Abstract
Studies over the last two decades have revealed profound immunomodulatory aspects of vitamin D on various aspects of the immune system. This review will provide an overview of Vitamin D metabolism, a description of dendritic cell subsets, and highlight recent advances on the effects of vitamin D on dendritic cell function, maturation, cytokine production and antigen presentation. The active form of vitamin D, 1,25(OH)2D3, has important immunoregulatory and anti-inflammatory effects. Specifically, the 1,25(OH)2D3-Vitamin D3 complex can affect the maturation and migration of many dendritic cell subsets, conferring a special immunoregulatory role as well as tolerogenic properties affecting cytokine and chemokine production. Furthermore, there have been many recent studies demonstrating the effects of Vitamin D on allergic disease and autoimmunity. A clear understanding of the effects of the various forms of Vitamin D will provide new opportunities to improve human health.
Keywords: vitamin D, vitamin D receptor, dendritic cells, innate and adaptive immunity, interleukins, cytokines, inflammation
1. Overview of Vitamin D Metabolism
Vitamin D plays a key role in maintaining mineral homeostasis. However, over the last several years, non-classic actions of vitamin D have been described. There are two main sources of vitamin D, including dietary intake and its synthesis in the skin exposed to sunlight [1]. During sunlight exposure, 7-dehydrocholesterol (7-DHC) in the skin is converted to the previtamin precholecalciferol that is then converted into activated 7-dehydrocholesterol or vitamin D3 [2,3]. Dietary or cutaneous vitamin D has to undergo two metabolic modifications in the liver and kidney to be converted into the bio-active form [4]. Vitamin D3 is transported to the liver where it undergoes hydroxylation by the enzyme 25-hydroxylase encoded by the cytochrome P450 (CYP) isoform family 2, subfamily R, polypeptide 1 (CYP2R1), but this reaction can also be mediated by other CYP isoforms including CYP27A1, CYP3A4 and CYP2J3, which results in the formation of 25-hydroxyvitamin D (25(OH)D) [5,6,7]. 25(OH)D has a very long half-life of several weeks and also is one of the major circulating metabolites, which is used to measure vitamin D status in humans [2]. The second step in metabolism is mainly in the kidneys, in which the 1α-hydroxylation (mediated by CYP27B1) occurs and is stimulated by the calcium/phosphorus regulatory hormone, parathyroid hormone (PTH) [1]. Conversion by CYP27B1 generates the most active metabolite, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) [2,8]. 1,25(OH)2D3 strongly induces gene expression of CYP24A1 to produce the enzyme 25-Hydroxyvitamin D3-24-hydroxylase that initiates catabolic degradation, resulting in the formation of 1,24,25(OH)3vitamin D3 and ultimately in the formation of 1α-hydroxy-23-carboxy-24,25,26,27-tetranorvitamin D3 [9]. This enzyme also promotes the formation of 24,25(OH)2 vitamin D3 via negative feedback by decreasing the 25(OH)D substrate available for 1α hydroxylation [9,10]. 1,25(OH)2D3 has different functions including regulation of intestinal calcium and phosphate absorption, calcium mobilization from bone, and reabsorption of calcium in the kidney. It also has different immune effects in the body [1,11]. 1,25(OH)2D3 binds to the vitamin D receptor (VDR), which is a member of the superfamily of nuclear receptors for steroid hormones [12,13,14]. The VDR complex can interact with different gene transcription factors leading to both activation and repression of genes that control inflammatory responses [15,16]. VDR can be activated by nanomolar concentrations of a ligand [17]. The nuclear receptors for the steroid hormones estradiol (ERα and ERβ), androgen receptor (AR), progesterone receptor (PR), glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) also share this property, as well as for the vitamin A derivative all-trans retinoic acid receptors (RARα, RARβ and RARγ) and for the thyroid hormone triiodothyronine (TRα and TRβ) [18,19]. VDR binding can also be facilitated by the transcription factor activator protein 1 (AP1) [20]. Other transcription factors including Forkheadbox protein A1 (FOXA1) or the hematopoetic transcription factor PU.1 encoded by the Spi-1 proto-oncogene (SPI-1) can act as pioneer factors for the VDR [18]. VDR agonists can act as an immunosuppressive molecule that can promote the intrinsic tolerogenic capacity of dendritic cells (DCs) in mouse and humans [21,22]. Given the evidence that VDR is expressed in many immune cells, including monocytes/macrophages, B and T cells [10,23,24,25,26] as well as DCs, along with the ability of DCs to produce 1,25(OH)2D3 [25], this review will focus on the function of VDR in dendritic cells.
2. Dendritic Cell Subsets
DCs are replenished from bone marrow (BM) precursors, but may also arise from blood monocytes under inflammatory conditions [27]. They play a critical role in the cellular immune response to self and foreign antigens and have a central role in the orchestration of the regulatory elements of immune homeostasis [28,29]. Dendritic cells specialize in capturing, processing, and presenting antigens to the adaptive immune system. Dendritic cells express lymphocyte co-stimulatory molecules, then migrate to lymphoid organs and secrete cytokines for the regulation of immune responses. Furthermore, DCs are important in the development of immunological memory and tolerance [27,30]. In the context of infection or exposure to non-self antigens, these cells can recognize both pathogen-associated molecular patterns (PAMPs), as well as cellular damage via pattern recognition receptors (PRRs). Activation of these receptors on DCs results in increased expression of antigen presentation machinery including the major histocompatibility complex type II (MHC-II) proteins, as well as co-stimulatory molecules [31,32,33,34]. This signaling allows for efficient antigen presentation to T cells followed by promotion and proliferation of distinct T helper (Th) cell subsets [31,32,33,34].
In mice and humans, DCs can be sub-classified based on morphology, origin, function and anatomical location [28,35,36]. Resident DCs are localized in lymphoid tissue (LT), where antigen uptake occurs from the lymph and bloodstream and they present it to local naïve T cells [36,37]. Non-lymphoid tissue (NLT) DCs, constitute cells that reside in tissues, then migrate to the lymph nodes and present antigens derived from mucosal sites to T cells [36]. Dendritic cell populations in the peripheral blood of humans have also been identified based on the human leukocyte antigen-D related (HLA-DR)+ lineage found on their surface marker expression [36,38,39]. Studies on human peripheral blood analyzed the transcriptome of classical and non-classical monocytes (CD14+CD16− and CD14+CD16+, respectively) against DCs defined as HLA-DR+ positive and negative for markers of other leukocyte lineages [39]. They found that the DCs clustered into three distinct populations with expression profiles clearly unique from both monocyte populations [40]. These DCs have been further classified as Plasmacytoid DCs (pDCs) and two subsets of myeloid DCs (mDCs). In humans, plasmacytoid DCs circulate in the blood and lymph node (LN) compartments and are characterized by CD123 interleukin-3 receptor (IL-3R), CD303 (BDCA-2), and CD304 (BDCA-4 or Neuropilin-1) expression [28]. The two myeloid DC (mDC) subsets are also referred to as conventional DCs (cDCs) and are identified by their surface markers: CD1c+/BDCA-1+ (CD1c+ cDC) or CD141+/BDCA-3+ (CD141+ cDC). The total blood DC population consists of about 5%–10% CD141+ cDCs, and the rest divided equally among pDCs and CD1c+ cDCs [28,36,41]. These subsets can also be found in the spleen and tonsils, however, it has not been reported in humans if there are differences in VDR expression among these subsets [42]. It has been demonstrated that some human DC subsets are also found in the mouse [36,40,43]. Comparison of the gene expression patterns using cross-presentation assays of all known human and mouse DC subsets revealed the following similarities as described in Table 1: human blood pDCs are equivalent to mouse pre-conventional DCs (pre-cDCs), CD141+ cDC are comparable to mouse CD8α+ DC, and human CD1c+ cDC are comparable to mouse CD11b+ DC [36,40,43,44,45]. Human analysis of NLT DCs in the skin, lung, and liver identified two cDCs subsets identical to CD1c+ and CD141+ blood cDCs. Furthermore, this study showed that pDCs were absent in skin, lung and liver in humans under steady-state conditions [46,47]. Transcriptome analysis comparing human and mouse DCs found that human CD1c+ and CD141+ tissue-resident DCs correspond to mouse NLT DCs, CD11b+ and CD103+ DCs, respectively [46,47].
Table 1.
