Abstract
The microenvironment formed by surface active compounds is being recognized as the active site of lipid oxidation. Trace amounts of water occupy the core of micro micelles and several amphiphilic minor components (e.g., phospholipids, monoacylglycerols, free fatty acids, etc.) act as surfactants and affect lipid oxidation in a complex fashion dependent on the structure and stability of the microemulsions in a continuous lipid phase such as bulk oil. The structures of the triacylglycerols and other lipid-soluble molecules affect their organization and play important roles during the course of the oxidation reactions. Antioxidant head groups, variably located near the water-oil colloidal interfaces, trap and scavenge radicals according to their location and concentration. According to this scenario, antioxidants inhibit lipid oxidation not only by scavenging radicals via hydrogen donation but also by physically stabilizing the micelles at the microenvironments of the reaction sites. There is a cut-off effect (optimum value) governing the inhibitory effects of antioxidants depending inter alias on their hydrophilic/lipophilic balance and their concentrations. These complex effects, previously considered as paradoxes in antioxidants research, are now better explained by the supramolecular chemistry of lipid oxidation and antioxidants, which is discussed in this review.
Keywords: Antioxidants, Amphiphilic compounds, Bulk oils, Critical micelle concentration, Lipid oxidation
Introduction
Unsaturated fatty acids play important roles as food components and nutrients, and contribute to food's stability and sensory properties such as texture and flavor 1–3. Specifically, the highly unsaturated omega-3 fatty acids of fish lipids undergo instantaneous oxidation, which limits their shelf-lives and restricts their applications as health promoting fatty acids in the diet 4–8. Attempts have continuously been made to find ways to protect unsaturated fatty acids from oxidative deterioration but no satisfactory solutions have been found 9.
Lipid oxidation is known to occur in three phases: initiation, propagation, and termination 4,9,10. The theory that was developed in the 1940s 11 about the autocatalysis of lipid oxidation by-produced hydroperoxides (LOOH) is largely acceptable and provides logical description of the chemical reactions involved in the oxidative changes of fatty acids during the propagation and termination periods 6,12–14 as well as the products of oxidation and their significance especially as off-flavor compounds 3,6,15–17. The hydroperoxide theory outlined by Farmer et al. 11 provides a general description of the role of antioxidants in inhibiting lipid oxidation. However, this description is often not precise and sometimes suffers from inconsistencies and paradoxical outcomes when it comes to certain details 1,3,18–20. Knowledge that was accumulated during the last two decades emphasize that lipid oxidation cannot be explained merely by chemical reactions but also by considering molecular positions in space, especially at the interfaces of nanoemulsions. This paper reviews the current knowledge of lipid oxidation with emphasis on the effects of antioxidants and the physical microenvironments on the oxidation of bulk oils. Bulk oils are considered as water-in-oil nanoemulsions rather than pure lipid phases.
Antioxidants and their mechanisms
An antioxidant is defined as “Any substance that, when present at low concentrations compared with those of an oxidizable substrate, delays or prevents the oxidation of that substrate” 21. Two kinds of antioxidants, primary and secondary antioxidants, have been classified based on their mechanisms of action in inhibiting lipid oxidation reaction 6,20,22.
Primary antioxidants (mainly phenolic compounds)
They react with lipid hydroperoxyl radicals producing lipid hydroperoxides and more stable, low energy, antioxidant radicals (Aglyph6) 22,23, which are significantly much less reactive in propagation reactions 19. By acting as hydrogen donors or radicals scavengers, primary antioxidants prolong the IP and delay the propagation period 3,22. Primary antioxidants (e.g., BHA, BHT, TBHQ, tocopherols, and flavonoids) are generally mono- or polyhydroxy phenols with hydrogen-donating substitutions on the ring 6,19,24. It is currently believed that the most important factor governing the antioxidant potency of phenolic compounds relates to their hydrogen donating powers, that is, to the number of O−H groups in ortho and para positions, their bond dissociation enthalphies (BDE), and whether these phenolic hydrogens are hydrogen bonded 4,14,25. Some primary antioxidants, called multiple-function antioxidants, combine more than one of the following antioxidant functionalities; free radical scavenging, oxygen sequestering, metal chelation, and light energy absorption. Examples of these antioxidants include propyl gallate, proanthocyanidins, and ascorbic acid 14.
Secondary antioxidants (or retarders)
These are preventive antioxidants that enhance the inhibitory activity of primary antioxidants. This class of antioxidants includes sequestrants or chelating agents (e.g., phytic acid, EDTA, and citric acid), oxygen scavengers, and reducing agents (e.g., ascorbates), and other factors whose effect is not completely explained (e.g., amino acids and phospholipids) 6,19. The exact mechanism of action of the wide variety of secondary antioxidants have not been properly understood but some of their speculated activities include chelating prooxidants or catalysts, providing hydrogen to primary antioxidants, decomposing LOOH to nonradical species, scavenging ground state and singlet oxygens, and absorbing UV light 22. It has been debated by Brimberg 26,27 that the role of these retarders relies on their effects on micellization but unfortunately this work has not been noticed in time.
Combinations of primary and secondary antioxidants are often found more effective in retarding lipid oxidation than the sum of their single actions 1,3,28. It was shown that the synergism between these two classes of antioxidants effectively increases the length of the IP and reduces reaction rates 13,29. This synergism has been shown, for example, between tocopherols and ascorbic acid and between mixtures of natural tocopherols and citric acid 19. A good method to evaluate the efficiency of inhibitors and retarders, according to which the “antioxidant” efficacy can be measured by considering the length of the IP as well as the rate of oxidation during the IP was presented by Yanishlieva and Marinova [29 and references cited therein]. Three descriptive parameters are considered:
Effectiveness, which is the ability of an antioxidant to inhibit the oxidation chain reaction by donating hydrogens and inactivating RO2• during the IP. Effectiveness is measured by stabilization factor, F = IPinh/IPo, where IPinh is the IP of an inhibited oxidation (with an antioxidant), and IPo is the IP of the uninhibited oxidation (no antioxidant present).
Strength, which is the inverse measure of the participation of an antioxidant in the side reactions that may results in the change of oxidation rate during the IP. The oxidation rate ratio ORR = Winh/Wo, where Winh is the rate of oxidation of an inhibited oxidation (with an antioxidant) and Wo is the rate of oxidation of an uninhibited oxidation (no antioxidant present) is an inverse measure of strength, ORR > 1 indicates that an antioxidant causes a faster oxidation rate than the rate without antioxidant.
Antioxidant activity (A = F/ORR), which indicates the capability of an antioxidant in terminating autoxidation chain and in affecting the rate of oxidation during IP 13,29.
Until recently, most of the explanations given for observed synergistic interactions have been based mainly on unfounded assumptions related to possible chemical interferences of primary and secondary antioxidants. The addition of primary antioxidant and synergists often increase the IP and decrease the rate of oxidation during the IP (Winh), for example, the inhibition of autoxidation of fish oil at 20°C by 1000 ppm ascorbyl palmitate and 5 ppm lecithin 28 (Fig. 1) of fish oil at 20°C with 500 ppm ascorbyl palmitate and 2000 ppm lecithin 30, of soybean oil at 110°C with 4000 ppm α-tocopherol and 15 000 ppm phospholipids 31, and of peanut oil at 110°C with 1000 ppm α-tocopherol and 1500 ppm phospholipids 32. Some indogenous minor components in refined bulk oils, such as phospholipids, can act as synergists to tocopherols and contribute to protecting the oils against oxidation while others, such as monoacylglycerols, may act as prooxidants and decrease the IP and/or increase the rate of oxidation during the IP 8,14,20,33–35. Besides synergism, there are more examples of unexplained phenomena related to reaction rates of inhibited oxidations. One permanent case is the loss of antioxidant efficacy with increased primary antioxidant concentration 4,25, which is well known, for example, α-tocopherol 18. The concept of side reactions was used to account for such paradoxical outcomes of antioxidants such as the loss of efficiency at increased concentrations [9 and references cited therein].
Figure 1.
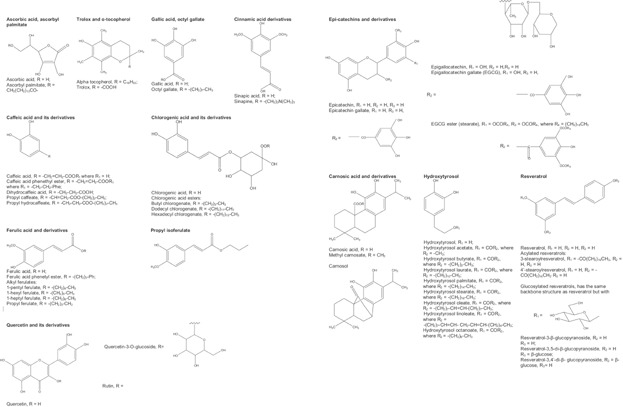
Structures of antioxidants with different polarity discussed in this paper.
The polar paradox and interfacial phenomena
It is clear from the previous section that the pure chemical model failed to adequately describe the effects of primary and secondary antioxidants on the rates of lipid oxidation reactions and their synergistic interactions. Knowledge has gradually developed to strongly suggest that effects related to molecular orientation and self-assembly of different molecular species are important and can help explain phenomena that are not yet well understood. The historical developments in the understanding of the role of physical location of lipid-soluble components on lipid oxidation are presented in (Table1).
Table 1.
Historical developments in the understanding of the physical effects of components and additives on lipid oxidation in bulk oils
| References | Observations | Conclusions |
|---|---|---|
| Porter 36,37 and Porter et al. 38 | The general rule of the polar paradox was proposed and confirmed stating that polar antioxidants (e.g., propyl gallate, tert-Butylhydroquinone (TBHQ), and Trolox C) are more effective in food systems with low surface-to-volume ratio or nonpolar lipids such as bulk vegetable oils while nonpolar antioxidants (e.g., BHA, BHT, and α-tocopherol) work better in foods with high surface-to-volume ratio or polar lipid emulsion such as o/w emulsion | The antioxidant activity is oppositely related to the polarity of antioxidants in relation to food lipids |
| Brimberg 26,27 | Lipid hydroperoxides (LOOH) are surface-active agents that form micelles at above their critical micelle concentration (CMC). O2 is maximumly solubilized in lipids when hydroperoxide CMC is attained | Micelles formed by hydroperoxides are the site of lipid oxidation reaction |
| Frankel et al. 39–41 | The interfacial phenomenon was proposed to explain the polar paradox. Lipophilic antioxidants (e.g., α-tocopherol and ascorbyl palmitate) were more effective in o/w emulsion system than in bulk oil because they had more affinities toward water-oil interface, while the opposite was true for hydrophilic antioxidants (Trolox, ascorbic acid, rosmarinic acid, carnosic acid, and rosemary extract), which were more oriented in air-oil interfaces in bulk oil. Mixtures of α-tocopherol and ascorbic acid were more active in bulk oils than in o/w emulsions | Interfacial phenomenon is related to the kinds of interfaces at which the antioxidants are more oriented, which may explain the polar paradox |
| Koga and Terao 54 | In the aqueous microenvironments in bulk lipids (15:85 by mol/mol mixture of methyl linoleate and methyl laurate), phospholipid aggregates enhanced the accessibility of α-tocopherol to radicals and hence the interruption of chain initiation. The polar OH group of α-tocopherol is located not too deeply in hydrophobic region of phospholipid bilayer membrane but just near by the membrane surface | Interfacial microenvironment is the place where interactions among surfactants, antioxidants, and radicals take place |
| Huang et al. 60 | Linoleic acid competed with Trolox for Tween 20 in the polar region of the micelles and at the o/w interface. Trolox diffused in the water phase and the mixed micelles and thus was a better antioxidant than α-tocopherol that was diffused in the oil phase | Micelle is where the oxidation and interactions of antioxidants and surfactants take place |
| Carlotti et al. 123 | An emulsion was known to contain micellar structure. l-tryptophan was a very effective synergist with α-tocopherol because it was distributed in the micellar core or in the o/w interface | Micelle core and interface have different roles in autoxidation |
| Endo et al. 106,116,118 | A mixture of trieicosapentaenoylglycerol and tripalmitoylglycerol (2:1, mol/mol) was most susceptible to oxidation than other ratios. The triacylglycerol (TAG) structure affected the oxidation rate of unsaturated fatty acids. TAGs with unsaturated fatty acids at sn-2 positions were more stable than those having unsaturated fatty acids at sn-1 and sn-3 positions | Physical structures, such as the position of fatty acids on TAG, have an effect on lipid oxidation |
| Hamilton et al. 28 | Lecithin solubilizes ascorbyl palmitate and enhances its physical interactions with α-tocopherol which form reversed micelles. This versatile network had an ability to interrupt free-radical propagation by inhibiting the participation of ascorbyl radical in promoting LOOH scission | Reversed micelles are formed in w/o emulsions |
| Frankel and Meyer 124 | The effectiveness of antioxidants in a system is influenced by several factors including the partitioning behavior of antioxidants between lipid and aqueous phase, the oxidation conditions, and the physical state of the oxidizable substrate. Surface-active substances influence the interfacial interactions between the system and antioxidant. The oil-water partition coefficients influence the distribution of relatively polar antioxidants in the lipid and aqueous phase of a food emulsion. Trolox, which is very polar, works very well in bulk oil and is more effective in o/w emulsions of linoleic acid compared to those of TAG. Unlike TAG, linoleic acid is more polar and forms micelles in aqueous system. Micelle-forming substrates enhance the activity of hydrophilic and polar antioxidant | O/w partition coefficient can explain the affinity of a compound in lipid and aqueous phase |
| Khan and Shahidi 84 | The synergistic interactions of tocopherols and phospholipids in borage and evening primrose TAG can be explained partly by phosphatidylcholine increasing the accessibility of α-tocopherol in the aqueous microenvironment where the induction of lipid oxidation occurs | Phospholipid synergists support antioxidants by modifying the reaction environment |
| Schwarz et al. 43 | Antioxidants (Trolox, propyl gallate, gallic acid, methyl carnosoate, and carnosic acid) had either moderate or higher activity in bulk oil than in emulsions. The most polar antioxidants (propyl gallate and gallic acid) exhibited either prooxidant or no antioxidant activity in polar medium (i.e., o/w emulsions). Emulsifiers (Cetheareth-15, glyceryl stearate, and polyglyceryl glucose methyl distearate) form lamellar structure in bulk oil causing a higher solubilization of polar antioxidants in nonpolar medium. Antioxidant actions in bulk oil, except gallic acid which was not influenced by polysiloxan polyalcohol polyether copolymer, are enhanced by emulsifiers including α-tocopherol | The activity of antioxidants can be enhanced or reduced by emulsifiers. Mesophase structures depend on molecular structure and critical packing parameter (CPP) of the compound |
| Gupta et al. 87 | Inverse micellar structures (∼60 Å in diameter) were formed by phospholipids in a hexane-oil mixtures containing <0.3% water. The principal domains of the phase behavior include micellar solution, two phase dispersion, and dense micellar solution. A smooth transition to dense micellar phase was observed with increased phospholipids concentration. Dynamic light scattering measurements showed that aggregate sizes were affected by the amount of phospholipids and >1.5% water, below which the available water is very limited to significantly affect core sizes | Reversed micelles are formed in w/o nanoemulsions. The size of aggregates depends on the amount of surfactants and water |
| Kortenska et al. 81 | Polar products of lipid oxidation with oxygen containing groups (e.g., LOOH, fatty alcohols, acids, and water) tend to associate in non-polar media to form complexes and aggregates. Fatty alcohols may play a role as an initiation of formation of these aggregates and hence influence lipid oxidation rate | Polar products of lipid oxidation affect the oxidation rate by modulating the reaction environment |
| Kortenska et al. 68 | Relatively high concentrations of polar compounds (e.g., LOOH, lipid peroxyl radical [LOO•], and BHT) form microaggregate (micelles) in the presence of fatty alcohols. This leads to an increase of the rate of termination and causes a decrease in the efficiency of BHT to protect purified sunflower oil (SFO) as LOOH decompose faster inside the polar interior of the micro aggregate | Fatty alcohols or BHT might act as surfactants and form microaggregates (micelles) in the w/o system |
| Velasco and Dobarganes 12 | Cloudy OO was more oxidatively stable than filtered OO. Suspended and dispersed materials in cloudy olive oil (OO) play a physical stabilization role by acting as antioxidants and/or as a buffer and preventing acidity increases | Polar constituents in oils, for example, unsaponifiable materials, may play a physical role in oil solubilization |
| Brimberg and Kamal-Eldin 125 | LOOH formed during methyl linoleate oxidation are surface-active and can form micelles. When LOOH concentration reaches CMC, lipid oxidation enters the propagation period | CMC of hydroperoxides marks the beginning of propagation period |
| Brimberg and Kamal-Eldin 55 | The amount of oxygen solubilized in lipid is comparative to the number of micelles formed during oxidation. When lipid medium has conjugated double bonds is oxidized, no hydroperoxides are formed but instead cyclic peroxides that are not surface-active and do not form micelles, hence there is no propagation period | Organic peroxides (not hydroperoxides) are not surface active and do not atfect the oxidation rate |
| El-Shattory et al. 69 | Reversed micelles were formed with surfactant aggregates in organic solvents, for example, LOOH, methylglucose dioleate, polyglyceryl-3-oleate, and lecithin | Reversed micelles are formed in organic system in the presence of surfactants |
| Kiokias and Gordon 71 | The activity of norbixin as antioxidant in bulk oil is consistent with the polar paradox. Norbixin is soluble in water as aggregates and is probably oriented at the oil-water interface in the emulsion due to its massive hydrocarbon backbone but it is insoluble in oil | Norbixin is an example to supports the polar paradox |
| Decker et al. 72 | Differences in the effectiveness of the antioxidants in oil systems are mainly due to their physical location in the system, namely the antioxidant paradox. Polar (hydrophilic) antioxidants are more effective in bulk oil because they can accumulate at the air-oil interface or in reversed micelles within the oil, where lipid oxidation occurs. On the other hand, nonpolar (lipophilic) antioxidants are more effective in o/w emulsions because they accumulate in the oil droplets and/or may accumulate at the oil-water interface, where interactions between LOOH at the droplet surface and pro-oxidants (e.g., transition metals) take place | Antioxidant effectiveness depends on how and where they are partitioned in the system. In bulk oil, lipid oxidation occurs at the air-oil interface as well as in the reversed micelle (oil-water) interface |
| Calligaris and Nicoli 126 | Salts with the antichaotropic anionic species were able to form weak bonds may form a “hydrophilic” structure around them and inhibit the solubility of other substances with lower polarities. Thus, these salts may enhance the activity of certain antioxidants | Hydrophobic structure formed by the salts might salt-out amphiphilic molecules and affect lipid oxidation |
| Becker et al. 44 | Antioxidant activity in bulk oil was related to the polarity of the antioxidants, within the order: quercetin >α-tocopherol ≫ astaxanthin = rutin. Rutin was an exception in that it is relatively hydrophilic but had the lowest activity in bulk oil. This indicated that it is not only the polarity that govern the effectiveness of antioxidants. Poor solubility of rutin in bulk oil or degradation of its glycoside at high temperature also influenced its effects | Hydrophilicity (or lipophilicity) do not always correlate with the antioxidant effectiveness in bulk oil |
| Chaiyasit et al. 14 | Edible oils contain polar lipids (e.g., monoacylglycerol (MAG), diacylglycerol (DAG), free fatty acid (FFA), phospholipids, sterols, cholesterols, phenolic compounds, aldehydes, and ketones), which have amphiphilic nature. Components with especially low HLB can self-assemble due to hydrophobic interactions and form association colloids, including lamellar structures and reversed micelles. These surface active molecules partition at the o/w interface and induce the concentration of antioxidants at the surface of colloids, thus increasing interactions between antioxidants and/or prooxidants with metal at the interface or water core | The term association colloids, include geometric forms such as lamellar structures and reversed micelles, which are formed by surfactants was proposed |
| Chaiyasit et al. 33 | Edible oils contain surface-active compounds and water that can form physical structures such as reversed micelles. Both phosphatidylcholine and oleic acid were suggested to be located at the o/w interface by 5-dodecanoylaminofluorescein probe measurement, and phosphatidylcholine was found to increase the accessibility of α-tocopherol to radicals while oleic acid acted as prooxidants | More examples on the effects of surface-active compounds and reversed micelles on lipid oxidation were presented |
| Kasaikina et al. 70 | LOOH do not form classical micelles but form associates (1–500 nm in size) alongside water, surfactants, alcohols, acids, ketones, and other oxidation products. LOOH is amphiphilic and concentrates on the boundary of micelle and water. In a natural olefin (limonene), cationic surfactant promotes oxidation, whereas anionic and nonionic surfactants did not have any influence | Associates rather than micelles were suggested. Charges of surfactants affect the role of the surfactants as antioxidant or prooxidant |
| Koprivnjak et al. 73 | Bipolar molecules such as lecithin form reversed micelle where their polar groups are pointed toward the interior and their nonpolar tails are directed toward the exterior (oil). Lecithin ability to increase oxidative stability was due to its bipolar character and its ability to entrap hydrophilic antioxidants to concentrate on the micellar interface | On the role of phospholipids as stabilizers of reversed micelles |
