Abstract
N-3 polyunsaturated fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) have protective effects against atherosclerosis. Monocyte chemotactic protein (MCP)-1 is a major inflammatory mediator in the progression of atherosclerosis. However, little is known about the regulation of Mcp-1 by DHA and EPA in vessels and vascular smooth muscle cells (VSMCs). In this study, we compared the effect of DHA and EPA on the expression of Mcp-1 in rat arterial strips and rat VSMCs. DHA, but not EPA, suppressed Mcp-1 expression in arterial strips. Furthermore, DHA generated 4-hydroxy hexenal (4-HHE), an end product of n-3 polyunsaturated fatty acids (PUFAs), in arterial strips as measured by liquid chromatography-tandem mass spectrometry. In addition, 4-HHE treatment suppressed Mcp-1 expression in arterial strips, suggesting 4-HHE derived from DHA may be involved in the mechanism of this phenomenon. In contrast, Mcp-1 expression was stimulated by DHA, EPA and 4-HHE through p38 kinase and the Keap1-Nuclear factor erythroid-derived 2-like 2 (Nrf2) pathway in VSMCs. In conclusion, there is a dual effect of n-3 PUFAs on the regulation of Mcp-1 expression. Further study is necessary to elucidate the pathological role of this phenomenon.
Keywords: monocyte chemotactic protein 1, 4-hydroxy hexenal, docosahexaenoic acid, eicosapentaenoic acid
1. Introduction
Atherosclerosis is characterized by accumulation of oxidized fat, thickening of vessel walls by collagens secreted by proliferating vascular smooth muscle cells, and macrophage filtration [1]. There are several steps in the progression of atherosclerosis: (1) endothelial dysfunction, (2) migration of leukocytes and smooth muscle cells into the vessel wall, (3) foam cell formation, and (4) degradation of extracellular matrix. Epidemiologically, fish consumption negatively correlates with cardiovascular events, suggesting beneficial effects of n-3 polyunsaturated fatty acids (PUFA) [2,3]. Other clinical studies have indicated that n-3 PUFAs improved the carotid intima-media thickness and endothelial function [4,5], suggesting that n-3 PUFAs attenuate atherosclerosis by decreasing migration of leukocytes and proliferation of smooth muscle cells. This is supported by animal experiments that showed n-3 PUFAs attenuated VCAM-1 expression and macrophage filtration [6]. N-3 PUFAs mainly consist of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). However, the difference between EPA and DHA in terms of their anti-atherosclerotic effect is still unclear.
Monocyte chemotactic protein (MCP)-1/chemokine (C-C motif) ligand 2 (CCL2) is expressed in inflammatory cells and stromal cells such as endothelial and smooth muscle cells, and its expression is regulated by proinflammatory stimuli and tissue injury. Mcp-1 is regulated both by the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway and stress-activated kinases including p38, ERK and JNK [7]. There are several potential mechanisms that explain the anti-inflammatory effect of EPA and DHA. A recent report revealed that G-protein coupled receptor 120 (GPR120) is a receptor for DHA that mediates anti-inflammatory and insulin-sensitizing effects in rodents [8]. Other reports have suggested that resolvins and protectins—which are derived from EPA and DHA—are mediators of the anti-inflammatory effects [9]. We have recently reported that 4-hydroxy hexenal (4-HHE)—an end product of n-3 PUFA peroxidation—activates the nuclear factor erythroid 2-related factor 2 (Nrf2)-Kelch-like ECH-associated protein 1 (Keap1) pathway in human umbilical vein endothelial cells (HUVECs), contributing to endothelial function and antioxidative activity [10,11].
Nrf2 is a redox-sensitive master regulatory transcription factor regulated by Keap1. Electrophiles, shear stress, and reactive oxygen species (ROS) stimulate modification of the cysteine residues of Keap1, which allows its translocation to the nucleus. Nrf2 induces antioxidant enzymes such as heme oxygenase-1 (Hmox1) through the antioxidant response element (ARE) consensus sequence [12,13]. The 4-HHE induces Nrf2-mediated Hmox1 expression in multiple organs [14,15]. In addition, it has also been reported that DHA induces Nrf2-mediated Hmox1 expression in human vascular smooth muscle cells (VSMCs) isolated from small pulmonary artery or endothelial cells [16,17].
Therefore, we examined the regulation of Mcp-1 by DHA and EPA in arterial strips and VSMCs. Furthermore, we measured the 4-HHE content by a liquid chromatography-tandem mass spectrometry (LC-MS/MS) and tested its role in these tissues.
2. Methods
2.1. Reagents
Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were obtained from Life Technologies (Grand Island, NY, USA). EPA, DHA, and 4-HHE were purchased from Cayman (Ann Arbor, MI, USA). The MTT assay kit, anti-β-actin (A5316) antibody and N-acetyl-l-cysteine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fatty acid-free bovine serum albumin (BSA) was purchased from Nacalai Tesque (Kyoto, Japan). Anti-p38 (#9112), anti-phospho-p38 (#9211), anti-ERK1/2 (#9102), anti-phospho-ERK1/2 (#9106), anti-JNK (#9252), anti-phospho-JNK (#9251), and anti-caspase-3 (#9661) antibodies were purchased from Cell Signaling (Danvers, MA, USA). Horseradish peroxidase-linked anti-mouse and anti-rabbit antibodies were purchased from Amersham Biosciences Corp. (Piscataway, NJ, USA). 2′7′-Dichlorodihydrofluorescein diacetate (H2DCFDA) and small interfering RNA (SiRNA) reagents were purchased from Life Technologies (Tokyo, Japan). SB203580, PD98059 and SP600125 were purchased from Calbiochem (Cambridge, UK).