DC development, subsets and lineage-specific markers.
| Dendritic cells | Location | Human | Mice | ||
|---|---|---|---|---|---|
| Alternative Subset Name | Surface Markers | Alternative Subset Name | Surface Markers | ||
| Conventional DCs (cDCs) | Myeloid (Blood) | CD1c/BDCA-1+ | CD1c+, CD11c+++, CX3CR1+, CD172a+, CD64+ | CD11b+/CD103+ | CD103 integrin marker (aE b7), IRF8 |
| CD141+/BDCA-3+ | CD141+, CD11c+++, CLE9A+, XCR1+, BDCA-3+ | CD8+ | CD8α+, NECL2 (CADM1), CLE9A, BATF3, XCR1 | ||
| Non-lymphoid tissue (NLT) skin, liver, lung and intestine |
CD1c+ | CD11c++, CD1c+, CD172a+, CD11b+, CD206+, CD64+, Lower expression of FLT3 and CLEC9A and intermediatelevels of M-CSFR and CX3CR1, compared with CD141+ | CD11b+/CD103+ | CD11c++, CD11b++, CD103 integrin marker (aEb7), CD24++, CD209a+, IRF8 | |
| CD141+/CLEC9A+ | CD11c+, XCR1+, TLR3, CLEC9+, CD141+, CADM1, CCR7 | CD11b+/CD103 | CD11c++, CD103 integrin marker (aEb7), CD24+, XCR1+, IRF8 | ||
| CD141+ | CD11c++, CD141+, CX3CR1+, CD1c+, CD172a+, CD11b+, CD206+, CD14+ | ||||
| Lymphoid tissue (LT) | CD141+ | CD141+, CD11c++, CLEC9A+, XCR1+ | CD11b+ | CD4, Endothelial cell-selective adhesion molecule (ESAM), EB12 | |
| CD1c+ | CD1c+, CD11c+++ | CD8a- | CD11c++, CD11b++, CD4, SIRPa+, DCAL2, Clec 12a, CD209a+, F4/80+ | ||
| CD11c+ | CD123 (IL-3R), CD303 (BDCA-2), CD304 (BDCA-4 or Neuropilin-1) | CD8a+ | CD8++, CD11c++, CD11b++, CD103+, CD86+, CD24+, Xcr1+, TLR3, T. gondii sensor, TLR11 | ||
| Plasmacytoid (pDcs) | Blood and lymph node (LN) | CD11c+ | CD123 (IL-3R), CD303 (BDCA-2), CD304 (BDCA-4 or Neuropilin-1) | Pre-conventional DCs (pre-cDCs) | PDCA-1 |
| Langerhans cells (LCs) | Epidermal | CD1a | Langerin (CD207+), CD11c+, BDCA1+, CD172a+, CD11b+, CD1a++, E-cadherin+, CD326+, XCR1, CSF1R | LCs | CD11c+, Langerin (CD207+), XCR1+ |
| DCs | Dermal | CD1a+ CD14− | CD1a+ CD14− | CD103+CD207+ | IRF8, ID2, BATF3, CLEC9A, XCR1 |
| CD103+CD207+ | |||||
| CD14+ | M-CSFR, CX3CR1, CD209 (DC-SIGN) | CD207− CD11b+ | |||
| CD207−, CD11b−, CD103− | |||||
DCs can also reside in the dermis of human skin and represent a large subset of dermal DCs involved in tissue homeostasis [48]. The human skin has three main cutaneous DC populations: epidermal Langerhans cells (LCs), CD1a+CD14− dermal DCs and CD14+ dermal DCs as shown in Table 1 [48,49]. In humans, LCs highly express the non-classical MHC class I molecule CD1a [50,51]. CD14+ dermal DCs express a prominent “mixed” DC/macrophage phenotype [46]. CD14+ dermal DCs express low levels of CD80 and CD86 and are poor inducers of naïve T-cell proliferation [52,53], however they can efficiently take up antigen [54] and they can induce CD25+ T regulatory cells (Tregs) through production of interleukin-10 (IL-10) [55]. CD141+ mDCs are less immunogenic and may be able to differentiate into the Langerhans cells of the skin in response to transforming growth factor β (TGF-β) [56,57]. In vitro, human CD141+ dermal DCs are efficient at cross-presenting soluble antigens as compared to other interstitial DCs and epidermal LCs.
In mice, mature DCs show a high level of expression of MHC II and the co-stimulatory molecules CD80 and CD86 and induce differentiation of naive CD4+ T cells, while immature DCs with low expression of these molecules are more endocytic and efficient at antigen processing [58,59]. mDCs produce high levels of interleukin-12 (IL-12), whereas pDCs have the ability to quickly produce high levels of type I interferons-α (IFN-α) [36,60] in response to viral infections in humans [61]. In response to bacterial and viral stimulation, human pDCs and mDCs produce different patterns of chemokines [27,62]. mDCs preferentially produce very high levels of the chemokine ligand 17 (CCL17) and chemokine ligand 22 (CCL22), whereas pDCs show minimal production of these chemokines [15]. pDCs can produce the pro-inflammatory chemokine ligand 3 (CCL3), whereas chemokine ligand 4 (CCL4) and chemokine ligand 8 (CCL8) can be produced by both subsets [27,62]. pDCs express endosomal toll-like receptors (TLR) 7, 8, and 9 which are able to detect nucleic acids derived from viruses, bacteria, and unmethylated CpG sequences in DNA molecules respectively. In humans, activation of TLR7 or TLR9 triggers a signaling cascade and upregulates the expression of interferon-α (IFN-α), interferon-β (IFN-β) [63] and interferon-λ (IFN-λ) [64]. pDCs contribute to the rapid and large amount of type I IFN production in response to viral infection and are critical in anti-viral immmunity [65].
3. Effects of Vitamin D on DC Function
3.1. DCs Maturation–Co-Stimulation
The modifications in phenotype as well as the functional plasticity of DCs varies through multiple signal codes that are generated by different stimuli in humans [66]. There are two major phases in the life of DCs, an immature stage, which is highly effective in terms of antigen uptake and processing, and the mature stage where the antigen uptake capacity is lost and the cell migrates toward regional lymph nodes, shifting the function to become a potent antigen-presenting cell (APC) [67].
1,25(OH)2D3 and VDR can regulate human DC maturation [68]. Exposure of differentiating human and mouse monocytes to 1,25(OH)2D3 increased the expression of molecules involved in antigen capture and inhibited DC differentiation and maturation, which impaired stimulatory capacity for the antigen specific CD8 T cells [29,69,70,71,72,73]. Furthermore, they stimulate an increase in the number of Tregs, directly affect CD4+ T cells, up-regulate IL-10, as well as reduce tumor necrosis factor-α (TNF-α) and interferon γ (IFN-γ) levels [29,69,70,71,72,73,74]. These molecular changes may play a role in the inhibition and interaction between DCs and T cells in mice and humans [70].
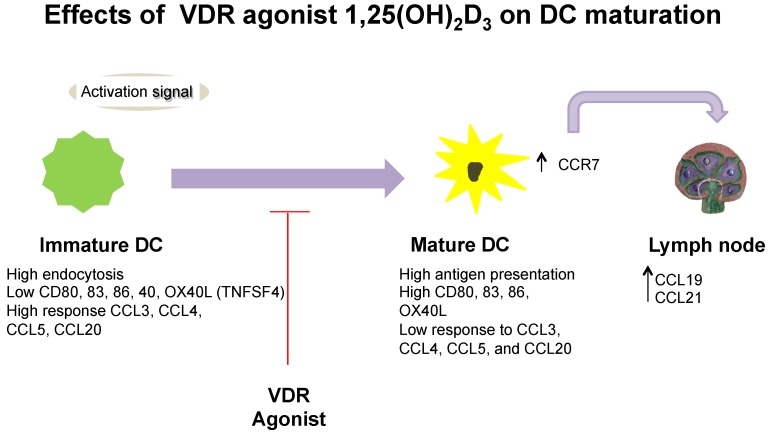
In vivo and in vitro experiments have shown that 1,25(OH)2D3 can induce mouse and human mDCs that have a tolerogenic phenotype, characterized by decreased CD40, CD80, and CD86, low interleukin-12 (IL-12), and enhanced IL-10 secretion, as shown in Figure 1 [69,75]. Specifically, immature monocyte-derived DCs were generated from buffy coat monocytes, activated by lipopolysaccharide (LPS) and stimulated with 1,25(OH)2D3, which resulted in the inhibition of pro-inflammatory cytokines such as the heterodimeric molecule interleukin-12p70 (IL-12p70) in both humans and mice [69,72]. The intrinsic production or exogenous stimulation by 1,25(OH)2D3 can arrest the differentiation and inhibit maturation of mDCs, resulting in the decreased expression of maturation markers CD40, CD80, CD86 and retention of antigen uptake, as shown in Figure 1 [29,72,76,77]. The expression of maturation markers CD40, CD80 and CD86 were inhibited, along with decreased IL-12 and upregulation of IL-10 production after mDCs were stimulated with 1,25(OH)2D3, and furthermore, 1,25(OH)2D3 stimulation led to decreased activation of CD4+ T cells in humans [21] and an increase in iTreg cells in mice [78]. Using genetic approaches to study VDR function, VDR-deficient mice compared with wild-type mice were found to demonstrate subcutaneous lymph node hypertrophy with an increase in mature DCs [76]. In addition to these effects, 1,25(OH)2D3 has marked effects by suppressing chemokines CCL17 and CCL22 in human mDCs, as shown in Figure 1 [79,80]. There are two pathways that can explain the anti-inflammatory effects of 1,25(OH)2D3 in mDCs [16,81]. In early inflammation, 1,25(OH)2D3 has a primary and direct effect on the up-regulation of the chemokine ligand (CXCL) gene expression via direct binding of VDR to the CXCL cluster locus in humans [16]. For the latter phase of inflammation, the secondary effect may demonstrate an overreactive inflammatory response that is controlled by 1,25(OH)2D3 via the repression of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [16,81]. In mice, DCs can be mobilized from the skin to the draining LN in response to a subcutaneous latex microsphere injection, but when 1,25(OH)2D3 is added to the microsphere inoculum, the DCs bypassed the draining LN and enter non-draining secondary lymphoid organs, including Peyer’s patches [82]. Furthermore, measurement by surface phenotype of the microsphere in human and murine myeloid/conventional DCs exposed to 1,25(OH)2D3 showed reduced chemotaxis toward a chemokine receptor 7 (CCR7) and chemokine ligand 21 (CCL21) [80,83], which are required for DC emigration from inflamed tissues to draining lymph nodes [79,80,83]. A similar effect has been observed in LCs where stimulation with 1,25(OH)2D3 decreased the chemotaxis of LCs towards the chemokine ligand 21 (CCL21), likely due to the inhibition of CCR7 expression, as shown in Figure 1 [84].
Figure 1.
Overview of vitamin D on dendritic cell (DC) function.