| Laguerre et al. 17 | Not all nonpolar antioxidants behave as antioxidant in polar medium; the antioxidant capacity of homologous series of chlorogenic acid esters in o/w emulsions increased as the alkyl chain length increased until dodecyl chain. Further chain extension caused a drastic drop of antioxidant capacity (a cut-off effect) | The Polar Paradox is not linear. As the alkyl chain length increase, the hydrophilicity and the antioxidant activity in o/w emulsions increase to a certain extent, but further increase reduces the antioxidant activity (a cut-off effect) |
| Belhaj et al. 103 | The size of nanoemulsions was influenced by the pressure, oil composition, and the surface-active properties of surfactants. Changes of α-tocopherol antioxidative effect in bulk oil was more significant than that in emulsions | The importance of nanoemulsions in lipid oxidation was proposed |
| Bendini et al. 127 | When virgin OO was subjected to temperature close to 0°C, changes in the physical state happened leading to destabilization of the microdroplets of water and the concentration of polar phenolic compounds and finally the lost of antioxidant activity | At lower temperatures (close to 0°C), destabilized microdroplets in bulk oils may accelerate the rate of lipid oxidation |
| Chen et al. 7 | When the phospholipid concentration exceeds their CMC, reversed micelles were formed. Dioleoylphosphatidylcholine and water formed spherical association colloids in SBO, and they were prooxidative because more (small) non-scattering association colloids were formed. 1,2-dibutyryl-sn-glycero-3-phoshocholineformed cylindrical structures and had no impact on oxidation rates | As amount of surfactant increased, CMC was affected and so the formation of reversed micelle. The kinds of physical structures affect oxidation differently. Spherical shapes of association colloids were prooxidants, while cylindrical shapes had no impact on oxidation rates |
| Gramza-Michalowska and Stachowiak 128 | Astaxanthin causes no protection of bulk oils, which indicates that antioxidant activity was correlated with its polarity. Astaxanthin is hydrophobic, it is located in the oil not at the air-oil interface protecting o/w emulsions but not bulk oils and liposome | Lipophilic compounds do not affect the oxidation in bulk oils |
| Kasaikina et al. 86 | Primary amphiphilic products of the oxidation of LOOH and lipids, and cationic surfactants form mixed micelles, which accelerated the decomposition of LOOH and other polar components (e.g., metal-containing compounds, inhibitors etc.) | Mixed micelles with different geometric forms were detected in w/o emulsions that enhance the decomposition of LOOH |
| Medina et al. 104 | The effectiveness of antioxidants relies on its chemical reactivity (as radical scavenger or metal chelator), its interaction with other food components, their concentration and physical location in homogeneous or heterogeneous system. For instance, resveratrol had a low activity in inhibiting lipid autoxidation in w/o emulsions and bulk oil because it has a low incorporation in the droplet interface and its poor solubilty in water, thus probably located far away from the air-oil interface | On the importance of physical effects of antioxidants |
| Chen et al. 7 | Amphiphilic surface active compounds, which exist after oil refining (such as MAG, DAG, phospholipids, sterol, and FFA), interact with water to form association colloids (in the forms of reversed micelles, microemulsions, lamella, and cylindrical aggregates). Increasing water concentration had very little impact on the IP of lipid oxidation (by hexanal) at 55°C. MAG formed ordered lamellar structures in hazelnut oil. Association colloids impact on lipid oxidation depends on the additives ability to form the colloids and how the additives are partitioned in the micelles | Different surfactants form different kinds of mesophase structures that affect lipid oxidation. Water concentration had a limited effect on oxidation at 55°C |
| Chen et al. 20 | Lipid oxidation is not only influenced by the traditional chemical factors, such as lipid compositions, transition metals; but also by the existence of physical structures. Phospholipids formed microstructures known as association colloids within soybean oil (SBO). Reversed micelle of dioleoylphosphatidylcholine shorthened the IP of SBO at 55°C | Physical structures are important affectors of lipid oxidation |
| An et al. 129 | Antioxidative and prooxidative properties are determined by internal factors (i.e., the oxidation substrates, structural organization and the microenvironment for the bioactive compound) and external factors (i.e., heat, pressure, and exposure to light). Hydrophobic alkyl chain increased water insolubility of 7-n-alkoxydaidzeins: daidzein, 7-n-butyloxy-daidzein, 7-n-octyloxy-daidzein, 7-n-dodecyloxy-daidzein, and 7-n-hexadecyloxy-daidzein. Daidzein increased membrane fluidity, but 7-n-butyloxy-daidzein until 7-n-hexadecyloxy-daidzein decreased fluidity. The compounds were suggested to present in the central domain of the liposome bilayer in the order 7-n-dodecyloxy-daidzein > 7-n-octyloxy-daidzein > 7-n-butyloxy-daidzein > 7-n-hexadecyloxy-daidzein > daidzein, leaving 7-n-dodecyloxy-daidzein as the most effective antioxidant, as monitored by fluoresence spectroscopy using a fluorescence probe | Changes in the hydrophilicity of an antioxidant affect its inhibitory activity of lipid oxidation |
| Shahidi and Zhong 53 | In this review, the polar paradox was re-examined. The distribution of polar antioxidants at the oil-air interface was questioned because air is much less polar than oil. Antioxidants action was influenced by various micro- or nanoenvironments (such as lamellar and reversed micelles) which are formed by water, amphiphilic compounds, and oxidation products (e.g., LOOH, aldehydes, and ketones) alter the physical location of antioxidants. The association colloids are the site of lipid oxidation in bulk oil. A cutoff effect was observed, a non-linear phenomenon occurred wherein antioxidant activity increases as the alkyl chain lengthens until a threshold is achieved, then further increased of chain length caused a drastic collapse on activity. Molecular size also influenced antioxidant effectiveness and causing a cutoff effect, antioxidants with bulky structures (e.g., phenolic derivatives with long alkyl chains) have steric hindrance thus lower mobility than those of smaller size, therefore lower diffusibility toward reactive centers | A cut-off effect was found for hydrophilic antioxidant in nonpolar medium |
| Sorensen et al. 61 | W/o emulsion resemble bulk oil, of which water is located in micelles and aqueous phase is surrounded by emulsifier. The efficacy of antioxidants in emulsions of water in omega-3 lipids follow polar paradox hypothesis, but not for the o/w emulsion. In the case of w/o, at pH7, ascorbic acid had negative charges and repulsive forces existed between the interface and ascorbic acid, thus it was located away from the interface. The polar paradox was insufficient to explain antioxidant effects in multiphase systems such as emulsions, as there are interactions between iron, emulsifiers, and antioxidants | W/o emulsions resemble bulk oils in their response to the polarity of compounds |
| Sun et al. 23 | Polar antioxidants with higher affinity were known to concentrate on oil/air or oil/water interface of the reversed micelle. Thus the antioxidant polar paradox does not always prevail, as some research found different results. Thus the influencing factors of antioxidant activity in reversed micelle were not solely based on antioxidant polarity | Polar paradox is affected by other factors contributing to non-linearity in the effect of antioxidants in lipid oxidation |
| Sun-Waterhouse et al. 74 | Caffeic acid and p-coumaric acid are hydrophilic. They tend to partition into the water phase, locate outside of the oil droplets and chelate metal ions which exist in the oils. Both antioxidants stabilized oil against autoxidation but facilitated the hydrolysis of TAG in the oils | Antioxidants may cause other adverse effects, for example, hydrolysis of TAG |
| Chen et al. 34 | Soybean oil is found in seeds inside micro-sized oil bodies, which consist of a central neutral lipid core (94–98% w/w) and is surrounded by phospholipids monolayer (0.5–2% w/w) and a coat of strong amphiphilic oleosin (0.5–3.5% w/w). These soybean oil bodies had a better physicochemical stability than emulsified soybean oil. Heat treatment (up to 55°C) did not affect the LOOH and hexanal content of oil body suspensions (2% wt at pH 3). | Natural organization protects unsaturated fatty acids. Water exists as nano-scale droplets in w/o emulsions |
| Rukmini et al. 130 | W/o microemulsion exist in bulk oil with nano-scale droplets of water inside. Formulation and stabilization of water-in-virgin coconut oil were prepared with food grade nonionic surfactants (Span 80, Span 20, and Tween 20). Cosurfactants may not be suitable for foods because of the toxicity and irritation induced by short- and medium-chain alcohols. Nonionic surfacts permitted stabilization of such w/o emulsion without the use of cosurfactants, but phase separation was observed when the microemulsion was heated at 70°C or higher | Nonionic surfactants offer an alternative solution as it stabilizes w/o emulsions and do not contribute to oxidation |
The initial observations related to the effects of molecular properties other than BDE were presented in the break through papers by late William Porter 36–38. He explained the role of a polar paradox by stating that polar (hydrophilic) antioxidant (e.g., Trolox C, ascorbic acid, propyl gallate, and TBHQ) are more effective in bulk lipids with a low surface/volume ratio whereas nonpolar (lipophilic) antioxidants (e.g., α-tocopherol, ascorbyl palmitate, BHA, and BHT) are more effective in oil-in-water emulsions (o/w) having a high surface/volume ratio. Shortly after, Frankel et al. 39–41 explained the polar paradox by the interfacial phenomena that hydrophilic antioxidants were more oriented at the air-oil interfaces in bulk lipids, while lipophilic antioxidants had more affinities toward water-oil interfaces in o/w. The interfacial phenomenon was first studied in o/w emulsions because of the wide availability of the methods needed to characterize these emulsions 14. Table2 presents results of investigations of the antioxidant potency of pairs of antioxidants shown in Fig. 1 having different polarities and effectiveness in bulk lipids confirming the polar paradox theory (e.g., carnosic acid vs. methyl carnosate 42,43, and quercetin vs. rutin 25,44). Examples for the effects of antioxidant polarity and steric hindrance on their effectiveness in bulk oils include the decrease in radical-scavenging activity by esterification of sinapic acid 45 and caffeic, dihydrocaffeic, and rosmarinic acids 46. Similarly, alkylation of ferulic acid 48 and hydroxytyrosol 51,52, and methylation of carnosic acid 42,43 cause their lipophilicity to increase and their antioxidant effectiveness in bulk oil to decrease. In addition, the presence of a double bond in the acyl substituent reduces the polarity and antioxidant capacity of propyl caffeate compared to hydrocaffeate and of ferulate compared to isoferulate 47. Catechin, with a trans configuration, has a better antioxidant activity than epicatechin, with a cis configuration 50. However, it was shown with epigallocatechin-gallate (EGCG) and its esters and with the glycosylation of quercetin (quercetin-3-O-glucoside and rutin) 25,44,49 that this effect is variable, that is, lipophilic antioxidants are sometimes more active in bulk oils (at lower levels) because the effects of lipid solubility on the antioxidant efficiency are stronger than those caused by the interfacial phenomenon. This suggests that the polar paradox might be applied only when antioxidant is added at high concentrations (above a critical concentration) where the interfacial phenomenon is dominant over solubility effects 53. This controversy is discussed below.
Table 2.
Results on antioxidants potency of pairs of antioxidants of similar structure and different hydrophilicity in bulk oil
| References | Antioxidants | Substrates and conditions | Results |
|---|---|---|---|
| Frankel et al. 39 | Ascorbic acid vs. ascorbyl palmitate | Stripped corn oil, added antioxidant (232 and 1161 μM), 60°C | Ascorbic acid was a more potent antioxidant than ascorbyl palmitate based on LOOH and hexanal formation |
| Carelli et al. 131 | SFO; each additive is at 200, 400, 600, and 800 ppm; 30 and 68°C (Oven); and 130°C (Rancimat) | Ascorbic acid was a more effective antioxidant than ascorbyl palmitate, according to Rancimat, and peroxide value (PV) (30°C), para-anisidine value (p-AV), total content, and distribution of polar compounds and residual α-tocopherol | |
| Sorensen et al. 61 | W/o emulsion (98% of 1:1 fish oil:rapeseed oil, stripped), 1% polyglycerol polyricinoleateemulsifier; antioxidants added at 100 mM; 37°C | Ascorbic acid was more effective antioxidant than ascorbyl palmitate on the basis of LOOH and propanal formation. Ascorbyl palmitate exhibited pro-oxidative effects toward the end of storage period | |
| Frankel et al. 39 | Trolox vs. α-tocopherol | Stripped corn oil, antioxidants added at 232 and 1161 μM, 60°C | Trolox was a better antioxidant than α-tocopherol on the basis of LOOH and hexanal formation. At the high concentration, α-tocopherol had a prooxidant effect |
| Schwarz et al. 43 | Stripped corn oil; w/o emulsion; cetheareth-15 and glyceryl stearate, polyglyceryl glucose methyl distearate, polysiloxan polyalcohol polyether copolymer and polyglyceryl-3 oleateas emulsifers each at 20% level; antioxidants added at 100 mM (based on the oil phase), 37 and 60°C | Trolox had higher activity than α-tocopherol based on LOOH and hexanal formation in both bulk oil and w/o emulsion with and without emulsifers. With a few exceptions, α-tocopherol showed better activity for w/o polysiloxan polyalcohol polyether copolymer emulsion based on LOOH and w/o polyglyceryl-3 oleate emulsion based on hexanal formation at 37°C. α-tocopherol showed a prooxidative effect in bulk oil with polyglyceryl-3 oleate at 60°C | |
| Huang et al. 60 | Linoleic acid, methyl linoleate, corn oil TAG, α-tocopherol are at 65 and 130 ppm and Trolox are at 38 and 76 ppm, 37 or 60°C in a shaking water bath | Trolox was a better antioxidant than α-tocopherol, in terms of LOOH and hexanal formation. With a few exceptions, α-tocopherol showed a better activity than Trolox (38 ppm), at 37°C, in bulk methyl linoleate and corn oil TAG based on hexanal formation; and at 60°C in bulk corn oil TAG, Trolox caused a pro-oxidative effect by hexanal results | |
| Chen et al. 20 | Stripped SBO; 1,2-dioleoyl-sn-glycero-3-phosphocholine, 1,2-dibutyryl-sn-glycero-3-phosphocholine each at 1000 μM; antioxidants at 10 and 100 μM; 55°C | Trolox was a more effective antioxidant than α-tocopherol with and without the addition of phospholipids (except 1,2-dibutyryl-sn-glycero-3-phosphocholine). 1,2-dioleoyl-sn-glycero-3-phosphocholine improved, while 1,2-dibutyryl-sn-glycero-3-phosphocholine decreased the α-tocopherol and Trolox activity | |
| Thiyam et al. 45 | Sinapic acid and sinapine | Purified rapeseed oil, sinapic acid, and sinapine at 50 and 500 μmol/kg oil, 40°C | Sinapic acid was better in reducing LOOH and propanal compared to its derivatives sinapine |
| Chen and Ho, 132 | Caffeic acid vs. caffeic acid phenethyl ester | Lard and corn oil each at 2 mM, Rancimat (110°C and 20 mL/min) | In lard, caffeic acid had better activity in extending the IP than caffeic acid phenethyl ester did. In corn oil, the activities of both antioxidants were the same |
| Nenadis et al. 46 | Caffeic acid vs. dihydrocaffeic acid | Triolein, each additive is at 10 ppm, 45°C in the dark | Based on the PV, the activity dihydrocaffeic acid was higher than caffeic acid and control. The presence of the conjugated double bond in the side chain of caffeic acid, makes its less polar and also decrease its hydrogen-donating properties, compared to dihydrocaffeic acid |
| Silva et al. 47 | Propyl caffeate and hydrocaffeate | Refined SFO, propyl hydrocaffeate, and propyl caffeate each at 160 and 200 ppm, Rancimat 110°C and 20 L/h | The antioxidant effectiveness of propyl hydrocaffeate was higher than that of propyl caffeate |
| Leonardis et al. 133 | Caffeic acid vs. chlorogenic acid (ester of caffeic acid and quinic acid) | Cod liver oil, antioxidants added at 0.005–0.05% by weight, Rancimat at 80 and 100°C and 20 L/h | Caffeic acid was a better antioxidant compared to chlorogenic acid. The later exhibited weak antioxidative effects |
| Laguerre et al. 134 | Chlorogenic acid and its esters | Stripped corn oil, each additive is at 200 μmol/kg, 55°C in the dark | Hydrophobicity of chlorogenic acid and its butyl, dodecyl, and hexadecyl esters did not correlate well with their antioxidant capacity in bulk oil. With and without dioleoylphosphatidylcholine, conjugated dienes test showed longer IP as the akyl chain increased |
| Chen and Ho 132 | Ferulic acid vs. ferulic acid phenetyl ester | Lard and corn oil, 2 mM, Rancimat (110°C and 20 mL/min) | In lard, ferulic acid and ferulic acid phenetyl ester had the same activity in extending the IP. In corn oil, both compounds had no significant effects in improving the oxidative stability |
| Fang et al. 48 | Ferulic acid vs. alkyl ferulates: 1-pentyl, 1-hexyl, 1-heptyl ferulates | Linolec acid; each additive is at 1.0 × 10−4, 3.0 × 10−4, 1.0 × 10−3, 3.0 × 10−3 molar ratios of the additive to linoleic acid; 37, 50, 65, and 80°C in the dark | Alkyl ferulate (1-pentyl, 1-hexyl and 1-heptyl ferulates) slightly increased the antioxidant activity compared to ferulic acid but their activities were not significantly different |
| Silva et al. 47 | Propyl ferulate and isoferulate | Refined SFO, propyl isoferulate, and propyl ferulate each at 160 and 200 ppm, Rancimat 110 °C and 20 L/h | Propyl isoferulate had a better antioxidant activity than propyl ferulate |
| Huber et al. 49 | Quercetin and quercetin-3-O-glucoside | Fish oil without antioxidant; each additive is at 100, 500, 1000, and 5000 μM; 70°C | Quercetin-3-O-glucoside had a higher antioxidant activity than quercetin at 100 and 500 μM. At 1000 μM, their activities were equal |
| Becker et al. 44 | Quercetin vs. rutin | Purified high-oleic SFO; each additive is at 0.25, 0.5, 1.0, and 2.0 mmol /kg oil; Rancimat 100°C and 20 L/h | The antioxidant activity of quercetin was higher than rutin |
| Wanasundara and Shahidi 25 | Refined-bleached and deodorized seal blubber oil and menhaden oil, each additive is at 200 ppm, 65°C in Schaal oven | The antioxidant activity of quercetin was higher than rutin in all substrates, as monitored by weight gain, PV, and thiobarbituric acid reactive substances (TBARS) | |
| Huang and Frankel 50 | Catechins | Corn oil TAG, each additive is at 140 μM, 50°C | According to LOOH formation: gallic acid had more antioxidant activity than epicatechin gallate and epigallocatechin gallate (EGCG) had more antioxidant activity than epigallocatechin |
| Shahidi and Zhong 53 | EGCG and its esters | Stripped corn oil; 1 mL/3 g, Rancimat (100°C and 20 L/h) | At lower concentrations, the antioxidant activity of EGCG was lower than its lipophilic ester derivative (stearate); but the effects were reversed at higher concentrations |
| Huang et al. 42 | Carnosic acid and methyl carnosate | Corn oil TAG, each additive is at 150 and 300 μM, 60°C | The antioxidant activity of methyl carnosate was higher than carnosic acid on basis of LOOH and hexanal formation |
| Schwarz et al. 43 | Tocopherol-stripped corn oil; w/o emulsion; cetheareth-15 and glyceryl stearate, polyglyceryl glucose methyl distearate, polysiloxan polyalcohol polyether copolymer, and polyglyceryl-3 oleate emulsifers each is at 20% level; antioxidants added at 100 mM (based on oil phase), 37 and 60°C | Methyl carnosate had higher antioxidant activity compared to carnosic acid in both bulk oil and w/o emulsions according to LOOH and hexanal formation, which does not agree with the polar paradox | |
| Frankel et al. 40 | Carnosic acid and carnosol | Stripped corn oil, each additive is at 50 ppm, 60°C in a shaker oven | Carnosic acid had a higher antioxidant activitiy than carnosol in bulk oil on the basis of LOOH and hexanal formation |
| Frankel et al. 41 | Corn oil, SBO, peanut oil, and fish oil. Each additive is at 30 and 50 ppm, 60°C | Carnosic acid had a higher antioxidant activitiy than carnosol in bulk corn oil, SBO, peanut oil, and fish oil on the basis of conjugated dienes and hexanal formation | |
| Hopia et al. 59 | Methyl linoleate, linoleic acid, corn oil TAG, additives at 150 and 300 μM, 37 and 60°C | Carnosic acid was a better antioxidant than carnosol in methyl linoleate and corn oil but not in bulk linoleic acid where carnosol had better activity than carnosic acid. The substrate seems to affect the performance of antioxidants | |
| Trujillo et al. 51 | Hydroxytyrosol and its fatty acid esters | Glyceridic matrix, each additive is at 1 and 5 mM, Rancimat 90°C | Hydroxytyrosol had higher antioxidant activity than hydroxytyrosyl acetate, palmitate, oleate, and linoleate |
| Medina et al. 52 | Fish oil, each additive is at 10, 25, 50, 100, 150, and 200 ppm, 40°C | Hydroxytyrosol had better antioxidant activity than its esters with increasing size of alkyl chain (i.e., hydroxytyrosol acetate, butyrate, octanoate, laurate, and octyl gallate) | |
| Medina et al. 104 | Resveratrol vs. acylated and glucosylated resveratrol | Cod-liver oil, each additive is at 100 ppm, 40°C | Resveratrol fatty acid esters with increasing size of alkyl chain (i.e., 3-stearoylresveratrol, 3-stearoylresveratrol, and 4′-stearoylresveratrol) and glucosylation (i.e., resveratrol-3-β-d-glucopyranoside, resveratrol-3, 5-di-β-d-glucopyranoside, and resveratrol-3,4′-di-β-d-glucopyranoside) had reduced antioxidant effectiveness compared to original phenol |
The role of micelles and association colloids
Koga and Terao 54 suggested that phospholipids enhance the antioxidant activity of α-tocopherol in bulk lipids because they aggregate to form microemulsions thus bringing the tocopherol closer to the oxidation site (or increased partition in the water phase of reversed micelles). Accordingly, the phenolic group of α-tocopherol and the polar head of the phospholipids are positioned near the polar region of the reversed micelles where radicals are formed and trapped while the nonpolar acyl chains are located in oil phase. Thus, these authors recognized the lipid oxidation reaction sites as the interfaces formed between traces of water and the continueous lipid phase rather than the air-oil interfaces suggested by Frankel et al. 39–41. It has also already been evident for Ulla Brimberg 26,27 that the transition of lipid oxidation from the initiation to the propagation phase is governed by the critical micelle concentration (CMC) of hydroperoxides and its modification by other amphiphiles acting as antioxidants, prooxidants, or modifiers (synergists or antagonists). Brimberg proposed a set of empirical equations that was also able to successfully describe the oxidation of different oil/additive combinations 26,27. Brimberg and Kamal-Eldin 56 proposed that lipid oxidation in bulk oil starts by pseudo-first order slow build-up of hydroperoxides until these reach their CMC and start aggregation to form reversed micelles. At this point, the reaction rate change to a second order reaction and the oxidation enters the propagation phase (Fig. 2). Accordingly, the main effects of anti- and prooxidants depend on their modulation of the CMC of lipid hydroperoxides.
