NT) is a neuropeptide implicated in aspects of motivation that also is noted for its strong interactions with dopamine [3, 4]. Dopaminergic projections from the ventral tegmental area (VTA) are key to mediating highly motivated, goal-directed behaviors [5, 6], including sexually-motivated birdsong [7–9]. NT and NT1 receptors (NTSR1; the only known NT receptor in birds [10]) co-localize with dopamine neurons in VTA [11–13]. NT neurons are also present in regions containing dopaminergic projections from VTA [14] that are strongly implicated in sexually-motivated birdsong (reviewed below). NT immunolabeling in VTA positively relates to courtship song [15], suggesting that NT may modulate dopaminergic circuits to influence vocal communication and other motivated behaviors, but this has not been well-studied.
VTA connects reciprocally to multiple brain regions involved in social behavior, including the medial preoptic area (often abbreviated as POM in birds) and lateral septum (LS). POM is an area that is an important regulator of male sexual behavior and sexual motivation [16]. Dopamine in POM plays a complex modulatory role in male sexually-motivated behaviors [see 17 for review], including courtship song. There is some evidence for positive associations between D1 dopamine receptor activation and D1 receptor measures in POM and song production [18, 19]; however, in other studies dopamine markers relate negatively to singing [20, 21]. Though seemingly inconsistent, these results may reflect an inverted U-shaped effect of D1 receptor stimulation in POM on song. In support of this hypothesis, sexually-motivated song in male starlings is facilitated at intermediate levels of D1 receptor stimulation, but reduced at lower and higher levels of receptor stimulation [19]. Furthermore, both male starlings with the highest and lowest D1 expression in the POM sing significantly less than males with intermediate levels of expression [18]. Dopamine in LS has not been previously implicated in courtship song, but the only study to date on NT and vocal communication did show a relationship between NT immunolabeling in LS and courtship song [15]. No associations were seen for NT labeling in POM and song, but given the complex association between dopamine in this region and song, other studies with additional measures of NT are needed to fully understand the role of NT in POM in singing behavior.
The songbird brain contains a specialized group of regions that is necessary for song development and production, known as the song control system [22] [reviewed in 23]. VTA directly projects to the song control nucleus Area X [24], which is part of the avian striatum and is involved in song learning [25, 26] and modifying structural elements of song [27, 28]. Area X has dense concentrations of dopamine and dopamine receptors [29, 30], and dopaminergic activity in Area X is related positively to sexually-motivated song production [21, 31–34]. Additionally, the song control nucleus HVC (acronym used as the proper name) also expresses NT [35, 36] and receives dopaminergic projections from VTA [37]. The role of NT in brain regions involved in vocal control and sensorimotor processing is not known, but given the relationship between singing behavior and dopamine in Area X, and the fact that HVC receives dopaminergic projections from VTA, these regions are potential targets for NT to modify vocal communication.
VTA, POM, and LS are components of the “social behavior network,” a group of reciprocally connected nuclei in which neuropeptides have been hypothesized to act to influence behavior [38–40]. In addition to vocal behavior in starlings (reviewed above), NT in these regions has been implicated in the mediation of other social behaviors in rodents. In mice, NT expression in the medial preoptic area is associated inversely with maternal defense of offspring [41], intracerebroventricular injections of NT reduce this agonistic behavior and increase expression of the immediate early gene cFOS in the medial preoptic area and LS [42], and maternal mice demonstrate altered NT mRNA expression profiles in POM and LS [43]. These findings highlight that NT may be a neuromodulator for multiple social behaviors, including song.
To further explore the association between NT and vocal communication, and to provide insight into the role of NT in social behavior more broadly, we examined NT and NTSR1 relative mRNA expression using quantitative PCR (qPCR) in VTA, POM, LS, Area X, and HVC, and related these measures to singing and other social behaviors in male European starlings (Sturnus vulgaris).
2. Materials and methods
2.1 Animals
Twenty male and 4 female starlings were captured in the winter of 2009–10 on a farm in Madison, WI with baited fly-in traps and brought to the University of Wisconsin-Madison. Birds were housed indoors in stainless steel cages (91 cm × 47 cm × 47 cm) in single sex groups of 5. Food and water were provided ad libitum. All procedures and protocols followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee.
2.2 Housing Conditions
Birds were placed indoors on photoperiods of 18 h light (L):6 dark (D) for 6 weeks, and then 8L:16D for 6 weeks. This sequence of photoperiods induces photosensitivity in starlings, which means that males exposed to day lengths of more than 11 h of light respond with increased gonad volume and plasma T concentrations indicative of the spring breeding season [44]. Males were randomly assigned to outdoor aviaries (2.13 m × 2.4 m × 1.98 m) containing 5 birds each; aviaries contained nest boxes, perches, nesting material, a bird bath, and ad libitum food and water. The natural day length was approximately 13L:11D. Birds habituated to the aviaries for 12 days prior to observations.
2.3 Behavioral Observations
Behavioral observations were conducted by a single observer for 4 consecutive days prior to tissue collection. To observe male courtship behavior, a female was released into an aviary, and fresh nesting material (grass clippings and green leaves) was also placed in the aviary. The same female was used across all aviaries on a given day, but a novel female was used for each new observation day. Aviaries were observed in a rotating order across days for 20 min each day. Singing behaviors observed included the number of complete songs and total time singing (s). Agonistic behavior was measured by recording displacements, which were defined as the number of times an individual approached within 5 cm of another individual who then departed. Non-vocal courtship behavior was defined as the sum of the number of times males entered a nest box, landed on a nest box, gathered nesting material, and wing waved. These behaviors are indicators of sexual motivation because males only perform them during the spring breeding season as a component of courtship behavior [45, 46]. Non-specific behaviors recorded included bouts of feeding and drinking, with bouts separated by at least 2 s from a previous behavior, and calling, which is a non-song vocalization. Only males with nesting sites respond with high rates of courtship song when presented with females [47], indicating that these males are the most highly sexually motivated. In the present study, males were classified as nest box owners or non-owners based their propensity to remain on nest box perches and to enter the box, in order to identify the most highly sexually-motivated individuals.
2.4 Tissue Preparation for qPCR
Following final observations, males were rapidly decapitated and brains were removed and placed in isopentane (Cat. #277258; Sigma, St. Louis, MO) over dry ice for 30 s, and then stored at −80 °C. Brains were sectioned coronally at 250 µm on a cryostat (−15 °C) on slides over dry ice. Samples from VTA, POM, LS, Area X, and HVC were punched using a Stoelting brain punch set (Cat. #57401; Stoelting, Wood Dale, IL) as in [48]. Punches were transferred to centrifuge tubes on dry ice and stored at −80 °C.