Immunoglobulin-like transcript 3 (ILT3) expression by DCs is required to induce CD4+Foxp3+ regulatory T cells [77,80,85]. One study found that 1,25(OH)2D3 was able to induce up-regulation of ILT3 expression on immature and mature human DCs [85]. Furthermore, NF-κB activity has been shown to regulate the production of IL-12, type I IFNs, CCL7, chemokine receptor 22 (CCL22), and the expression of MHC class II molecules, CD40, CD80, CD86, and ILT3 [15]. NF-κB is a regulator of the immune system, inflammatory genes and is also a target for many anti-inflammatory and immunosuppressive agents [69], including glucocorticoids and anti-inflammatory medications that bind to the same family of nuclear receptor as the VDR [86,87]. Human mDCs treated with 1,25(OH)2D3 showed decreased nuclear translocation of the p65 subunit of NF-κB, which may explain some of the anti-inflammatory effects of 1,25(OH)2D3 [80].
Type I IFN mediates and induces the differentiation of monocytes to DCs (type 1 IFN DCs). Freshly isolated monocytes treated with 1,25(OH)2D3, inhibited the generation of type 1 IFN DCs [88]. Monocytes that were freshly isolated and cultured with GM-CSF and IFN-β along with 1,25(OH)2D3 compared with control IFN-DCs, showed that IFN-DCs cultured in the presence of 1,25(OH)2D3, failed to up-regulate the differentiation marker CD1a or the maturation marker CD83 [88]. IFN-DCs also had significantly impaired functional activities. For example, IFN-DCs exhibited a potent allostimulatory capacity, while cells cultured with 1,25(OH)2D3 had limited capability to stimulate T cell proliferation [88]. Additionally, when 1,25(OH)2D3 was added to human IFN-generated DCs, these cells could not produce interleukin-1α (IL-1α) and demonstrated impaired chemotaxis in response to both CCL4 and CCL19 [88,89].
As stated above, pDCs are major producers of type 1 interferon especially following viral infection [60]. 1,25(OH)2D3 treatment of pDCs resulted in no effect on T helper 1 (Th1) development or Treg activity [80]. All-trans-retinoic acid (RA) plays a critical role in maintaining intestinal immune homeostasis [90]. Experiments demonstrated that human blood CD1c+ mDCs, but not CD141+ mDCs or plasmacytoid DCs responded to 1,25(OH)2D3 by promoting the production of RA by highly expressing retinaldehyde dehydrogenase (RALDH2) mRNA and aldehyde dehydrogenase (ALDH) activity [91]. RALDH2 is an enzyme that converts retinol to retinoic acid and promotes CD4+ T cells to acquire the ability to produce T helper 2 (Th2) cytokines in an RA-dependent and an IL-4-independent manner [92]. Murine experiments also demonstrated that CD103+ DCs found in the lamina propria and mesenteric lymph nodes (MLNs) can produce RA and promote the conversion of naïve T cells to Foxp3+ T regulatory cells in the intestine, contributing to the maintenance of intestinal immune homeostasis [93].
3.2. DC Cytokine Production
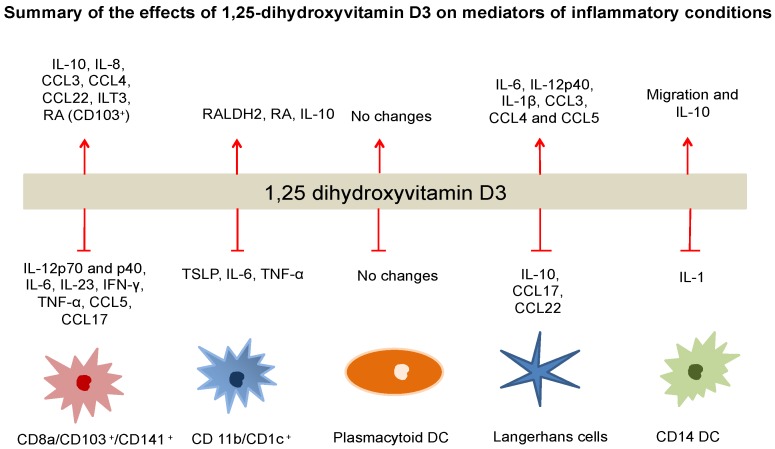
Studies have shown that DCs stimulated with 1,25(OH)2D3 showed increased IL-10 production and an inhibition of the release of pro-inflammatory cytokines including TNF-α, IFN-γ and IL-12 [74,77,94,95]. It has been demonstrated that IL-12 production in DC cultures is inhibited by intrinsic 25(OH)D conversion in humans [70]. IL-12 is important to the induction of Th1 responses via inhibition of TNF-α production [70]. The expression of CD14, which is a monocyte/macrophage marker, was increased after 1,25(OH)2D3 exposure, indicating that the developing DC differentiates towards a more macrophage-like and less antigen-presenting pathway [70]. By a functional assay, 1,25(OH)2D3 reduced the ability of DCs to induce proliferation of human tetanus toxoid (TT)-specific T lymphocytes [70]. Human blood derived mDCs exposed to 1,25(OH)2D3 showed inhibition of CD40-triggered production of both interleukin-12p70 (IL-12p70) and interleukin-12p40 (IL12-p40), but not IL-10 [96]. The resultant inhibition of the IL-12p40 chain, which heterodimerizes to form both IL-12 and IL-23, resulted in a marked reduction in the development of Th1 and T helper 17 (Th17) cells [80]. Mouse LCs exposed to 1,25(OH)2D3 demonstrated up-regulation of the production of IL-1β, CCL3, CCL4 and CCL5 [84]. 1,25(OH)2D3 stimulation also affects mDCs, by inhibiting the development of Th1 and favoring Th 2 induction, resulting in transcriptional repression of IL-2 and IFN-γ [74,75,97]. Human myeloid DCs stimulated with 1,25(OH)2D3 showed up-regulation in the expression of CCL3 and CCL4, but down-regulation of CCL5 [98,99]. LCs exposed to 1,25(OH)2D3 had suppressed production of Th 2 type chemokines, CCL17 and CCL22 upon activation through CD40 ligation, which are largely secreted during LC maturation [80]. Chemokines such as CCL22 were up-regulated, whereas the CCR4 ligand, CCL17 was down-regulated by 1,25(OH)2D3 in mDCs as shown in Figure 2 [80].
Figure 2.
The influence of 1,25(OH)2D3 on the expression of interleukins, cytokines and regulatory molecules in different DC subsets.
There have been several polymorphisms of the human VDR gene identified, specifically resulting in VDR proteins with different structures, either a long f-VDR or a shorter F-VDR [100]. Shorter VDR protein of 424 amino acids (aa) or the long isoform with 427 aa have been shown to influence IL-12 expression in DCs [100]. A study evaluating the IL-12 promoter activity in human mDCs showed that the presence of the shorter F-VDR led to an increase in the expression of NF-κB and nuclear factor of activated T-cells (NFAT)-driven transcription, as well as higher IL-12p40 promoter activity [100]. It was also found that the levels of IL-12p35, the other component of IL-12p70, were higher in antigen-presenting cells from F-VDR genotype [100].
The 1,25(OH)2D3-VDR complex may have effects in different group of cells, including DC interactions with transcription factors such as NF-κB, NFAT, or the glucocorticoid receptor (GCR) leading to anti-inflammatory effects [101,102]. Ubiquitination-mediated proteolysis of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor α (IκBα) by the 26S proteasome is a critical step in NF-κB activation [103]. In a human monocyte-like THP-1 cell line and in DCs upon direct binding of 1,25(OH)2D3 to VDR, NF-κB activation is inhibited by interactions with a specific inhibitor IκB, which allow NF-κB to remain in the cytosol [15,16]. When there is an inflammatory event that stimulates the cells, IκB gets phosphorylated, ubiquitinated, and subsequently degraded by the IκB kinase [104]. Free NF-κB translocates to the nucleus, where it initiates the transcription of pro-inflammatory cytokines and promotes apoptotic events, as well as activates enzymes involved in pro-inflammatory mediator generation such as cyclooxygenase-2 (COX-2) [105]. This cascade of inflammatory events may be affected by the repressive effects of the 1,25(OH)2D3-VDR complex on NF-κB. Six ChIP-seq data sets found 21,776 non-overlapping VDR binding sites, whereas only 54 sites were common in all six data sets. This suggests that VDR binding is cell and stimulus-specific. Only 17.5% of the non-overlapping binding sites contain a DR3-type VDRE, whereas the percentage of DR3-type response elements is enriched in highly ligand-responsive loci. These data suggest that the VDR interacts with other transcription factors and that these interactions may be only in part ligand dependent [101,105].
3.3. DCs Migration/Antigen Presentation: in Vivo Studies
A murine model of a vaccination with the Toll-like receptor 4 (TLR4) adjuvant Monophosphoryl Lipid A (MPLA) in wild-type (WT) and 1α-hydroxylase (1αOHase)-deficient mice showed that in the presence of 1,25(OH)2D3, mDCs were incapable of migrating beyond the draining LNs following vaccination [106]. These data suggest that a local production of 1,25(OH)2D3 is required for the migration of DCs beyond the draining LNs [106]. The presence of 1,25(OH)2D3 during in vitro DC maturation of immature DCs of human monocytes cultured with granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4 resulted in promotion of spontaneous DC apoptosis [77]. DCs generated from human monocytes showed decreased DC survival along with significantly lower levels of HLA-DR and CD86 [107], affecting the persistence of antigen presentation (measured by flow cytometry) an important prerequisite for proper T cell (re)activation [107]. Moreover, another study reported that DCs had a dose dependent response to 1,25(OH)2D3, where CD80 and HLA-DR were down-regulated after stimulation with a high concentration of the Vitamin D analogue TX527 (19-nor-14,20-bis-epi-23-yne-1α,25(OH)2D3) at the highest doses of 10−7 M and 10−8 M. However, this response was lost when using 1,25(OH)2D3 at a lower concentration of 10−10 M [68]. 1,25(OH)2D3 and 25(OH)D-treated LCs and dermal DCs express elevated IL-10 levels and promote the development of IL-10-producing Treg cells in humans [108]. Interestingly, 25(OH)D-treated DCs had persistent production of IL-12 that led to the development of IFN-γ-producing T cells, however vitamin D3 had no direct effect on IFN-γ production by T cells and 1,25(OH)2D3 inhibited IL-12 production [109].
