Abstract
The association between maternal smoking during pregnancy and autism spectrum disorder (ASD) risk in offspring has been investigated in several studies, but the evidence is not conclusive. We, therefore, conducted this meta-analysis to explore whether an association exists between maternal smoking during pregnancy and ASD risk in offspring. We searched PubMed, Embase, Web of Science, and the Cochrane Library for studies of maternal smoking during pregnancy and ASD risk in offspring up to 10 June 2015. The random-effects model was used to combine results from individual studies. 15 observational studies (6 cohort studies and 9 case-control studies), with 17,890 ASD cases and 1,810,258 participants were included for analysis. The pooled odds ratio (OR) was 1.02 (95% confidence interval (CI): 0.93–1.13) comparing mothers who smoked during pregnancy with those who did not. Subgroup and sensitivity analysis suggested the overall result of this analysis was robust. Results from this meta-analysis indicate that maternal smoking during pregnancy is not associated with ASD risk in offspring. Further well-designed cohort studies are needed to confirm the present findings.
Keywords: maternal smoking, risk factor, autism spectrum disorder, meta-analysis
1. Introduction
Autism Spectrum Disorders (ASDs) are a group of heterogeneous neurodevelopmental conditions characterized by social and communication difficulties, and by restricted and repetitive interests and behaviors [1]. The ASDs include autistic disorder, Asperger syndrome, atypical autism, and other or unspecified pervasive developmental disorders [2]. The global prevalence of ASDs is estimated to be 1 person in 132, accounting for more than 7.7 million disability-adjusted life years worldwide in 2010 [3]. Although the cause of ASDs still remains unknown, they are regarded as multifactorial, with many risk factors acting together to produce the phenotype [4]. The identification of modifiable environmental risk factors is of great importance for the primary prevention of ASDs.
Smoking during pregnancy may lead to numerous adverse outcomes in the offspring, such as low birth weight, placenta abruption, and sudden infant death syndrome [5]. Maternal smoking is also a risk factor for several neurodevelopmental disorders, including attention-deficit, conduct disorder, and antisocial behavior [6]. Several studies have explored the relationship of maternal smoking during pregnancy and ASDs risk, but the results were inconclusive. A recent meta-analysis by Roson et al. concluded that there was no association between maternal prenatal smoking and ASD in offspring based on 15 observational studies [7]. However, Roson et al. [7] only searched two databases which missed several eligible studies. In addition, Rosen et al. misunderstood the difference between the period of “prenatal” and “pregnancy”, and they inappropriately included studies that only reported smoking during pregnancy but not specified whether it was during the prenatal period or not. We therefore performed this study to systematically assess the association of maternal smoking during pregnancy (including both prenatal and perinatal smoking) and ASDs risk.
2. Methods
2.1. Data Sources and Searches
This meta-analysis was performed according to the Meta-Analysis of Observational Studies in Epidemiology guidelines [8]. A systematic search of PubMed, EMBASE, Web of Science, and Cochrane Library up to 10 June 2015 was conducted to identify relevant studies regarding the association between maternal smoking during pregnancy and ASDs risk. A reference list of the related studies were further screened for any additional literatures. The search terms for PubMed were: (smoke OR smoking OR tobacco OR risk factors) AND (autism spectrum disorder or autism) AND (pregnancy OR prenatal OR perinatal OR maternal). Similar search strategies were used in other databases. We applied no language restriction in the process of literature search and selection.
2.2. Study Selection
Studies were included if they met the following criteria: (i) had a cohort or case-control design; (ii) reported the association between maternal smoking during pregnancy (including prenatal or perinatal smoking) and ASD risk in their offspring; and (iii) reported risk estimates and 95% confidence interval (CI). Only the study with the most complete data was included if data was reported more than once in different literatures. Non-human studies, letters, case reports, conference abstract, review, and studies that with insufficient information were excluded.