RNA was extracted with the Bio-Rad Aurum Total RNA Fatty and Fibrous Tissue Kit (Cat. #732-6830; Bio-Rad, Hercules, CA). Tissue was homogenized with a Dremel tool, RNA was isolated with PureZOL, and then treated with DNase. RNA was eluted with 30 µl nucleotide free water and the RNA concentration was measured with a NanoDrop system (Thermo Scienfitic, Wilmington, DE). RNA integrity was validated with Agilent 2100 BioAnalyzer and Agilent RNA 6000 Pico Kit (Agilent Technologies, Santa Clara, CA).
RNA was converted to single stranded cDNA with the Invitrogen SuperScript III First- Strand Synthesis System (Cat. #18080-051; Life Technologies, Carlsbad, CA). RNA starting amounts for synthesis were 100 ng, except the VTA which was 50 ng due to low yield RNA from tissue punches, following manufacturer instructions. cDNA pooled from the robust nucleus of the arcopallium (RA) was used for standards. Relative gene expression for NT and NTSR1 was quantified in HVC, Area X, POM, VTA, and LS as a normalized ratio to reference genes as described below.
2.5 qPCR
Methods for qPCR follow [48] and are summarized here. The BioRad CFX96 Touch Real-Time PCR Detection System (Cat. #185-5195; Bio-Rad, Hercules, CA) was used for qPCR. For cDNA amplification, samples were mixed with Sso Fast Evagreen Supermix (Cat. #172-5201; Bio-Rad, Hercules, CA), nucleotide free H2O, and 5 µM forward and reverse primers (prepared by University of Wisconsin, Biotech Center). Samples were run along with 5 amplification standards in a 1:4 dilution series, beginning with 500 ng/µl, and a negative control that contained nucleotide free water instead of cDNA. All samples and standards were run in triplicate. Runs had an initiation step at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, followed by a 30 s annealing phase based on the annealing temperature for the primer, and a 20 s elongation phase at 72 °C. Plates went through a melt curve from 60 °C to 88 °C, at 0.5 °C for each 5 s step. Plates were read at each elongation step and each melt curve step. Standard amplification curves assayed the efficiency of the reaction (required efficiency between 90–110% and an R2 of at least 0.990), and a melt curve with a single peak verified the specificity of the PCR products.
NT and NTSR1 primers were designed and screened with NCBI Primer-BLAST using the zebra finch (Taeniopygia guttata) genome (see [48]). The quality of the primers was examined on NetPrimer (Premier Biosoft) for secondary structures. Reference genes for HVC were hydroxymethylbilane synthase (HMBS) and beta actin (BA). Reference genes for Area X, POM, LS, and VTA were hypoxanthine-guanine phosphoribosyltransferase (HPRT) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Table 1 contains the details of primers and runs. Sanger sequencing with both forward and reverse primers at the University of Wisconsin Biotechnology Center was used to sequence the reaction product for the reference genes and NTSR1 (Table 2). Using NCBI BLAST, all sequences match the intended targets. Typical Sanger sequencing could not be used to validate specificity of the NT primer because the relatively small size of the amplicon (70 bp) was below the threshold needed for Sanger sequencing. Therefore, to validate the specificity of the primer, we developed two additional primer sets. One primer set used the original forward primer but had a reverse primer which was intended to amplify a much larger segment of the NT gene (196 bp), while the other primer set contained the original reverse primer and a forward primer designed to amplify a 169 bp product. Both of these primer sets returned Sanger sequences which identified NT as the gene being amplified. Both forward and reverse primers from the original data were contained within these two large sequences, indicating that the original primers were specific for NT (Table 3).
Table 1.
Information for primers used. BA, GAPDH, HMBS, and HPRT were reference genes.
| Gene | Direction | Sequence | Annealing | Product |
|---|---|---|---|---|
| NT | Forward | ATGACCCTGCTAAATGTCTG | 58 °C | 70 bp |
| NM_001245684 | Reverse | CTACCTCTACTGTTTCCCCC | ||
| NTSR1 | Forward | CGTCAACACCGACATCTACTC | 60 °C | 117 bp |
| XM_002194650 | Reverse | AGCGACTTCTTCCTCACCAG | ||
| BA | See Cordes et al., 2015 | |||
| GAPDH | See Riters et al., 2014 | |||
| HMBS | See Cordes et al., 2015 | |||
| HRPT | See Riters et al., 2014 | |||
Table 2.
Product sequencing of primers. BA, GAPDH, HMBS, and HPRT were reference genes.
| Gene | Species for primers | Sequence |
|---|---|---|
| NT | Taeniopygia guttata | See Table 3 |
| NTSR1 | Taeniopygia guttata | GNANNCTGGCTCTTTTCTTGGTGGGCACCGCTGGGCAACTCC ATCACGGCGCTACACGCTGGTGAGGAANAAGTCGCATA |
| BA | Taeniopygia guttata | CNTNGTGCCCTGGGATTTCGAGCAGGAGATGGCCACAGCTGC CTCTAGCTCTTCCCTGGAGAAGAGCTACGAACTCCCTGATGG CCAGGTCATCACCATTGGCAATGAGAGGTTCAGGTGCCCCGA GGCCCTGTTCCAGCCATCTTTCNAA |
| GAPDH | See Riters et al., 2014 | |
| HMBS | Taeniopygia guttata | GGCCCANGGCNTCGCAAAGGGAAACCCACCTTGNATGCTGTT GTCTTTCATCCCAAAAACTGTGGAAAAACACTGAGCCTCCTT CCTGAAAAGAGTGTGATTGGAACNAA |
| HPRT | See Riters et al., 2014 |
Table 3.