Intradermal 1,25(OH)2D3 injection influences the different human skin DC subsets, CD1a(+), langerin(+) Langerhans cells, CD14(+) dermal DCs and CD1a(+) and selectively enhanced the migration of CD14(+) DCs, a subset known for the induction of tolerance [82,110]. Furthermore, intradermal 1,25(OH)2D3 repressed the LPS-induced T cell stimulatory capacity of migrating DCs in mice [110]. These migrating DCs induced T cells with suppressive activity and eliminated IFN-γ productivity and promoted the development of Foxp3(+) Tregs [111]. In addition, the postmigrational DCs, macrophage-like CD14(+)CD1a(−) DC subset showed poor T cell-stimulatory abilities [112]. Mature and immature DCs are not known for their capacity to kill ingested bacteria [113], however, the mixed DC-macrophage phenotype observed after stimulation of CD14(+)/CD1a(−) DCs with 1,25(OH)2D3, support the finding that 1,25(OH)2D3 can enhance the production of the human cathelicidin LL-37 [65,114,115].
4. Implications for Human Disease
4.1. Allergic Disease
Allergic bronchopulmonary aspergillosis (ABPA) is caused by a Th2 immune response to antigens derived from Aspergillus fumigatus (A. fumigatus). Patients with ABPA have an increased IL-13 response in blood CD4+ T-cells when stimulated with autologous CD11c+ DCs and pulsed with Aspergillus antigens [116,117]. Addition of vitamin D3 can suppress this A. fumigatus-specific Th2 response in peripheral CD4+ T cells in patients with cystic fibrosis (CF) and ABPA [116]. As a result of this specific Th2 suppression, there was an increase in TGF-β+ regulatory T cells, and suppression of OX40 ligand (OX40L), a costimulatory molecule on dendritic cells that is regulated by thymic stromal lymphopoietin (TSLP), an epithelial cell cytokine that can drive Th2 differentiation [116,118].
As mentioned above, the 1,25(OH)2D3-VDR complex can decrease the maturation of DCs and decrease the DCs capacity to activate alloreactive T cells [69,79]. DCs have an important role in initiating and maintaining allergic Th2 immune cell responses to inhaled allergens [50]. CD11c+ mDCs also express receptors for TSLP [117,119], and is required for the development of inflammatory allergic responses [120]. TSLP-activated DCs express OX40L through the activation of NF-κB components [66], which is responsible for triggering Th2 inflammation in the lung [118,121]. Blockade of OX40L inhibits antigen-specific Th2 inflammation [122]. Another study reported that in the lungs of vitamin D-deficient mice, their lung CD11c+ DCs have increased expression of OX40L and stimulation with vitamin D3 inhibits the promoter activity of OX40L [123]. This study demonstrates that vitamin D3 leads to VDR binding to the OX40L promoter and represses OX40L promoter activity [123]. Specifically, this study found that VDR and the p50 and p65 subunits of NF-κB bind to the promoter region of OX40L, which down-regulate the expression of OX40L. In addition, they found that treatment with vitamin D3 inhibited OX40L promoter activity that was induced by TNF-α [123].
4.2. Autoimmunity
Studies have suggested that Vitamin D status plays an important role in the initiation, progression and/or severity of different autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, asthma, systemic lupus erythematous and inflammatory bowel disease (IBD) [2,124,125,126,127,128,129,130]. IBD such as Crohn’s disease and ulcerative colitis, are chronic, idiopathic inflammatory disorders of the gastrointestinal tract [131]. In the context of IBD, DCs play an important role in directing immunity and regulating intestinal mucosal inflammation by modulation of many cell types including Tregs, Th17 cells as well as natural killer (NK) cells, monocyte and macrophages [132]. Activated and mature DCs in Crohn’s disease induce the production of inflammatory cytokines such as IL-12, IL-18, TNF-like 1A and IFN-γ, which stimulates macrophages to release IL-1α, TNF-α, and IL-6 [133,134].
Additionally, murine studies reported the development of experimental colitis in IL-10 knock-out (KO) mice that were exposed to a Vitamin D-deficient diet. Vitamin D-deficient IL-10 KO mice start dying at 7 weeks of age and by 9 weeks of age, 58% (15/26) of the vitamin D-deficient IL-10 KO mice were dead [135]. After 9 wks of age, the remaining vitamin D-deficient IL-10 KO mice had persistent weight loss [135]. In contrast, the vitamin D-sufficient IL-10 KO (n = 10) and the vitamin D-deficient WT mice (n = 20) appeared healthy, even up to 13 wks of age [135].
VDR activation by the intrinsic production of 1,25(OH)2D3 in type 1 IFN DCs, macrophages and intestinal epithelial cells, can promote transcription of the Nucleotide-binding oligomerization domain protein 2/caspase recruitment domain-containing protein 15 (NOD2/CARD15), a cytosolic protein involved in intracellular recognition of microbes by sensing peptidoglycan fragments (e.g., muramyl dipeptide) [136,137,138]. VDR activation by the intrinsic production of 1,25(OH)2D3 in monocyte-derived cells and epithelial cells promoted the transcription of NOD2 and expression of genes encoding for antimicrobial peptide defensin β2 (DEFB2)/Human β-defensin-2 (HBD2) and the antimicrobial cathelicidins in the presence of muramyl dipeptide [139]. This signaling pathway was defective in cells expressing the major variant of NOD2 present in a subset of patients with Crohn’s disease [140]. NOD2 may affect the intestinal microbiome and can also potentiate autophagy, which is the process by which damaged organelles, proteins and intracellular microorganisms are removed through engulfment into an autophagosome and are then degraded by lysosomes [141]. Intestinal microbiota is a main driver for the development of the mucosal immune system. A dysregulated immune response to infection may cause perturbations of this interaction with the intestinal microbiota that may lead to disorders such as IBD [142,143,144,145]. Immune responses elicited by intestinal DCs induce anti-inflammatory and tolerogenic responses to harmless antigens such as those derived from the resident microflora in mice [146]. 1,25(OH)2D3 can activate signaling programs in DCs that yield in priming of regulatory and anti-inflammatory T cell responses [146]. Murine vitamin D deficiency results in the overproduction of Th1 and Th17 immune responses [147] and a reduction in the amount of tolerogenic DCs and regulatory T-cells [148]. Vitamin D and the VDR inhibit Th1, Th17, and inflammatory cytokine production in the gastrointestinal tract that serve to reduce inflammation, shift the microbiome, and maintain tolerance within the intestine [146]. Additional functions of 1,25(OH)2D3 and VDR in IBD include functioning as a regulator of T cell function, which has been reported specifically as having the ability to turn off chronically activated T cells [149]. Other additional roles of 1,25(OH)2D3 and VDR include providing protection in mucosal barrier homeostasis by contributing to the maintenance of the integrity of the tight junction proteins zonula occludens-1 and claudin-1 in mice [146]. In addition, 1,25(OH)2D3 also contributes to the healing of the colonic mucosa [150,151] and maintenance of the gut microbiome [146]. A recent study reported dysbiosis in different mice fed a Vitamin D-deficient diet, VDR knock out (VDR KO) mice, and Cyp27b1 knockout (Cyp KO) mice [146]. Cyp KO and VDR KO mice had more bacteria from the Bacteroidetes and Proteobacteria phyla and fewer bacteria from the Firmicutes and Deferribacteres phyla in the feces compared with wild-type mice. There was an increase in the Helicobacteraceae family in Cyp KO compared with wild-type mice [146]. This study also showed that depletion of the gut bacterial flora using antibiotics protected mice from colitis [146]. Providing 1,25(OH)2D3 treatment (125 μg/100 g diet) to Cyp KO mice decreased colitis severity and reduced the numbers of Helicobacteraceae in the feces compared to the feces of untreated Cyp KO mice [146]. The mechanisms by which the dysbiosis occurs in VDR KO and Cyp KO mice included lower expression of E-cadherin on gut epithelial cells and immune cells as well as fewer tolerogenic dendritic cells, resulting in more gut inflammation in VDR and Cyp KO mice compared with wild-type mice [146]. Several studies suggest that Vitamin D has a potential role in the therapy of IBD [152,153,154,155]. Randomized controlled trials have reported that patients with IBD may remain in remission longer when treated with oral treatment with 25(OH)D 1200 IU daily [152]. Suboptimal Vitamin D status is common in IBD, and studies suggest that this factor is associated with increased disease severity [156]. The 1,25(OH)2D3 anti-inflammatory role has been reported and one of the recommendations in the clinical management of Crohn’s disease is to prevent Vitamin D deficiency [153].