2.3. Data Extraction and Quality Assessment
Two reviewers independently extracted data, any disagreements were resolved by discussion. The following data were extracted: last name of the first author, publication year, study design, study location, age, sex, number of cases and total population, risk estimates adjusted for the maximum number of confounding variables with corresponding 95% CIs, and confounding factors that were being adjusted for in the analysis. We also extracted any dose-relationship data for smoking and ASDs risk.
The methodological quality was assessed using the Newcastle-Ottawa Scale (NOS) [9]. The NOS has been widely used for quality assessment of cohort and case-control studies, a maximum of nine scores were assigned to eight items which indicated the methodological quality of each study. We divided the study quality into three categories (1) high quality (scored 7–9); (2) moderate quality (scored 4–6); and (3) low quality (scored 0–3).
2.4. Data Analysis
We combined the results using the OR as a measure of the association between smoking and ASD risk. Heterogeneity was investigated by the Cochrane Q statistic (significance level at p < 0.10) and the I2 statistic [10,11]. The random effect model was used to calculate the pooled OR if p > 0.10 and I2 ≤ 50%, otherwise the fixed effect model was chosen [12]. Sensitivity analysis was performed to assess whether any individual study significantly affected pooled estimates by omitting one study in each turn. Additionally, subgroup analyses were performed according to study design, study location, and whether they were adjusted for confounding variables to examine the source of potential heterogeneity. We used both Begg’s test [13] and Egger’s test [14] to detect publication bias. Stata version 11.0 (Stata Corporation) was used for all analysis. All statistical tests in this study were two-sided with a significance level of 0.05, unless otherwise specified.
3. Results
3.1. Literature Search and Selection
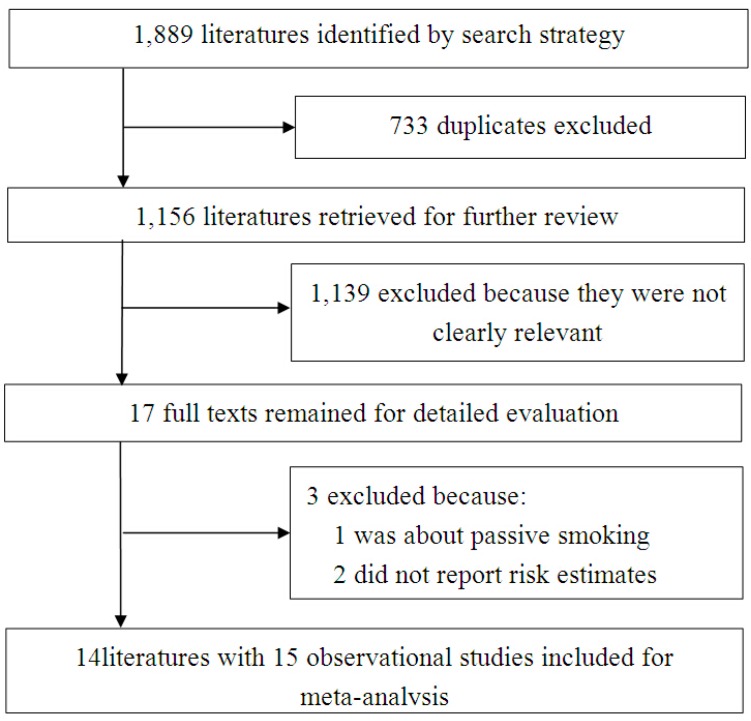
Figure 1 shows a flow diagram of the selection process. A total of 1889 literatures were identified by the search strategy. After removal of duplicate literatures, 1156 articles were left for screening. By screening the titles and abstracts, 1140 articles were excluded as they were not clearly relevant. After detailed evaluation of the 16 full texts, one article [15] that reported passive smoking and two articles [16,17] that did not reported risk estimates was excluded. Due to the fact that one article [18] met the inclusion criteria reported results based on two separate cohort studies, 14 articles [6,18,19,20,21,22,23,24,25,26,27,28,29,30] with 15 studies were included for meta-analysis.