Product sequences for the elongated primers derived from the original forward and reverse. See “Materials and Methods: qPCR” for detailed explanation.
| Direction | Primer Sequence | Product | Product Sequence | |
|---|---|---|---|---|
| NT Original |
Forward | ATGACCCTGCTAAATGTCTG | 70 bp | CAATGNNTANCANNNGAAGGGG GAANNGGTAAAGGTAAAA |
| Reverse | CTACCTCTACTGTTTCCCCC | NNCCCGGTNNNGTGNTTGNNGGA ATGGCCGGANCATTTTT |
||
| NT Elongated Derived from Original Forward |
Forward | ATGACCCTGCTAAATGTCTG | 196 bp | ACATAACATCAGNATGGGGGAAA CAGNTAGAGGTAGGATGAGGAGG GATCTGGNTTCCAGGGAAGACAGT TCCCTGCTGCTCTGGACGGCTTCA GCTTGGAAGCAATGCTGACAATAT ATCAACTCCAGAAAGTTTGCCACA GCAGAGCCTTTCAGCATTGGGAGT A |
| Reverse | ACTCCCAATGCTGAAAGGC | |||
| NT Elongated Derived from Original Reverse |
Forward | TGCTCAGATTCAGAAGAGGA | 169 bp | CGATTTATTGACCACTATGGTNAC ATCAAAGATTAACAGAGCAAAACT TCCTTACTGGAAAATGACCCTGCTA AATGTCTGCAATCTTGTCAACAACA TAAACAATCAGATGGGGGAAACA GTAGAGGTAGA |
| Reverse | CTACCTCTACTGTTTCCCCC |
Mean Ct values, the cycle number when a sample crossed the amplification threshold set at 200 RFU, were transformed for each sample through use of the Pfaffl Method [48, 49]. The geometric mean of the Ct values for the two reference genes for each region was used to transform the Ct values for each gene analyzed to a normalized ratio.
2.6 Statistics
Data were analyzed in R v 3.1.1 (x86_64) in Windows 7 [50]. A multiple regression analysis was run for each behavior, with the behavioral measure as the dependent variable and mRNA expression in the brain regions as independent variables. Separate analyses were run for NT expression and NTSR1 expression. The independent variables were not correlated with each other. Model assumptions were tested with residual plots. If assumptions were violated, the dependent variable underwent the transformation log10(x + 1) to satisfy assumptions (specific variables indicated in Results). Outliers were removed if they were highly influential (i.e., the presence or absence of a single point was entirely responsible for significant or non-significant results). In the analyses of complete songs and time singing, one individual was an influential outlier with behavioral values of more than 2 standard deviations outside the mean and was removed (see Results). For NT expression, 1 value was missing in Area X and LS, and these values were replaced with the mean expression for each area. However, 3 values were missing in VTA, and rather than mean replacing these points, NT expression in VTA was removed from the model and analyzed separately. We found that retaining VTA in the analyses with mean replacement, without mean replacement, and removing VTA entirely did not drastically alter the models, but we report here results from models with VTA removed as they best modeled the data. Thus, for each behavior, one model was run that included mRNA expression in POM, LS, Area X, and HVC; a separate model was run that included only mRNA expression in VTA. NTSR1 expression was analyzed in a similar manner, but no significant models were produced (see Results).
Student’s t-tests were used to compare expression in brain regions between the most highly sexually-motivated birds (nest box owners) and those who demonstrated less sexual motivation (non-owners). Measures were log-transformed as described above if they violated assumptions (for normality: Shapiro-Wilk test; for equal variance: Levene’s test).
3. Results
3.1 Singing Behaviors
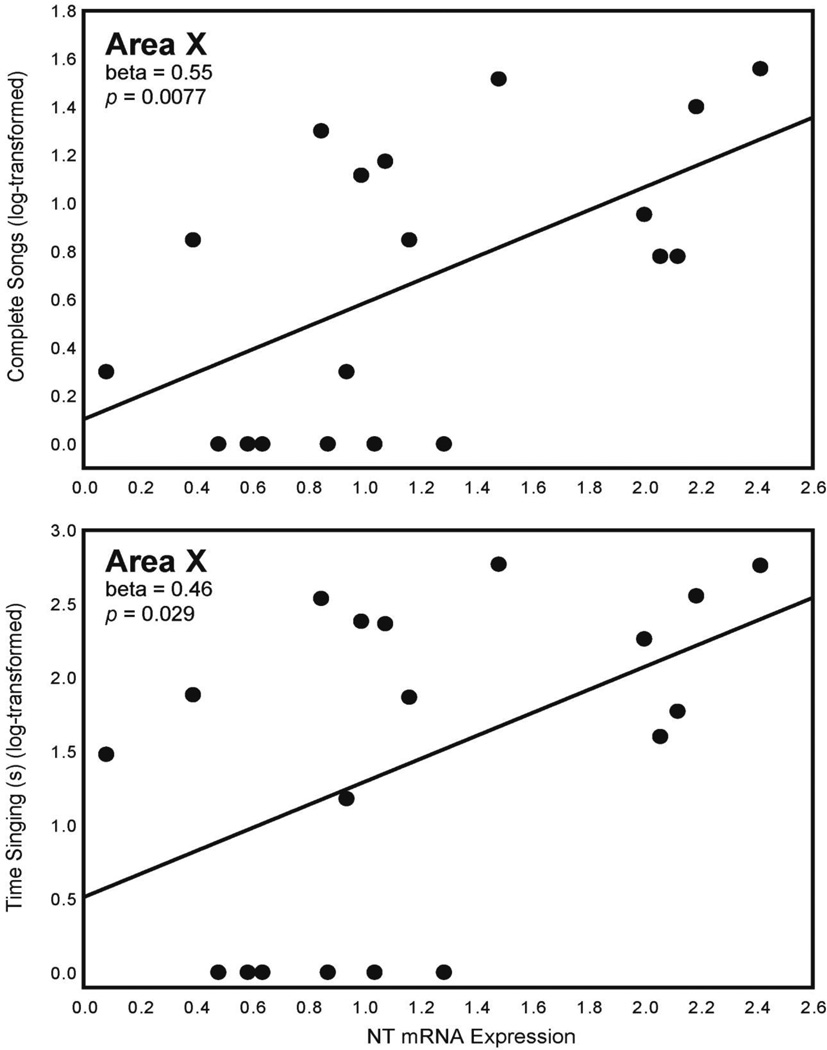
Multiple regression analyses for singing behaviors with NT expression in Area X, HVC, POM, and LS as independent variables were significant (Table 4). NT expression in Area X statistically explained the number of complete songs (log-transformed) (Fig. 1; Table 4). Similarly, NT expression in Area X statistically explained time singing (log-transformed) (Fig. 1; Table 4). One individual was dropped from these analyses because its behavioral measures were more than 2 standard deviations outside the mean for complete songs (value = 39; average = 10.35±12.76) and time singing (value = 712; average = 175.70±225.37); n = 19 for both analyses). No relationships were seen between singing behaviors and NT expression in separate analyses of VTA.