Figure 2.
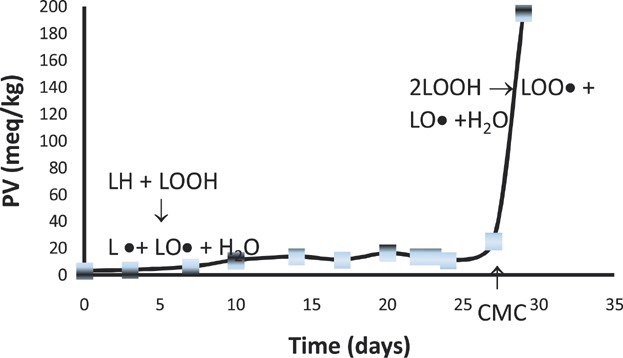
Peroxide value (PV) evolution during the autoxidation of flaxseed oil at 40°C. The first period (lag phase or induction period) is dominated by reactions between unsaturated fatty acids (LH) and low concentrations of hydroperoxides (LOOH). This reaction is first order with respect to LH and LOOH but because of the relatively very high concentration of LH and the very low concentration of LOOH, it is often described as zero order. When the concentration of LOOH reaches its critical micelle concentration (CMC), formation of micelles become significant and the reaction enter the bimolecular phase with respect to hydroperoxides. Attainment of CMC marks the end of the induction period (IP).
It started to become clear that lipid oxidation is affected by several properties of emulsion droplets and interface properties including droplet size, interfacial area, charge, thickness, and permeability 14,57,160. For example, cationic charge caused transition metals to be excluded from the interface hence lessening oxidation rates in o/w 14. This finding was later verified in bulk oils when the research group of Decker et al. studied these phenomena in depth and reported several supporting findings 14,22,160. The conclusion is that compounds that are surface active change the physical location or the state of other compounds that form reversed micelles in bulk oils such as hydroperoxides and metals 20,33. When water, other polar compounds, and amphiphilic molecules (e.g., lecithin) coexist in oil, association colloids (e.g., reversed micelles) are formed providing a reaction site for oxidation to take place 14,33. Oxygen, the primary catalyst of lipid oxidation, is 3–10 times more soluble in oil than in water and in fact, air (dielectric constant = 1.0) is much nonpolar than oil (dielectric constant = 3.0). Thus hydrophilic antioxidants are more likely to partition at the oil-water interface than at the air-oil interface supporting the assumption that the microemulsions in the oil are the site of oxidation 14,42,53,59–61. These polar antioxidants aggregate to form microemulsions and create a shield to protect micelles 14,62. In the same way, emulsifiers enhance the formation of micelles that entrap amphiphilic antioxidants and bring them to the interphase 63,64. On the other hand, lipophilic antioxidants are less effective in retarding oxidation in bulk lipids because they disperse in the continuous oil phase far away from the catalytic sites of the micelles while they are effective in protecting o/w emulsions because they orient more at the water-oil interface 33,39,61,169.
Table3 presents collated results on the effect of different additives on lipid oxidation in bulk oil. The more hydrophilic Trolox was found to be a better antioxidant than α-tocopherol because it concentrates in the water phase of the mixed micelles 7,65. On the other hand, trace metal ions act as prooxidants by migrating to the oil-water interface of the micelles 57,66,67. Similarly, free fatty acids and monoacylglycerols act as prooxidants by a “micellar effect” by concentrating at the oil-water interface and accelerating the decomposition of hydroperoxides 33,39,61,169. On the other hand, phospholipids act as synergists by enabling the antioxidants at the interface 14,33, where the polar heads of the antioxidants are located at the water-oil interface and their hydrogen atoms are donated to the radicals. Thus, the antioxidants and phospholipids (synegist) trap the radicals in a cage (i.e., microenvironment) 54 and prevent their diffusion into the bulk oil; the so called volume cage effect 68. The combination of antioxidants and surfactants can act synergistically or antagonistically depending on their types (Fig. 3) and concentrations 69. The effects of surface-active agents are influenced by their hydrocarbon chain length, hydrophilic lipophilic balance, and concentrations 63,64.
Table 3.
The effects of different types of additives on the stability of bulk lipids
| References | Substrates and oxidation conditions | Additive(s) | Results | Conclusions |
|---|---|---|---|---|
| Fatty alcohols | ||||
| Yanishlieva and Kortenska 135 | SFO, OO, lard, tristearin, olive oil methyl esters, 70–135°C | 1-Tetradecanol, 1-hexadecanol, 1-eicosanol (5–80 mmol/kg) | The pro-oxidative effects of fatty alcohols depend on the type, concentration, valency of the alcohols and LOOH, and the degree of unsaturation of the lipid media. The pro-oxidative effect was less in TAG than in fatty acid methyl esters | Fatty alcohols antagonized the antioxidant effect of phenolic inhibitors |
| Kortenska et al. 136 | SFO, methyl ester, 50°C | p-Methoxyphenol (0.1 M), 1-octadecanol (0.1 M), and 1-palmitoylglycerol (0.1 M) | 1-Octadecanol and 1-palmitoylglycerol acted as prooxidant, by decreasing the rate constant of chain termination, in the presence of inhibitor (p-methoxyphenol) | Fatty alcohols inhibited inhibitor by formation of H-bonds and complex formation with the inhibitor. 1-palmitoylglycerol had a stonger effect because of its two hydroxyl groups |
| Yanishlieva and Kortenska 137 | TAG of SFO and TAG of OO, 23 and 110°C | Hydroquinone (1 × 10−4 mol/L), 1-tetradecanol, 1-octadecanol ([0.5−9.0] × 10−2 mol/L) | Fatty alcohols accelerated the oxidation of lipids (in the presence of hydroquinone). Increasing unsaturation of substrate caused a lesser prooxidative effect of the alcohols | Shorter chain alcohols caused stronger complex formation (H-bond) with hydroquinone. However, longer chain alcohols had a higher prooxidative activity in the propagation, branching, and termination reactions |
| Kortenska and Yanishlieva 138 | TAG of SFO, 80°C | Hydroquinone, BHT, α-tocopherol (each at 0.1 mM); 1-tetradecanol, 1-octadecanol (5, 20, 40, and 60 mM) | Fatty alcohols acted as prooxidants. A linear dependence of oxidation of oil was seen, with hydroquinone, in the presence of 1-tetradecanol, α-tocopherol + 1-tetradecanol + 1-octadecanol. No interactions between BHT and 1-octadecanol in inhibiting oxidation | There was no interaction between 1-octadecanol and BHT, because 1-octadecanol participates in the process only by accelerating the decomposition of LOOH |
| Kortenska et al. 68,81 | TAG of SFO, 80°C | 2,6-Di-tert-butyl-4-methylphenol (0.1 mM), 1-tetradecanol (40 mM), 1-octadecanol (40 mM), and 1-monopalmitoylglycerol (40 mM) | Fatty alcohols decreased the IP, as measured by LOOH. 1-monopalmitoylglycerol caused a further reduction of IP. BHT + fatty alcohols also exhibited prooxidative effects, with no improvement on the IP | Polar compounds such as fatty alcohols and oxidation products associate in non-polar medium. LOOH decomposed faster inside the polar interior of the micro emulsion. Fatty alcohols alone and combined with BHT were prooxidants |
| Kortenska et al. 68 | SFO and lard, 100°C | α-Tocopherol (1.3 mM), 1-octadecanol (5, 40, and 80 mM) | The higher the 1-octadecanol level, the more the reduction of IP and increase of oxidation rate in both medium. The relative increase of oxidation rate in SFO was more than in lard | Fatty alcohols acted as prooxidant and inhibited α-tocopherol activity |
| Free Fatty Acids (FFA) | ||||
| Miyashita and Takagi 115 | Oleic acid, methyl oleate, linoleic acid, methyl linoleate, linolenic acid, methyl linolenate; 50°C in the dark | No additives | Methyl esters are more stable than their corresponding FFA (longer IP and have lower PV) | FFAs are more susceptible to oxidation than corresponding esterified fatty acids. The catalytic effect of the carboxyl groups of FFA on the formation of free radicals and decomposition of LOOH was thought to be the reason. According to the current theory a bulk of FFAs is different from a bulk of TAGs in the molecular assembly of the substrate itself and of any added additive. FFAs are surface active and contribute to micelle formation when added to TAGs |
| Methyl linoleate and SBO; 50°C in the dark | Stearic acid (0, 0.5, 1, 3, and 5%) | The addition of stearic acid to methyl linoleate or SBO did not affect the IP but increased the oxidation rate during this IP | ||
| Methyl linoleate hydroperoxides; 50°C in the dark | Stearic acid (0, 0.2, 0.5, and 1%) | Hydroperoxides (PV and conjugated diene content), decomposed faster in the presence of stearic acid | ||
| Mistry and Min 139 | SBO, forced air oven 55°C | Stearic, oleic, linoleic, linolenic, or octadecane (0, 0.5, and 1%) | FFA, but not octadecane, showed prooxidant activity in SBO (PV, volatile compounds, and oxygen in the headspace) | |
| Hamam and Shahidi 140 | Doxosahexaenoic acid single cell oil; Schaal oven 60°C | Capric acid (not added but due to acidolysis) | Acidolysis of the single cell oil, with capric acid, decreased the oxidative stability of the oil (Conjugated dienes and TBARS) compared to unmodified doxosahexaenoic acid single cell oil | |
| Frega et al. 141 | Virgin OO; Rancimat 110°C, 20 L/h; OO (cloudy untreated, cloudy paper-filtered, cloudy membrane-filtered, and cloudy bleached with clay); Rancimat 110°C, 20 L/h | Oleic acid or methyl oleate (0–3%) | Methyl oleate but not oleic acid (% acidity) decreased the IP of virgin OO. Oleic acid (% acidity) increased the IP of cloudy untreated oil, did not affect the IP of cloudy paper-filtered oil, and decreased the IP of cloudy membrane-filtered and cloudy bleached oils | The prooxidant effect of FFA is dependent on the matrix. For example, oleic acid had a prooxidant activity in membrane-filtered and bleached OO but not in cloudy oils; and methyl oleate but not oleic acid had a prooxidant effect on virgin OO |
| Chaiyasit et al. 14 | Methyl linolenate in model oil system containing sodium bis(2-ethylhexyl) sulfosuccinate, water-hexadecane; ferrous sulfate, 24°C in the dark | Oleic acid (0, 25, 50, and 100 mmol/kg lipid) | Oleic acid reduced the reversed micelle size and accelerated lipid oxidation (LOOH and TBARS) compared to a control and added phosphatidylcholine | The prooxidant effect of FFA seems to be related to their action as surfactants (increasing the number of small micelles) |
| Monoacylglycerols (MAG) and diacylglycerols (DAG) | ||||
| Mistry and Min 142 | Refined, bleached, and deodorized SBO, forced-air oven 55°C | 1-Monolinolein (0.01%) | MAG (1-monolinolein) had prooxidant activity in SBO (PV and volatile compounds) | MAG and DAG showed prooxidant effects in pure (but not unpure TAG) depending on polarity, concentration, and temperature |
| Mistry and Min 143 | SBO, forced-air oven 55°C | Monostearin, monolinolein, distearin, and dilinolein (0, 0.25, and 0.5%) | Monostearin, monolinolein, distearin, and dilinolein acted as prooxidants in SBO (decrease of headspace oxygen) in a concentration-dependant manner | |
| Caponio et al. 144 | Purified SBO, oven 60°C; measured at 4, 6, 9, 14, and 18 days | Unnamed MAG (0, 0.5, 1, 2, and 3%) | The effect of MAG in the oxidation of purified SBO is concentration-dependent. At low amount (0.5 and 1%), MAG increased the oxidation rate while at higher concentrations the IP was reduced | |
| Aubourg 65 | Cod liver oil containing citric acid; 15, 30, and 50°C | Unnamed MAG (0.1, 0.5, 2, and 5%) | MAG (2 and 5%) caused an inhibitory effect on the protective effect of citric acid at 50, but not at 15 and 30°C | MAG and DAG enhanced the oxidation of cod liver oil containing citric acid, which functions as a metal chelator. A longer chain length increased the prooxidant effect possibly because of increased surfactant activity |
| Cod liver oil containing citric acid; 50°C | Monolauroyl-glycerol, monomiristoyl-glycerol, monopalmitoyl-glycerol, and monostearoyl-glycerol (3.78 mM) | The prooxidant effect of MAG increased as the chain length of MAG increased | ||
| Cod liver oil containing citric acid; 30 and 50°C | Unnamed diacylglycerols (0.1, 0.5, 2, and 5%) | DAG showed an inhibitory effect on the protective effect of citric acid at 50 but not 30°C. There was no difference in effect between different DAG concentrations | ||
| Wang et al. 145 | Purified and natural corn oil; 28°C | 1-Monolinoleoyl-rac-glycerols (0, 0.1, 0.25, and 0.5%) | MAG decreased the IP for purified but not for unpurified oil | MAG may contribute pro-oxidant effect(s) in the oxidation of bulk oils |
| Natural and randomized corn oil, Oxidative Stability Index (OSI), 28°C | 1-Monolinoleoyl-rac-glycerols and 1,3-dilinoleoyl-rac-glycerol (conc. unknown) | MAG showed a higher prooxidant activity than DAG (reduced OSIIP) | ||
| Natural and randomized corn oil; 28°C | 1,3-Dilinoleoyl-rac-glycerol (5%) | There was an increase in the oxidation rate of purified oil with 5% DAG but the increases were not as great as that of randomized oil | ||
| Phospholipids (PL) | ||||
| King et al. 146 | Salmon oil, Fischer forced-draft oven, 180°C | Phosphatidylcholine (0.01, 0.1, and 1%) | Phosphatidylcholine improved the oxidative stability of the oil in a concentration-dependant manner (as measured by TBARS and polyene index) | The amine group of phosphatidylcholine and phosphatidylethanolamine and the reducing sugar of phosphatidylinositol can facilitate hydrogen or electron donation by α-tocopherol at 180°C. Nitrogen-containing phospholipids perform better in improving the oxidative stability of oil than those that do not contain nitrogen |
| Salmon oil, Fischer forced-draft oven, 180°C | Phosphatidylglycerol, phosphatidylinositol, phosphatidylserine, phosphatidylethanolamine, phosphatidylcholine, lysophosphatidylcholine, and sphingomyelin (1% each) | Nitrogen-containing phospholipids (i.e., phosphatidylethanolamine, phosphatidylcholine, lysophosphatidylcholine, and sphingomyelin showed higher antioxidant acitivity than phosphatidylglycerol and phosphatidylinositol (yielded the least activity). The slope of oxidation rate showed that sphingomyelin was the most effective and phosphatidylinositol was the least effective | ||
| Methyl linoleate, methyl laureate, 50°C in the dark, continuous shaking at 120 rpm | Dipalmitoylphosphatidylcholine (DPPC, 100 nM), dipalmitoylphosphatidylethanol-amine (DPPE, 100 nM), α-tocopherol (10 nM) | Without α-tocopherol, Dipalmitoylphosphatidylcholine gave a slower oxidation rate than dipalmitoylphosphatidylethanolamine. With α-tocopherol, IP was prolonged and methyl linoleate-OOH accumulated after α-tocopherol was consumed. DPPC and DPPE showed an insignificant effect in oxidation rate, in the presence or absence of α-tocopherol | DPPC and DPPE act synergistically with α-tocopherol, but the effect is insignificant | |
| Koga and Terao 54 | Methyl linoleate; methyl laureate; 2,2′-azobis(2-amidinopropyl) dihydrochloride (AAPH) (water soluble radical initiator); 2,2′-azobis(2,4-dimethylvaleronitrile) (AMVN) (lipid soluble radical initiator), 50°C in the dark, continuous shaking at 120 rpm | Dipalmitoylphosphatidylcholine, dibutyrylphosphatidylcholine, dicaprylphosphatidylcholine, and dimyristoylphosphatidylcholine (each 100 nM), α-tocopherol (10 nM) | With the use of 2,2′-azobis(2-amidinopropyl) dihydrochloride, phospholipids caused a more rapid consumption of α-tocopherol. With 2,2′-azobis(2,4-dimethylvaleronitrile), phospholipids did not affect the consumption of vit E. The IP increased with increasing hydrocarbon chain length of acyl moieties of phospholipids | Phospholipids accelerated the consumption of α-tocopherol when radicals are generated from water-soluble radical generators with a trace of water |
| Khan and Shahidi 84 | Borage oil TAG, dark, in a Schaal oven at 60°C | Phosphatidylcholine (500 ppm), phosphatidylethanolamine (500 ppm), α-tocopherol (500 ppm), δ-tocopherol (500 ppm) | Phosphatidylcholine lengthened the oxidation time more than phosphatidylethanolamine (based on conjugated dienes). Combinations of phosphatidylcholine + α-tocopherol., phosphatidylcholine + δ-tocopherol, phosphatidylethanolamine + α-tocopherol, phosphatidylethanolamine + δ-tocopherol (500 ppm phospholipids and 500 ppm tocopherol) lengthened the oxidation time than individually added phosphatidylcholine, phosphatidylethanolamine, α-tocopherol, and δ-tocopherol. Combination of α-tocopherol with each phospholipid was more effective than combination of δ-tocopherol. The most effective combination was that of phosphatidylcholine and α-tocopherol (on basis of TBARS) | Phosphatidylcholine is more effective than phosphatidylethanolamine alone and in combination with α-tocopherol in borage oil TAGs. Phosphatidylethanolamine was more effective than phosphatidylcholine in evening primose TAGs. Phospholipids increase the accessibility of the tocopherols to the aqueous environment (the micellar phase) |
| Evening primrose oil TAG, dark, in a Schaal oven at 60°C | Phosphatidylcholine (500 ppm), phosphatidylethanolamine (500 ppm), α-tocopherol (500 ppm), and δ-tocopherol (500 ppm) | Phosphatidylethanolamine lengthened the oxidation time more than phosphatidylcholine (based on conjugated dienes). Combinations of phosphatidylcholine + α-tocopherol, phosphatidylcholine + δ-tocopherol, phosphatidylethanolamine + α-tocopherol, phosphatidylethanolamine + δ-tocopherol (500 ppm phospholipids and 500 ppm tocopherol) with the combinations with phosphatidylethanolamine being more effective than those with phosphatidylcholine (on basis on TBARS) | ||
| Hidalgo et al. 147 | Refined SBO, heated in the dark under air at 60°C | Phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositol (each 200 ppm) | Phospholipids lengthened the IP (phosphatidylcholine > phosphatidylethanolamine > phosphatidylinositol) and the protection was better with oxidized phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositol (by polymeric pyrroles). Phosphatidylethanolamine showed max. antioxidative activity when the pyrrole content was between 800–1400 nmol of pyrrole/mmol of phosphatidylethanolamine | Phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol improved the oxidative stability of the oil. Lysine activity as antioxidant was improved in combination with phosphatidylethanolamine or phosphatidylcholine (synergism). No synergism was observed for phosphatidylcholine plus phosphatidylethanolamine |
| Refined OO (ROO), Rancimat 110°C | Phosphatidylethanolamine, phosphatidylcholine, lysine, and BHT (each added at 4 levels: 100, 200, 300, and 400 ppm) | Phosphatidylethanolamine, phosphatidylcholine, lysine, and BHT increased oxidative stability of the oil at 200 ppm or more. The IP of oil with 400 phosphatidylethanolamine had similar stability as that of oil with 200–400 ppm BHT | ||
| Phosphatidylethanolamine and/or lysine (combinations at 100/300,200/200, and 300/100 ppm) | Phosphatidylethanolamine and lysine showed a better protection to the oil than when each was added alone. The IP increased in the following sequence 100/300 < 200/200 < 300/100 ppm | |||
| Phosphatidylcholine and/or lysine (combinations at 100/300, 200/200, and 300/100 ppm) | Phosphatidylcholine and lysine showed a better protection to the oil than when each was used alone. The IP increased in the following sequence 200/200 ≤ 300/100 < 100/300 ppm | |||
| Phosphatidylcholine and/or phosphatidylethanolamine (combination at 100/300, 200/200, and 300/100 ppm) | Phosphatidylcholine and phosphatidylethanolamine did not exhibit any synergism | |||