5. Summary/Perspective
The active form of vitamin D, 1,25(OH)2D3 has in addition to its central role in calcium and bone metabolism, has important immunoregulatory and anti-inflammatory effects. This secosteroid hormone affects the growth, differentiation and molecular expression of many cell types. The biological effects of 1,25(OH)2D3 are mediated by the extrinsic or intrinsic cell activation or 1,25 hydroxylation of vitamin D3. The VDR is a member of the superfamily of nuclear hormone receptors. 1,25(OH)2D3-VDR complex formation leads to interactions with various transcription factors within the immunomodulatory response and is reported to have anti-inflammatory and allogenic effects. VDR is present in most cell types of the immune system, in particular in antigen presenting cells (APCs) such as macrophages and DCs, as well as in both CD4+ and CD8+ T cells. DCs have an important role in capturing and processing antigens; they express lymphocyte co-stimulatory molecules, migrate to lymphoid organs and secrete cytokines to initiate immune responses. Specifically, the 1,25(OH)2D3-VDR complex affects the maturation and migration of many subsets of DCs, conferring a special immunoregulatory role along with tolerogenic properties affecting cytokine and chemokine production. These vitamin D3 immunoregulatory activities have been an intense area of investigation in allergic and autoimmune diseases and it remains to be determined if these activities are directly related to serum 25-OH vitamin D levels which are currently being used to assess vitamin D sufficiency. Additional basic studies as well as well-designed clinical studies will clarify the role of vitamin D3 in DC function in humans.
DCs are a heterogeneous population of immune cells and DC precursors develop in the bone marrow. While plasmacytoid DCs complete development in the bone marrow, most DCs complete development in lymphoid and peripheral tissues. DCs can be sub-classified based on morphology, origin, function and anatomical location. Several phenotypic and functional DCs subsets have been identified based on the HLA-DR+ lineage found on their surface marker expression including mDCs which are also known as cDCs, pDCs, inflammatory or monocyte-derived DCs (moDCs), LCs and two dermal DCs subsets. Three subsets of DCs have been identified in human blood and tissues that are either CD1c/BDCA-1+ or Thrombomodulin/CD141/BDCA-3+ mDCS, CD123 (IL-3R), CD303 (BDCA-2) mDCS, and CD304 (BDCA-4 or Neuropilin-1) pDCs. There is functional homology between human and mouse DCs. Multiple cDC subsets have been identified in mice including CD4−CD8+ cDCs, CD4+CD8− cDCs, CD4−CD8− cDCs, Integrin alpha E/CD103+ cDCs, and Integrin alpha M/CD11b+ cDCs. Inflammatory or moDCs develop from monocytes at sites of inflammation and are identified by their expression of Ly-6C in mouse. Langerhans cells can be identified in both human and mouse by the presence of Langerin/CD207-containing Birbeck granules. Two subsets of human and mouse dermal-resident DCs have also been characterized that are defined by the presence or absence of CD14 in human or Langerin/CD207 in mice. Unlike other DCs, pDCs are inefficient antigen-presenting cells and have low MHC class II expression.
Macrophages, DCs and T cells can synthesize 1,25(OH)2D3 and contribute to the regulation of immune responses. VDR activation by 1,25(OH)2D3 stimulation or intrinsic hydroxylation of 25(OH)D arrests DC maturation induced by different stimuli, maintaining them in an immature state, in terms of phenotype and functional plasticity. VDR agonists have the capacity to inhibit expression of surface co-stimulatory molecules (e.g., CD40, CD80, CD83 and CD86) and MHC class I and II molecules in several DC subsets including mDCs, cDCs and LCs. 1,25(OH)2D3-VDR complex inhibits the promoter activity of OX40L or tumor necrosis factor (ligand) superfamily, member 4 (TNFSF4) in CD11c+ DCs.
The 1,25(OH)2D3-VDR complex can inhibit the expression of IL-12, IL-23, IL-6, TNFα and INF-γ, CCL5 and CCL17 in both mDCs and cDCs. In contrast, IL-10 and IL-8 expression can be enhanced by 1,25(OH)2D3. A shift from a Th1 profile towards a Th2 type and a decrease in Th17 responses is to be anticipated from these changes. In contrast, minimal immunomodulatory effects seem to be exerted by 1,25(OH)2D3 on circulating plasmacytoid DCs. In mDCs, the expression of surface inhibitory molecules such as ILT3 and of inhibitory cytokines such as IL-10, were markedly upregulated. DCs expressing high levels of inhibitory molecules, such as ILT3, favor induction and/or enhancement of regulatory/suppressor T cells. CD1c+ and CD103+ mDCs but not CD141+ mDCs or plasmacytoid DCs responded to 1,25(OH)2D3 by promoting the production of retinoic acid (RA) by highly expressing RALDH2. RA production can promote the conversion of naïve T cells to Foxp3+ T regulatory cells. Divergent responses have been observed in LCs, such as the expression of IL-10 was down-regulated, but the expression of IL-6 and IL-12p40 was up-regulated, leading to decreased production of Th2 type chemokines, CCL17 and CCL22. In CD14+ DCs, the expression of IL10 was upregulated, but IL-1 expression was down-regulated, enhancing the migration of these DCs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.DeLuca H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004;80(Suppl. 6):1689s–1696s. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 2.Holick M.F. The vitamin D epidemic and its health consequences. J. Nutr. 2005;135:2739s–2748s. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 3.Tavera-Mendoza L.E., White J.H. Cell defenses and the sunshine vitamin. Sci. Am. 2007;297:62–72. doi: 10.1038/scientificamerican1107-62. [DOI] [PubMed] [Google Scholar]
- 4.White J.H. Vitamin D metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 2012;13:21–29. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 5.Nissen J., Vogel U., Ravn-Haren G., Andersen E.W., Madsen K.H., Nexo B.A., Andersen R., Mejborn H., Bjerrum P.J., Rasmussen L.B., et al. Common variants in CYP2R1 and GC genes are both determinants of serum 25-hydroxyvitamin D concentrations after UVB irradiation and after consumption of vitamin D3-fortified bread and milk during winter in Denmark. Am. J. Clin. Nutr. 2015;101:218–227. doi: 10.3945/ajcn.114.092148. [DOI] [PubMed] [Google Scholar]
- 6.Prosser D.E., Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem. Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Shinkyo R., Sakakib T., Kamakurab M., Ohtac M., Inouyea K. Metabolism of vitamin D by human microsomal CYP2R1. Biochem. Biophys. Res. Commun. 2004;324:451–457. doi: 10.1016/j.bbrc.2004.09.073. [DOI] [PubMed] [Google Scholar]
- 8.Hewison M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Investig. 2012;243:92–102. doi: 10.3109/00365513.2012.682862. [DOI] [PubMed] [Google Scholar]
- 9.Christakos S., DeLuca H.F. Minireview: Vitamin D: Is there a role in extraskeletal health? Endocrinology. 2011;152:2930–2936. doi: 10.1210/en.2011-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLuca H.F. Evolution of our understanding of vitamin D. Nutr. Rev. 2008;66:S73–S87. doi: 10.1111/j.1753-4887.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 11.Lang P.O., Aspinall R. Can we translate vitamin D immunomodulating effect on innate and adaptive immunity to vaccine response? Nutrients. 2015;7:2044–2060. doi: 10.3390/nu7032044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun R.F., Gacad M., Nguyen L., Hewison M., Adams J.S. Co-chaperone potentiation of vitamin D receptor-mediated transactivation: A role for Bcl2-associated athanogene-1 as an intracellular-binding protein for 1,25-dihydroxyvitamin D3. J. Mol. Endocrinol. 2007;39:81–89. doi: 10.1677/JME-07-0042. [DOI] [PubMed] [Google Scholar]
- 13.Haussler M.R., Whitfield G.K., Haussler C.A., Hsieh J.C., Thompson P.D., Selznick S.H., Dominguez C.E., Jurutka P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 14.Norman A.W., Ishizuka S., Okamura W.H. Ligands for the vitamin D endocrine system: Different shapes function as agonists and antagonists for genomic and rapid response receptors or as a ligand for the plasma vitamin D binding protein. J. Steroid Biochem. Mol. Biol. 2001;76:49–59. doi: 10.1016/S0960-0760(00)00145-X. [DOI] [PubMed] [Google Scholar]
- 15.Li Q., Verma I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 16.Ryynanen J., Carlberg C. Primary 1,25-dihydroxyvitamin D3 response of the interleukin 8 gene cluster in human monocyte- and macrophage-like cells. PLoS ONE. 2013;8:e78170. doi: 10.1371/journal.pone.0078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molnar F., Perakyla M., Carlberg C. Vitamin D receptor agonists specifically modulate the volume of the ligand-binding pocket. J. Biol. Chem. 2006;281:10516–10526. doi: 10.1074/jbc.M513609200. [DOI] [PubMed] [Google Scholar]
- 18.Carlberg C., Campbell M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids. 2013;78:127–136. doi: 10.1016/j.steroids.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chawla A., Repa J.J., Evans R.M., Mangelsdorf D.J. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 20.Schule R., Umesono K., Mangelsdorf D.J., Bolado J., Pike J.W., Evans R.M. Jun-Fos and receptors for vitamins A and D recognize a common response element in the human osteocalcin gene. Cell. 1990;61:497–504. doi: 10.1016/0092-8674(90)90531-I. [DOI] [PubMed] [Google Scholar]