Table 4.
Regression models demonstrating brain areas in which NT expression statistically explains variation in behavior. Significant predictor variables are in bold.
| Behavior | Model Statistics | Variables | Beta | p-Values |
|---|---|---|---|---|
| Complete songs (log-transformed); n = 19 | Adjusted R2 = 0.48, F4,14 = 5.12, p = 0.0094 | Area X | 0.55 | 0.0077 |
| POM | 0.31 | 0.10 | ||
| HVC | −0.27 | 0.15 | ||
| LS | 0.26 | 0.16 | ||
| Time singing (log-transformed); n = 19 | Adjusted R2 = 0.42, F4,14 = 4.30, p = 0.018 | Area X | 0.46 | 0.029 |
| POM | 0.30 | 0.12 | ||
| HVC | −0.34 | 0.088 | ||
| LS | 0.25 | 0.19 | ||
| Non-vocal courtship behavior (log-transformed); n = 20 | Adjusted R2 = 0.31, F4,15 = 3.09, p = 0.048 | Area X | 0.049 | 0.81 |
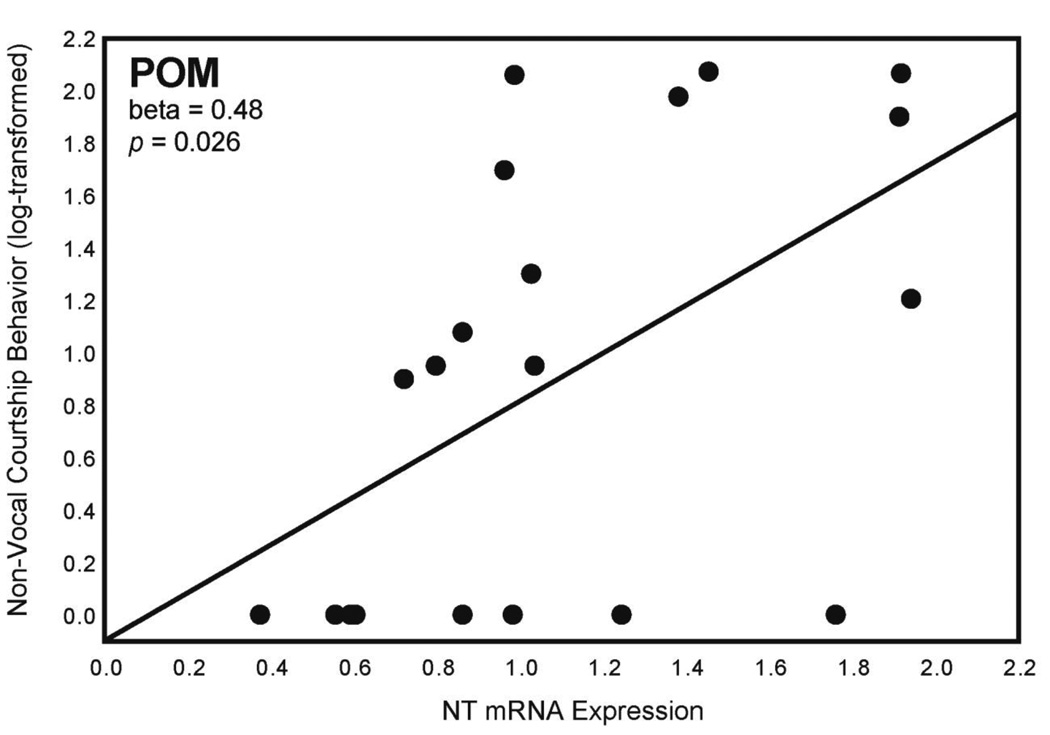
| POM | 0.48 | 0.026 | ||
| HVC | 0.33 | 0.12 | ||
| LS | 0.23 | 0.25 | ||
| Agonistic behavior (log-transformed); n = 20 | Adjusted R2 = 0.31, F4,15 = 3.11, p = 0.048 | Area X | 0.19 | 0.35 |
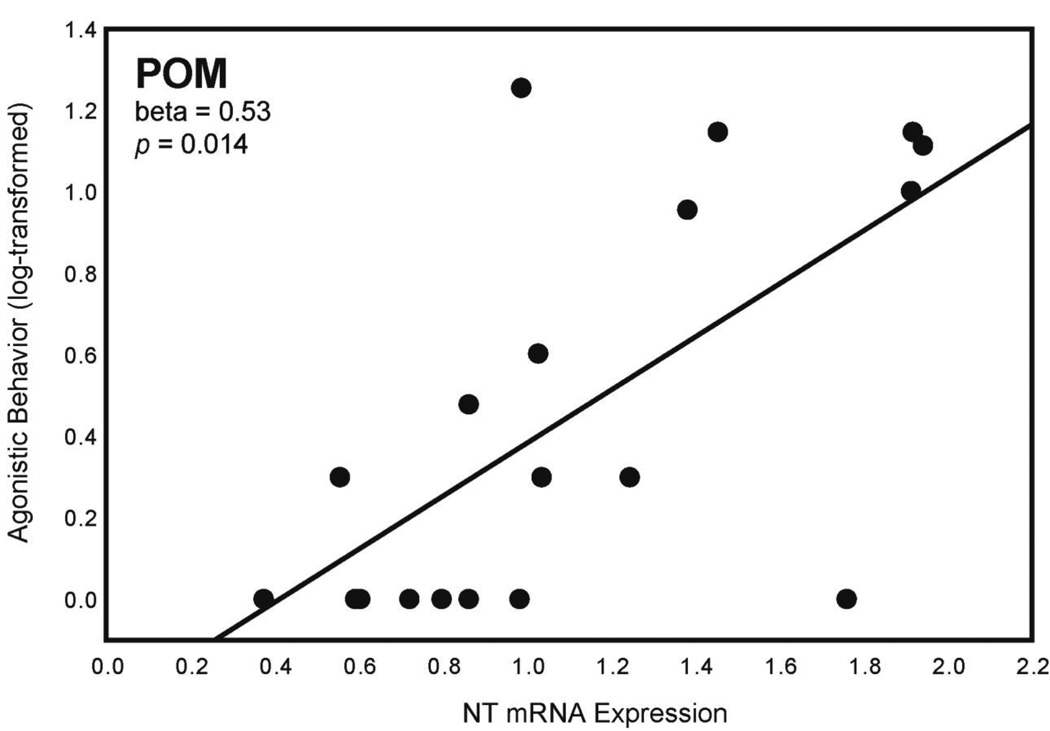
| POM | 0.53 | 0.014 | ||
| HVC | −0.083 | 0.68 | ||
| LS | 0.31 | 0.13 | ||
| Calling | No significant model | (none) | ||
| Feeding | No significant model | (none) | ||
| Preening | No significant model | (none) |
Figure 1. NT expression in Area X and singing behavior.