Figure 1.
Flow diagram of the study selection process.
3.2. Study Characteristics
Table 1 listed the characteristics of the included studies. Of the 15 include studies, there were six cohort studies [18,20,21,26,30] and nine case-control studies [6,19,22,23,24,25,27,28,29], with a total of 17,890 cases and 1,810,258 participants involved. 10 studies [6,18,22,23,25,26,27,28,29] were from Europe (Denmark, Sweden, Finland, Norway and Poland) while five studies [19,20,21,24,30] were from America (Canada and US). The information about smoking was generally collected during pregnancy, and ASDs were ascertained by medical records in most of the included studies. Overall, the methodological quality was good (average score: 7.3), with 12 studies [6,18,19,21,22,23,24,25,27,28,30] in high quality and three studies [20,26,29] in moderate quality (Supplemental Table 1).
Table 1.
Characteristics of included studies of maternal smoking during pregnancy and ASD risk in the meta-analysis.
| Study | Location | Design a | ASD Cases/Total Participants | Age(y), Sex | Ascertainment of Smoking | Ascertainment of ASDs | OR(95%CI) | Confounding |
|---|---|---|---|---|---|---|---|---|
| Hultman 2002 [23] | Sweden | CC | 408/2448 | <10, M&F b | Collected by midwives at registration for antenatal care | Medical record | 1.4(1.1,1.8) | Maternal age, parity, mothers’ country of birth, hypertensive diseases, diabetes, mode of delivery, pregnancy bleeding, season of birth, gestational age, birth weight for gestational age, Apgar at 5 min, congenital malformations |
| Larsson 2005 [25] | Denmark | CC | 249/6474 | ≤24, M&F | Reported at the first antenatal visit | Medical record | 1.06(0.80,1.39) | NA |
| Maimburg 2006 [28] | Denmark | CC | 473/5203 | <10, M&F | Collected by midwives at the first antenatal visit | Medical record | 0.9(0.7,1.4) | Mother and fathers age, mothers citizenship, birth weight and gestational age, Apgar, birth defect and irregular foetal position |
| Bilder 2009 [19] | US | CC | 132/13,332 | 8, M&F | NAc | Clinician review of medical and school record | 0.506(0.222,1.152) | NA |
| Larsson 2009 [26] | Sweden | C | 72/4779 | 6–8, M&F | Parent-report collected for pregnancy when child was age 1–3 years | Parent-report collected by follow-up questionnaire | 2.09(1.08,4.03) | Unadjusted |
| Burstyn 2010 [20] | Canada | C | 1138/218,890 | ≤9, M&F | Collected on admission to hospital for delivery | Medical registry record | 0.86(0.72,1.02) | Maternal age, maternal weight, maternal height, pre-pregnancy diabetes, gestational diabetes, bleeding, weight gain, parity, socio-economic status, pre-eclampsia, presentation, type of labour, delivery by caesarian section, gestational age, birth weight, apgar at 1 min, Apgar at 5 min, birth year |
| Dodds 2010 [21] | Canada | C | 924/129,733 | 1–17, M&F | Investigated by a standardized questionnaire | Administrative databases with relevant diagnostic information | 0.93(0.81,1.08) | Unadjusted |