| Koprivnjak et al. 73 | Filtered virgin OO, Rancimat, 120°C | Phospholipids (lecithin) (0, 2.5, 5, 7.5, and 10 g/kg) | As the amount of lecithin increased, IP lengthened. The addition of lecithin also increased total tocopherols but decreased the α/γ tocopherol ratio | |
| Lee and Choe 148 | Tocopherol-stripped SFO; Water in oil emulsion consists of methylene chloride, n-butanol, Na2MoO4·2H2O, sodium dodecyl sulfate; rubrene (a singlet oxygen quencher); 25°C for 24 h | Phosphatidylcholine (0, 250, and 1000 ppm) | Phosphatidylcholine extended the IP of the oil. Different phospholipids concentrations have the same effects | |
| Chen et al. 7 | Stripped SBO, 25°C for 24 h | Dioleoylphoshatidylcholine, dibutyrylphosphatidylcholine, (each 0–1270 mmol/kg) | The association colloids formed by dioleoylphoshatidylcholine and water were prooxidative, while those formed by dibutyrylphosphatidylcholine were comparable to control | The structure of association colloids may influence lipid oxidation in bulk oils. Dioleoylphoshatidylcholine and dibutyrylphosphatidylcholine have identical choline groups but different physical structure in oil. SAXS measurement revealed that dioleoylphoshatidylcholine formed spherical structures while dibutyrylphosphatidylcholine formed cylindrical structures |
| Chen et al. 20 | Stripped SBO, 50°C | Dioleoylphoshatidylcholine (1000 μM), α-tocopherol (10 and 100 μM), and Trolox (10 and 100 μM) | Dioleoylphoshatidylcholine formed reversed micelles in oil and shortened the IP. Dioleoylphoshatidylcholine improved the activity of low α-tocopherol or Trolox concentrations (10 μM) but decreased the activity at high concentrations (100 μM) | |
| OH-containing carotenoids | ||||
| Haila et al. 149 | TAG from low-erucic acid rapeseed oil, under dark at 40°C | Lutein (5, 20, 30, and 40 ppm), lycopene (20 ppm), annatto (20, 30, and 60 ppm as bixin), β-carotene (20 ppm), γ-tocopherol (10 and 15 ppm), lutein + γ-tocopherol (20 + 15 ppm, 1:1 in molar ratio), or lutein + γ-tocopherol (20 + 10 ppm, 1.5:1 in molar ratio) | Lutein caused more LOOH. Lutein (20 ppm) + γ-tocopherol (15 ppm) were antioxidants. Lutein was consumed faster and slower (higher retention), without and with γ-tocopherol, respectively. The consumption of γ-tocopherol was not affected by lutein. Total of 30 and 60 ppm bixin (annatto) was significantly reduced LOOH levels | Lutein was prooxidant both in the dark and light. When lutein is combined with tocopherol, they significantly increase oxidative stability. Lycopene and β-carotene were prooxidants |
| Henry et al. 150 | Purified safflower seed oil, OSI, 75°C for 24 h, 85°C for 12 h, 95°C for 5 h | Lycopene (35 μM), lutein (66 μM), 9-cis-β-carotene (54 μM), and all-trans-β-carotene (150 μM) | The orders of the rate of degradation were lycopene >9-cis-β-carotene = all-trans-β-carotene > lutein | Geometric configurations do not effect the decomposition rate, as in 9-cis and all-trans carotene. OSI in hours and lipid oxidation measurements (e.g., PV, TBARS) need to be performed to study the effects of carotenoids on safflower oil oxidation |
| Subagio and Morita 151 | Purified corn-oil TAG, paraffin, 40°C, dark | α-Tocopherol, β-carotene, and lutein (each at 5, 10, and 30 ppm and combination of 30 and 30 ppm) | Lutein increased the amount of LOOH. β-carotene + lutein caused more degradation of β-carotene. Lutein was more unstable than β-carotene in paraffin (medium similar to TAG) | The antioxidative effect of β-carotene was dose-dependent, at higher concentration it became prooxidant. When combined, β-carotene protected lutein, as β-carotene degraded more than lutein in oil. But in paraffin it is the opposite |
| Kiokias and Gordon 71 | Purified OO, oven, 60°C | β-carotene (1 g/L), annatto oil-soluble (bixin; 1 g/L), and annatto water-soluble (norbixin) (1 and 2 g/L), virgin olive oil polar extract (0.2 g/L), α-tocopherol (0.1 mM), γ-tocopherol (0.1 mM), ascorbic acid (0.1 mM), and ascorbyl palmitate (0.1 mM) | Norbixin showed synergisms with ascorbic acid, ascorbyl palmitate, and tocopherols, which were beyond the effect of phenolic antioxidants in oils and emulsions. These effects are better than that of β-carotene or β-carotene and polar extract | The presence of polar carboxylic acid groups in the norbixin molecule may contribute to chelation of metal ions or other polar initiating species, thus retarding autoxidation of oil |
| Becker et al. 44 | Purified SFO; Rancimat (OSI), 100°C, 20 L/h | α-Tocopherol, rutin, astaxanthin, quercetin (each at 0.25, 0.5, 1.0, and 2.0 μmol antioxidant/g oil, and their combination of 1.0 + 1.0, 0.5 + 0.5, and 0.25 + 0.25 μmol/g) | The antioxidant ranking in bulk oil: quercetin > α-tocopherol ≫ astaxanthin = rutin | Astaxanthin exerts antioxidant activity in bulk oil |
| Zeb and Murkovic 152 | Refined OO, Rancimat, 110°C, 1–14 h | β-Carotene, E-astaxanthin (300 ± 0.5 ppm) in olive oil | E-astaxanthin protected olive oil from oxidation (reduced epoxides) and inhibited β-carotene degradation | 9-Z-astaxanthin showed a higher antioxidant effects among E and Z-astaxanthin. β-Carotene acted as prooxidant after prolonged heating |
| Amino acids | ||||
| Ahmad et al. 153 | Safflower oil, a mixture of sunflower and cottonseed oil, active oxygen method at 97.8°C | Cysteine, proline, tryptophan, methionine, glutamic acid, lysine, and arginine (each at 0.01, 0.02, 0.04, 0.07, 0.10, 0.40, 0.70, and 1.00%) | Cysteine and glutamic acid were prooxidants in the oil mix, and glutamic acid was prooxidants in the safflower oil. The highest protection activity in the safflower oil was due to methionine, proline, lysine, and cysteine. The highest protection activity in the mix was due to lysine, arginine, glutamic acid, methionine, and hydroxyproline. However the amino acid protection activities were very low, low, or medium | Antioxidative activity of amino acids in such oils was low. It could be that they do not contribute as antioxidant by themselves, but requiring primary antioxidants |
| Alaiz et al. 154 | SBO, air in the dark at 60°C | N-(Carbobenzyloxy)-1(3)-[1′-(formylmethyl)hexyl]-l-histidine dihydrate (compound 1) formed from reaction of histidine and (E)-2-octenal (50, 100, and 200 ppm), Z-histidine (50, 100, and 200 ppm) | The order of stability: Compound 1 > Z-Hitidine > Control. The protection index of compound 1 and Z-histidine increased with concentration | Reactions of products from lipid oxidation (aldehydes) and amino acids exhibited an antioxidant property. The polymerization of these compounds produces melanoidin-like polymers which cause changes in color and fluorescence |
| Carlotti et al. 123 | Linoleic acid in sodium dodecylsuphate micellar solutions (with ethylenediaminetetraacetic acid (EDTA) and azo-initiator added), pH 5.0 and 7.0, 45 and 56°C | α-Tocopherol (1.0−6.0 × 10−6 M), α-tocopherol: 5.0 × 10−6 M, and ascorbic acid: 0.4−1.0 × 10−4 M, α-tocopherol: 5.0 × 10−6 M, and ascorbic acid: 0.5 × 10−4 M, l-tryptophan (8.5 × 10−5 M − 1.0 × 10−4 M), l-alanine (1.0 − 1.2 × 10−4 M), l-cysteine (8.5 × 10−4 M − 1.0 × 10−4 M), glycine (1.0 − 1.5 × 10−4 M), and gluthathione reduced form (7.5 − 8.5 × 10−5 M) | Glutathione, l-tryptophan, l-alanine, l-cysteine, and glycine prolonged the IP of micellar solution (containing 5.0 × 10−6 M α-tocopherol + 5.0 × 10−6 M vit C) at pH 5.0 and 7.0 (at 45°C). The synergistic action was particularly significant for l-cysteine, l-tryptophan, and Glutathione | Amino acids exhibited antioxidative effects, either added alone or combination with other amino acids or α-tocopherol |
| Hidalgo et al. 155 | Refined OO, Rancimat, 110°C | Phosphatidylethanolamine, phosphatidylcholine, lysine, BHT (each 0, 100, 200, 300, and 400 ppm and combination of phospholipids of 100, 200, or 300 ppm with amino acid of 100, 200, or 300 ppm) | Lysine (200 ppm or more) increased IP, which was superior to those of oil with phosphatidylethanolamine, phosphatidylcholine, and BHT of the same concentration. A total of 300 ppm phosphatidylethanolamine + 100 ppm lysine caused 185% increase of IP compared to control, and when both were used alone. Phosphatidylcholine and lysine increased the IP | Lysine and phosphatidylethanolamine or phosphatidylcholine exhibited synergism. The amino group of lys reacted with oxidized lipids to form hydrophilic pyrroles, which are good for oil (polar paradox) |
| Papadopoulou and Roussis 156 | Corn oil; 50, 120, and 180°C | N-acetyl cysteine and gluthathione (10, 20, and 40 mg/L),butylated hydroxyanisole (BHA; 200 mg/L) | N-acetyl cysteine and gluthathione reduced the formation of conjugated dienes, trienes, and PV at 50, 120, and 180°C. The antioxidative ranking were BHA > N-acetyl cysteine > gluthathione; except that at 180°C, N-acetyl cysteine were more effective than BHA | N-acetyl cysteine and gluthathione are hydrophilic compounds, which have more affinities toward the air-oil interface in bulk oil, thus more effective than lipophilic ones in bulk oil |
| Hidalgo et al. 157 | Stripped virgin OO, β-sitosterols added stripped virgin OO, Rancimat, 90°C, 10 L/h | Phosphatidylethanolamine (0, 100, 200, 300, and 400 ppm), phosphatidylcholine (0, 100, 200, 300, and 400 ppm), lysine (0, 100, 200, 300, and 400 ppm), and β-sitosterols (1500 μg) | Phosphatidylethanolamine, phosphatidylcholine, lysine, and their combinations cause a significantly higher antioxidative effects in β-sitosterol added virgin OO, compared to virgin OO. The combined phosphatidylethanolamine/phosphatidylcholine gave a shorter IP but phosphatidylethanolamine/lysine and phosphatidylcholine/lysine increased the IP, more than when the components were added alone | Amino groups of lysine react with oxidized lipids to form hydrophilic pyrroles, which may contribute to the stabilization of oil |
| Citric acid and ethylenediaminetetraacetic acid (EDTA) | ||||
| Hras et al. 158 | Stripped SFO, oven, 60°C | Rosemary extract (0.02%), α-tocopherol(0.01%), ascorbyl palmitate (0.01%), citric acid (0.01%), and their combinations | Citric acid alone reduced peroxide value (PV) and anisidine value (AV), but the activity was lower than the extract and ascorbyl palmitate. The order of antioxidant activity: extract + ascorbyl palmitate > extract + citric acid > extract > extract + AT > control | Citric acid is a chelating agent, by forming bonds between the metal and the carboxyl or hydroxyl groups of the citric acid molecule. Citric acid alone had antioxidant role. The effect was greater when citric acid was combined with extract |
| Jaswir et al. 159 | Fresh, cold-pressed, unrefined, and antioxidant-free flaxseed oil, heated to frying temp. 165 ± 5°C for 3.5 min, then 165°C for 6 min., and allowed to reach 60°C at room T, flushed with N2 gas and kept in a cold room at 4°C until analysis | Oleosin rosemary extract (0, 0.05, and 0.1%), sage extract (0, 0.05, and 0.1%), and citric acid (0, 0.025, and 0.05%) | Antioxidants (extracts and their combinations) significantly reduced PV, p-AV, FFA, color yellow, C18:1, C18:2, and C18:3, reduced some of absorbances at 232 and 268 nm, but increased C16:0 and C18:0 | Antioxidants added to the oil before frying were effectively retarding lipid oxidation and reducing oil hydrolysis during deep frying |
| Wang et al. 145 | Natural and randomized corn oil; OSI, 100°C | Citric acid (100 and 200 ppm) | Randomized corn oil had a much lower OSI than natural corn oil does. Citric acid (200 ppm) partially restored the OSI of randomized oil | Citric acid protective effect is not related to chelation of transition metals |
| Drusch et al. 32 | Stripped refined fish oil, 20°C | α-Tocopherol (100 ppm), δ-tocopherol (1000 ppm), ascorbyl palmitate (50 and 500 ppm), lecithin (500 and 2000 ppm), and citric acid(100 and 500 ppm) | 500 ppm ascorbyl palmitate + 500 ppm citric acid reduced LOOH compared to control and sample with 500 ppm ascorbyl palmitate + 200 ppm lecithin + (100 or 500 ppm) citric acid | Citric acid effects are due to its metal ions chelation ability, the action is greater when trace metal content is high and is trivial when trace metal content is low. Citric acid showed a more synergism with ascorbyl palmitate, but less with lecithin |
| Yi et al. 160 | Mixture of yellow palm olein/fish oil, mixture of red palm olein/fish oil, 30°C in the dark | Ascorbyl palmitate (200 and 500 ppm), citric acid (50 ppm), phosphatidylethanolamine (500 ppm), and phosphatidycholine (500 ppm) | Citric acid and ascorbyl palmitate displayed a pronounced inhibiting effect on the formation of radicals, IP, PV, and TBARS (Phosphatidylethanolamine or phosphatidylcholine) + (ascorbyl palmitate + citric acid), the phospholipids exhibited a prooxidant effect | Citric acid did not show synergisms with phospholipids |
| Wang et al. 145 | Natural and randomized corn oil; OSI, 100°C | EDTA (100 ppm) | Randomized corn oil had a much lower OSI than natural corn oil does. EDTA (100 ppm) completely restored the OSI of randomized oil | The protective effect of EDTA is not related to chelation of transition metals |
| Ascorbyl palmitate | ||||
| Frankel et al. 39 | Stripped corn oil, 60°C in a shaker oven | α-Tocopherol, trolox, ascorbic acid, and ascorbyl palmitate (each at 100 and 500 ppm) | α-Tocopherol and ascorbyl palmitate were more effective in o/w emulsion than in bulk oil. Ascorbic acid, Trolox and α-tocopherol + ascorbic acid, or α-tocopherol + ascorbyl palmitate were more active in bulk oil, but ascorbic acid alone was better than α-tocopherol + ascorbic acid. LOOH and hexanal were measured | Lipophilic antioxidants (α-tocopherol and ascorbyl palmitate) were more effective in o/w emulsions than bulk oil (or w/o emulsions). The opposite exists for hydrophilic antioxidants (ascorbic acid and Trolox). There is a strong synergism between α-tocopherol + ascorbyl palmitate and α-tocopherol + ascorbic acid, but α-tocopherol + ascorbic acid were not significantly better than ascorbic acid alone |
| Gordon and Kourkimska 161 | Rapeseed oil, heating at 80°C, deep fat frying, Rancimat 100°C | TBHQ (0.2 g/kg), lecithin (1 g/kg), ascorbyl palmitate (0.2 g/kg), rosemary extract (1 g/kg), BHT (0.2 g/kg), BHA (0.2 g/kg), and d-δ-tocopherol (0.2 g/kg) | Rancimat results gave the order of antioxidant activity: TBHQ > lecithin > ascorbyl palmitate > rosemary extract > BHT, BHA, and δ-tocopherol | The effect on microenvironment modulation may sometimes be more important than hydrogen donation |
| Hamilton et al. 28 | Refined-deodorized Chilean anchovy fish oil, 20°C, free access to air, RH 45% | α-Tocopherol, δ-tocopherol, γ/δ-tocopherols (each was 0.006, 0.2, 1, and 2%), ascorbyl palmitate (0.1 %), and lecithin (0.5%) | Ascorbyl palmitate and lecithin alone gave a small improvement in oxidative stability (unlike tocopherols). Ascorbyl palmitate exhibited a pro-oxidant effect in the presence of 0.2 and 2% δ-tocopherol or γ/δ-tocopherol. Ascorbyl palmitate + lecithin and ascorbyl palmitate + 0.2 or 1% α-tocopherol (not at other level) displayed strong synergy | Ascorbyl palmitate promotes LOOH scissions. The function of lecithin is merely for solubilizing ascorbyl palmitate, which causes ascorbyl palmitate to partition in the o/w interface. The action of lecithin as an antioxidant is due to its phosphatidyl part which sequesters trace of heavy metals, its ability to inhibit ascorbyl palmitate in hydroperoxide scission and to react with free ascorbyl radicals |
| Hras et al. 158 | α-Tocopherol free SFO, oven, 60°C | Rosemary extract (0.02%), α-tocopherol (0.01%), ascorbyl palmitate (0.01%), citric acid (0.01%), and their combinations | Ascorbyl palmitate significantly reduced PV and p-AV, compared to other additives and control, but lower than extract. Combination of extract and ascorbyl palmitate resulted in the lowest PV and p-AV | Rosemary extract contains phenolic diterpenes such as carnosic acid, carnosol, rosmanol; and other phenolic acid such as rosmarinic acid. Extract and tocopherol act as radical scavengers. Ascorbyl palmitate is ascorbic acid derivative that is oil-soluble. Ascorbyl palmitate plays a role as oxygen scavenger. Ascorbyl palmitate alone acted as antioxidant but not when combined with extract |
| Frankel et al. 162 | Algal oil containing 5.1–12.7% eicosapentaenoic acid (EPA), 10.5–52.4% docosahexaenoic acid (DHA), 11–19.37% α-tocopherol, and carotenoids (577–2823 ppm); 40, 50, and 60°C in a shaker oven | Ascorbyl palmitate (0.025%) | Ascorbyl palmitate caused an increase in the oxidative stability (PV and propanal) of the oil. Carotenoids at high concentration may have pro-oxidant effect by lowering the relative stability of certain algae oil | Ascorbyl palmitate might have an antioxidant synergism with tocopherols |
| Kiokias and Gordon 71 | Tocopherol-stripped OO, oven, 60°C | β-Carotene (1 g/L), annatto oil-soluble (bixin; 1 g/L), and annatto water-soluble (norbixin; 1 and 2 g/L), virgin OO polar extract (0.2 g/L), α-tocopherol (0.1 mM), γ-tocopherol (0.1 mM), ascorbic acid (0.1 mM), and ascorbyl palmitate (0.1 mM) | Ascorbyl palmitate reduced PV of oil. Ascorbyl palmitate showed synergism with norbixin, which were beyond the effect of phenolic antioxidants, but lower than ascorbyl palmitate alone | Ascorbyl palmitate significantly modulates the antioxidant activity of phenolic inhibitors |
| Carelli et al. 131 | SFO; stored at 30°C, Rancimat 130°C | Ascorbic acid, δ-tocopherol, ascorbyl palmitate, α-tocopherol, and citric acid (each at 0, 100, 200, 400, 600, and 800 ppm) | Ascorbic acid, ascorbyl palmitate, and δ-tocopherol significantly increased IP. Ascorbic acid gave the most effect. Samples containing 100 ppm of each additive and control had similar PV, p-AV, and residual tocopherol. Polar compound showed an antioxidative synergism with ascorbic palmitate and δ-tocopherol | There was an absence of linearity of OSI and concentration of ascorbic acid and ascorbyl palmitate, because they are consumed or participated in chain termination reactions and in one or more side reactions. α-tocopherol showed the greatest efficacy at <700 ppm but not at higher level because of its participation in side reactions |
| SFO; stored at 68°C; Rancimat 130°C | Ascorbic acid, δ-tocopherol, ascorbyl palmitate, α-tocopherol, and citric acid (each at 0, 100, 200, 400, 600, and 800 ppm) | Results of rancimat test of IP were the same as above. No significant differences in p-AV of all treatments. Antioxidant effectiveness in terms of PV and phosphatidylcholine was δ-tocopherol > ascorbyl palmitate > ascorbic acid > citric acid. δ-Tocopherol was the only antioxidant present. Oxidized triglyceride monomers were lower from oil with δ-tocopherol, at a longer storage time | At high temperature, oxygen has lower solubility in oil thus autoxidation rate is lower and becomes gradually replaced with polymerization, showed by formation of triglyceride dimer (At 68°C). Ascorbyl palmitate and δ-tocopherol protect the oil at higher temperatures | |
| SFO; stored at 130°C; Rancimat 130°C | Ascorbic acid, δ-tocopherol, ascorbyl palmitate, α-tocopherol, and citric acid (each at 0, 100, 200, 400, 600, and 800 ppm) | Results of rancimat test of IP were the same as above. The p-AVshowed antioxidant effects of ascorbyl palmitate and δ-tocopherol. Ascorbyl palmitate spared the highest tocopherol residual. Ascorbyl palmitate and δ-tocopherol gave the lowest polar compounds. δ-tocopherol showed lower oxidized triglyceride monomers | Ascorbic acid could deteriorate at high temperature, which lessens its antioxidant activity. Both oxidative and thermal degradation took place at 130°C, as shown by a significant increase in polar triglyceride and triglyceride dimer | |