2.2. Animals and Experimental Procedures
All animal experimentation was approved by the committee for Animal Research of Shiga University of Medical Science (No. 2014-4-8, 7 May 2014). The experimental procedure for artery strips was performed as previously reported [18]. Briefly, eight-week-old male Sprague-Dawley rats (Japan SLC, Shizuoka, Japan) were housed in an environmentally controlled room with a 12 h light/dark cycle and free access to food and water. Rats were fed a regular diet (Dyets Inc., Bethlehem, PA, USA) for 12 weeks. After 12 h of fasting, rats were sacrificed by bleeding from the abdominal aorta under deep anesthesia. The thoracic aorta was dissected, excised, and cut into strips with special care being taken to preserve the endothelium. The strips were then fixed vertically between hooks in a muscle bath (10-mL capacity) containing modified Ringer-Locke solution bubbled with a gas mixture of 95% O2 and 5% CO2, pH 7.4 at 37 ± 0.3 °C. After treatment with DHA, EPA or 4-HHE for 6 h, the arterial strips were immediately freeze-clamped by liquid nitrogen, and stored at −80 °C. For lipid extraction, the frozen tissues were pulverized into a fine powder using a Cryo Press disruptor (Microtec Co., Ltd., Chiba, Japan). This fine powder was weighed on an ME235 electronic balance (Sartorius AG, Göttingen, Germany), homogenized in 490 µL of chloroform/methanol (1:1, v/v) and 10 μL of dibutylhydroxytoluene solution (10 mg/mL in ethanol), and incubated at 36 °C for 1 h [19]. The resulting solution was used for measuring the 4-HHE content.
2.3. Cell Culture
VSMCs were isolated from the aortas of male Sprague-Dawley rats (150–200 g) by enzymatic digestion as previously described [20]. Briefly, cells were maintained in DMEM supplemented with 10% FBS, and used between the 4th–12th passages except for primary cells, showing a dramatic growth rate because of transformation. Cells were grown to confluence in 12-well plates, and cell growth was arrested for 24 h in DMEM supplemented with 1% FBS before the real-time quantitative polymerase chain reaction (RT-qPCR) experiments.
2.4. Fatty Acid Treatment
DHA or EPA was administered as a complex with fatty acid-free BSA as previously described [15]. Briefly, 0.3 mM DHA or EPA was dissolved in ethanol (2.5 mL), and gradually solubilized in an 8.4% BSA solution (14.3 mL) at 37 °C. The 4-HHE was dissolved in dimethyl sulfoxide and then in serum-containing medium.
2.5. Messenger RNA (mRNA) Extraction and Real-Time RT-qPCR Analysis
Total RNA was extracted from cells and tissues using a Total RNA Mini Kit (Bio-Rad, Hercules, CA, USA). Single-stranded cDNA was synthesized from 1.5 μg of total RNA using the Prime Script RT Reagent Kit (Takara Bio, Shiga, Japan), and endogenous genomic DNA was degraded by DNase I (Life Technologies, CA, USA). RT-qPCR experiments were carried out with SYBR Green PCR master mix (Life Technologies, CA, USA) and the ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). All the quantitative data were normalized against the expression levels of 18S rRNA (18S). RT-qPCR conditions were 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The primers for the RT-qPCR are listed in Table 1.
Table 1.
Candidate genes, primer sequences and accession numbers.
| Forward Primer | Reverse Primer | Accession Number | |
|---|---|---|---|
| Mcp-1 | GCTGCTACTCATTCACTGGCAA | TGCTGCTGGTGATTCTCTTGTA | NM_031530.1 |
| Hmox-1 | TCTATCGTGCTCGCATGAAC | AAGGCGGTCTTAGCCTCTTC | NM_012580.2 |
| Nrf2 | GGAGCAATTCAACGAAGCTC | ACAGTTCTGAGCGGCAACTT | NM_031789.2 |
| 18S | TTCCGATAACGAACGAGACTCT | TGGCTGAACGCCACTTGTC | NR_046237.1 |
2.6. Quantitative Analysis of 4-HHE in Biological Samples
The 4-HHE in aorta and VSMCs was quantitatively analyzed using LC-MS/MS procedure as described previously [15,21]. Briefly, a standard solution of 4-HHE (Cayman Chemical Co., Ann Arbor, MI, USA) was used for the calibration curve. Solid-phase extraction was done using a mixed-mode anion exchange solid-phase extraction (SPE) cartridge (Oasis MAX, Waters, Milford, MA, USA). An ACQUITY CSH C18 column (Waters) was used for separating 4-HHE. Electrospray ionization (ESI) was carried out with API4000 operating in the positive ionization and SRM mode. The SRM transitions for CHD-derivatized 4-HHE were m/z 284-216.
2.7. MTT Assay for Cell Viability
Rat VSMCs were seeded on 24-well plates. To determine the cell toxicity of DHA, EPA and 4-HHE, confluent cells were exposed to these reagents for 24 h, and then washed with phosphate-buffered saline (PBS). Cell viability was determined by the conventional MTT assay as previously described [11]. The absorbance of BSA-treated cells was used as the control.
2.8. Reactive Oxygen Species (ROS) Measurement Assay
Intracellular ROS production was determined using the fluorescent probe H2DCFDA in VSMCs incubated with 20 µM H2DCFDA for 20 min as previously described [11]. Following washing with PBS, cells were incubated with 50 µM DHA or 50 µM EPA. The fluorescence emitted from the cells was recorded immediately at 492 nm (excitation) and 525 nm (emission) using a fluorescent microplate reader (Tecan, Männedorf, Switzerland) over a 2-h period.
2.9. Western Blot Analysis
Total protein samples from VSMCs were prepared as previously descried [11], and were resolved by SDS-PAGE before being transferred to PVDF membranes. Membranes were incubated with antibodies against p38, ERK, JNK, their phosphorylated forms, caspase-3, or β-actin. Blots were then incubated with horseradish peroxidase-linked second antibody (Amersham, Buckinghamshire, UK), followed by chemiluminescence detection (PerkinElmer, Waltham, MA, USA).
2.10. Statistical Analysis
Data are presented as mean ± SE, unless otherwise stated. Differences between more than three groups were analyzed by Tukey–Kramer test. When two groups were compared, differences were analyzed by two-tailed Student’s t-test. P < 0.05 was considered statistically significant.