| Haglund 2011 [22] | Sweden | CC | 157/68,964 | 8–15, M&F | Swedish Medical Birth Registry | Medical registry | 0.7(0.5,1.0) | Year of birth, maternal age at delivery, parity , sex, gestational age at birth, standard deviations scores, obstetrical risk factor |
| Kalkbrenner 2012 [24] | US | CC | 3315/633,989 | 8, M&F | Birth certificate data | Surveillance-ascertained | 0.9(0.8,1.01) | Maternal education, race and ethnicity, marital status, maternal age, county population size, birth year and surveillance site. |
| Lee 2012 [27] | Sweden | CC | 3958/42,941 | 4–17, M&F | Recorded by midwives at the first prenatal visit | Medical registry | 1.10(1.01,1.20) | Unadjusted |
| Tran 2013 [6] | Finland | CC | 4019/20,601 | ≤7, M&F | Collected by maternity clinic nurses during routine obstetric visits | Medical registry | 1.0(0.9,1.2) | Maternal age, maternal mental diagnosis, socioeconomic status and weight for gestational age |
| Nilsen 2013 [18] | Norway | C | 2072/507,856 | 3–11, M&F | Recorded by check boxes at the beginning and the end of pregnancy | Medical registry | 1.20(0.84,1.71) | Year of birth, maternal age, marital status, hospital size. |
| Norway | C | 234/89,836 | 3–11, M&F | The same as above | Medical registry | 1.17(1.04,1.31) | The same as above | |
| Mrozek-Budzyn 2013 [29] | Poland | CC | 96/288 | 2–15, M&F | Investigated by a standardized questionnaire | Medical record | 3.32(1.23,9.82) | Unadjusted |
| Xiang 2015 [30] | US | C | 643/64,924 | 1.5–2 M&F | Extracted from medical records and birth certificate records | Screened by a modified version of CHAT d and diagnosed by pediatric developmental specialist evaluation | 0.83(0.33,2.09) | Birth year |
a C: cohort study CC: case-control study; b NA: not available; c M&F: male and female; d CHAT: Checklist for Autism in Toddlers.
3.3. Main Analysis
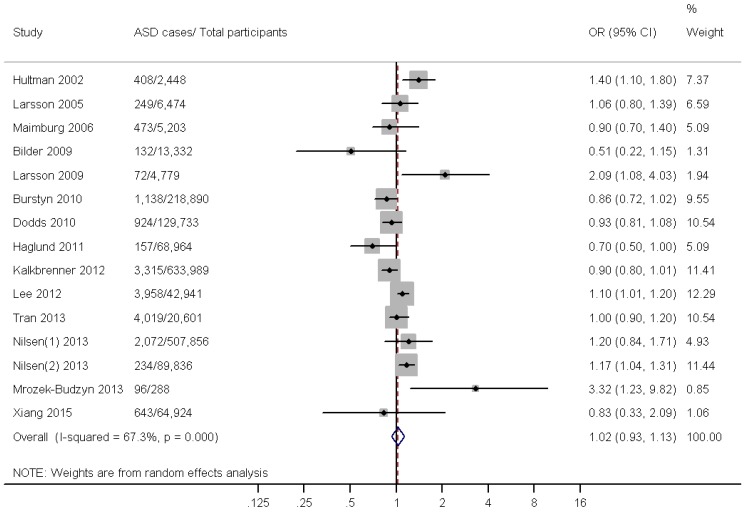
The meta-analysis data that explored the effect of maternal smoking during pregnancy on the risk of ASDs is shown in Figure 2. A statistically significant heterogeneity was found across studies (pheterogeneity < 0.01, I2 = 67.3%). The pooled OR estimates based on 15 studies showed that maternal smoking during pregnancy was not associated with an increased risk of ASDs (OR = 1.02; 95% CI: 0.93–1.13).
Figure 2.
A forest plot of the association between maternal smoking during pregnancy and ASD risk.
3.4. Subgroup and Sensitivity Analysis
Table 2 presents the results of subgroup analysis stratified by study design, study location, and adjustment for confounders. Maternal smoking during pregnancy was associated with significantly reduced risk of ASDs in the North American populations (OR = 0.90, 95%CI: 0.83–0.97), but not European populations (OR = 1.11, 95%CI: 0.99–1.24). In subgroup analysis by study design and adjustment for confounders, the difference in pooled ORs across strata was not statistically significant. For the sensitivity analysis, the pooled ORs did not vary materially ranging from 1.00(95% CI: 0.91–1.10) when omitted Hultman et al. study [23] to 1.05(95% CI: 0.95–1.15) when excluded Dodds et al. study [21].