| Olsen et al. 163 | Refined and deodorized cod liver oil, 25°C in dark, Rancimat is done at 20 mL/min and 80°C | Tocopherol concentrate (800 ppm), ascorbyl palmitate (200 ppm) | The order of decreasing PV was tocopherol concentrate > control > tocopherol concentrate + ascorbyl palmitate. The p-AV increased significantly with time. Tocopherol concentrate + ascorbyl palmitate caused a more grass/cucumber-like, than herring oil and paint odor and flavor, which was perceived as more acceptable by consumers. Tocopherol conc. + ascorbyl palmitate inhibited the formation of most volatile oxidation products, except hexanal groups. IP did not significantly changed during storage, compared to initial IP | Tocopherol + ascorbyl palmitate can be used to stabilize cod liver oil, in terms of odor and flavor, when the oil is stored in the dark at 25°C. Volatile compounds and IP did not give useful information about the resistance of the oils to autoxidation at 25°C |
| Drusch et al. 32 | Stripped refined fish oil, 20°C | α-Tocopherol (100 ppm), δ-tocopherol (1000 ppm), ascorbyl palmitate (50 and 500 ppm), and lecithin (500 and 2000 ppm) | Order of reduction of LOOH: 500 ppm ascorbyl palmitate > 2000 ppm lecithin > 500 ppm lecithin > 50 ppm ascorbyl palmitate. 500 ppm ascorbyl palmitate + 2000 ppm lecithin + (100 ppm α-tocopherol and 1000 ppm δ-tocopherol) gave the most effect in LOOH reduction, but increased propanal content. 500 ppm lecithin used in combination with others caused a lower effect than a single effect of lecithin | The synergisms of ascorbyl palmitate, lecithin, and α-tocopherol are owing to the antioxidative effect of lecithin (by regeneration of α-tocopherol from its oxidized radical or by interaction with ascorbyl radicals), a chelating effect of lecithin, or physical phenomena that might occured (better solubilization of ascorbyl palmitate and formation of reversed micelles of lecithin with tocopherol and ascorbyl palmitate) |
| Karabulut 164 | Butter oil TAG, oven, 60°C | Ascorbyl palmitate (5, 50, 100, and 200 ppm), α-tocopherol (10, 25, and 50 ppm), and β-carotene (5, 10, 25, and 50 ppm) | β-car. and ascorbyl palmitate had higher oxidation rate (no IP) than that with α-tocopherol, but lower than control; measured by PV and p-AV. There was synergism between ascorbyl palmitate + α-tocopherol, but not ascorbyl palmitate + β-car. (had no effects) | The synergism of α-tocopherol and ascorbyl palmitate was due to α-tocopherol that was spared at the expense of ascorbyl palmitate during oxidation or ascorbyl palmitate is used to regenerate tocopherols. Ascorbyl palmitate donates hydrogen to tocopheroxyl radical |
| Sorensen et al. 61 | Water in oil emulsion containing 98% oil (fish oil and rapeseed oil, 1:1), 37°C in the dark | Ascorbic acid, ascorbyl palmitate, ascorbyl conjugated linoleic acid (ascorbyl conjugated linoleic acid), and conjugated linoleic acid, each additive at 50, 100, 150, 200, and 250 ppm; polyglycerol polyricinoleate (1%) | The IP as measured by LOOH showed that all additives acted as antioxidants. Propanal and hexanal concentration results were: ascorbyl palmitate > ascorbyl conjugated linoleic acid > ascorbic acid > conjugated linoleic acid | Ascorbic acid, ascorbyl palmitate, and ascorbyl conjugated linoleic acid owing their antioxidative properties mostly due to their ascorbyl group. Ascorbic acid is known as a radical scavenger of hydrophilic radicals and to have a reducing power due to its ability to donate an electron to reactive free radicals |
| Yi et al. 160 | Mixture of yellow palm olein/fish oil, mixture of red palm olein/fish oil, 30°C in the dark, 100°C | Ascorbyl palmitate (200 and 500 ppm), citric acid (50 ppm), phosphatidylethanolamine (500 ppm), and phosphatidylcholine (500 ppm) | Ascorbyl palmitate (200 and 500 ppm) alone or with citric acid displayed a pronounced inhibiting effect on the formation of radicals, lengthening IP, PV, and TBARS in both oil. (Phosphatidylethanolamine or phosphatidylcholine) along with (ascorbyl palmitateorcitric acid), the phospholipids exhibited a prooxidant effect | Ascorbyl palmitate synergists with citric acid, but not with phosphatidylethanolamine and phosphatidylcholine |
| Sterols | ||||
| Soupas et al. 165 | Tripalmitin, 80°C for 1–8 wk, 100°C for 3–48 h, 140°C for 0.5–6 h, 180°C for 0.5–6 h; purified rapeseed oil, 60°C for 1–7 days, 100°C for 3–48 h, 140°C for 0.5–24h, 180°C for 0.5–6 h | Stigmasterol, sitostanol (each 1%) | Stigmasterol and sitostanol oxides (formed more) increased during all heat treatments in both medium, except stigmasterol oxides during heating at 100°C for 0–6 h and sitostanol oxides during heating at 80°C for 0–4 wk. At low T., the stigmasterol oxides had lower oxidation in tripalmitin than in the rapeseed oil. At all T., sitostanol was oxidized more at both matrix | At high temperature, the unsaturated matrix is more readily oxidized than the stigmasterols, thus protecting the sterols; while saturated matrix forces the stigmasterols to react. There is no relationship between sitostanol, matrix, and temperatures, as sitostanol oxidized faster than both matrix |
| Soupas et al. 166 | Rapeseed oil, hydrogenated coconut oil, and refined palm kernel oil, 4°C for 12 months | Microcrystalline phytosterol suspensions contains of 77% sitosterol and 8% campesterol (18 and 30%) | 30% phytosterol caused more phytosterol oxides than that of 18% phytosterol. 18 and 30% phytosterol, caused the sitosterol to be more oxidized in hydrogenated coconut oil and refined palm kernel oil, and rapeseed oil, respectively. The phytosterol content at the beginning and after 12 months of cold storage did not change significantly | The differences in the susceptibility of phytosterol oxidation in different matrix are due to initial oxide contents in phytosterol preparation and lipid matrix. A higher level sitosterol is oxidized more readily than the unsaturated matrix (at 4°C) |
| Cercaci et al. 167 | Corn oil, 55°C in the dark; phytosterol-oxide was made at 150°C for 2 h in an oven; hexadecane, 30°C for 24 h | Cholesterol, stigmasterol, β-sitosterol, 5-alfa-cholestane (1, 2, 3, 4, and 5 mmol/kg hexadecane) | 7-keto derivatives of phytosterols increased with time, the most being 7-ketositosterol. The ability of sterols to reduce interfacial tension was in the order: stigmasterol > cholesterol > β-sitosterol > 5α-cholestane | Oxidation of phytosterols results in ketones, alcohols, epoxides and dienes. The 7-keto derivative is the major phytosterol oxidation product. Sterols have a planar rigid structure which causes them to pack together tightly, and with their ability to be surface active, they can concentrate at o/w interface |
| Winkler-Moser et al. 102 | SBO, high-oleic SFO, stripped SBO, stripped high-oleic SFO, Rancimat (OSI), 110°C | Mixed phytosterols consist of brassicasterol (3.8%), campesterol (26.9%), campestanol (0.6%), stigmasterol (17.2%), β-sitosterol (48.2%), sitostanol (1.1%), Δ5-avenasterol (1.3%), Δ7-stigmastenol (0.8%) (0.25, 0.5, 1, and 2.5%) | Phytosterol was prooxidants, by increasing dimers and polymerized triacylglycerol in stripped SBO and high-oleic SFO, but not after 4 h. In stripped oil, phytosterol caused lower OSI | At lower temperature and in less unsaturated matrix (e.g., stripped high oleic SFO), phytosterols oxidized quicker than the matrix, causes higher dimers and polymerized TAG formation. But at higher T and in more unsaturated matrix (e.g., stripped SBO), polyunsaturated fatty acids (PUFA) are oxidized preferentially over sterols and protect the sterols from oxidation |
| Hidalgo et al. 157 | Stripped virgin OO, β-sitosterols added stripped virgin OO, Rancimat, 90°C, 10 L/h | Phosphatidylethanolamine (0, 100, 200, 300, and 400 ppm), phosphatidylcholine (0, 100, 200, 300, and 400 ppm), lysine (0, 100, 200, 300, and 400 ppm), and β-sitosterols (1500 μg) | Phosphatidylethanolamine, phosphatidylcholine, lysine, and their combinations caused a significantly higher antioxidative effects in phytosterol added OO, compared to stripped OO. Phosphatidylethanolamine/lysine and phosphatidylcholine/lysine increased the IP compared to when they are added alone, but not phosphatidylethanolamine/phosphatidylcholine. The effects were higher for lysine (200 ppm or more) and phosphatidylethanolamine/lysine (300/100 ppm) in stripped OO and phytosterol added OO | There was a synergism among β-sitosterol and phosphatidylethanolamine; β-sitosterol and phosphatidylcholine; β-sitosterol and lysine; β-sitosterol and phosphatidylethanolamine + lysine; and β-sitosterol and phosphatidylcholine + lysine |
| Soupas et al. 166 | Heat treated non-fat milks, long term storage at room T. and 4°C | Phytosteryl esters containing 45% sitosterol, 25% campesterol, and 18% stigmasterol; phytostanyl esters containing 65% sitostanol and 33% campestanol (the sterol ester were added at 0.5%) | Phytosteryl esters oxidized more than phytostanyl esters. Temperature did not influence the oxidation effects of both antioxidants | As phytosteryl esters oxidized more than phytostanyl esters, it could be that if phytosteryl esters were used as antioxidant then it will protect the matrix more. The effects on oil matrix must be investigated |
| Salts | ||||
| Calligaris and Nicoli 126 | SBO; Rancimat, 120°C at 20 L/h | Potassium carbonate, potassium acetate, acetic acid, sodium acetate, sodium chloride, and potassium chloride (all salts were added at 10% w/w) | Potassium carbonate and potassium acetate significantly reduced PV and hexanal. Others (acetic acid, sodium acetate, sodium chloride, and potassium chloride) reduced hexanal and increased IP by Rancimat | The antioxidant activity was attributed to the antichaotropic anionic species, of which could interact and form H bonds with LOOH |
| Gurkov et al. 168 | Water/air and water/oil (n-hexadecane and SBO) emulsions | Sodium dodecyl sulfate (SDS) (10−5, 10−4, 10−3, and 10−2), NaCl (10 and 150 mM) | 150 mM NaCl had a lower surface tension than 10 mM NaCl. SDS and NaCl caused a lower surface tension of SBO/water than in C16/water. The coverage of interface of the nanoemulsions at CMC was lower than 90%. The surface coverage of SBO (with SDS and NaCl) was lower than that of hexadecane (with SDS and NaCl). There was an absence of saturation of the ionic surfactants at the CMC | Salt decreased CMC. The size and other parameters of nanoemulsions might need to be studied. The surface coverage did not depend on the type of fluid interface (air/water, oil/water with different hydrocarbons) and salt concentration |
Figure 3.
Synergistic effects of binary mixtures of ascorbyl palmitate and lecithin on the autoxidation of fish oil at 20°C; ♦: no antioxidant, ÿ: 0.1% ascorbyl palmitate, Δ: 0.5% lecithin, ◊: 0.1% ascorbyl palmitate +0.5% lecithin. Data from 28.
In summary, microemulsions are the site of oxidation and can be considered as microreactors for autocatalysis by hydroperoxides. The oxidation is prevalent at the water-oil interface and the antioxidants positioned near the polar region will scavenge radicals in this region 70–74. The nature of microenvironments is affected by and affects the physical location of prooxidants (including oxidation products), antioxidants, and secondary modifiers, and thus the rate of lipid oxidation 14.
The supramolecular chemistry of lipid oxidation
Micelles are formed in a heterophase system in order to achieve a minimum free energy state with the driving force is the increase of entropy that accompany the withdrawal of hydrophobic regions of surfactants from water and the accompanying disorder. In the microemulsions, the hydrophilic head groups of antioxidants and surfactants are oriented towards the water and the hydrophobic hydrocarbon tails are more oriented towards the oil 7,14,75. In bulk lipids (Fig. 2), trace amounts of water and other polar compounds like salts and acids are located in the core of the micelles, while surfactants occupy the interface as a monolayer and is separated from the core of the micelle by a depletion layer, that is, a layer of water of a few molecular diameter that almost does not contain surfactants, occurs next to the monolayer 75. The microemulsion is defined as a thermodynamically stable single phase system of water, oil, and an amphiphile, which varies in size and is capable of solubilizing significant amount of polar and non-polar compounds 56,76–80. The model in Fig. 4 considering bulk oil as a nanoemulsion would better explain available results on lipid oxidation 53,68,81.
Figure 4.
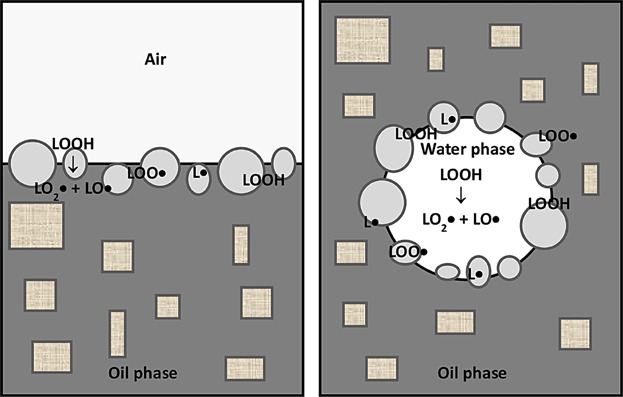
Schematic representation of the distribution of hydrophilic  and hydrophobic
and hydrophobic  antioxidants in bulk oil and the microenvironment with hydroperoxides and water as explained by Frankel et al. in 1994 39 by the antioxidant partitioning at the oil-air interface (left) and later modified by Chaiyasit et al. in 2007 14 to antioxidant and surfactant partioning at the oil-water interface of micelles/association colloids (right).
antioxidants in bulk oil and the microenvironment with hydroperoxides and water as explained by Frankel et al. in 1994 39 by the antioxidant partitioning at the oil-air interface (left) and later modified by Chaiyasit et al. in 2007 14 to antioxidant and surfactant partioning at the oil-water interface of micelles/association colloids (right).
Water activity influences lipid oxidation rates due to its relation to metal reactivity and hydroperoxide stability 82. In food products, the rate of lipid oxidation is lowest at water activity of 0.2–0.4 and it increases with increased water activity 14,69. When water activity is increased, metals are mobilized and oil viscosity is reduced hence oxidation is accelerated 69. Different water types or concentrations and levels of emulsifiers produce different shapes of association colloids and affect lipid oxidation differently 20,83. It was found that phospholipids synergize the activity of α-tocopherol only in the presence of small amounts of water and catalysis by water-soluble radical generators 54. It was also found that as an increase of the length of the acyl moieties of phospholipids increases the induction period 54,84. The kinds of fatty acid composition and functional groups of the phosphate groups of phospholipids also influence oxidation differently 20. This research implies that the aqueous microenvironment influences lipid oxidation and must be controlled 85 as discussed in the previous part. It is possible that water causes some hydrolysis of triacylglycerols (TAG) resulting in the formation of mono- and diacylglycerols and free fatty acids, which would influence the microemulsion in a negative way and act as prooxidants as in many studies (Table3).
At low levels (e.g., in the range of 230–240 ppm) water seem to have no effects on the oxidation of an oil despite a high interfacial tension and more oxidation 33. The same results was found when the amount of water is increased (up to 1000 ppm) possibly because water here is bound to polar compounds and is trapped in multilayer association colloids 20. Water in refined oils (approximately 300 ppm) originates from the water present in oilseeds, and the water used during neutralization and degumming processes 7,14,20. Throughout oxidation, more water is formed by the bimolecular decomposition of hydroperoxides during the propagation phase.
The minor components of vegetable oils (such as FFA, MAG, and phospholipids) and lipid oxidation products (e.g., hydroperoxides, aldehydes, ketones, and epoxides) are amphiphilic and surface active. In the presence of small amounts of water and/or surfactants, these minor constitutents will be able to form microemulsions or association colloids, which influence oxidation 7,14,20,33,70. Stripped corn oil had higher interfacial tension (31.5 ± 0.68 mN/m) than original corn oil (20.1 ± 0.09 mN/m) indicating that minor components act as surfactants by reducing the interfacial tension and the overall energy of the system 14,33,80. The size of the reversed micelles in bulk oils is in the range of 1–500 nm 70,86. Refining of vegetable oils effectively removes minor components (some minor components are undesireable such as free fatty acids, and cause foaming and reduce smoke point of oils and chlorophyll, which acts as photosensitizers) but increases the rate of lipid oxidation in bulk oils because antioxidants such as tocopherols and emulsifiers are removed 14,20,33. Although refining removes minor components to a significant extent, there are still traces of these compounds in the refined oil that can still affect oxidation in oil 20. Techniques commonly used to investigate the structure of microemulsions include cryo-transmission electron microscopy (cryo-TEM), dynamic light scattering (DLS), small-angle neutron scattering (SANS), wide-angle X-ray scattering (WAXS), and small-angle X-ray scattering (SAXS) 87.
The effect of antioxidants and other surface-active additives on microemulsion formation depends on their hydrocarbon chain length, that is, their hydrophilic lipophilic balance (HLB) 54 and quantities 146,151. From the empirical point of view, the term HLB is used to indicate the solubility of an antioxidant in lipid systems 88. Surfactants with low HLB (3–6) favor the formation of w/o emulsions whereas those with high HLB (8–18) enhance o/w emulsions 90,91. Free fatty acids, mono- and diacylglycerol have low HLBs (1.0, 3.4–3.8, and 1.8, respectively) and, thus, prefer to form and stabilize reversed micelles in w/o 14. Phospholipids with intermediate HLB (around 8.0) can form variety of structures; spherical reversed micelles in bulk oil with small amount of water (<0.3%) and lamellar structures in combination with other surfactants 7,14.
Another factor that determines surfactants activity is the surfactant packing parameter (Sp) which use a more quantitative approach, relates to the geometry of the surfactant and determines the curvature preference of a surfactant molecule (particularly when co-surfactant is used) 76,78,91. Sp is related to solubilization of microemulsions in the solvent 78,170. Additional information of additives, such as log P, topological polar surface area and volume are presented in (Table4). In summary, the octanol-water partition coefficient (or log P), which is a measure of the lipophilicity of a compound, can be used to estimate transport properties of a compound across an interface 171 and to predict the structure-activity relationship of compounds 172. Molecular volume, the volume of 1 mol of a substance at a specific temperature and pressure, also predicts the transport characteristics of substances across an interface 173. Another property that is used to predict the transport characteristics of a molecule is topological or molecular total polar surface area (TPSA) 174, which is the quantity of surface contributions of polar atoms, such as oxygens, nitrogens, and hydrogens in a molecule 175.
Tabel 4.