Two-dimensional visualization of multiple regression models with 1 significant variable showing the relationship between NT mRNA expression (X-axis; presented as a ratio as described in Section 2.5) in Area X and the number of complete songs (log-transformed; Y-axis, top) and time singing (log-transformed; Y-axis, bottom). Statistics were computed in a multiple regression model and do not indicate values of individual correlations (see Table 4). n = 19 for each analysis.
3.2 Non-vocal courtship behavior
A multiple regression analysis for non-vocal courtship behavior with NT expression in Area X, HVC, POM, and LS as independent variables was significant (Table 4). NT expression in POM statistically explained variation in non-vocal courtship behavior (log-transformed) (Fig. 2; Table 4); n = 20. No relationships were seen between non-vocal courtship behavior and NT expression in a separate analysis of VTA.
Figure 2. NT expression in POM and non-vocal courtship behavior.
Two-dimensional visualization of a multiple regression model with 1 significant variable showing the relationship between NT mRNA expression (X-axis; presented as a ratio as described in Section 2.5) in POM and non-vocal courtship behavior (log-transformed; Y-axis). Statistics were computed in a multiple regression model and do not indicate values of individual correlations (see Table 4). n = 20.
3.3 Agonistic behavior
A multiple regression analysis for agonistic behavior with NT expression in POM, Area X, HVC, and LS as independent variables was significant (Table 4). NT expression in POM statistically explained variation in agonistic behavior (log-transformed) (Fig. 3; Table 4); n = 20. No relationships were seen between agonistic behavior and NT expression in VTA.
Figure 3. NT expression in POM and agonistic behavior.
Two-dimensional visualization of a multiple regression model with 1 significant variable showing the relationship between NT mRNA expression in POM (X-axis; presented as a ratio as described in Section 2.5) and agonistic behavior (log-transformed; Y-axis). n = 20.
3.4 Non-specific behaviors
Multiple regression analyses with NT expression in POM, LS, Area X, and HVC as independent variables did not significantly explain calling, feeding, or preening (Table 4). There also were no relationships between these behaviors and NT expression in VTA.
3.5 Sexual motivation as indicated by nest box ownership
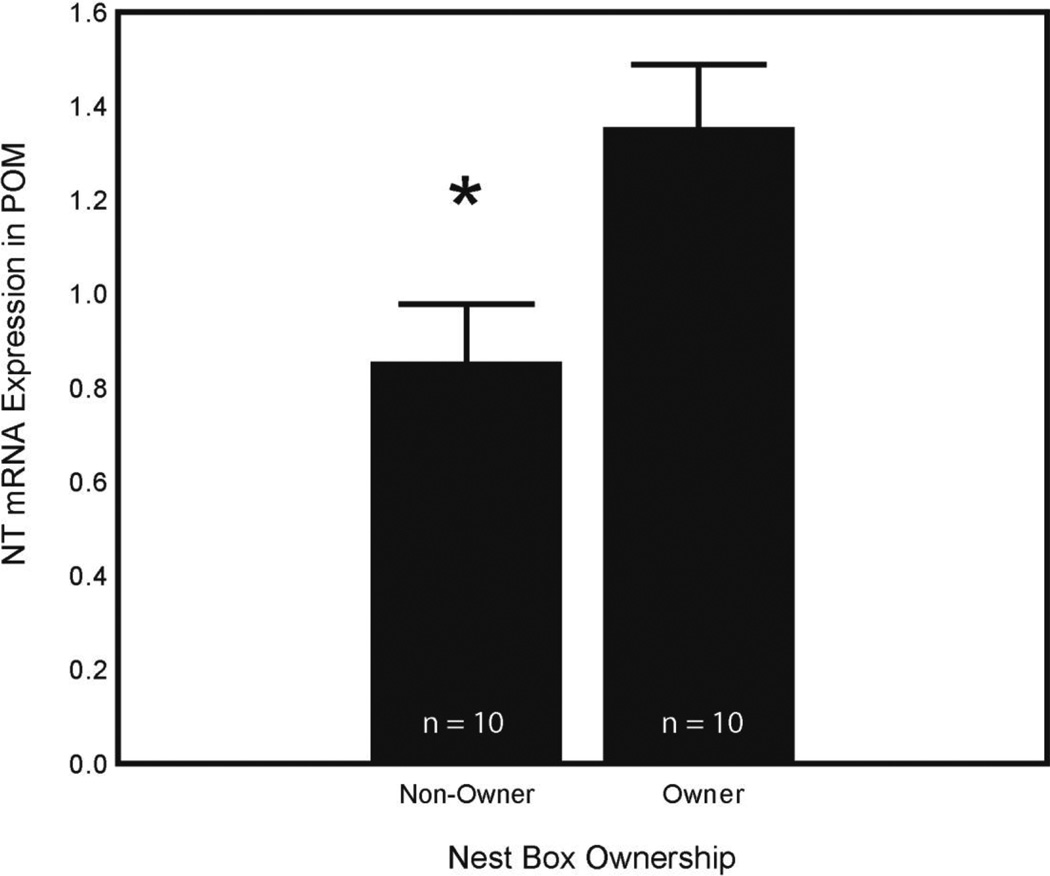
A Student’s t-test revealed that individuals who owned a nest box (n = 10), presumably indicating high levels of sexual motivation, had higher levels of NT expression in POM compared to those without nest boxes (n = 10) (t = 2.65; df = 18; p = 0.016) (Fig. 4). Comparisons between owners and non-owners for NT expression in other regions were not significant (p > 0.05 in all cases).
Figure 4. NT expression in POM and nest box ownership.
Mean plot (+SEM) showing NT mRNA expression (presented as a ratio as described in Section 2.5) in POM in next box owners (n = 10) and non-owners (n = 10). Asterisk indicates a significant difference between nest box owners and non-owners (p = 0.016).