- 21.Adorini L., Giarratana N., Penna G. Pharmacological induction of tolerogenic dendritic cells and regulatory T cells. Semin. Immunol. 2004;16:127–134. doi: 10.1016/j.smim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Hackstein H., Thomson A.W. Dendritic cells: Emerging pharmacological targets of immunosuppressive drugs. Nat. Rev. Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 23.Bhalla A.K., Amento E.P., Clemens T.L., Holick M.F., Krane S.M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in T lymphocytes following activation. J. Clin. Endocrinol. Metab. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 24.Bouillon R., Okamura W.H., Norman A.W. Structure-function relationships in the vitamin D endocrine system. Endocr. Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 25.Hewison M., Burkeb F., Evansb K.N., Lammasb D.A., Sansomb D.M., Liuc P., Modlinc R.L., Adamsa J.S. Extra-renal 25-hydroxyvitamin D3–1α-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 26.Zehnder D., Bland R., Williams M.C., McNinch R.W., Howie A.J., Stewart P.M., Hewison M. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J. Clin. Endocrinol. Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 27.De Kleer I., Willems F., Lambrecht B., Goriely S. Front. Immunol. Vol. 5. Willems; 2014. Ontogeny of myeloid cells; p. 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boltjes A., van Wijk F. Human dendritic cell functional specialization in steady-state and inflammation. Front. Immunol. 2014;5:131. doi: 10.3389/fimmu.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piemonti L., Monti P., Sironi M., Fraticelli P., Leone B.E., Cin E.D., Allavena P., Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 30.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 31.Pulendran B. The Varieties of Immunological Experience: Of Pathogens, Stress, and Dendritic Cells. Annu. Rev. Immunol. 2015;33:563–606. doi: 10.1146/annurev-immunol-020711-075049. [DOI] [PubMed] [Google Scholar]
- 32.Thiele F., Tao S., Zhang Y., Muschaweckh A., Zollmann T., Protzer U., Abele R., Drexler I. MVA-infected Dendritic Cells Present CD4+ T-Cell Epitopes by Endogenous MHC Class II Presentation Pathways. J. Virol. 2015;89:2698–2709. doi: 10.1128/JVI.03244-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volchenkov R., Sprater F., Vogelsang P., Appel S. The 2011 Nobel Prize in physiology or medicine. Scand. J. Immunol. 2012;75:1–4. doi: 10.1111/j.1365-3083.2011.02663.x. [DOI] [PubMed] [Google Scholar]
- 34.Wagner H. Innate immunity’s path to the Nobel Prize 2011 and beyond. Eur. J. Immunol. 2012;42:1089–1092. doi: 10.1002/eji.201242404. [DOI] [PubMed] [Google Scholar]
- 35.Haniffa M., Collin M., Ginhoux F. Ontogeny and functional specialization of dendritic cells in human and mouse. Adv. Immunol. 2013;120:1–49. doi: 10.1016/B978-0-12-417028-5.00001-6. [DOI] [PubMed] [Google Scholar]
- 36.Schlitzer A., Ginhoux F. Organization of the mouse and human DC network. Curr. Opin. Immunol. 2014;26:90–99. doi: 10.1016/j.coi.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Segura E., Durand M., Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J. Exp. Med. 2013;210:1035–1047. doi: 10.1084/jem.20121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzionek A., Fuchs A., Schmidt P., Cremer S., Zysk M., Miltenyi S., Buck D.W., Schmitz J. BDCA-2, BDCA-3, and BDCA-4: Three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D.N., Leenen P.J.M., Liu Y.J., MacPherson G., Randolph G.J. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 40.Robbins S.H., Walzer T., Dembélé D., Thibault C., Defays A., Bessou G., Xu H.C., Vivier E., Sellars M., Pierre P. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9 doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geissmann F., Manz M.G., Jung S., Sieweke M.H., Merad M., Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velasquez-Lopera M.M., Correa L.A., Garcia L.F. Human spleen contains different subsets of dendritic cells and regulatory T lymphocytes. Clin. Exp. Immunol. 2008;154:107–114. doi: 10.1111/j.1365-2249.2008.03734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachem A., Güttler S., Hartung E., Ebstein F., Schaefer M., Tannert A., Salama A., Movassaghi K., Opitz C., Mages H.W., et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jongbloed S.L., Kassianos A.J., McDonald K.J., Clark G.J., Ju X., Angel C.E., Chen C.J., Dunbar P.R., Wadley R.B., Jeet V., et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poulin L.F., Salio M., Griessinger E., Anjos-Afonso F., Craciun L., Chen J.L., Keller A.M., Joffre O., Zelenay S., Nye E., et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells. J. Exp. Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haniffa M., Shin A., Bigley V., McGovern N., Teo P., See P., Wasan P.S., Wang X.N., Malinarich F., Malleret B., et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlitzer A., McGovern N., Teo P., Zelante T., Atarashi K., Low D., Ho A.W., See P., Shin A., Wasan P.S., et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nestle F.O., Di Meglio P., Qin J.Z., Nickoloff B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaba L.C., Krueger J.G., Lowes M.A. Resident and “inflammatory” dendritic cells in human skin. J. Investig. Dermatol. 2009;129:302–308. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammad H., Lambrecht B.N. Dendritic cells and epithelial cells: Linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 51.Merad M., Ginhoux F., Collin M. Origin, homeostasis and function of langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 52.Angel C.E., George E., Brooks A.E., Ostrovsky L.L., Brown T.L., Dunbar P.R. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J. Immunol. 2006;176:5730–5734. doi: 10.4049/jimmunol.176.10.5730. [DOI] [PubMed] [Google Scholar]
- 53.Haniffa M., Ginhoux F., Wang X.N., Bigley V., Abel M., Dimmick I., Bullock S., Grisotto M., Booth T., Taub P., et al. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J. Exp. Med. 2009;206:371–385. doi: 10.1084/jem.20081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angel C.E., Lala A., Chen C.J., Edgar S.G., Ostrovsky L.L., Dunbar P.R. CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts. Int. Immunol. 2007;19:1271–1279. doi: 10.1093/intimm/dxm096. [DOI] [PubMed] [Google Scholar]
- 55.Chu C.C., Ali N., Karagiannis P., Di Meglio P., Skowera A., Napolitano L., Barinaga G., Grys K., Sharif-Paghaleh E., Karagiannis S.N., et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J. Exp. Med. 2012;209:935–945. doi: 10.1084/jem.20112583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klechevsky E., Morita R., Liu M., Cao Y., Coquery S., Thompson-Snipes L., Briere F., Chaussabel D., Zurawski G., Palucka A.K., et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larregina A.T., Morelli A.E., Spencer L.A., Logar A.J., Watkins S.C., Thomson A.W., Falo L.D. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2001;2:1151–1158. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- 58.Manh T.P., Alexandre Y., Baranek T., Crozat K., Dalod M. Plasmacytoid, conventional, and monocyte-derived dendritic cells undergo a profound and convergent genetic reprogramming during their maturation. Eur. J. Immunol. 2013;43:1706–1715. doi: 10.1002/eji.201243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piccioli D., Sammicheli C., Tavarini S., Nuti S., Frigimelica E., Manetti A.G., Nuccitelli A., Aprea S., Valentini S., Borgogni E., et al. Human plasmacytoid dendritic cells are unresponsive to bacterial stimulation and require a novel type of cooperation with myeloid dendritic cells for maturation. Blood. 2009;113:4232–4239. doi: 10.1182/blood-2008-10-186890. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y.J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 61.Cella M., Jarrossay D., Facchetti F., Alebardi O., Nakajima H., Lanzavecchia A., Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 62.Penna G., Vulcano M., Roncari A., Facchetti F., Sozzani S., Adorini L. Cutting edge: Differential chemokine production by myeloid and plasmacytoid dendritic cells. J. Immunol. 2002;169:6673–6676. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- 63.Mathan T.S., Figdor C.G., Buschow S.I. Human plasmacytoid dendritic cells: From molecules to intercellular communication network. Front. Immunol. 2013;4:372. doi: 10.3389/fimmu.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin Z., Dai J., Deng J., Sheikh F., Natalia M., Shih T., Lewis-Antes A., Amrute S.B., Garrigues U., Doyle S., et al. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J. Immunol. 2012;189:2735–2745. doi: 10.4049/jimmunol.1102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greiller C.L., Martineau A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients. 2015;7:4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arima K., Watanabe N., Hanabuchi S., Chang M., Sun S.C., Liu Y.J. Distinct signal codes generate dendritic cell functional plasticity. Sci. Signal. 2010;3 doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J., Schuler-Thurner B., Schuler G., Huber C., Seliger B. Bipartite regulation of different components of the MHC class I antigen-processing machinery during dendritic cell maturation. Int. Immunol. 2001;13:1515–1523. doi: 10.1093/intimm/13.12.1515. [DOI] [PubMed] [Google Scholar]
- 68.Ferreira G.B., Overbergh L., Verstuyf A., Mathieu C. 1α,25-Dihydroxyvitamin D3 and its analogs as modulators of human dendritic cells: A comparison dose-titration study. J. Steroid Biochem. Mol. Biol. 2013;136:160–165. doi: 10.1016/j.jsbmb.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Adorini L. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting autoimmune diabetes. Ann. N. Y. Acad. Sci. 2003;987:258–261. doi: 10.1111/j.1749-6632.2003.tb06057.x. [DOI] [PubMed] [Google Scholar]