3. Results
3.1. Docosahexaenoic Acid (DHA)—Though Not Eicosapentaenoic Acid (EPA)—Inhibits Mcp-1 mRNA Expression in Rat Aorta
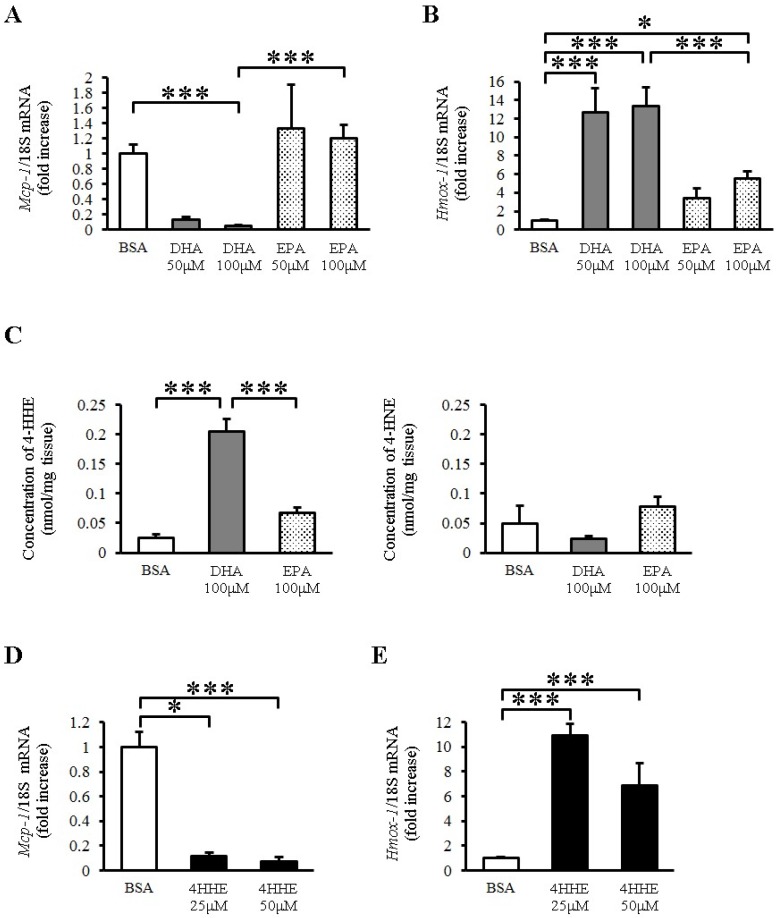
To explore the direct effects of EPA and DHA on vessels, we examined the expression of Mcp-1 mRNA in rat arterial strips. DHA (50–100 μM) but not EPA (50–100 μM) almost completely inhibited the expression of Mcp-1 mRNA compared with BSA (Figure 1A). In contrast, DHA increased the expression of heme oxygenase 1 (Hmox-1) (Figure 1B), which is a known antioxidative gene in vessels. EPA also increased the expression of Hmox-1, but to a lesser extent than DHA did (Figure 1B). Because Hmox-1 is a target gene of the Keap1-Nrf2 pathway, we measured the lipid peroxidation product levels in rat arterial strips by LC-MS/MS with or without n-3 PUFA incubation. We found that DHA but not EPA increased the tissue 4-HHE content, whereas it did not change the content of 4-hydroxy 2-noneral (4-HNE), a lipid peroxidation product derived from n-6 PUFA (Figure 1C). To test the role of 4-HHE, we exposed the arterial strips to 4-HHE and found that it inhibited the expression of Mcp-1 (Figure 1D) and increased that of Hmox-1 (Figure 1E) in rat aortic strips, suggesting that DHA regulates Mcp-1 and Hmox-1 expression through 4-HHE.
Figure 1.
Docosahexaenoic acid (DHA)-derived DHA generated 4-hydroxy hexenal (4-HHE) inhibits the expression of Mcp-1 Messenger RNA (mRNA), but induces heme oxygenase 1 (Hmox-1) mRNA in rat aorta. Rat arterial strips were treated with bovine serum albumin (BSA), DHA (50–100 μM), EPA (50–100 μM) or 4-HHE (25–50 μM) for 6 h under ex vivo conditions. (A,B) Relative mRNA expression of Mcp-1 (A) and Hmox-1 (B) in arterial strips was quantitated using the real-time quantitative polymerase chain reaction (RT-qPCR). Results were normalized against 18S rRNA and expressed as fold increase over control. (C) 4-HHE and 4-HNE content were measured by a liquid chromatography-tandem mass spectrometry (LC-MS/MS). (D,E) Relative mRNA expression of Mcp-1 (D) and Hmox-1 (E) in arterial strips was quantitated using RT-qPCR. Results were normalized as above. Results are expressed as mean ± SE of 4–8 animals (n = 3–22; A,B,D,E), or a single experiment (n = 3; C). * P < 0.05, *** P < 0.001, compared with BSA control. NS, no significant difference.