3.6 NTSR1
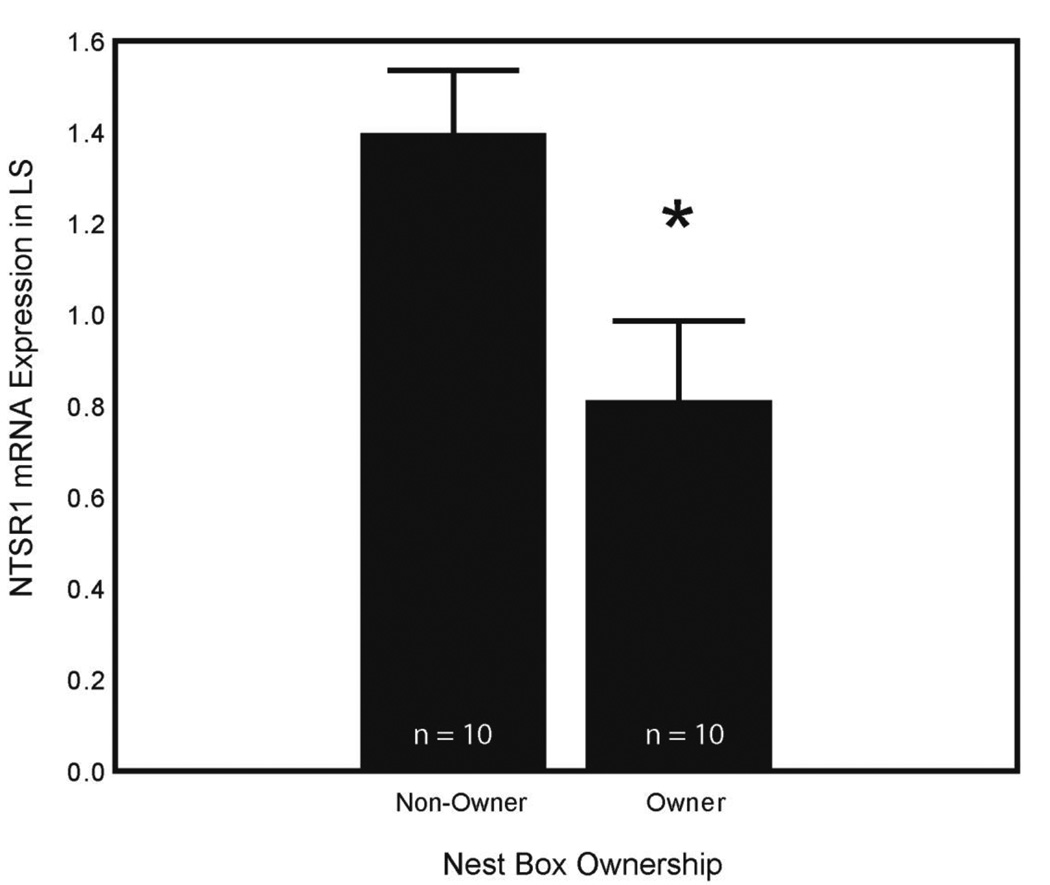
Multiple regression analyses for each parameter were run for NTSR1 expression as they were for NT expression. No significant models resulted. A comparison between NTSR1 expression in LS for nest box owners and non-owners was significant (t = 2.54; df = 18; p = 0.020) (Fig. 5), but similar comparisons for other regions were not significant.
Figure 5. NTSR1 expression in LS and nest box ownership.
Mean plot (+SEM) showing NTSR1 mRNA expression (presented as a ratio as described in Section 2.5) in LS in next box owners (n = 10) and non-owners (n = 10). Asterisk indicates a significant difference between nest box owners and non-owners (p = 0.020).
4. Discussion
The results further highlight a role for NT in the mediation of vocal communication and other highly-motivated behaviors, and support the hypothesis that neuropeptides in social brain regions may contribute to the regulation of social behavior. This study is the first to show a relationship between NT in a song-control nucleus and song production, and to demonstrate alterations in NT and NTSR1 expression along with changes in motivational state.
4.1 NT expression in Area X related to sexually-motivated song
NT expression in Area X correlated positively with measures of singing behavior, suggesting that NT synthesis in Area X may act in this area to modulate vocal communication. These findings are also consistent with the non-mutually exclusive possibility that singing induces NT expression in this region. Sexually-motivated song is positively linked to dopamine markers in Area X [21, 34], dopamine levels in Area X increase during courtship song [33], and activity of D1 receptors in Area X modifies the structure of song [31, 32]. NT up-regulation in Area X may contribute to these effects by modulating dopaminergic activity.
We did not observe any associations between NT expression in VTA, LS, or POM and courtship song production. For POM, these findings are consistent with results of a previous study that showed no significant relationships between NT protein immunolabeling in POM and courtship song; they are, however, somewhat inconsistent with past data showing that NT protein labeling in VTA and LS positively relates to sexually-motivated song [15]. The lack of results for NT expression in VTA and LS may indicate that NT is not produced in these areas in association with singing; however, this does not preclude a role for the protein in this area. qPCR is a measure of gene expression, and the final destination of the synthesized protein may differ from the site of synthesis. It is thus possible that NT could be produced in other regions and travel to VTA or LS to modify courtship song, as suggested by protein immunolabeling data. More research is needed to identify the locations in which NT acts to modify song. Additionally, we saw no relationships between NT expression in HVC and singing behavior. Dopamine in HVC has not been previously implicated in sexually-motivated song [21], which taken together with our results may indicate that changes in NT expression in HVC are not involved in song production.
4.2 NT expression in POM statistically explained non-vocal courtship behavior
NT expression in POM was positively associated with non-vocal courtship behaviors (i.e., the sum of perching on top of nest boxes, entering nest boxes, gathering nest material, and wing waving). This suggests a role for NT in POM in sexual motivation, or that these behaviors induce NT expression in this region; however, these results are somewhat inconsistent with a past study showing no relationship between NT immunolabeling in POM of male starlings and non-vocal courtship behaviors [15]. NT may not act directly in POM to modify these behaviors, but it could be produced in this area and travel to other regions where NT is associated with non-vocal courtship behavior, such as LS and the bed nucleus of the stria terminalis [15]. There also could be a more complex association between NT in POM and sexually-motivated behaviors, similar to what is observed for dopamine (described in the Introduction).