- 70.Bartels L.E., Hvasb C.L., Agnholtb J., Dahlerupb J.F., Aggera R. Human dendritic cell antigen presentation and chemotaxis are inhibited by intrinsic 25-hydroxy vitamin D activation. Int. Immunopharmacol. 2010;10:922–928. doi: 10.1016/j.intimp.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Rook G.A., Steele J., Fraher L., Barker S., Karmali R., O’Riordan J., Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–163. [PMC free article] [PubMed] [Google Scholar]
- 72.Sochorova K., Budinsky V., Rozkova D., Tobiasova Z., Dusilova-Sulkova S., Spisek R., Bartunkova J. Paricalcitol (19-nor-1,25-dihydroxyvitamin D2) and calcitriol (1,25-dihydroxyvitamin D3) exert potent immunomodulatory effects on dendritic cells and inhibit induction of antigen-specific T cells. Clin. Immunol. 2009;133:69–77. doi: 10.1016/j.clim.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 73.Van Halteren A.G.S., Tysma O.M., van Etten E., Mathieu C., Roep B.O. 1α,25-Dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J. Autoimmun. 2004;23:233–239. doi: 10.1016/j.jaut.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Cantorna M.T., Snyder L., Lin Y.D., Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mathieu C., Adorini L. The coming of age of 1,25-dihydroxyvitamin D3 analogs as immunomodulatory agents. Trends Mol. Med. 2002;8:174–179. doi: 10.1016/S1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 76.Griffin M.D., Lutz W., Phan V.A., Bachman L.A., McKean D.J., Kumar R. Dendritic cell modulation by 1α,25 dihydroxyvitamin D3 and its analogs: A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Penna G., Adorini L. 1α,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 78.Ferreira G.B., Gysemans C.A., Demengeot J., da Cunha J.P., Vanherwegen A.S., Overbergh L., Van Belle T.L., Pauwels F., Verstuyf A., Korf H., et al. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J. Immunol. 2014;192:4210–4220. doi: 10.4049/jimmunol.1302350. [DOI] [PubMed] [Google Scholar]
- 79.Penna G., Amuchastegui S., Giarratana N., Daniel K.C., Vulcano M., Sozzani S., Adorini L. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J. Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 80.Penna G., Amuchastegui S., Laverny G., Adorini L. Vitamin D receptor agonists in the treatment of autoimmune diseases: Selective targeting of myeloid but not plasmacytoid dendritic cells. J. Bone Miner. Res. 2007;22:V69–V73. doi: 10.1359/jbmr.07s217. [DOI] [PubMed] [Google Scholar]
- 81.Maxwell P.J., Coulter J., Walker S.M., McKechnie M., Neisen J., McCabe N., Kennedy R.D., Salto-Tellez M., Albanese C., Waugh D.J. Potentiation of inflammatory CXCL8 signalling sustains cell survival in PTEN-deficient prostate carcinoma. Eur. Urol. 2013;64:177–188. doi: 10.1016/j.eururo.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Enioutina E.Y., Bareyan D., Daynes R.A. Vitamin D3-mediated alterations to myeloid dendritic cell trafficking in vivo expand the scope of their antigen presenting properties. Vaccine. 2007;25:1236–1249. doi: 10.1016/j.vaccine.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 83.Enioutina E.Y., Bareyan D., Daynes R.A. TLR ligands that stimulate the metabolism of vitamin D3 in activated murine dendritic cells can function as effective mucosal adjuvants to subcutaneously administered vaccines. Vaccine. 2008;26:601–613. doi: 10.1016/j.vaccine.2007.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fujita H., Asahina A., Komine M., Tamaki K. The direct action of 1α,25(OH)2-vitamin D3 on purified mouse Langerhans cells. Cell. Immunol. 2007;245:70–79. doi: 10.1016/j.cellimm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 85.Penna G., Roncari A., Amuchastegui S., Daniel K.C., Berti E., Colonna M., Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 86.Auphan N., DiDonato J.A., Rosette C., Helmberg A., Karin M. Immunosuppression by glucocorticoids: Inhibition of NF-κB activity through induction of I κB synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 87.Scheinman R.I., Cogswell P.C., Lofquist A.K., Baldwin A.S. Role of transcriptional activation of I κBα in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 88.Gauzzi M.C., Purificato C., Donato K., Jin Y., Wang L., Daniel K.C., Maghazachi A.A., Belardelli F., Adorini L., Gessani S. Suppressive effect of 1α,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: Impairment of functional activities and chemotaxis. J. Immunol. 2005;174:270–276. doi: 10.4049/jimmunol.174.1.270. [DOI] [PubMed] [Google Scholar]
- 89.Ward S.G., Bacon K., Westwick J. Chemokines and T lymphocytes: More than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/S1074-7613(00)80583-X. [DOI] [PubMed] [Google Scholar]
- 90.Mora J.R., Iwata M., von Andrian U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato T., Kitawaki T., Fujita H., Iwata M., Iyoda T., Inaba K., Ohteki T., Hasegawa S., Kawada K., Sakai Y., et al. Human CD1c(+) myeloid dendritic cells acquire a high level of retinoic acid-producing capacity in response to vitamin D3. J. Immunol. 2013;191:3152–3160. doi: 10.4049/jimmunol.1203517. [DOI] [PubMed] [Google Scholar]
- 92.Iwata M., Eshima Y., Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int. Immunol. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 93.Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bartels L.E., Jorgensen S.P., Agnholt J., Kelsen J., Hvas C.L., Dahlerup J.F. 1,25-dihydroxyvitamin D3 and dexamethasone increase interleukin-10 production in CD4+ T cells from patients with Crohn’s disease. Int. Immunopharmacol. 2007;7:1755–1764. doi: 10.1016/j.intimp.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 95.Willheim M., Thien R., Schrattbauer K., Bajna E., Holub M., Gruber R., Baier K., Pietschmann P., Reinisch W., Scheiner O., et al. Regulatory effects of 1α,25-dihydroxyvitamin D3 on the cytokine production of human peripheral blood lymphocytes. J. Clin. Endocrinol. Metab. 1999;84:3739–3744. doi: 10.1210/jcem.84.10.6054. [DOI] [PubMed] [Google Scholar]
- 96.D’Ambrosio D., Cippitelli M., Cocciolo M.G., Mazzeo D., Di Lucia P., Lang R., Sinigaglia F., Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-κB downregulation in transcriptional repression of the p40 gene. J. Clin. Investig. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Staeva-Vieira T.P., Freedman L.P. 1,25-dihydroxyvitamin D3 inhibits IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J. Immunol. 2002;168:1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 98.Griffin M.D., Xing N., Kumar R. Gene expression profiles in dendritic cells conditioned by 1α,25-dihydroxyvitamin D3 analog. J. Steroid Biochem. Mol. Biol. 2004;89–90:443–448. doi: 10.1016/j.jsbmb.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 99.Xing N., Maldonado M., Bachman L.A., McKean D.J., Kumar R., Griffin M.D. Distinctive dendritic cell modulation by vitamin D3 and glucocorticoid pathways. Biochem. Biophys. Res. Commun. 2002;297:645–652. doi: 10.1016/S0006-291X(02)02262-3. [DOI] [PubMed] [Google Scholar]
- 100.Van Etten E., Verlinden L., Giulietti A., Ramos-Lopez E., Branisteanu D.D., Ferreira G.B., Overbergh L., Verstuyf A., Bouillon R., Roep B.O., et al. The vitamin D receptor gene FokI polymorphism: Functional impact on the immune system. Eur. J. Immunol. 2007;37:395–405. doi: 10.1002/eji.200636043. [DOI] [PubMed] [Google Scholar]
- 101.Wöbke T.K., Sorg B.L., Steinhilber D. Vitamin D in inflammatory diseases. Front. Physiol. 2014;5:244. doi: 10.3389/fphys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu X.P., Bellido T., Manolagas S.C. Down-regulation of NF-κB protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA. 1995;92:10990–10994. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 104.Karin M., Lin A. NF-κB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 105.Tsatsanis C., Androulidaki A., Venihaki M., Margioris A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 106.Enioutina E.Y., Bareyan D., Daynes R.A. TLR-induced local metabolism of vitamin D3 plays an important role in the diversification of adaptive immune responses. J. Immunol. 2009;182:4296–4305. doi: 10.4049/jimmunol.0804344. [DOI] [PubMed] [Google Scholar]
- 107.Van Halteren A.G., van Etten E., de Jong E.C., Bouillon R., Roep B.O., Mathieu C. Redirection of human autoreactive T-cells Upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D3. Diabetes. 2002;51:2119–2125. doi: 10.2337/diabetes.51.7.2119. [DOI] [PubMed] [Google Scholar]
- 108.Van der Aar A.M., Sibiryak D.S., Bakdash G., van Capel T.M., van der Kleij H.P., Opstelten D.J., Teunissen M.B., Kapsenberg M.L., de Jong E.C. Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J. Allergy Clin. Immunol. 2011;127:1532–1540. doi: 10.1016/j.jaci.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 109.Bakdash G., van Capel T.M.M., Mason L.M.K., Kapsenberg M.L., de Jong E.C. Vitamin D3 metabolite calcidiol primes human dendritic cells to promote the development of immunomodulatory IL-10-producing T cells. Vaccine. 2014;32:6294–6302. doi: 10.1016/j.vaccine.2014.08.075. [DOI] [PubMed] [Google Scholar]
- 110.Gorman S., Kuritzky L.A., Judge M.A., Dixon K.M., McGlade J.P., Mason R.S., Finlay-Jones J.J., Hart P.H. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J. Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 111.Bakdash G., Schneider L.P., van Capel T.M., Kapsenberg M.L., Teunissen M.B., de Jong E.C. Intradermal application of vitamin D3 increases migration of CD14+ dermal dendritic cells and promotes the development of Foxp3+ regulatory T cells. Hum. Vaccines Immunother. 2013;9:250–258. doi: 10.4161/hv.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Gruijl T.D., Sombroek C.C., Lougheed S.M., Oosterhoff D., Buter J., van den Eertwegh A.J., Scheper R.J., Pinedo H.M. A postmigrational switch among skin-derived dendritic cells to a macrophage-like phenotype is predetermined by the intracutaneous cytokine balance. J. Immunol. 2006;176:7232–7242. doi: 10.4049/jimmunol.176.12.7232. [DOI] [PubMed] [Google Scholar]