3.2. Paradoxical Increase in Mcp-1 by DHA, EPA and 4-HHE in VSMCs
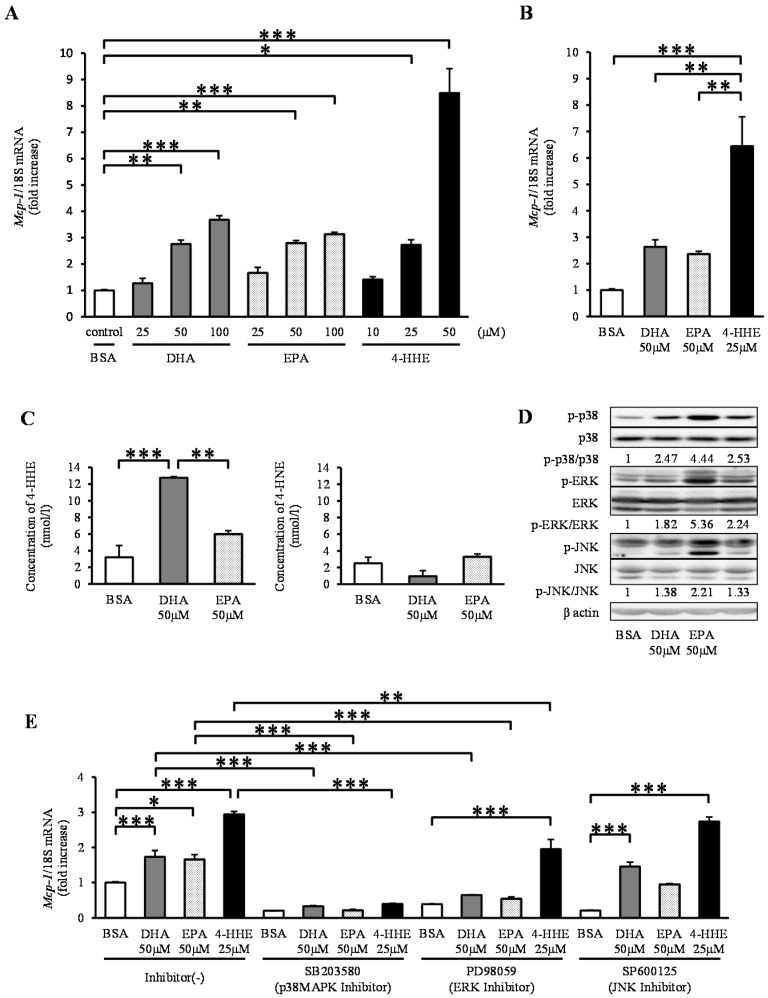
In contrast to the results observed for arterial strips, DHA, EPA and 4-HHE increased the expression of Mcp-1 mRNA in a dose-dependent manner in rat VSMCs (Figure 2A). To clarify the differences in Mcp-1 responses between rat arterial strips and VSMCs (Passage 4–12), we performed the same experiment using primary VSMCs (Passage 1). Similar to VSMCs (Passage 4–12), DHA, EPA, and 4-HHE increased the expression of Mcp-1 in primary VSMCs (Figure 2B). Similar to rat arterial strips, DHA (50 μM), but not EPA (50 μM), increased the content of 4-HHE in VSMCs (Figure 2C), whereas it did not change the 4-HNE content.
Figure 2.
DHA, EPA, and 4-HHE induce Mcp-1 expression through the p38 mitogen-activated protein kinase (MAPK) pathway in VSMCs. VSMCs (Passage 4–12) were treated with the indicated reagent for 6 h (A). (B) Primary vessels and vascular smooth muscle cells (VSMCs) (Passage 1) were treated with BSA, DHA (50 μM), EPA (50 μM) or 4-HHE (25 μM) for 6 h. Relative mRNA expression of Mcp-1 was quantitated using RT-qPCR. The results were normalized against 18S rRNA and expressed as fold increase over control. (C) 4-HHE and 4-HNE content in VSMCs were measured using LC-MS/MS. (D) p38, ERK, JNK and their phosphorylated forms, and β-actin were determined by Western blotting. DHA (50 μM), EPA (50 μM) or 4-HHE (25 μM) were added for 10 min. (E) Pretreatment with p38 kinase inhibitor (SB203580; 10 μM), ERK inhibitor (PD98059; 25 μM) or JNK inhibitor (SP600125; 10 μM) was performed for 30 min before BSA, DHA, EPA or 4-HHE incubation. The results were normalized against 18S rRNA and expressed as fold increase over corresponding control. (A) Values represent the mean ± SE of four independent experiments (n = 9); (B) a single experiment (n = 3); (C) a single experiment (n = 3); or (E) three independent experiments (n = 3–9). * P < 0.05, ** P < 0.01, *** P < 0.001, compared with corresponding control.
Because n-3 PUFAs are known activators of the mitogen-activated protein kinase (MAPK) family, we assessed the phosphorylation levels of p38 kinase, ERK and JNK. DHA, EPA and 4-HHE increased the phosphorylation levels of p38, ERK and JNK (Figure 2D). To understand the effect of the MAPK family on the Mcp-1 expression, we tested the effect of MAPK inhibitors on DHA-, EPA- or 4-HHE-induced Mcp-1 expression. Pre-incubation with the p38 kinase inhibitor SB203580 completely suppressed the induction of Mcp-1 expression (Figure 2E). The ERK inhibitor PD98059 had a partial inhibitory effect on Mcp-1 expression, whereas the JNK inhibitor SP600125 did not (Figure 2E).
3.3. 4-HHE Derived from DHA Induces Mcp-1 Expression through the Nrf2 Pathway in Human Vascular Smooth Muscle Cells (VSMCs)
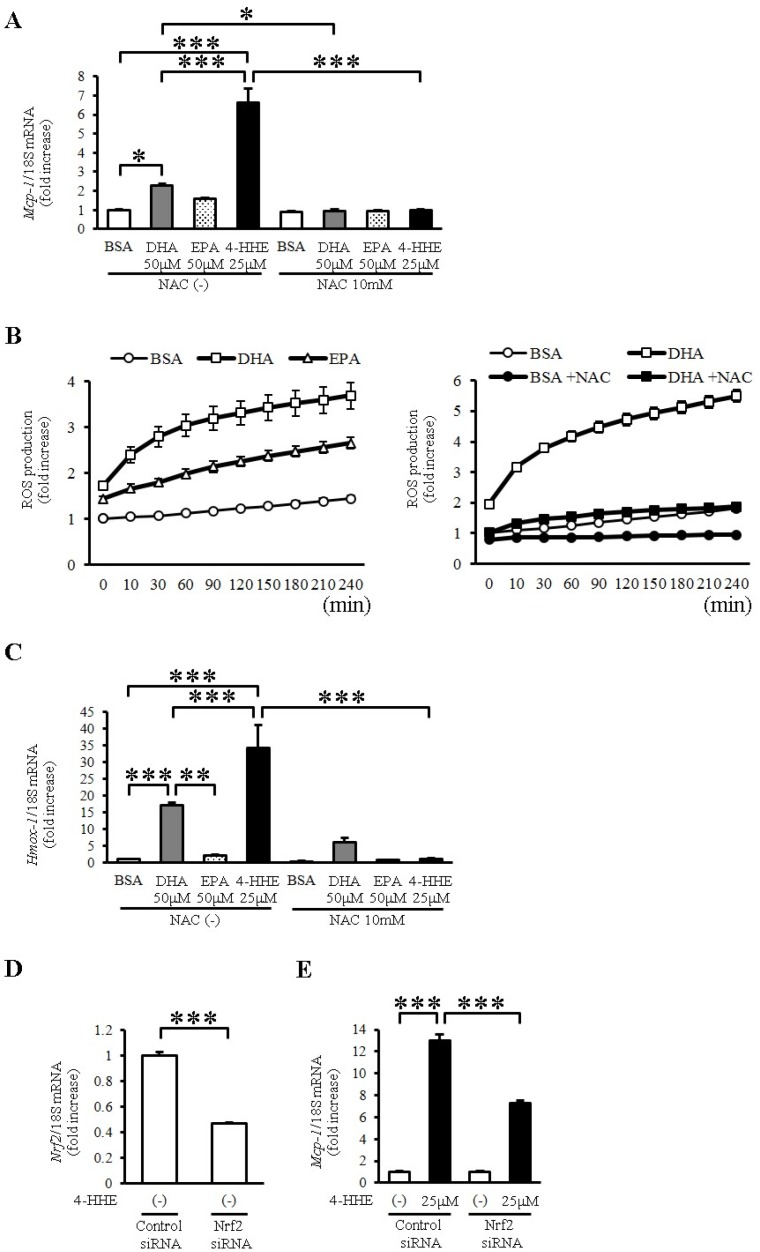
To evaluate the oxidative stress induced by DHA and 4-HHE, we used N-acetyl-l-cysteine (NAC), a known antioxidant that mimics glutathione. Pretreatment with NAC (10 mM) completely inhibited the DHA-, EPA- and 4-HHE-induced Mcp-1 expression (Figure 3A). Furthermore, DHA and EPA increased ROS production measured by H2DCFDA (Figure 3B). NAC pretreatment completely inhibited the DHA-induced ROS production in VSMCs (Figure 3B), supporting the role of oxidative stress in the DHA-induced Mcp-1 expression.