Structural feature of selected additives (polarity, volume, and topological polar surface area)
| Compound | Molinspirationsmiles (miSmiles) | CAS# | M.Wt (Da) | LogP (miLogP) | Volume (cubic Å) | Topological polar surface area (TPSA) |
|---|---|---|---|---|---|---|
| Dipalmitoylphosphatidyl ethanolamine (DPPE) | CCCCCCCCCCCCCCCC(=O)O[C@H](COC(=O)CCCCCCCCCCCCCCC)COP(O)(=O)OCCN | 5681-36-7 | 691.96 | 9.647 | 721.801 | 134.400 |
| Dipalmitoylphosphatidyl serine (DPPS) | CCCCCCCCCCCCCCCC(=O)O[C@H](COC(=O)CCCCCCCCCCCCCCC)COP(O)(=O)OC[C@H](N)C(O)=O | 40290-42-4 | 757.94 | 9.111 | 748.828 | 171.699 |
| Dipalmitoylphosphatidyl choline (Lecitin) | CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC | 2644-64-6 | 734.04 | 6.383 | 773.671 | 111.206 |
| Ascorbylpalmitate | CCCCCCCCCCCCCCCC(=O)OC[C@H](O)[C@H]1OC(=O)C(O)=C1O | 57233-83-7 | 414.5 | 6.284 | 411.443 | 113.294 |
| Glycerol-1,3-dipalmitate | CCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCC | 502-52-3 | 568.91 | 9.913 | 630.593 | 72.838 |
| Glycerol-1,2-dipalmitate | CCCCCCCCCCCCCCCC(=O)OCC(CO)OC(=O)CCCCCCCCCCCCCCC | 40290-32-2 | 568.91 | 9.913 | 630.593 | 72.838 |
| Glycerol monopalmitate | CCCCCCCCCCCCCCCC(=O)OCC(O)CO | 26657-96-5 | 330.50 | 6.090 | 358.856 | 66.761 |
| Astaxanthin | CC2=C(\C=C\C(C)=C\C=C \C(C)=C\C=C\C=C(C)\C=C\C=C(C)\C=C\C1=C(C)C(=O)C(O)CC1 (C)C)C(C)(C)CC(O)C2=O | 472-61-7 | 596.85 | 8.596 | 612.415 | 74.598 |
| β-Carotene | CC2=C(\C=C\C(C)=C\C=C \C(C)=C\C=C\C=C(C)\C=C\C=C(C)\C=C\C1=C(C)CCCC1(C)C)C(C) (C)CCC2 | 7235-40-7 | 536.88 | 9.843 | 591.964 | 0 |
| Stigmastanol | CCC(CCC(C)C3CCC4C2CCC1CC(O)CCC1(C)C2CCC34C)C(C)C | 19466-47-8 | 416.72 | 8.714 | 462.730 | 20.228 |
| β-Sitosterol | CCC(CCC(C)C3CCC4C2CC=C1CC(O)CCC1(C)C2CCC34C)C(C)C | 83-46-5 | 414.72 | 8.620 | 456.517 | 20.228 |
| Δ5—Avenasterol | CC=C(CCC(C)C3CCC4C2CC=C1CC(O)CCC1(C)C2CCC34C)C(C)C | 481-14-1 | 412.69 | 7.688 | 450.304 | 20.228 |
| Palmitic acid | CCCCCCCCCCCCCCCC(=O)O | 9006-59-1 | 256.42 | 7.059 | 291.422 | 37.299 |
| Oleic acid | CCCCCCCC/C=C\CCCCCCCC(O)=O | 112-80-1 | 232.50 | 7.583 | 318.839 | 37.299 |
| Methyl linoleate | CCCCC/C=C\C/C=C\CCCCCCCC(=O)OC | 112-63-0 | 294.47 | 7.165 | 330.18 | 26.305 |
| Linoleic acid | CCCCC/C=C\C/C=C\CCCCCCCC(O)=O | 60-33-3 | 280.45 | 6.858 | 312.652 | 37.299 |
| α-Linolenic acid | CC/C=C\C/C=C\C/C=C\CCCCCCCC(O)=O | 463-40-1 | 278.43 | 5.840 | 306.466 | 37.299 |
| Eicosapentaenoic acid | CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(O)=O | 10417-94-4 | 302.45 | 5.399 | 327.696 | 37.299 |
| Docosahexaenoic acid | CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(O)=O | 24880-45-3 | 330.50 | 5.684 | 355.112 | 37.299 |
| β-Sitosteryl palmitate | CCCCCCCCCCCCCCCC(=O)O[C@H]4CC[C@]3(C)[C@H]2CC[C@@]1(C)[C@@H](CC[C@@H]1 [C@H](C)CCC(CC)C(C)C)C2CC=C3C4 | 2308-85-2 | 653.14 | 10.256 | 728.254 | 26.305 |
| β-Sitosteryl ferulate | CCC(CCC(C)[C@H] 5CCC4C3CC=C2CC(OC(=O)\C=C/c1ccc(O)c(OC)c1)CC[C@]2(C) C3CC[C@@]45C)C(C)C | – | 590.87 | 9.397 | 608.856 | 55.767 |
| Squalene | CC(C)=CCCC(C)=CCCC(C)=CCCC=C(C)CCC=C(C)CCC=C(C)C | 111-02-4 | 410.72 | 9.622 | 477.642 | 000 |
| Squalane | CC(C)CCCC(C)CCCC(C)CCCCC(C)CCCC(C)CCCC(C)C | 111-01-3 | 422.81 | 9.568 | 514.918 | 000 |
| Chlorophyll a | CCc2c(C)c3cc6[n-]c(cc1nc ([C@@H](CCC(=O)OC/C=C(\C)CCC[C@H](C)CCC[C@H](C)CCCC(C)C) [C@@H]1C)c4[C@@H](C(=O)OC)C(=O)c5c(C)c(cc2n3)[n-]c45)c(C) c6C=C.[Mg+2] | 479-61-8 | 893.51 | 9.149 | 864.172 | 123.665 |
| Chlorophyll b | CCc2c(C=O)c3cc6[n-]c (cc1nc([C@@H](CCC(=O)OC/C=C(\C)CCC[C@H](C)CCC[C@H](C) CCCC(C)C)[C@@H]1C)c4[C@@H](C(=O)OC)C(=O)c5c(C)c(cc2n3)[n-] c45)c(C)c6C=C.[Mg+2] | 519-62-0 | 907.47 | 8.915 | 866.594 | 140.736 |
| α-Tocopherol | CC(C)CCC[C@@H](C)CCC [C@@H](C)CCC[C@]2(C)CCc1c(C)c(O)c(C)c(C)c1O2 | 59-02-9 | 430.71 | 9.043 | 474.499 | 29.462 |
| β-Tocopherol | CC(C)CCC[C@@H](C)CCC [C@@H](C)CCC[C@]2(C)CCc1cc(O)c(C)c(C)c1O2 | 54-28-4 | 416.68 | 8.982 | 457.938 | 29.462 |
| δ-Tocopherol | CC(C)CCC[C@@H](C)CCC [C@@H](C)CCC[C@]2(C)CCc1cc(O)cc(C)c1O2 | 119-13-1 | 402.65 | 8.602 | 441.377 | 29.462 |
| Linoleic acid-9-hydroperoxide | CCCCC/C=C\C=C/C(CCCCCCCC(O)=O)OO | – | 313.45 | 6.004 | 329.681 | 66.761 |
| Linoleic acid-13-hydroperoxide | CCCCCC(OO)C=C/C=C\CCCCCCCC(O)=O | – | 313.45 | 6.004 | 329.681 | 66.761 |
| BHT | Cc1cc(C(C)(C)C)c(O)c(C(C)(C)C)c1 | 128-37-0 | 220.35 | 5.435 | 240.996 | 20.228 |
| BHA | COc1ccc(O)c(C(C)(C)C)c1.COc1ccc(O)cc1C(C)(C)C | 25013-16-5 | 180.24 | 3.617 | 183.794 | 29.462 |
| TBHQ | CC(C)(C)c1cc(O)ccc1O | 1948-33-0 | 166.24 | 3.081 | 166.266 | 40.456 |
| Trolox | Cc2c(C)c1OC(C)(C(O)=O)CCc1c(C)c2O | 53188-07-1 | 250.29 | 3.071 | 233.557 | 66.761 |
| Curcumin | COc2cc(/C=C/C(=O)CC(=O)\C=C\c1ccc(O)c(OC)c1)ccc2O | 458-37-7 | 366.38 | 2.303 | 332.182 | 93.066 |
| Ferulic acid | COc1cc(/C=C/C(O)=O)ccc1O | 1135-24-6 | 194.18 | 1.249 | 172.025 | 66.761 |
| Caffeic acid | OC(=O)\C=C\c1ccc(O)c(O)c1 | 331-39-5 | 180.16 | 0.941 | 154.497 | 77.755 |
| Vanillin | COc1cc(C=O)ccc1O | 121-33-5 | 152.15 | 1.067 | 136.591 | 46.533 |
| Ascorbic acid | OC[C@H](O)C1OC(=O)C(O)=C1O | 50-81-7 | 176.12 | −1.402 | 139.707 | 107.217 |
| EDTA | C(CN(CC(=O)O)CC(=O)O)N(CC(=O)O)CC(=O)O | 60-00-4 | 292.24 | −5.193 | 247.019 | 155.672 |
| Phytic acid | [C@@H]1([C@@H]([C@@H] ([C@@H]([C@H]([C@@H]1OP(=O)(O)O)OP(=O)(O)O)OP(=O)(O)O)OP (=O)(O)O)OP(=O)(O)O)OP(=O)(O)O | 83-86-3 | 660.04 | −5.547 | 422.961 | 400.566 |
| Citric acid | C(C(=O)O)C(CC(=O)O)(C(=O)O)O | 77-92-9 | 192.12 | −1.983 | 151.764 | 132.125 |
aSimplified molecular-input line-entry specification (SMILES). Data on log P values, volume, and topological polar surface area are from www.molinspiration.com (accessed 1 November 2012).
A co-surfactant is a compound that can physically synergize the dissolution of surfactants in the organic solvent (increasing the solubility of surfactant) and facilitate the formation of reversed micelles and the stabilization of the microemulsions 92,93. Examples of co-surfactants commonly used in pharmaceutics include short-chain alcohols C3–C6, medium chain length alcohols C6–C12, glycerol, sorbitol, geraniol, and fatty acid sucrose esters 78,80. The location of cosurfactants in micelles is not clear, but they are also in the interface as they can partition between oil and water phase 21. In addition, co-surfactants can be located in the oil phase close and in between surfactant molecules (Fig. 5), and forms complex with surfactants 93. Co-surfactants are weak amphiphiles 94, that exert their effects by reducing electrostatic repulsion between surfactant head groups and causing weak hydrophobic interactions between surfactant tails leading to a modulated packing of surfactants in micelles because of the increased volume and mobility of surfactant tails (v) and reduced interfacial surface area (ao) 80,95,96. Co-surfactants, whose effects are dependent on their size and chain length 97,98, keep water in the micelles and reduce the size of these micelles 93. System conditions and molecular geometry of molecular aggregates in o/w and w/o emulsions, when surfactants and/or co-surfactants are employed, are illustrated in (Fig. 5). So far, the existence of molecules with co-surfactant properties and their role in lipid oxidation have not been discussed but it might explain the synergistic effect of compounds such as citric acid and amino acids 99.
Figure 5.
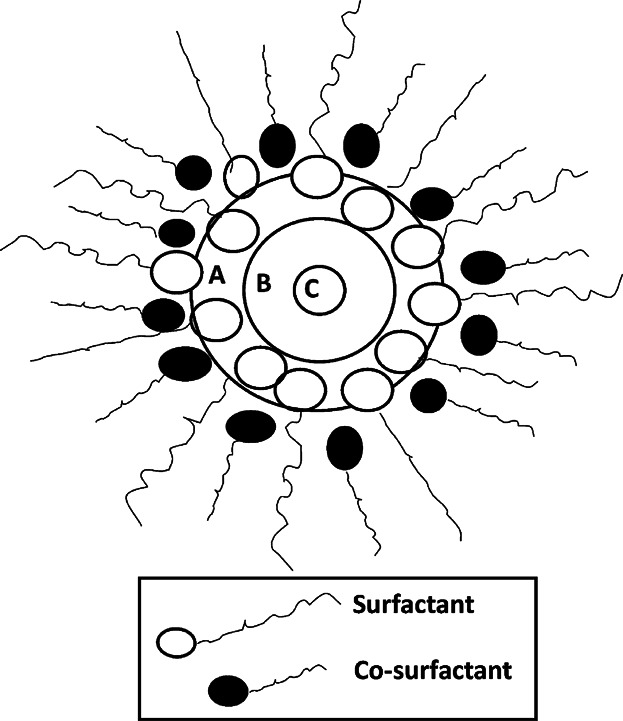
Structure representation of a reversed micelle stabilized by surfactants and co-surfactants. Symbols: A = monolayer of surfactants, B = depletion layer, and C = core (water).
The antioxidant mechanisms of some secondary antioxidants and minor components are not well known. It could be that they act as synergists by affecting the physical structures of the microemulsions, which are the site of oxidation. The historical developments in understanding the physical effects of components on lipid oxidation are presented in (Table1).
Hydrophilic–lipophilic balance and the cut-off effect
In a study in o/w emulsified systems, the antioxidant capacity (tested by conjugated autoxidizable triene assay) of homologous series of chlorogenic acid esters increased with alkyl chain length increased (lipophilicity increased), but up to C12 while further chain extension, starting from C16, caused a drastic drop of antioxidant capacity 17. In another study in o/w emulsions, the effect of hydroxytyrosol fatty acid esters on antioxidant capacity (measured by surfactant effectiveness) increased until hydroxytyrosol decanoate (C12) with further increase of chain length caused a drop in surfactant effectiveness 62. The cut-off effects are related to the molecular size of antioxidants and their mobility with bulkier structures compounds (i.e., compounds with long alkyl chain) having reduced mobility due to steric hindrance and lower diffusability in oxidation sites 100,101.
As mentioned above, the general rule described in the polar paradox is that “polar (hydrophilic) antioxidant are more effective in bulk oil with a low surface/volume ratio whereas nonpolar (lipophilic) antioxidants are more effective in oil in water emulsions.” However, lately it was discovered that there is a certain non linearity in this effect. Using EGCG and its esters as examples, Shahidi and Zhong 53 explained that as lipophilicity increases, the antioxidants are more active at lower levels in bulk oils because their effects on their solubility in lipids are stronger than those of interfacial phenomenon. So, it is possible that the polar paradox is applicable only when antioxidant is added at high concentration (reaching a critical concentration) making its effects on interfacial phenomena to dominate over solubility effects. These phenomena were also observed for Trolox/α-tocopherol, ascorbic acid/ascorbyl palmitate, and gallic acid/propyl gallate 53.
Thus, the polar paradox is influenced not only by the HLB of the antioxidants but also by their molecular size and configuration (Table4), and indeed concentrations. Therefore, polar paradox does not always prevail and can explain antioxidant effectiveness in the system from only a general perspective. Moreover, previous experimental inferences were the outcome of “pure chemical thinking” in the absence of the understanding of supramolecular chemistry.
Seeking further explanations in supramolecular chemistry
It is well understood that besides the degree of fatty acid unsaturation, several other factors influence antioxidant effectiveness in bulk oil, for example, diffusion of oxygen, temperature and light 6,18, interaction with prooxidants, amphiphilic minor components (e.g., phospholipids, MAG, FFA 7, the presence of compounds which act as surfactants (their HLB, nature, and ratio) 56, lipid composition-structure-position 102,103, other food components 104, physical structures 20, pH 34,105, interfacial characteristics 22,57,67, stability of EPA and DHA 3, and position of fatty acids on glycerol 106. A global conceptual framework has, however, never been reached due to frequent inconsistencies and paradoxes. It is clear from the above discussions that these effects should be revisited and may be explained by the new paradigm of supramolecular interactions and effects.This new paradigm needs to recognize and consider molecular shapes and the relative positions of hydrophilic and lipophilic regions.
In bulk lipids, several components are often surface active and form, together with water, many kinds of association colloids that affect oxidation by modulating prooxidative or antioxidative effects 33. These effects may be very sensitive to structural details. For example, 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dibutyryl-sn-glycero-3-phosphocholine (DC4PC) increased and reduced the oxidation rate of stripped soybean oil with Trolox added due to influences on the size and shape of micelles 20,107. DOPC formed reversed micelles and caused a prooxidative effect in stripped soybean oil while DC4PC formed cylindrical structures (not reversed micelles) and had no effect on oxidation in the same oil 7,8,20. Therefore, surfactants also affect antioxidant effectiveness. The reasons could be due to phospholipids aliphatic chain which influences the size and shape of micelles 107 and DOPC reversed micelle which may had negatively charged surface thus attracted metals 20. Similarly, phosphatidylcholine (1,2-dibutyl-sn-glycero-3-phosphocholine) does not form reversed micelles and is not prooxidative while the reversed micelles formed by DOPC lead to the concentration of endogenous iron and lipid hydroperoxides at the water-lipid interface and enhancement of the decomposition of the hydroperoxides 35. In addition, charge-related interactions with metals 20 where the zwitterionic effects of the phospholipid surfactants may have significant relevance 80.
Interfacial characteristics due to ionic surfactants can have variable impacts on oxidation. Anionic surfactants (e.g., sodium dodecylsulfate [SDS] and sodium dioctyl sulfosuccinate or [AOT]) form large micelles 68 and create negatively charged interfaces causing metals to bind to reversed micelles and increase the oxidation rates in o/w 14,33 but showed no effects on oxidation in lipophilic substrates (e.g., limonene) 86. Cationic surfactants, such as cetyltrimethylammonium bromide (CTAB) and quaternary ammonium alkyl salts, form many very small micelles 20,70,86 and promote oxidation in bulk oil and limonene while reducing it in o/w 70,78. The addition of a cosurfactant that increases the micelle sizes will mitigate these effects and improve stability 110. In bulk oils, cationic surfactants affect oxidation at the initiation phase but not at the propagation and termination phases 70. Nonionic surfactants (e.g., polyoxylene lauryl ether [Brij 35], sorbitan oleate [Span 80], sugar surfactants such as alkyl glucosides and sucrose fatty acid esters, mono- and diacylglycerol, medium chain triacylglycerides, fatty acid esters such as isopropyl myristate) may increase the oxidative stability of emulsions 23,64,80 but these findings are not consistent and are in contradiction with results of other studies 23,111. The trend seems to be clear but fine tuning of the concept is still needed. This gives possibilities for molecular modifications, for example, by esterifying free –OH group(s) MAG or DAG with other molecules such as citric acid.
The position of double bonds of unsaturated fatty acids also has an effect in oxidation of colloidal dispersions 112 with more stability when the double bond is closer to the methyl end of the molecule 22. Trans fatty acids, with a straight configuration causing their molecules to be tightly packed with higher melting points, are more stable than cis fatty acids 113. The pH also has an impact on oxidative stability of lipid. A study of oil/water emulsions showed that when the lipid droplets were coated with proteins, at pH lower than the isoelectric points of amino acids, droplets had cationic surface which repel transition metals thus lessening the oxidation 34,105. High pH causes precipitation of iron onto emulsion surface and increases the lipid oxidation rates 23,57.
The discussion of lipid oxidation in fish oils requires special considerations because of the high degree of unsaturation as well as the highly bended structure of their very long-chain omega-3 polyunsaturated fatty acids, namely eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) 114. The autoxidation rate of these PUFAs depends not only on their degree of unsaturation but also on the position of the fatty acids in the triacylglycerols 115. It should be mentioned here that sea mammalian oils, such as seal and whale oils, are more stable than fish oils because of different location of the fatty acids in their triacylglycerol molecules, that is, at sn-1 and sn-3 positions 106,116,117. In these mamalian oils, EPA and DHA are distributed mainly in the sn-1 and sn-3 positions while they are mainly in the sn-2 position in fish oils 176. In another study, it was shown that the stability of triacylglycerols is compromised when EPA was highly concentrated within rather than between the triacylglycerol molecules 118. However, in the case of soybean oil, it had achieved a higher oxidative stability when its unsaturated fatty acids are at sn-2 position 106. This shows that the type of the unsaturated fatty acids and substrate/lipid system also influence the oxidation. The bent structure of fish/mammalian fatty acids affects the interfacial space and the molecular distribution of lipids in a way different from that in other oils and fats. In dispersed systems, such as bulk lipids adopting a nano-emulsion structure, the size of the micelles and dispersion phase is critical with more stability for systems containing smaller particles 119. Certain kinds of fish lipids (such as fish roe lipids) with high amounts of EPA and DHA were more stable against oxidation than other kinds of fish lipids because their EPA and DHA present together with α-tocopherol and phospholipids. Similar observation was also found in perilla oil. Thus phospholipids act synergistically with α-tocopherol in protecting the oil 3,120–122.
Conclusions
Most lipid oxidation studies were hitherto concerned with chemical reactions, namely the free radical theory of lipid oxidation. Starting 1990s, the role of physical effects on lipid oxidation started to emerge. The model reviewed here considers that nanoemulsions of water in bulk oil act as nanoreactors or sites of lipid oxidation. Our hypothesis is that molecular organizations and nano-emulsifications are the most important factors affecting lipid oxidation during the initial stage (induction period) and that free radical reactions become important during the propagation and termination stages. The role of primary antioxidants and synergists in the initial phase of lipid oxidation is mainly to stabilize the micelles loaded with water, hydroperoxides, and other amphiphiles (Fig. 6) while their role during the propagation phase is to scavenge radicals. This shift in paradigm implies that by understanding both chemical and physical effects, there will be a perspective to control lipid oxidation in a more integrated manner. This approach is especially useful when we consider developing healthier food products with high content of polyunsaturated fatty acids.