4.3 NT expression in POM statistically predicted variation in agonistic behavior
NT expression in POM strongly positively related to agonistic behavior, measured as the displacements of other individuals. In contrast to the positive relationship found here for NT expression, in a past study NT protein immunolabeling in POM negatively related to agonistic behavior in male starlings [15], suggesting an inhibitory role for NT in POM. It may be that as activity depletes NT stores, NT synthesis subsequently is upregulated to account for this depletion. NT mRNA is upregulated in the medial preoptic area of maternal mice [43], indicating that changes in NT expression may be involved in the control of agonistic behavior. The present results further support a role for NT in POM in agonistic interactions, though it is also possible that agonistic behaviors alter NT expression.
4.4 NT and NTSR1 expression differed for individuals that acquired a nest box
Males that acquired nest boxes displayed up-regulated NT expression in POM, compared to males that did not. Male starlings defend nest sites during the spring breeding season [51], when they also have the largest POM volumes [47, 52]. When males acquire nesting sites, they begin to court females and behave agonistically toward other males, both of which they do not do prior to gaining the nesting sites [46, 51, 53]. Our data suggest that increased NT expression in POM may facilitate nest site acquisition, or that the acquisition of a nest site may increase NT expression to enable sexually- and agonistically-motivated behaviors. Research is now needed to determine whether sexual motivation drives, or is reflective of, altered NT expression in POM.
Expression of NTSR1 in LS was lower in males that owned nest boxes, compared to non-owners. This may suggest that NT in LS inhibits behaviors typical of nest owners. However, somewhat in contrast with these findings NT protein immunolabeling in LS is related positively to sexually-motivated song and non-vocal courtship behaviors [15]. Starlings that acquire nest boxes are presumably the most sexually-motivated individuals in a flock. It is possible that, if NT acts in LS to stimulate sexually-motivated behaviors, high levels of NT activity could lead to receptor down-regulation. Thus, the lower NTSR1 expression observed in males with nest boxes could be reflective of receptor down-regulation due to high levels of NT binding in LS. Future studies are now needed to test this hypothesis and to examine other factors that may be responsible for the observed differences in NTSR1 expression in LS.
4.5 NT and NTSR1 expression and non-specific behaviors
NT expression did not relate to calling, feeding or preening. The purpose of calling is not clear, so it is not known how highly motivated this behavior is, nor what its social function is [46]. Feeding and preening are motivated for homeostatic purposes, not social ones. Overall, the lack of associations between changes in NT expression and non-specific behaviors lend support for a role of NT in highly-motivated, goal-directed behaviors.
4.6 NTSR1 expression did not relate to behaviors
NTSR1 expression was not associated linearly with any behaviors. Changes in NTSR1 expression relating to motivational states have been reported in rodents. In mice, expression of NTSR1 in multiple brain regions differs between virgin females and postpartum females [43]. Our results suggest that modulation of NT ligand expression, rather than receptor expression, in the observed brain regions may be more pertinent to sexually- and agonistically-motivated behavior in starlings. However, in both the present study and the rodent study, the role of causality between behavior, motivational state, and NT system modulation is unclear.
5. Conclusions
Our results suggest region-specific roles for NT in vocal communication, agonistic behavior, and sexually-motivated behaviors. These findings support and expand upon initial explorations of the relationship between NT and social behavior. Research using site-directed pharmacological manipulations or gene silencing techniques is now needed to determine the directionality and function of the relationships between NT expression and behavior.
Highlights.
Neurotensin mRNA expression in Area X positively related to courtship song.
Neurotensin mRNA expression in POM positively linked to non-vocal courtship behavior.
Neurotensin mRNA expression in POM positively related to agonistic behavior.
Data implicate neurotensin synthesis in POM and Area X in highly-motivated behaviors.
Study suggests a role for neurotensin in vocal communication and social behaviors.
Acknowledgements
This material is based upon work supported by the National Institute of Mental Health (R01 MH080225 to LVR) and by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1256259. The authors thank Jeremy A. Spool and Jonathan D. Rodriguez for helpful manuscript edits, Caroline S. Angyal for superb technical assistance and manuscript comments, and Chris Elliott for starling care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Catchpole C, Slater PJB, Mann N. Bird song biological themes and variations. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 2.Zeigler HP, Marler P. Neuroscience of birdsong. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]
- 3.Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and Dopamine Interactions. Pharmacol Rev. 2001;53:453–486. [PubMed] [Google Scholar]
- 4.Cáceda R, Kinkead B, Nemeroff CB. Neurotensin: Role in psychiatric and neurological diseases. Peptides. 2006;27:2385–2404. doi: 10.1016/j.peptides.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 6.Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends in Neurosciences. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Simonyan K, Horwitz B, Jarvis ED. Dopamine regulation of human speech and bird song: A critical review. Brain and Language. 2012;122:142–150. doi: 10.1016/j.bandl.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubikova Ľ, Košťál Ľ. Dopaminergic system in birdsong learning and maintenance. Journal of Chemical Neuroanatomy. 2010;39:112–123. doi: 10.1016/j.jchemneu.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riters LV. The role of motivation and reward neural systems in vocal communication in songbirds. Frontiers in Neuroendocrinology. 2012;33:194–209. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Numao M, Sudo H, Yamamoto I, Nakao N, Kaiya H, Miyazato M, et al. Molecular characterization of structure and tissue distribution of chicken neurotensin receptor. General and Comparative Endocrinology. 2011;171:33–38. doi: 10.1016/j.ygcen.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Hökfelt T, Everitt BJ, Theodorsson-Norheim E, Goldstein M. Occurrence of neurotensinlike immunoreactivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. The Journal of Comparative Neurology. 1984;222:543–559. doi: 10.1002/cne.902220407. [DOI] [PubMed] [Google Scholar]
- 12.Palacios JM, Kuhar MJ. Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature. 1981;294:587–589. doi: 10.1038/294587a0. [DOI] [PubMed] [Google Scholar]
- 13.Seroogy K, Ceccatelli S, Schalling M, Hökfelt T, Frey P, Walsh J, et al. A subpopulation of dopaminergic neurons in rat ventral mesencephalon contains both neurotensin and cholecystokinin. Brain Research. 1988;455:88–98. doi: 10.1016/0006-8993(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 14.Moyse E, Rostène W, Vial M, Leonard K, Mazella J, Kitabgi P, et al. Distribution of neurotensin binding sites in rat brain: A light microscopic radioautographic study using monoiodo [125I]Tyr3-neurotensin. Neuroscience. 1987;22:525–536. doi: 10.1016/0306-4522(87)90350-2. [DOI] [PubMed] [Google Scholar]