Figure 3.
DHA-derived 4-HHE induces Mcp-1 expression partially through the oxidative stress-induced Nrf2 pathway in VSMCs. (A,C) VSMCs were treated with N-acetyl-l-cysteine (NAC; 10 mM) for 1 h before incubation with BSA, DHA (50 μM), EPA (50 μM) or 4-HHE (25 μM) for 6 h. Relative mRNA expression of Mcp-1 (A) and Hmox-1 (C) in VSMCs was quantitated using RT-qPCR. Results were normalized against 18S rRNA and expressed as fold increase over control. (B) Reactive oxygen species (ROS) production was measured by 2′7′-Dichlorodihydrofluorescein diacetate (H2DCFDA). BSA, DHA (50 μM) or EPA (50 μM) was added for 4 h (left panel). BSA or DHA (50 μM) was added with or without NAC (10 mM) for 4 h (right panel). (D,E) VSMCs were treated with Nrf2 siRNA (40 nM) or control siRNA (40 nM). After 24 h, VSMCs were treated with vehicle or 4-HHE (25 μM) for 6 h. Relative mRNA of Nrf2 (D) and Mcp-1 (E) was quantitated using RT-qPCR. Values represent the mean ± SE of three independent experiments (n = 9; A,C); a single experiment (n = 3; B); and two independent experiments (n = 6; D,E). * P < 0.05, *** P < 0.001, compared with the corresponding control.
To evaluate Nrf2 activation by DHA and 4-HHE, we examined the mRNA expression of Hmox1, a target of Nrf2, in VSMCs. We found that DHA and 4-HHE stimulated the expression of Hmox1 mRNA in VSMCs, and that NAC inhibited the DHA- and 4-HHE-induced Hmox-1 expression (Figure 3C). As expected, the 4-HHE-induced Mcp-1 expression was decreased by siRNA against Nrf2 (Figure 3D,E).
3.4. DHA Induces Apoptosis of VSMCs through 4-HHE
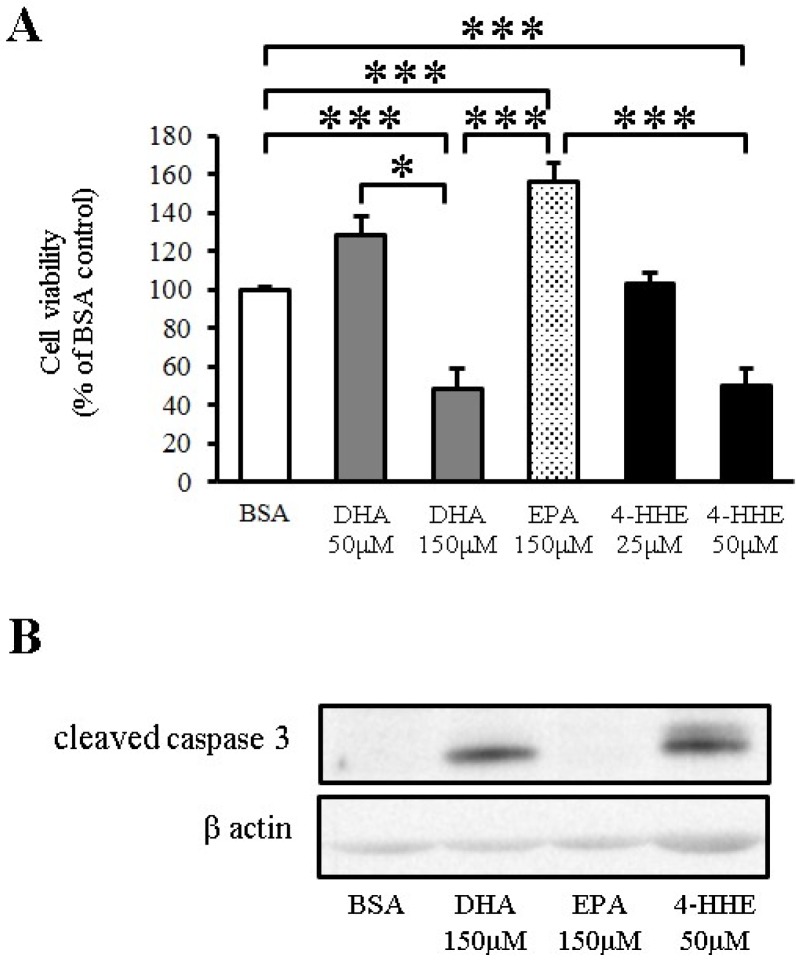
To test the toxicity of n-3 PUFA in VSMCs, VSMCs were incubated for 24 h with DHA, EPA or 4-HHE at a higher but physiological concentration, followed by measurement of cell viability by the MTT assay. DHA and 4-HHE only decreased cell viability at a high concentration (150 μM) compared with the BSA control (Figure 4A). In contrast, EPA did not decrease the cell viability of VSMCs (Figure 4A).