Table 2.
Summary results of association between maternal smoking during pregnancy and ASD risk.
| Variables | No. of Studies | I2% | pheterogeneity | OR(95%CI) |
|---|---|---|---|---|
| All studies | 15 | 67.3 | <0.001 | 1.02(0.93,1.13) |
| Location | ||||
| Europe | 10 | 60.1 | 0.007 | 1.11(0.99,1.24) |
| America | 5 | 0.00 | 0.671 | 0.90(0.83,0.97) |
| Design | ||||
| Cohort | 6 | 68.9 | 0.007 | 1.05(0.88,1.25) |
| Case-control | 9 | 70.0 | 0.001 | 1.01(0.88,1.15) |
| Study quality | ||||
| High | 12 | 60.4 | 0.003 | 1.02(0.92, 1.12) |
| Moderate | 3 | 83.1 | 0.003 | 1.65(0.73, 3.73) |
| Adjustment for confounders | ||||
| Yes | 9 | 69.0 | 0.001 | 1.00(0.88,1.13) |
| No/NA * | 6 | 68.2 | 0.008 | 1.08(0.89,1.32) |
* NA: not available.
3.5. Publication Bias
Begg’s test and Egger’s test suggested no significant publication bias among the included studies (all p > 0.05).
4. Discussion
The present study used a comprehensive search strategy and explored the relationship between maternal smoking during pregnancy and ASDs based on 15 observational studies. However, the overall results did not suggest a significant association between maternal smoking during pregnancy and ASDs risk.
Several hypotheses of association between maternal smoking during pregnancy and ASD are plausible. First, nicotine is the chemical that most probably has adverse effects on brain development among thousands of ingredients of tobacco smoke [31] and its effects are thought to occur via its action on nicotinic acetylcholine receptors [32]. Second, women with lower socioeconomic status (SES) have a higher tendency to smoke during pregnancy compared with those with higher SES [33,34]; nevertheless, low parental SES is associated with an increased risk of ASD [35]. A third explanation is the transmission of genetic risk from mothers to their offspring, as ASD is highly genetic [4] and smoking during pregnancy is more likely to happen in women with a predisposition to mental health and behavioral problems [36]. Another hypothesis was that maternal smoking related high levels of intrauterine testosterone might contribute to the increased ASD risk [37].
Despite all the hypotheses mentioned above, the pooled OR failed to find a positive association between maternal smoking during pregnancy and ASDs. However, in the subgroup analysis, a significant negative association was observed in the North America. Although the association was not statistically significant in the individual studies of this subgroup, the increased sample size narrowed the confidence interval of the pooled result and thus it became statistically significant. Nevertheless, further studies are needed to examine this contrary finding, considering that the results were not fully adjusted and potential bias may exist due to the design of included studies.
Our study has several limitations that deserve consideration. First, because this meta-analysis was based on observational studies, our results were prone to recall and selection bias inherent in the included studies. In addition, the potential confounding from other risk factors could not be ruled out, especially when considering that some studies only reported crude risk estimated without adjustments. Second, potential misclassification of maternal smoking may lead to incorrect estimates of associations. The smoking status was generally collected at early pregnancy and was based on self-report, there was a possibility that participants tend to narrow their smoking status and the smoking habit might change during pregnancy. Third, high statistical heterogeneity was found across the studies that may weaken the strength of our findings; however, the subgroup analysis, as well as sensitivity analysis, showed that results from our meta-analysis was robust. Finally, the included studies of our meta-analysis were mainly conducted in the North Europe and the North America. The findings of this study had limited implications for other populations, like the Africans and Asians, as both the genetic and environmental factors in relation to autism varied among different ethnicities.