- 15.Merullo DP, Cordes MA, Stevenson SA, Riters LV. Neurotensin immunolabeling relates to sexually-motivated song and other social behaviors in male European starlings (Sturnus vulgaris) Behav Brain Res. 2015;282C:133–143. doi: 10.1016/j.bbr.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Will RG, Hull EM, Dominguez JM. Influences of dopamine and glutamate in the medial preoptic area on male sexual behavior. Pharmacology, biochemistry, and behavior. 2014;121:115–123. doi: 10.1016/j.pbb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 18.DeVries MS, Cordes MA, Stevenson SA, Riters LV. Differential relationships between D1 and D2 dopamine receptor expression in the medial preoptic nucleus and sexually-motivated song in male European starlings (Sturnus vulgaris) Neuroscience. 2015;301:289–297. doi: 10.1016/j.neuroscience.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riters LV, Pawlisch BA, Kelm-Nelson CA, Stevenson SA. Inverted-U shaped effects of D1 dopamine receptor stimulation in the medial preoptic nucleus on sexually motivated song in male European starlings. European Journal of Neuroscience. 2014;39:650–662. doi: 10.1111/ejn.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience. 2009;159:962–973. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiology & Behavior. 2008;95:258–266. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. The Journal of Comparative Neurology. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 23.Bertram R, Daou A, Hyson RL, Johnson F, Wu W. Two neural streams, one voice: Pathways for theme and variation in the songbird brain. Neuroscience. 2014;277:806–817. doi: 10.1016/j.neuroscience.2014.07.061. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. The Journal of Comparative Neurology. 1981;196:347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- 25.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behavioral and neural biology. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi K, Uno H, Okanoya K. Partial lesions in the anterior forebrain pathway affect song production in adult Bengalese finches. Neuroreport. 2001;12:353–358. doi: 10.1097/00001756-200102120-00034. [DOI] [PubMed] [Google Scholar]
- 28.Kubikova L, Bosikova E, Cvikova M, Lukacova K, Scharff C, Jarvis ED. Basal ganglia function, stuttering, sequencing, and repair in adult songbirds. Scientific reports. 2014;4:6590. doi: 10.1038/srep06590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol. 1993;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- 30.Casto JM, Ball GF. Characterization and localization of D1 dopamine receptors in the sexually dimorphic vocal control nucleus, area X, and the basal ganglia of European starlings. J Neurobiol. 1994;25:767–780. doi: 10.1002/neu.480250703. [DOI] [PubMed] [Google Scholar]
- 31.Leblois A, Perkel DJ. Striatal dopamine modulates song spectral but not temporal features through D1 receptors. The European Journal of Neuroscience. 2012;35:1771–1781. doi: 10.1111/j.1460-9568.2012.08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leblois A, Wendel BJ, Perkel DJ. Striatal Dopamine Modulates Basal Ganglia Output and Regulates Social Context-Dependent Behavioral Variability through D1 Receptors. The Journal of Neuroscience. 2010;30:5730–5743. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heimovics SA, Salvante KG, Sockman KW, Riters LV. Individual differences in the motivation to communicate relate to levels of midbrain and striatal catecholamine markers in male European starlings. Hormones and Behavior. 2011;60:529–539. doi: 10.1016/j.yhbeh.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato M, Okanoya K. Molecular characterization of the song control nucleus HVC in Bengalese finch brain. Brain Res. 2010;1360:56–76. doi: 10.1016/j.brainres.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Lovell PV, Clayton DF, Replogle KL, Mello CV. Birdsong "transcriptomics": neurochemical specializations of the oscine song system. PLoS One. 2008;3:e3440. doi: 10.1371/journal.pone.0003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. Journal of Chemical Neuroanatomy. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- 38.Newman SW. The Medial Extended Amygdala in Male Reproductive Behavior A Node in the Mammalian Social Behavior Network. Annals of the New York Academy of Sciences. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 39.Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Hormones and Behavior. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Connell LA, Hofmann HA. The Vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. The Journal of Comparative Neurology. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- 41.Gammie SC, Auger AP, Jessen HM, Vanzo RJ, Awad TA, Stevenson SA. Altered gene expression in mice selected for high maternal aggression. Genes, Brain and Behavior. 2007;6:432–443. doi: 10.1111/j.1601-183X.2006.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gammie SC, D'Anna KL, Gerstein H, Stevenson SA. Neurotensin inversely modulates maternal aggression. Neuroscience. 2009;158:1215–1223. doi: 10.1016/j.neuroscience.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Driessen TM, Zhao C, Whittlinger A, Williams H, Gammie SC. Endogenous CNS Expression of Neurotensin and Neurotensin Receptors Is Altered during the Postpartum Period in Outbred Mice. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0083098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawson A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. General and Comparative Endocrinology. 1983;49:286–294. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- 45.Eens M, Pinxten R, Verheyen RF. On the function of singing and wing-waving in the European Starling Sturnus vulgaris. Bird Study. 1990;37:48–52. [Google Scholar]
- 46.Feare C. The starling. Oxford: Oxford University Press; 1984. [Google Scholar]
- 47.Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal Changes in Courtship Song and the Medial Preoptic Area in Male European Starlings (Sturnus vulgaris) Hormones and Behavior. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- 48.Cordes MA, Stevenson SA, Driessen TM, Eisinger BE, Riters LV. Sexually-motivated song is predicted by androgen-and opioid-related gene expression in the medial preoptic nucleus of male European starlings (Sturnus vulgaris) Behavioural Brain Research. 2015;278:12–20. doi: 10.1016/j.bbr.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 51.Kessel B. A Study of the Breeding Biology of the European Starling (Sturnus vulgaris L.) in North America. American Midland Naturalist. 1957;58:257–331. [Google Scholar]
- 52.Pintér O, Péczely P, Zsebők S, Zelena D. Seasonal changes in courtship behavior, plasma androgen levels and in hypothalamic aromatase immunoreactivity in male free-living European starlings (Sturnus vulgaris) General and Comparative Endocrinology. 2011;172:151–157. doi: 10.1016/j.ygcen.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Gwinner H, Van't Hof T, Zeman M. Hormonal and Behavioral Responses of Starlings during a Confrontation with Males or Females at Nest Boxes during the Reproductive Season. Hormones and Behavior. 2002;42:21–31. doi: 10.1006/hbeh.2002.1795. [DOI] [PubMed] [Google Scholar]