Figure 4.
DHA-derived 4-HHE reduces cell viability through apoptosis in VSMCs. (A) VSMCs were treated with a high concentration of DHA (150 μM), EPA (150 μM) or 4-HHE (50 μM) for 24 h. Cell viability was determined by the MTT assay. Values are expressed as percentage of cell survival, and each value represents the mean ± SE of five experiments (n = 15). (B) Cleaved caspase-3 and β-actin were determined by Western blotting. DHA (150 μM), EPA (150 μM) or 4-HHE (50 μM) were added for 6 h. * P <0.05, *** P < 0.001, compared with BSA control.
Apoptosis is a known downstream process of ROS production. Increased cleaved caspase-3 expression measured by Western blot analysis indicated that the cell toxicity of DHA and 4-HHE was caused by the induction of apoptosis (Figure 4B).
4. Discussion
Our study has three important findings. First, DHA and 4-HHE, but not EPA, inhibited Mcp-1 expression in rat arterial strips. Second, DHA and 4-HHE inhibited cell survival by promoting apoptosis in VSMCs. Third, DHA, EPA and 4-HHE stimulated Mcp-1 expression via oxidative stress, p38 and the Keap1-Nrf2 pathway in VSMCs.
DHA and 4-HHE, but not EPA, inhibited Mcp-1 expression in rat arterial strips. Previous studies have shown that n-3 PUFAs affect inflammation and plaque stability [22,23], which is consistent with the inhibitory effect of DHA on Mcp-1 expression under ex vivo conditions in our study. In contrast, EPA had almost no effect on Mcp-1 expression (Figure 1A). We assume this difference was the result of Nrf2 activation by 4-HHE, because we observed a similar difference between DHA and EPA in HUVECs, which was explained by the generation of 4-HHE [10,11,14,15,16]. As expected, DHA preferentially increased intracellular 4-HHE content in rat arterial strips compared with EPA (Figure 1C). In addition, 4-HHE directly inhibited Mcp-1 expression in rat arterial strips (Figure 1D). Although the molecular mechanism underlying this phenomenon is not clear, 4-HHE may be a mediator of the anti-inflammatory effect of DHA.
We also found that DHA and 4-HHE at a higher concentration and longer incubation inhibited cell survival by promoting apoptosis in VSMCs. In agreement with our study, previous reports have shown that DHA induced apoptosis in VSMCs or cancer cells through p38 MAPK activation at 24 hours [24,25]. Another report demonstrated that 4-HHE induced cytotoxic and negative effects on YPEN-1 prostatic endothelial cells at 24 hours [26]. Because migration of transformed VSMCs is one of the main features of atherosclerosis [27], 4-HHE-induced apoptosis, followed by macrophage clearance via Mcp-1 expression may be beneficial. Conversely, apoptosis in advanced plaque lesions may be detrimental. This discrepancy might explain the inconsistent effects of n-3 PUFAs on cardiovascular events in a secondary prevention study [28].
Both DHA and EPA stimulated Mcp-1 expression in VSMCs. Our preliminary data suggest that other fatty acids including palmitic and arachidonic acid also stimulate Mcp-1 expression. We speculate that this was due to oxidative stress induced by fatty acids—known as lipotoxicity [29,30]—rather than being an n-3 PUFA-specific effect. In addition to lipotoxicity, DHA preferentially degrades to 4-HHE via peroxidation. 4-HHE has an aldehyde residue that causes a Michael reaction with proteins, forming protein adducts [13]. N-acetyl-l-cysteine—a known antioxidant—protected VSMCs from 4-HHE-induced Nrf2 action through the formation of 4-HHE-NAC adducts. Nrf2 siRNA inhibited the 4-HHE-induced Mcp-1 expression, suggesting that DHA stimulated Mcp-1—at least in part—through the 4-HHE-Nrf2 pathway.
The opposite effects caused by DHA on Mcp-1 mRNA expression between arterial strips and VSMCs were observed in this study. VSMCs were cultured in a different environment as compared to arterial strips: culture media, growth factors, and monolayer. These biological factors may explain the discrepancy between arterial strips and VSMCs. Other possibilities are that endothelial cells are a major source of Mcp-1 mRNA and that endothelial cells induce smooth muscle cells to suppress Mcp-1 mRNA in response to DHA. To test this possibility, DHA-induced Mcp-1 expression were analyzed in VSMCs with the condition media from rat aortic endothelial cells, and aortic strips without endothelial cells. Our preliminary data suggest that DHA-induced Mcp-1 is not affected by endothelial cells.
DHA but not EPA produces 4-HHE in rat arterial strips and VSMCs. Previous reports from our group and others have shown that the 4-HHE content or 4-HHE adducts increased after fish oil treatment in heart, liver and other tissues [15,31,32]. Furthermore, previous reports have shown that plasma 4-HHE levels increased following supplementation with DHA or fish-based diet intervention in humans [33,34]. The reason for the difference in 4-HHE generation between DHA and EPA is not clear; hence, further experiments are necessary to elucidate this phenomenon.
Concentrations of EPA and DHA (25–150 μM) used in this study are similar to previous studies [3,11,14,16]. These concentrations are relatively low compared to the reported concentrations in human plasma (200–400 μM) [3]. As shown in Figure 4, high concentrations of DHA had a cytotoxic effect compared to high concentrations of EPA. This phenomenon was consistent with a previous study that showed DHA but not EPA had a profound growth inhibitory effect on HPV16 immortalized cells but not on normal cells [35]. In addition, DHA has strong inhibitory effects on multiple cancer cell lines [26]. We speculated that 4-HHE preferentially generated by DHA might explain the difference between DHA and EPA.