- 113.Nagl M., Kacani L., Mullauer B., Lemberger E.M., Stoiber H., Sprinzl G.M., Schennach H., Dierich M.P. Phagocytosis and killing of bacteria by professional phagocytes and dendritic cells. Clin. Diagn. Lab. Immunol. 2002;9:1165–1168. doi: 10.1128/CDLI.9.6.1165-1168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van der Does A.M., Kenne E., Koppelaar E., Agerberth B., Lindbom L. Vitamin D3 and phenylbutyrate promote development of a human dendritic cell subset displaying enhanced antimicrobial properties. J. Leukoc. Biol. 2014;95:883–891. doi: 10.1189/jlb.1013549. [DOI] [PubMed] [Google Scholar]
- 115.Gunville C.F., Mourani P.M., Ginde A.A. The role of vitamin D in prevention and treatment of infection. Inflamm. Allergy Drug Targets. 2013;12:239–245. doi: 10.2174/18715281113129990046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kreindler J.L., Steele C., Nguyen N., Chan Y.R., Pilewski J.M., Alcorn J.F., Vyas Y.M., Aujla S.J., Finelli P., Blanchard M., et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J. Clin. Investig. 2010;120:3242–3254. doi: 10.1172/JCI42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Montagnoli C., Bozza S., Gaziano R., Zelante T., Bonifazi P., Moretti S., Bellocchio S., Pitzurra L., Romani L. Immunity and tolerance to Aspergillus fumigatus. Novartis Found. Symp. 2006;279:66–219. [PubMed] [Google Scholar]
- 118.Ito T., Bozza S., Gaziano R., Zelante T., Bonifazi P., Moretti S., Bellocchio S., Pitzurra L., Romani L. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soumelis V., Reche P.A., Kanzler H., Yuan W., Edward G., Homey B., Gilliet M., Ho S., Antonenko S., Lauerma A., et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 120.Zhou B., Headley M.B., Aye T., Tocker J., Comeau M.R., Ziegler S.F. Reversal of thymic stromal lymphopoietin-induced airway inflammation through inhibition of Th2 responses. J. Immunol. 2008;181:6557–6562. doi: 10.4049/jimmunol.181.9.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salek-Ardakani S., Song J., Halteman B.S., Jember A.G., Akiba H., Yagita H., Croft M. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J. Exp. Med. 2003;198:315–324. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seshasayee D., Lee W.P., Zhou M., Shu J., Suto E., Zhang J., Diehl L., Austin C.D., Meng Y.G., Tan M., et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Investig. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nguyen N.L., Chen K., McAleer J., Kolls J.K. Vitamin D regulation of OX40 ligand in immune responses to Aspergillus fumigatus. Infect. Immun. 2013;81:1510–1519. doi: 10.1128/IAI.01345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cantorna M.T. Vitamin D and multiple sclerosis: An update. Nutr. Rev. 2008;66:S135–S138. doi: 10.1111/j.1753-4887.2008.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O’Sullivan M. Session 3: Joint Nutrition Society and Irish Nutrition and Dietetic Institute Symposium on ‘Nutrition and autoimmune disease’ Nutrition in Crohn’s disease. Proc. Nutr. Soc. 2009;68:127–134. doi: 10.1017/S0029665109001025. [DOI] [PubMed] [Google Scholar]
- 126.Shapira Y., Agmon-Levin N., Shoenfeld Y. Geoepidemiology of autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2010;6:468–476. doi: 10.1038/nrrheum.2010.86. [DOI] [PubMed] [Google Scholar]
- 127.Stojanovic O.I., Lazovic M., Lazovic M., Vuceljic M. Association between atherosclerosis and osteoporosis, the role of vitamin D. Arch. Med. Sci. 2011;7:179–188. doi: 10.5114/aoms.2011.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Summerday N.M., Brown S.J., Allington D.R., Rivey M.P. Vitamin D and multiple sclerosis: Review of a possible association. J. Pharm. Pract. 2012;25:75–84. doi: 10.1177/0897190011421839. [DOI] [PubMed] [Google Scholar]
- 129.Szekely J.I., Pataki A. Effects of vitamin D on immune disorders with special regard to asthma, COPD and autoimmune diseases: A short review. Expert Rev. Respir. Med. 2012;6:683–704. doi: 10.1586/ers.12.57. [DOI] [PubMed] [Google Scholar]
- 130.Theodoratou E., Tzoulaki I., Zgaga L., Ioannidis J.P. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coskun M. Intestinal epithelium in inflammatory bowel disease. Front. Med. (Lausanne) 2014;1:24. doi: 10.3389/fmed.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baumgart D.C., Carding S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 133.Neves A.R., Castelo-Branco M.T., Figliuolo V.R., Bernardazzi C., Buongusto F., Yoshimoto A., Nanini H.F., Coutinho C.M., Carneiro A.J., Coutinho-Silva R., et al. Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn’s disease. Inflamm. Bowel Dis. 2014;20:444–457. doi: 10.1097/01.MIB.0000441201.10454.06. [DOI] [PubMed] [Google Scholar]
- 134.Whittall T., Wang Y., Kelly C.G., Thompson R., Sanderson J., Lomer M., Soon S.Y., Bergmeier L.A., Singh M., Lehner T. Tumour necrosis factor-α production stimulated by heat shock protein 70 and its inhibition in circulating dendritic cells and cells eluted from mucosal tissues in Crohn’s disease. Clin. Exp. Immunol. 2006;143:550–559. doi: 10.1111/j.1365-2249.2006.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cantorna M.T., Munsick C., Bemiss C., Mahon B.D. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J. Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 136.Girardin S.E., Boneca I.G., Viala J., Chamaillard M., Labigne A., Thomas G., Philpott D.J., Sansonetti P.J. NOD2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 137.Inohara N., Ogura Y., Fontalba A., Gutierrez O., Pons F., Crespo J., Fukase K., Inamura S., Kusumoto S., Hashimoto M., et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J. Biol. Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 138.Yuk J.M., Shin D.M., Lee H.M., Yang C.S., Jin H.S., Kim K.K., Lee Z.W., Lee S.H., Kim J.M., Jo E.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 139.Wang T.T., Dabbas B., Laperriere D., Bitton A.J., Soualhine H., Tavera-Mendoza L.E., Dionne S., Servant M.J., Bitton A., Seidman E.G., et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Voss E., Wehkamp J., Wehkamp K., Stange E.F., Schroder J.M., Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human β-defensin-2. J. Biol. Chem. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 141.Kaser A., Blumberg R.S. Autophagy, microbial sensing, endoplasmic reticulum stress, and epithelial function in inflammatory bowel disease. Gastroenterology. 2011;140:1738–1747. doi: 10.1053/j.gastro.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Halme L., Paavola-Sakki P., Turunen U., Lappalainen M., Farkkila M., Kontula K. Family and twin studies in inflammatory bowel disease. World J. Gastroenterol. 2006;12:3668–3672. doi: 10.3748/wjg.v12.i23.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hewison M. Vitamin D and immune function: An overview. Proc. Nutr. Soc. 2012;71:50–61. doi: 10.1017/S0029665111001650. [DOI] [PubMed] [Google Scholar]
- 145.Ruemmele F.M., Garnier-Lengline H. Transforming growth factor and intestinal inflammation: The role of nutrition. Nestle Nutr. Inst. Workshop Ser. 2013;77:91–98. doi: 10.1159/000351390. [DOI] [PubMed] [Google Scholar]
- 146.Ooi J.H., Li Y., Rogers C.J., Cantorna M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J. Nutr. 2013;143:1679–1686. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bruce D., Yu S., Ooi J.H., Cantorna M.T. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int. Immunol. 2011;23:519–528. doi: 10.1093/intimm/dxr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cantorna M.T. Why do T cells express the vitamin D receptor? Ann. N. Y. Acad. Sci. 2011;1217:77–82. doi: 10.1111/j.1749-6632.2010.05823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cantorna M.T., Waddell A. The vitamin D receptor turns off chronically activated T cells. Ann. N. Y. Acad. Sci. 2014;1317:70–75. doi: 10.1111/nyas.12408. [DOI] [PubMed] [Google Scholar]
- 150.Kong J., Zhang Z., Musch M.W., Ning G., Sun J., Hart J., Bissonnette M., Li Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G208–G216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 151.Zhao H., Zhang H., Wu H., Li H., Liu L., Guo J., Li C., Shih D.Q., Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57. doi: 10.1186/1471-230X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jorgensen S.P., Agnholt J., Glerup H., Lyhne S., Villadsen G.E., Hvas C.L., Bartels L.E., Kelsen J., Christensen L.A., Dahlerup J.F. Clinical trial: Vitamin D3 treatment in Crohn’s disease—A randomized double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2010;32:377–383. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 153.O’Sullivan M. Vitamin D as a novel therapy in inflammatory bowel disease: New hope or false dawn? Proc. Nutr. Soc. 2015;74:5–12. doi: 10.1017/S0029665114001621. [DOI] [PubMed] [Google Scholar]
- 154.Shepherd D., Day A.S., Leach S.T., Lopez R., Messenger R., Woodhead H.J., Ledder O., Lemberg D.A. Single High Dose Oral Vitamin D3 Therapy (Stoss)—A Solution to Vitamin D Deficiency in Children with Inflammatory Bowel Disease? J. Pediatr. Gastroenterol. Nutr. 2015 doi: 10.1097/MPG.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 155.Zhu T., Liu T.J., Shi Y.Y., Zhao Q. Vitamin D/VDR signaling pathway ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis by inhibiting intestinal epithelial apoptosis. Int. J. Mol. Med. 2015;35:1213–1218. doi: 10.3892/ijmm.2015.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Raftery T., O’Sullivan M. Optimal vitamin D levels in Crohn’s disease: A review. Proc. Nutr. Soc. 2015;74:56–66. doi: 10.1017/S0029665114001591. [DOI] [PubMed] [Google Scholar]