Figure 6.
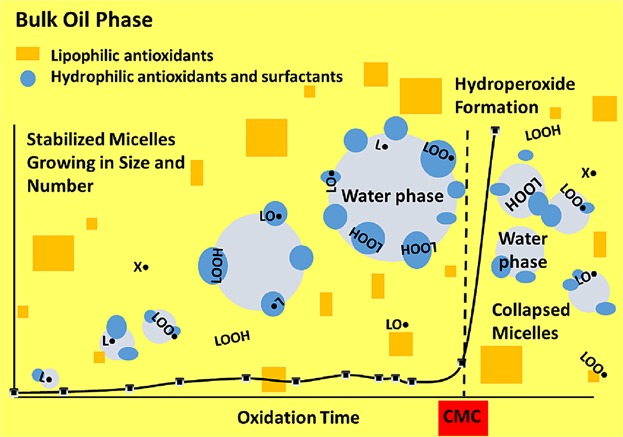
The relation between stabilization of reversed micelles by antioxidants and synergists and the transition from the initiation phase to the propagation phase. This transition occurs when the micelles reach their critical micelle concentration (CMC).
So far, there have been few antioxidants that are approved and used by the food industries to retard oxidation in bulk oils 14. Thus, in order to solve the rancidity problem, antioxidants must be delivered to the oxidation sites. Especially as microemulsions are capable of containing significant amount of water (of which causing hydration of metal ions in the core of the micelles and thus decreasing the formation of hydrogen bonds with hydroperoxides 69; as water concentration increases, the core and net size of the micelles increase 87; thus stabilizing these microemulsions, and understanding their physical properties can open new research opportunities to retard oxidation in bulk oils. Indeed, there is a correlation between the chemical properties relevant to primary antioxidants, mainly BDE, and important physical properties, such as HLB, as both are influenced by the substitution in the antioxidant phenolic ring(s). This indirect correlation might have concealed the physical factors governing antioxidant activity. It is now time to reconsider the antioxidant structure-activity relationships as a first step to design more effective antioxidants for future applications.
Acknowledgments
This work was supported by the United Arab Emirates University PhD study grant to the first author.
The authors declare no conflicts of interest.
Glossary
- BHA
butylated hydroxyanisole
- BHT
butylated hydroxytoluene
- CMC
critical micelle concentration
- CPP
critical packing parameter
- DAG
diacylglycerol
- DHA
docosahexaenoic acid
- DOPC
dioleoylphosphatidyl-choline
- DPPC
dipalmitoylphosphatidyl-choline
- DPPE
dipalmitoylphosphatidyl-ethanolamine
- DPPS
dipalmitoylphosphatidyl-serine
- EDTA
ethylenediaminetetraacetic acid
- EGCG
epigallocatechin gallate
- EPA
eicosapentaenoic acid
- FFA
free fatty acid
- HLB
hydrophilic lipophilic balance
- IP
induction or initiation period
- LOOH
lipid hydroperoxide
- LOO
lipid peroxyl radical
- MAG
monoacylglycerol
- OO
olive oil
- OSI
oil stability index
- o/w
oil in water emulsion
- p-AV
para-Anisidine value
- PUFA
polyunsaturated fatty acids
- PV
peroxide value
- SAXS
small angle X-ray scattering
- SBO
soybean oil
- SDS
sodium dodecyl sulfate
- SFO
sunflower oil
- SMILES
simplified molecular-input line-entry specification
- TAG
triacylglycerols
- TBARS
thiobarbituric acid reactive substances
- TBHQ
tert-butylhydroquinone
- w/o
water in oil emulsion
References
- Bandarra NM, Campos RM, Batista I, Nunes ML, Empis JM. Antioxidant synergy of α-tocopherol and phospholipids. J. Am. Oil Chem. Soc. 1999;76:905–913. [Google Scholar]
- Jacobsen C, Bruni Let M, Nielsen NS, Meyer AS. Antioxidant strategies for preventing oxidative flavour deterioration of foods enriched with n-3 polyunsaturated lipids: A comparative evaluation. Trends Food Sci. Tech. 2008;19:76–93. [Google Scholar]
- Shahidi F, Zhong Y. Novel antioxidantsinfood quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010;112:930–940. [Google Scholar]
- Schaich KM. In: Bailey's Industrial Oil and Fat Products. 6th Edn. Shahidi F, editor. Hoboken (NJ): John Wiley & Sons; 2005. p. 269. , in:, (Ed.),, 6 Volume Set. [Google Scholar]
- Pinedo AT, Peñalver P, Pérez- Victoria I, Rondón D, Morales JC. Synthesis of new phenolic fatty acid ester and their evaluation as lipophilic antioxidants in an oil matrix. Food Chem. 2007;105:657–665. [Google Scholar]
- Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. F. 2006;5:169–186. [Google Scholar]
- Chen B, Han A, McClements DJ, Decker EA. Physical structures in soybean oil and their impact on lipid oxidation. J. Agric. Food Chem. 2010;58:11993–1 1999. doi: 10.1021/jf102763p. [DOI] [PubMed] [Google Scholar]
- Chen B, McClements DJ, Decker EA. Minor components in food oils: A critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Crit. Rev. Food Sci. Nutr. 2011;51:901–916. doi: 10.1080/10408398.2011.606379. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A, Makinen M, Lampi AM. In: Lipid Oxidation Pathways. Kamal-Eldin A, editor. Champaign (IL): American Oil Chemist's Society Publishing; 2003. pp. 1–36. , in:, (Ed.) [Google Scholar]
- Shahidi F, Chandrasekara A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem. Rev. 2010;9:147–170. [Google Scholar]
- Farmer EH, Koch HP, Sutton DAJ. The course of autoxidation reactions in polyisoprenes and allied compounds, part VII, rearrangement of double bonds during autoxidation. J. Chem. Soc. 1943:541–547. [Google Scholar]
- Velasco J, Dobarganes C. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2002;104:661–676. [Google Scholar]
- Yanishlieva NV, Kamal-Eldin A, Marinova EM, Toneva AG. Kinetics of antioxidant action of α- and γ-tocopherols in sunflower and soybean triacylglycerols. Eur. J. Lipid Sci. Technol. 2002;104:262–270. [Google Scholar]
- Chaiyasit W, Elias RJ, McClements DJ, Decker EA. Role of physical structures in bulk oils on lipid oxidation. Crit. Rev. Food Sci. Nutr. 2007;47:299–317. doi: 10.1080/10408390600754248. [DOI] [PubMed] [Google Scholar]
- Wijeratne SSK, Amarowicz R, Shahidi F. Antioxidant activity of almonds and their by-products in food model systems. J. Am. Oil Chem. Soc. 2006;83:223–230. [Google Scholar]
- Tabee E, Azadmard-Damarchi S, Jagerstad M, Dutta PC. Effects of α-tocopherol on oxidative stability and phytosterol oxidation during heating in some regular and high-oleic vegetable oils. J. Am. Oil Chem. Soc. 2008;85:857–867. [Google Scholar]
- Laguerre M, Giraldo LJL, Lecomte J, Figueroa-Espinoza MC, et al. Chain length affects antioxidant properties of chlorogenate estersinemulsion: The cutoff theory behind the polar paradox. J. Agric. Food Chem. 2009;57:11335–11342. doi: 10.1021/jf9026266. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A, Appelqvist L-A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- Wanasundara PKJPD, Shahidi F. In: Bailey's Industrial Oil and Fat products. 6th Edn. Shahidi F, editor. Hoboken (NJ): John Wiley & Sons; 2005. p. 431. , in:, (Ed.),, 6 Volume Set. [Google Scholar]
- Chen B, Han A, Laguerre M, McClements DJ, Decker EA. Role of reverse micelles on lipid oxidation in bulk oils: Impact of phospholipids on antioxidant activity of α-tocopherol and Trolox. Food Funct. 2011;2:302–309. doi: 10.1039/c1fo10046g. [DOI] [PubMed] [Google Scholar]
- Halliwell B. How to characterize a biological antioxidant. Free Rad. Res. Com. 1990;9:1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000;65:1270–1282. [Google Scholar]
- Sun YE, Wang WD, Chen HW, Li C. Autoxidation of unsaturated lipids in food emulsion. Crit. Rev. Food Sci. Nutr. 2011;51:453–466. doi: 10.1080/10408391003672086. [DOI] [PubMed] [Google Scholar]
- Maqsood S, Benjakul S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010;119:123–132. [Google Scholar]
- Wanasundara UN, Shahidi F. Stabilization of marine oils with flavonoids. J. Food Lipids. 1998;5:183–196. [Google Scholar]
- Brimberg U. On the kinetics of the autoxidation of fats. J. Am. Oil Chem. Soc. 1993;70:249–254. [Google Scholar]
- Brimberg U. On the kinetics of the autoxidation of fats II. Monounsaturated substrates. J. Am. Oil Chem. Soc. 1993;70:1063–1067. [Google Scholar]
- Hamilton RJ, Kalu C, McNeill GP, Padley FB, Pierce JH. Effects of tocopherols, ascorbyl palmitate, and lecithin on autoxidation of fish oil. J. Am. Oil Chem. Soc. 1998;75:813–822. [Google Scholar]
- Yanishlieva NV, Marinova EM. In: Lipid Oxidation Pathways. Kamal-Eldin A, editor. Urbana (IL): American Oil Chemist's Society Publishing; 2003. pp. 85–110. , in:, (Ed.) [Google Scholar]
- Drusch S, Gross N, Schwarz K. Efficient stabilization of bulk fish oil rich in long-chain polyunsaturated fatty acids. Eur. J. Lipid Sci. Technol. 2008;110:351–359. [Google Scholar]
- Hildebrand DH, Terao J, Kito M. Phospholipids plus tocopherols increase soybean oil stability. J. Am. Oil Chem. Soc. 1984;61:552–555. [Google Scholar]
- Chu Y-H, Hsu H-F. Effects of antioxidants of peanut oil stability. Food Chem. 1999;66:29–34. [Google Scholar]
- Chaiyasit W, McClements DJ, Weiss J, Decker EA. Impact of surface-active compounds on physicochemical and oxidative properties of edible oil. J. Agric. Food Chem. 2008;56:550–556. doi: 10.1021/jf072071o. [DOI] [PubMed] [Google Scholar]
- Chen B, McClements DJ, Gray DA, Decker EA. Physical and oxidative stability of pre-emulsified oil bodies extracted from soybeans. Food Chem. 2012;132:1514–1520. doi: 10.1016/j.foodchem.2011.11.144. [DOI] [PubMed] [Google Scholar]
- Chen B, Panya A, McClements DJ, Decker EA. New insights into the role of iron in the promotion of lipid oxidation in bulk oils containing reverse micelles. J. Agric. Food Chem. 2012;60:3524–3532. doi: 10.1021/jf300138h. [DOI] [PubMed] [Google Scholar]
- Porter WL. In: Autoxidation in Food and Biological Systems. Simic MG, Karel M, editors. New York (NY): Plenum Press; 1980. pp. 295–365. , in:, (Eds.) [Google Scholar]
- Porter WL, Black ED, Drolet AM. Use of polyamide oxidative fluorescence test on lipid emulsions: Contrast in relative effectiveness of antioxidants in bulk versus dispersed systems. J. Agric. Food Chem. 1989;37:615–624. [Google Scholar]
- Porter WL. In: Antioxidants: Chemical, Physiological, Nutritional and Toxicological Aspects. Williams GM, editor. Princeton (NJ): Princeton Scientific; 1993. pp. 93–122. , in:, (Ed.) [Google Scholar]
- Frankel EN, Huang S-W, Kanner J, German JB. Interfacial phenomena in the evaluation of antioxidants: Bulk oil vs. emulsion. J. Agric. Food Chem. 1994;42:1054–1059. [Google Scholar]
- Frankel EN, Huang SW, Aeschbach R, Prior E. Antioxidant activity of a rosemary extract and its constituents, carnosic acid, carnosol, and rosmarinic acid, in bulk oil and oil-in-water emulsion. J. Agric. Food Chem. 1996;44:131–135. [Google Scholar]
- Frankel EN, Huang S-W, Prior E, Aeschbach R. Evaluation of antioxidant activity of rosemary extracts, carnosol and carnosic acid in bulk vegetable oils and fish oil and their emulsions. J. Sci. Food Agric. 1996;72:201–208. [Google Scholar]
- Huang S-W, Frankel EN, Schwarz J, Aeschbach R, German JB. Antioxidant activity of carnosic acid and methyl carnosate in bulk oils and oil-in-water emulsions. J. Agric. Food Chem. 1996;44:2951–2956. [Google Scholar]
- Schwarz K, Huang SW, German JB, Tiersch B, et al. Activities of antioxidants are affected by colloidal properties of oil-in-water and water-in-oil emulsions and bulk oils. J. Agric. Food Chem. 2000;48:4874–4882. doi: 10.1021/jf991289a. [DOI] [PubMed] [Google Scholar]
- Becker EM, Ntouma G, Skibsted LH. Synergism and antagonism between quercetin and other chain-breaking antioxidants in lipid systems of increasing structural organisation. Food Chem. 2007;103:1288–1296. [Google Scholar]
- Thiyam U, Stockmann H, Felde TZ, Schwarz K. Antioxidative effect of the main sinapic acid derivatives from rapeseed and mustard oil by products. Eur. J. Lipid Sci. Technol. 2006;108:239–248. [Google Scholar]
- Nenadis N, Boyle S, Bakalbassis EG, Tsimidou M. An experimental approach to structure-activity relationships of caffeic and dihydrocaffeic acids and related monophenols. J. Am. Oil Chem. Soc. 2003;80:451–458. [Google Scholar]
- Silva FA, Borges F, Ferreira MA. Effects of phenolic propyl esters on the oxidative stability of refined sunflower oil. J. Agric. Food Chem. 2001;49:3936–3941. doi: 10.1021/jf010193p. [DOI] [PubMed] [Google Scholar]
- Fang X, Shima M, Kadota M, Tsuno T, Adachi S. Suppressive effect of alkyl ferulate on the oxidation of linoleic acid. Biosci. Biotechnol. Biochem. 2006;70:457–461. doi: 10.1271/bbb.70.457. [DOI] [PubMed] [Google Scholar]
- Huber GM, Rupasinghe HPV, Shahidi F. Inhibition of oxidation of omega-3 polyunsaturated fatty acids and fish oil by quercetin glycosides. Food Chem. 2009;117:290–295. [Google Scholar]
- Huang S-W, Frankel EN. Antioxidant activity of tea catechins in different lipid systems. J. Agric. Food Chem. 1997;45:3033–3038. [Google Scholar]
- Trujillo M, Mateos R, De Teran LC, Espartero JL, et al. Lipophilic hydroxytyrosyl eters. Antioxidant activity in lipid matrices and biological systems. J. Agric. Food Chem. 2006;54:3779–3785. doi: 10.1021/jf060520z. [DOI] [PubMed] [Google Scholar]
- Medina I, Lois S, Alcantara R, Lucas R, Morales JC. Effect of lipophilization of hydroxytyrosol on its antioxidant activity in fish oils and fish oil-in-water emulsions. J. Agric. Food Chem. 2009;57:9733–9799. doi: 10.1021/jf9023867. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Zhong Y. Revisiting the polar paradox theory: A critical overview. J. Agric. Food Chem. 2011;59:3499–3504. doi: 10.1021/jf104750m. [DOI] [PubMed] [Google Scholar]
- Koga T, Terao J. Phospholipids increase radical-scavenging activity of vitamin E in a bulk oil model system. J. Agric. Food Chem. 1995;43:1450–1454. [Google Scholar]
- Brimberg U, Kamal-Eldin A. On the kinetics of the autoxidation of fats: Influence of pro-oxidants, antioxidants and synergists. Eur. J. Lipid Sci. Technol. 2003;105:83–91. [Google Scholar]
- Garti N. Microemulsions as microreactors for food applications. Curr. Opin. Colloid Interface Sci. 2003;8:197–211. [Google Scholar]
- Mancuso JR, McClements DJ, Decker EA. The effects of surfactant type, pH and chelators on the oxidation of salmon oil- in-water emulsions. J. Agric. Food Chem. 1999;47:4112–4116. doi: 10.1021/jf990203a. [DOI] [PubMed] [Google Scholar]
- Waraho T, McClements DJ, Decker EA. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Tech. 2011;22:3–13. [Google Scholar]
- Hopia A, Huang S-W, Schwarz K, German JB, Frankel EN. Effect of different lipid systems on antioxidant activity of rosemary constituents carnosol and carnosic acid with and without α- tocopherol. J. Agric. Food Chem. 1996;44:2030–2036. [Google Scholar]
- Huang S-W, Hopia A, Schwarz K, Frankel EN, German JB. Antioxidant activity of α-Tocopherol and Trolox in different lipid substrates: Bulk oils vs oil-in-water emulsions. J. Agric. Food Chem. 1996;44:444–452. [Google Scholar]
- Sorensen MA-D, Nielsen NS, Decker EA, Let MB, et al. The efficacy of compounds with different polarities as antioxidants in emulsions with omega-3 lipids. J. Am. Oil Chem. Soc. 2011;88:489–502. [Google Scholar]
- Lucas R, Comelles F, Alcantara D, Maldonado OS, et al. Surface-active properties of lipophilic antioxidants tyrosol and hydroxytyrosol fatty acid esters: A potential explanation for the nonlinear hypothesis of the antioxidant activity in oil-in-water emulsions. J. Agric. Food Chem. 2010;58:8021–8026. doi: 10.1021/jf1009928. [DOI] [PubMed] [Google Scholar]
- Pena A, Miller C. Solubilization rates of oils in surfactant solutions and their relationship to mass transport in emulsions. Adv. Colloid Interface Sci. 2006;123–126:241–257. doi: 10.1016/j.cis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Oehlke K, Heins A, Stockmann H, Schwarz K. Impact of emulsifier microenvironments on acid-base equilibrium and activity of antioxidants. Food Chem. 2010;118:48–55. [Google Scholar]
- Aubourg PS. Effect of partially hydrolyzed lipids on inhibition of oxidation of marine lipids. Eur. Food Res. Technol. 2001;212:540–545. [Google Scholar]
- Mei LY, Decker EA, McClements DJ. Evidence of iron association with emulsion droplets and its impact on lipid oxidation. J. Agric. Food Chem. 1998;46:5072–5077. [Google Scholar]
- Mei LY, McClements J, Wu JN, Decker EA. Iron-catalyzed lipid oxidation as affected by surfactant, pH and NaCl. Food Chem. 1998;61:307–312. [Google Scholar]
- Kortenska VD, Yanishlieva NV, Kasaikina OT, Totzeva IR, et al. Phenol antioxidant efficiency in various lipid substrates containing hydroxy compounds. Eur. J. Lipid Sci. Technol. 2002;104:513–519. [Google Scholar]
- El-Shattory YA, Saadia MA, Hamed SF, Schwarz K. Effect of different reversed micelles on autoxidation and photooxidation of stripped corn oil. Grasas Aceites. 2003;54:24–29. [Google Scholar]
- Kasaikina OT, Kartasheva ZS, Pisarenko LM. Effect of surfactants on liquid-phase oxidation of hydrocarbons and lipids. Russ. J. Gen. Chem. 2008;78:1533–1544. [Google Scholar]
- Kiokias S, Gordon MH. Antioxidant properties of annatto carotenoids. Food Chem. 2003;83:523–529. [Google Scholar]
- Decker EA, Warner K, Richards MP, Shahidi F. Measuring antioxidant effectiveness in food. J. Agri. Food Chem. 2005;53:4303–4310. doi: 10.1021/jf058012x. [DOI] [PubMed] [Google Scholar]
- Koprivnjak O, Skevin D, Valic S, Majetic V, et al. The antioxidant capacity and oxidative stability of virgin olive oil enriched with phospholipids. Food Chem. 2008;111:121–126. [Google Scholar]
- Sun-Waterhouse D, Thakorlal J, Zhou J. Effects of added phenolics on the storage stability of avocado and coconut oils. Int. J. Food Sci. Technol. 2011;46:1575–1585. [Google Scholar]
- Smit B, Hilbers PAJ, Esselink K, Rupert LAM, van Os NM. Structure of a water/oil interface in the presence of micelles: A computer simulation study. J. Phys. Chem. 1991;95:6361–6368. [Google Scholar]
- Oldfield C. Enzymes in water-in-oil microemulsions (‘Reversed Micelles’): Principles and applications. Biotechnol. Genet. Eng. Rev. 1994;12:255–327. doi: 10.1080/02648725.1994.10647914. [DOI] [PubMed] [Google Scholar]
- Winsor PA. Hydrotropy olubilisation and related emulsification processes. Trans. Faraday Soc. 1948;44:376–398. [Google Scholar]
- Surabhi K, Op K, Atul N, Arun G. Microemulsions developmental aspects. Res. J. Pharm., Biol. Chem. Sci. 2010;1:683–706. [Google Scholar]
- Mehta SK, Kaur G. In: Thermodynamics. Tadashi M, editor. Rijeka (Croatia): Intech; 2011. pp. 381–406. , in:, (Ed.) [Google Scholar]
- Lawrence MJ, Rees GD. Microemulsion- based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000;45:89–121. doi: 10.1016/s0169-409x(00)00103-4. [DOI] [PubMed] [Google Scholar]
- Kortenska VD, Yanishlieva NV, Kasaikina OT, Totzeva IR, Boneva MI. Effect of lipid hydroxy compounds on the phenol antioxidant efficiency in lipid autoxidation. Cr. Acad. Bulg. Sci. 2001;54:29–34. [Google Scholar]
- Frankel EN. Lipid Oxidation. 1st Edn. Dundee (Scotland): The Oily Press; 1998. [Google Scholar]
- Chen CH, Terentjev EM. Effects of water on aggregation and stability of monoglycerides in hydrophobic solutions. Langmuir. 2010;26:3095–3105. doi: 10.1021/la904139b. [DOI] [PubMed] [Google Scholar]
- Khan MA, Shahidi F. Tocopherols and phospholipids enhance the oxidative stability of borage and evening primrose triacylglycerols. J. Food Lipids. 2000;7:143–150. [Google Scholar]
- Nicholson M. Additives Improve Fuel Oil Properties. Bunker World: Marine Additives Special. , August/September 2005. http://i.pmcdn.net/p/bw/magazine/2005/09/Additives.pdf. (accessed September 10, 2012) [Google Scholar]
- Kasaikina OT, Golyavin AA, Krugovov DA, Kartasheva ZS, Pisarenko LM. Micellar catalysis in the oxidation of lipids. Moscow University Chemistry Bulletin. 2010;65:206–209. [Google Scholar]
- Gupta R, Muralidhara HS, Davis HT. Structure and phase behavior of phospholipid-based micelles in nonaqueous media. Langmuir. 2001;17:5176–5183. [Google Scholar]
- Griffin W. Calculation of HLB values of non-ionic surfactants. J. Soc. Cosmet. Chem. 1954;5:249–256. [Google Scholar]
- Davies JT , A quantitative kinetic theory of emulsion type. 1. Physical chemistry of the emulsifying agent, in: Gas/Liquid and Liquid/Liquid Interfaces, Proceedings of the 2nd International Congress Surface Activity, Vol. 1, Butterworths (London), 1959, pp. 426–438.