There were some limitations in this study. First, we could not identify the molecular mechanism of the DHA-induced Mcp-1 decrease in artery strips, although our data suggest that 4-HHE-induced Nrf2 activation may play a role. Second, the 4-HHE content measured was free 4-HHE. Because 4-HHE generates 4-HHE adducts—especially with glutathione—the total 4-HHE content in the tissues may be higher. We incubated VSMCs with 4-HHE at 25 μM based on its ability to stimulate Hmox1. Third, the clinical significance of the DHA-induced Mcp-1 expression is still not clear.
5. Conclusions
DHA had contrasting effects on Mcp-1 expression in vessels and VSMCs. We suggest a possible role for Nrf2 activation by DHA-derived 4-HHE. Furthermore, 4-HHE derived from DHA decreased cell viability by inducing apoptosis in VSMCs. These findings may explain the different effects of EPA and DHA on vessels.
Acknowledgments
We thank Chisato Kusunoki, Megumi Matsuo, Keiko Kosaka and Keiko Kondo for their technical help. Sources of founding: this study was funded by Shiga University of Medical Science. The Department of Medicine, Shiga University of Medical Science receives research promotion grants (Shogaku Kifukin) from Astellas Pharma, AstraZeneca, Boehringer-Mannheim, Daiichi-Sankyo, Dainippon-Sumitomo Pharma, MSD, Kowa, Sunstar, Takeda Pharmaceutical Company, Mitsubishi-Tanabe Pharma Corporation, Novartis, Novo Nordisk, Kyowa-Hakko-Kirin, Taisho-Toyama, Teijin Pharma. However, the research topics of these grants are not restricted. This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan to Y. Nishio (#23591336). This study was performed in collaboration between Shiga University of Medical Science and JCL Bioassay Corporation. F. Nakagawa is an employee of JCL Bioassay Corporation and a graduate student at Shiga University of Medical Science; however, this does not alter the authors’ adherence to all of the policies of Nutrients regarding the sharing of data and materials.
Abbreviations
Monocyte chemotactic protein 1 (Mcp-1), nuclear factor erythroid 2-related factor 2 (Nrf2), 4-hydroxy hexenal (4-HHE), polyunsaturated fatty acids (PUFAs), docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), heme oxygenase-1 (Hmox1), vascular cell adhesion protein 1 (VCAM-1), extracellular signal-regulated kinase (ERK), c-JUN N-terminal kinase (JNK), p38 mitogen-activated protein kinase (p38).
Author Contributions
H.M. and Y.N. conceived and supervised the study; K.N, K.M. and O.S. designed experiments; K.N., F.N., A.I., H.I., T.O. and M.T. performed experiments; K.N., K.M. and D.S. wrote the manuscript; S.U., T.I., A.K., T.O. and H.M. made manuscript revisions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.CIR.104.3.365. [DOI] [PubMed] [Google Scholar]
- 2.Kromhout D., Bosschieter E.B., de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N. Engl. J. Med. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 3.Iso H., Kobayashi M., Ishihara J., Sasaki S., Okada K., Kita Y., Kokubo Y., Tsugane S. Intake of fish and n-3 fatty acids and risk of coronary heart disease among Japanese: The Japan public health center-based (JPHC) study cohort I. Circulation. 2006;113:195–202. doi: 10.1161/CIRCULATIONAHA.105.581355. [DOI] [PubMed] [Google Scholar]
- 4.Yagi S., Aihara K.I., Fukuda D., Takashima A., Hara T., Hotchi J., Ise T., Yamaguchi K., Tobiume T., Iwase T., et al. Effects of docosahexaenoic acid on the endothelial function in patients with coronary artery disease. J. Atheroscler. Thromb. 2015;22:447–454. doi: 10.5551/jat.26914. [DOI] [PubMed] [Google Scholar]
- 5.Yamada H., Yoshida M., Nakano Y., Suganami T., Satoh N., Mita T., Azuma K., Itoh M., Yamamoto Y., Kamei Y., et al. In vivo and in vitro inhibition of monocyte adhesion to endothelial cells and endothelial adhesion molecules by eicosapentaenoic acid. Arterioscler. Thromb. Vasc. Biol. 2008;28:2173–2179. doi: 10.1161/ATVBAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto M., Sata M., Fukuda D., Tanaka K., Soma M., Hirata Y., Nagai R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis. 2008;197:524–533. doi: 10.1016/j.atherosclerosis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Orr A.W., Hastings N.E., Blackman B.R., Wamhoff B.R. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J. Vasc. Res. 2010;47:168–180. doi: 10.1159/000250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W., Li P., Lu W.J., Watkins S.M., Olefsky J.M. Gpr120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwab J.M., Chiang N., Arita M., Serhan C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikado A., Nishio Y., Morino K., Ugi S., Kondo H., Makino T., Kashiwagi A., Maegawa H. Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010;402:99–104. doi: 10.1016/j.bbrc.2010.09.124. [DOI] [PubMed] [Google Scholar]
- 11.Ishikado A., Morino K., Nishio Y., Nakagawa F., Mukose A., Sono Y., Yoshioka N., Kondo K., Sekine O., Yoshizaki T., et al. 4-hydroxy hexenal derived from docosahexaenoic acid protects endothelial cells via Nrf2 activation. PLoS ONE. 2013;8:e69415. doi: 10.1371/journal.pone.0069415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh K., Tong K.I., Yamamoto M. Molecular mechanism activating Nrf2-keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi N., Dinkova-Kostova A.T., Holtzclaw W.D., Kang M.I., Kobayashi A., Yamamoto M., Kensler T.W., Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusunoki C., Yang L., Yoshizaki T., Nakagawa F., Ishikado A., Kondo M., Morino K., Sekine O., Ugi S., Nishio Y., et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/Ho-1 pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2013;430:225–230. doi: 10.1016/j.bbrc.2012.10.115. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa F., Morino K., Ugi S., Ishikado A., Kondo K., Sato D., Konno S., Nemoto K., Kusunoki C., Sekine O., et al. 4-hydroxy hexenal derived from dietary n-3 polyunsaturated fatty acids induces anti-oxidative enzyme heme oxygenase-1 in multiple organs. Biochem. Biophys. Res. Commun. 2014;443:991–996. doi: 10.1016/j.bbrc.2013.12.085. [DOI] [PubMed] [Google Scholar]
- 16.Stulnig G., Frisch M.T., Crnkovic S., Stiegler P., Sereinigg M., Stacher E., Olschewski H., Olschewski A., Frank S. Docosahexaenoic acid (DHA)-induced heme oxygenase-1 attenuates cytotoxic effects of DHA in vascular smooth muscle cells. Atherosclerosis. 2013;230:406–413. doi: 10.1016/j.atherosclerosis.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y.C., Lii C.K., Wei Y.L., Li C.C., Lu C.Y., Liu K.L., Chen H.W. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-κB pathways. J. Nutr. Biochem. 2013;24:204–212. doi: 10.1016/j.jnutbio.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Okamura T., Tawa M., Geddawy A., Shimosato T., Iwasaki H., Shintaku H., Yoshida Y., Masada M., Shinozaki K., Imamura T. Effects of atorvastatin, amlodipine, and their combination on vascular dysfunction in insulin-resistant rats. J. Pharmacol. Sci. 2014;124:76–85. doi: 10.1254/jphs.13178FP. [DOI] [PubMed] [Google Scholar]
- 19.Folch J., Lees M., Stanley G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Obata T., Kashiwagi A., Maegawa H., Nishio Y., Ugi S., Hidaka H., Kikkawa R. Insulin signaling and its regulation of system A amino acid uptake in cultured rat vascular smooth muscle cells. Circ. Res. 1996;79:1167–1176. doi: 10.1161/01.RES.79.6.1167. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien-Coker I.C., Perkins G., Mallet A.I. Aldehyde analysis by high performance liquid chromatography/tandem mass spectrometry. Rapid. Commun. Mass Spectrom. 2001;15:920–928. doi: 10.1002/rcm.324. [DOI] [PubMed] [Google Scholar]
- 22.Calder P.C. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol. Nutr. Food Res. 2012;56:1073–1080. doi: 10.1002/mnfr.201100710. [DOI] [PubMed] [Google Scholar]
- 23.Thies F., Garry J.M., Yaqoob P., Rerkasem K., Williams J., Shearman C.P., Gallagher P.J., Calder P.C., Grimble R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 24.Jeong S., Jing K., Kim N., Shin S., Kim S., Song K.S., Heo J.Y., Park J.H., Seo K.S., Han J., et al. Docosahexaenoic acid-induced apoptosis is mediated by activation of mitogen-activated protein kinases in human cancer cells. BMC Cancer. 2014;14:481. doi: 10.1186/1471-2407-14-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diep Q.N., Touyz R.M., Schiffrin E.L. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension. 2000;36:851–855. doi: 10.1161/01.HYP.36.5.851. [DOI] [PubMed] [Google Scholar]
- 26.Lee J.Y., Je J.H., Kim D.H., Chung S.W., Zou Y., Kim N.D., Yoo M. A., Suck Baik H., Yu B.P., Chung H.Y. Induction of endothelial apoptosis by 4-hydroxyhexenal. Eur. J. Biochem. 2004;271:1339–1347. doi: 10.1111/j.1432-1033.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- 27.Yoo A.R., Koh S.H., Cho G.W., Kim S.H. Inhibitory effects of cilostazol on proliferation of vascular smooth muscle cells (VSMCs) through suppression of the ERK1/2 pathway. J. Atheroscler. Thromb. 2010;17:1009–1018. doi: 10.5551/jat.4309. [DOI] [PubMed] [Google Scholar]
- 28.Kwak S.M., Myung S.K., Lee Y.J., Seo H.G., the Korean Meta-analysis Study Group Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double-blind, placebo-controlled trials. Arch. Intern. Med. 2012;172:686–694. doi: 10.1001/archinternmed.2012.262. [DOI] [PubMed] [Google Scholar]
- 29.Dong X., Bi L., He S., Meng G., Wei B., Jia S., Liu J. FFAs-ROS-ERK/P38 pathway plays a key role in adipocyte lipotoxicity on osteoblasts in co-culture. Biochimie. 2014;101:123–131. doi: 10.1016/j.biochi.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L., Cai X., Han X., Ji L. P38 plays an important role in glucolipotoxicity-induced apoptosis in INS-1 cells. J. Diabetes Res. 2014;2014 doi: 10.1155/2014/834528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson E.J., Thayne K., Harris M., Carraway K., Shaikh S.R. Aldehyde stress and up-regulation of Nrf2-mediated antioxidant systems accompany functional adaptations in cardiac mitochondria from mice fed n-3 polyunsaturated fatty acids. Biochem. J. 2012;441:359–366. doi: 10.1042/BJ20110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gladine C., Roy N.C., Rigaudière J.P., Laillet B., da Silva G., Joly C., Pujos-Guillot E., Morio B., Feillet-Coudray C., McNabb W.C., et al. Increasing intake of long-chain n-3 PUFA enhances lipoperoxidation and modulates hepatic gene expression in a dose-dependent manner. Br. J. Nutr. 2012;107:1254–1273. doi: 10.1017/S0007114511004259. [DOI] [PubMed] [Google Scholar]
- 33.Calzada C., Colas R., Guillot N., Guichardant M., Laville M., Véricel E., Lagarde M. Subgram daily supplementation with docosahexaenoic acid protects low-density lipoproteins from oxidation in healthy men. Atherosclerosis. 2010;208:467–472. doi: 10.1016/j.atherosclerosis.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 34.Kondo K., Morino K., Nishio Y., Kondo M., Nakao K., Nakagawa F., Ishikado A., Sekine O., Yoshizaki T., Kashiwagi A., et al. A fish-based diet intervention improves endothelial function in postmenopausal women with type 2 diabetes mellitus: A randomized crossover trial. Metabolism. 2014;63:930–940. doi: 10.1016/j.metabol.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Chen D.Z., Auborn K. Fish oil constituent docosahexa-enoic acid selectively inhibits growth of human papillomavirus immortalized keratinocytes. Carcinogenesis. 1999;20:249–254. doi: 10.1093/carcin/20.2.249. [DOI] [PubMed] [Google Scholar]