- Israelachvilli J, Mitchell DJ, Ninham BW. Theory of self assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2. 1976;72:1525–1528. [Google Scholar]
- Mitchell DJ, Ninham BW. Micelles, vesicles and microemulsions. J. Chem. Soc., Faraday Trans. 2. 1981;77:601–629. [Google Scholar]
- Krei G, Meyer U, Borner B, Hustedt H. Extraction of α-amylase using BDBAC reversed micelles. Bioseparation. 1995;5:175–183. [Google Scholar]
- Mathew DS, Juang R-S. Role of alcohols in the formation of inverse microemulsions and back extraction of proteins/enzymes in a reverse micellar system. Sep. Purif. Technol. 2007;53:199–215. [Google Scholar]
- Kahlweit M, Strey R, Busse G. Effect of alcohols on the phase behavior of microemulsions. J. Phys. Chem. 1991;95:5344–5352. [Google Scholar]
- Karpe P, Ruckenstein E. The enzymatic superactivity in reverse micelles: Role of the dielectric constant. J. Colloid Interf. Sci. 1991;141:534–552. [Google Scholar]
- Leodidis EB, Hatton TA. Special ion effects in electrical double layers: Selective solubilization of cations in aerosol-OT reversed micelles. Langmuir. 1989;5:741–753. [Google Scholar]
- Graciaa A, Lachaise J, Cucuphat C, Bourrel M, Salager JL. Improving solubilization in microemulsions with additives. 1. The lipophilic linker role. Langmuir. 1993;9:669–672. [Google Scholar]
- Graciaa A, Lachaise J, Cucuphat C, Bourrel M, Salager JL. Improving solubilization in microemulsions with additives. 2. Long chain alcohols as lipophilic linkers. Langmuir. 1993;9:3371–3374. [Google Scholar]
- Ahmad MM, Al-Hakin S, Adel A, Shehata AAY. The effect of amino acids on the activity of synergists and antioxidants in two vegetable oils. J. Am. Oil Chem. Soc. 1983;60:468. [Google Scholar]
- Takahashi M, Komura E, Niki E, Tanaka K. Action of fatty acid esters of l-ascorbic acid as antioxidants in phosphatidylcholine liposomal membranes. Bull. Chem. Soc. Jpn. 1992;65:679–684. [Google Scholar]
- Stockmann H, Schwarz K, Huynh-Ba T. The influence of various emulsifiers on the partitioning and antioxidant activity of hydroxybenzoic acids and their derivatives in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2000;77:535–542. [Google Scholar]
- Winkler J-K, Warner K. The effect of phytosterol concentration on oxidative stability and thermal polymerization of heated oils. Eur. J. Lipid Sci. Technol. 2008;110:455–464. [Google Scholar]
- Belhaj N, Arab-Tehrany E, Linder M. Oxidative kinetics of salmon oil in bulk and in nanoemulsion stabilized by marine lecithin. Process Biochem. 2010;45:187–195. [Google Scholar]
- Medina I, Alcantara D, Gonzalez MJ, Torres P, et al. Antioxidant activity of resveratrol in several fish lipid matrices: Effect of acylation and glucosylation. J. Agric. Food Chem. 2010;58:9778–9786. doi: 10.1021/jf101472n. [DOI] [PubMed] [Google Scholar]
- Kellerby SS, McClements DJ, Decker EA. Role of proteins in oil-in-water emulsions on the stability of lipid hydroperoxides. J. Agric. Food Chem. 2006;54:7879–7884. doi: 10.1021/jf061340s. [DOI] [PubMed] [Google Scholar]
- Endo Y, Hoshizaki S, Fujimoto K. Autoxidation of synthetic isomers of triacylglycerol containing eicosapentaenoic acid. J. Am. Oil Chem. Soc. 1997;74:543–548. [Google Scholar]
- Fanun M. Microstructure of mixed nonionic surfactants microemulsions studied by SAXS and DLS. J. Dispers. Sci. Technol. 2009;30:115–123. [Google Scholar]
- Castro MJM, Cabral JMS. Reversed micelles in biotechnological processes. Biotechnol. Adv. 1988;6:151–167. doi: 10.1016/0734-9750(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Kadam KI. Reversed micelles as a bioseparation tool. Enzyme Microb. Technol. 1986;8:266–273. [Google Scholar]
- Pessoa A, Vitola M. Recovery of inulinase using BDBAC reversed micelles. Process Biochem. 1988;33:291–297. [Google Scholar]
- Mei L, Decker EA, McClements DJ. Lipid oxidation in emulsions as affected by charge status of antioxidants and emulsion droplets. J. Agric. Food Chem. 1999;47:2267–2273. doi: 10.1021/jf980955p. [DOI] [PubMed] [Google Scholar]
- Miyashita K, Azuma G, Ota T. Oxidative stability of geometric and positional isomers of unsaturated fatty acids in an aqueous solution. J. Jpn. Oil Chem. Soc. 1995;44:425–430. [Google Scholar]
- Talbot G. Trans Fatty Acids—The Options. IOI Loders Croklaan: 2013. http://www.ioigroup.com/Business/download/Transfat.pdf (accessed May 26, 2013) [Google Scholar]
- Cho SY, Miyashita K, Miyazawa T, Fujimoto K, Kaneda T. Autoxidation of ethyl eisosapentaenoate and docosahexaenoate. J. Am. Oil Chem. Soc. 1987;64:876–879. [Google Scholar]
- Miyashita K, Takagi T. Autoxidation rates of various esters of safflower oil and linoleic acid. J. Am. Oil Chem. Soc. 1988;65:1156–1158. [Google Scholar]
- Endo Y, Hoshizaki S, Fujimoto K. Oxidation of synthetic triacylglycerols containing eicosapentaenoic and docosahexaenoic acids: Effect of oxidation system and triacylglycerol structure. J. Am. Oil Chem. Soc. 1997;74:1041–1045. [Google Scholar]
- Kimoto H, Endo Y, Fujimoto K. Influence of interesterification on the oxidative stability of marine oil triacylglycerols. J. Am. Oil Chem. Soc. 1994;71:469–473. [Google Scholar]
- Endo Y, Hoshizaki S, Fujimoto K. Kinetics for the autoxidation of triacylglycerols containing eicosapentaenoic acid. Biosci., Biotechnol. Biochem. 1997;61:1036–1037. [Google Scholar]
- Adachi S, Minten S, Kobayashi T. Oxidation of lipid in bulk and dispersion systems. Jpn. J. Food Eng. 2009;10:9–15. [Google Scholar]
- Kashima M, Cha GS, Isoda Y, Hirano J, Miyazawa T. The antioxidant effects of phospholipids on perilla oil. J. Am. Oil Chem. Soc. 1984;61:950–954. [Google Scholar]
- King MF, Boyd LC, Sheldon BW. Effects of phospholipids on lipid oxidation of a salmon oil model system. J. Am. Oil Chem. Soc. 1992;69:237–242. [Google Scholar]
- Hara S, Okada N, Hibino H, Totani Y. Antioxidative behaviour of phospholipids for polyunsaturated fatty acids of fish oil. J. Jpn. Oil Chem. Soc. 1992;41:130–135. [Google Scholar]
- Carlotti ME, Gallarate M, Gasco MR, Morel S, et al. Synergistic action of vitamin C and amino acids on vitamin E in inhibition of the lipoperoxidation of linoleic acid in disperse systems. Int. J. Pharm. 1997;155:251–261. [Google Scholar]
- Frankel EN, Meyer AS. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000;80:1925–1941. [Google Scholar]
- Brimberg U, Kamal-Eldin A. On the kinetics of the autoxidation of fats: Substrates with conjugated double bonds. Eur. J. Lipid Sci. Technol. 2003;105:17–22. [Google Scholar]
- Calligaris S, Nicoli MC. Effect of selected ions from lyotropic series on lipid oxidation rate. Food Chem. 2006;94:130–134. [Google Scholar]
- Bendini A, Cerretani L, Salvador MD, Frepagane G, Lercker G. Stability of the sensory quality of virgin olive oil during storage: An overview. Italian Food & Beverage Technology. 2010:5–18. , LX. [Google Scholar]
- Gramza- Michalowska A, Stachowiak B. The antioxidant potential of carotenoid extract from Phaffia Rhodozyma. ACTA Sci. Pol. Technol. Aliment. 2010;9:171–188. [Google Scholar]
- An CB, Li D, Liang R, Bu YZ, et al. Chain length effects in isoflavonoid daidzein alkoxy derivatives as antioxidants: A quantum mechanical approach. J. Agric. Food Chem. 2011;59:12652–12657. doi: 10.1021/jf2030314. [DOI] [PubMed] [Google Scholar]
- Rukmini A, Raharjo S, Hastuti P, Supriyadi S. Formulation and stability of water-in-virgin coconut oil microemulsion using ternary food grade nonionic surfactants. Int. Food Res. J. 2012;19:259–264. [Google Scholar]
- Carelli AA, Franco IC, Crapiste GH. Effectiveness of added natural antioxidants in sunflower oil. Grasas Aceites. 2005;56:303–310. [Google Scholar]
- Chen JH, Ho CT. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997;45:2374–2378. [Google Scholar]
- Leonardis AD, Pizzella L, Macciola V. Evaluation of chlorogenic acid and its metabolites as potential antioxidants for fish oils. Eur. J. Lipid Sci. Technol. 2008;110:941–948. [Google Scholar]
- Laguerre M, Chen B, Lecomte J, Villeneuve P, et al. Antioxidant properties of chlorogenic acid and its alkyl esters in stripped corn oil in combination with phospholipids and/or water. J. Agric. Food Chem. 2011;59:10361–10366. doi: 10.1021/jf2026742. [DOI] [PubMed] [Google Scholar]
- Yanishlieva NV, Kortenska VD. On the participation of fatty alcohols in the autoxidation of lipids. J. Sci. Food Agric. 1989;47:215–223. [Google Scholar]
- Kortenska VD, Yanishlieva NV, Roginskii VA. Kinetics of inhibited oxidation of lipids in the presence of 1-octadecanol and 1-palmitoylglycerol. J. Am. Oil Chem. Soc. 1991;68:888–890. [Google Scholar]
- Yanishlieva NV, Kortenska VD. On the participation of fatty alcohols in inhibited oxidation of lipids. Lipid/fett. 1993;95:35–40. [Google Scholar]
- Kortenska VD, Yanishlieva NV. Effect of the phenol antioxidant type on the kinetics and mechanism of inhibited lipid oxidation in the presence of fatty alcohols. J. Sci. Food Agric. 1995;68:117–126. [Google Scholar]
- Mistry BS, Min DB. Effects of fatty acids on the oxidative stability of soybean oil. J. Food Sci. 1987;52:831–832. [Google Scholar]
- Hamam F, Shahidi F. Synthesis of structured lipids via acidolysis of docosahexaenoic acid single cell oil (DHASCO) with capric acid. J. Agric. Food Chem. 2004;52:2900–2906. doi: 10.1021/jf035316f. [DOI] [PubMed] [Google Scholar]
- Frega N, Mozzon M, Lercker G. Effects of free fatty acids on oxidative stability of vegetable oil. J. Am. Oil Chem. Soc. 1999;76:325–329. [Google Scholar]
- Mistry BS, Min DB. Isolation of sn-α-monolinolein from soybean oil and its effect on oil oxidative stability. J. Food Sci. 1987;52:786–790. [Google Scholar]
- Mistry BS, Min DB. Prooxidant effects of monoglycerides and diglycerides in soybean oil. J. Food Sci. 1988;53:1896–1897. [Google Scholar]
- Caponio F, Paradiso VM, Bruno G, Summo C, et al. Do monoacylglycerols act as pro-oxidants in purified soybean oil? Evidence of a dose-dependent effect. Itl. J. Food Sci. 2011:239. [Google Scholar]
- Wang T, Jiang Y, Hammond EG. Effect of randomization on the oxidative stability of corn oil. J. Am. Oil Chem. Soc. 2005;82:111–117. [Google Scholar]
- King MF, Boyd LC, Sheldon BW. Antioxidant properties of individual phospholipids in a salmon oil model system. J. Am. Oil Chem. Soc. 1992;69:545–551. [Google Scholar]
- Hidalgo FJ, Nogales F, Zamora R. Changes produced in the antioxidative activity of phospholipids as a consequence of their oxidation. J. Agric. Food Chem. 2005;5:659–662. doi: 10.1021/jf0483220. [DOI] [PubMed] [Google Scholar]
- Lee Y, Choe E. Singlet oxygen quenching effects of phosphatidylcholine in emulsion containing sunflower oil. J. Food Sci. 2008;73:506–511. doi: 10.1111/j.1750-3841.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Haila KM, Lievonen SM, Heinonen MI. Effects of lutein, lycopene, annatto, and g-tocopherol on autoxidation of triglycerides. J. Agric. Food Chem. 1996;44:2096–2100. [Google Scholar]
- Henry LK, Catignani GL, Schwartz SJ. Oxidative degradation kinetics of lycopene, lutein, and 9-cis and all-trans- β-carotene. J. Am. Oil Chem. Soc. 1998;75:823–829. [Google Scholar]
- Subagio A, Morita N. Instability of carotenoids is a reason for their promotion on lipid oxidation. Food Res. Int. 2001;34:183–188. [Google Scholar]
- Zeb A, Murkovic M. Carotenoids and triacylglycerols interactions during thermal oxidation of refined olive oil. Food Chem. 2011;127:1584–1593. [Google Scholar]
- Ahmad MM, Al-Hakin S, Shehata AAY. The antioxidant activity of amino acids in two vegetable oils. J. Am. Oil Chem. Soc. 1983;80:837–840. [Google Scholar]
- Alaiz M, Zamora R, Hidalgo FJ. Antioxidative activity of (E)-2-octenal/amino acids reaction products. J. Agric. Food Chem. 1995;43:795–800. [Google Scholar]
- Hidalgo FJ, Leon MM, Zamora R. Antioxidative activity of amino phospholipids and phospholipid/amino acid mixtures in edible oils as determined by the Rancimat method. J. Agric. Food Chem. 2006;54:5461–5467. doi: 10.1021/jf060848s. [DOI] [PubMed] [Google Scholar]
- Papadopoulou D, Roussis IG. Inhibition of corn oil oxidation by N-acetyl-cysteine and glutathione. Food Chem. 2008;109:624–629. [Google Scholar]
- Hidalgo FJ, León MM, Zamora R. Effect of β-sitosterol in the antioxidative activity of oxidized lipid–amine reaction products. Food Res. Int. 2009;42:1215–1222. doi: 10.1021/jf070119s. [DOI] [PubMed] [Google Scholar]
- Hras AR, Hadolin M, Knez Z, Bauman D. Comparison of antioxidative and synergistic effects of rosemary extract with α-tocopherol, ascorbyl palmitate and citric acid in sunflower oil. Food Chem. 2000;71:229–233. [Google Scholar]
- Jaswir I, Kitts DD, Che Man YB, Hassan TH. Synergistic effect of rosemary, sage and citric acid on fatty acid retention of heated flaxseed oil. J. Oleo. Sci. 2004;53:581–591. [Google Scholar]
- Yi J, Andersen ML, Skibsted LH. Interactions between tocopherols, tocotrienols and carotenoids during autoxidation of mixed palm olein and fish oil. Food Chem. 2011;127:1792–1797. [Google Scholar]
- Gordon MH, Kourkimska L. The effects of antioxidants on changes in oils during heating and deep frying. J. Sci. Food Agric. 1995;68:347–353. [Google Scholar]
- Frankel EN, Satué-Gracia T, Meyer AS, German JB. Oxidative stability of fish and algae oils containing long-chain polyunsaturated fatty acids in bulk and in oil-in-water emulsions. J. Agric. Food Chem. 2002;50:2094–2099. doi: 10.1021/jf0111458. [DOI] [PubMed] [Google Scholar]
- Olsen E, Vogt G, Saarem K, Greibrokk T, Nilsson A. Autoxidation of cod liver oil with tocopherol and ascorbyl palmitate. J. Am. Oil Chem. Soc. 2005;82:97–103. [Google Scholar]
- Karabulut I. Effects of α-tocopherol, β-carotene and ascorbyl palmitate on oxidative stability of butter oil triacylglycerols. Food Chem. 2010;123:622–627. [Google Scholar]
- Soupas L, Juntunen L, Lampi A-M, Piironen V. Effects of sterol structure, temperature and lipid medium on phytosterol oxidation. J. Agric. Food Chem. 2004;52:6485–6491. doi: 10.1021/jf049168k. [DOI] [PubMed] [Google Scholar]
- Soupas L, Huikko L, Lampi A-M, Piironen V. Oxidative stability of phytosterols in some food applications. Eur. Food Res. Technol. 2006;222:266–273. [Google Scholar]
- Cercaci L, Rodriguez-Estrada MT, Lercker G, Decker EA. Phytosterol oxidation in oil-in-water emulsions and bulk oil. Food Chem. 2007;102:161–167. [Google Scholar]
- Gurkov TD, Dimitrova DT, Marinova KG, Bilke-Crause C, et al. Ionic surfactants on fluid interfaces: Determination of the adsorption; role of the salt and the type of the hydrophobic phase. Colloid Surf. A. 2005;261:29–38. [Google Scholar]
- Chaiyasit W, McClements DJ, Decker EA. Ability of antioxidants to alter interfacial tension and lipid oxidation in bulk oil and oil-in-water emulsions. J. Agric. Food Chem. 2005;53:4982–4988. doi: 10.1021/jf0501239. [DOI] [PubMed] [Google Scholar]
- Israelachvilli J. The science and applications of emulsions—An overview. Colloid Surf. A. 1994;91:1–8. [Google Scholar]
- Poole SK, Durham D, Kibbey C. Rapid method for estimating the octanol-water partition coefficient (log Pow) by microemulsion electrokinetic chromatography. J. Chromatogr. B Biomed. Sci. Appl. 2000;745:117–126. doi: 10.1016/s0378-4347(00)00072-4. [DOI] [PubMed] [Google Scholar]
- Wehmeyer KR, Tu J, Jin Y, King S, et al. The application of multiplexed microemulsion electrokinetic chromatography for the rapid determination of log Pow values for neutral and basic compounds. LCGC Asia Pacific. 2004;7:1078–1088. [Google Scholar]
- La- Scalea MA, Menezes CMS, Ferreira EI. Molecular volume calculation using AM1 semi- empirical method toward diffusion coefficients and electrophoretic mobility estimates in aqueous solution. J. Mol. Struct. 2005;730:111–120. [Google Scholar]
- Remko M. Molecular structure, lipophilicity, solubility, absorption and polar surface area of novel anticoagulant agents. J. Mol. Struct. 2009;916:76–85. [Google Scholar]
- Ertl P, Rohde B, Selzeer P. Fast calculation of molecular polar surface area as a sum of fragment based-contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- Wanasundara UN, Shahidi F. Positional distribution of fatty acids in triacylglycerols of seal blubber oil. J. Food Lipids. 1997;4:51–64. [Google Scholar]