5. Conclusions
This meta-analysis indicates that there is no association between maternal smoking during pregnancy and ASD. Given the limited number of cohort studies included in this study, the present findings should be confirmed in further prospective cohort studies.
Acknowledgments
The authors would like to thank the Renmin Hospital of Wuhan University for providing financial support for publishing this work in an open access journal.
Supplemental Material
Supplemental Table 1.
Quality assessment based on the Ottawa-Newcastle Scale.
| Case-Control Study | Definition of Cases | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Control * | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate | Total Score |
|---|---|---|---|---|---|---|---|---|---|
| Hultman 2002 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Larsson 2005 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Maimburg 2006 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Bilder 2009 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Haglund 2011 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Kalkbrenner 2012 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Lee 2012 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 7 |
| Tran 2013 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Mrozek-Budzyn 2013 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Cohor study | Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | outcome of interest was not present at start of study | Comparability of cohorts * | Assessment of outcome | follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | |
| Larsson 2009 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Burstyn 2010 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Dodds 2010 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Nilsen(1) 2013 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Nilsen(2) 2013 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Xiang 2015 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
* A maximum of 2 points were assigned to this item.
Author Contributions
Gaohua Wang conceived and designed the experiments; Shiming Tang and Ying Wang performed the experiments; Shiming Tang, Ying Wang and Xuan Gong analyzed the data; Xuan Gong contributed reagents/materials/analysis tools; Gaohua Wang, Shiming Tang and Ying Wang wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lai M.C., Lombardo M.V., Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 2.Christensen J., Gronborg T.K., Sorensen M.J., Schendel D., Parner E.T., Pedersen L.H., Vestergaard M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter A.J., Brugha T.S., Erskine H.E., Scheurer R.W., Vos T., Scott J.G. The epidemiology and global burden of autism spectrum disorders. Psychol. Med. 2014;45:601–613. doi: 10.1017/S003329171400172X. [DOI] [PubMed] [Google Scholar]
- 4.Levy S.E., Mandell D.S., Schultz R.T. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pineles B.L., Park E., Samet J.M. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am. J. Epidemiol. 2014;179:807–823. doi: 10.1093/aje/kwt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran P.L., Lehti V., Lampi K.M., Helenius H., Suominen A., Gissler M., Brown A.S., Sourander A. Smoking during pregnancy and risk of autism spectrum disorder in a Finnish National Birth Cohort. Paediatr. Perinat. Epidemiol. 2013;27:266–274. doi: 10.1111/ppe.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen B.N., Lee B.K., Lee N.L., Yang Y., Burstyn I. Maternal Smoking and Autism Spectrum Disorder: A Meta-analysis. J. Autism Dev. Disord. 2015;45:1689–1698. doi: 10.1007/s10803-014-2327-z. [DOI] [PubMed] [Google Scholar]
- 8.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Ji J., Liu Y.-J., Deng X., He Q.-Q. Passive Smoking and Risk of Type 2 Diabetes: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0069915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 14.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan G., Yao M., Ma Y., Zhang W. Perinatal and background risk factors for childhood autism in central China. Psychiatry Res. 2014;220:410–417. doi: 10.1016/j.psychres.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 16.Ronald A., Happe F., Dworzynski K., Bolton P., Plomin R. Exploring the relation between prenatal and neonatal complications and later autistic-like features in a representative community sample of twins. Child Dev. 2010;81:166–182. doi: 10.1111/j.1467-8624.2009.01387.x. [DOI] [PubMed] [Google Scholar]
- 17.Visser J.C., Rommelse N., Vink L., Schrieken M., Oosterling I.J., van der Gaag R.J., Buitelaar J.K. Narrowly versus broadly defined autism spectrum disorders: Differences in pre- and perinatal risk factors. J. Autism Dev. Disord. 2013;43:1505–1516. doi: 10.1007/s10803-012-1678-6. [DOI] [PubMed] [Google Scholar]
- 18.Nilsen R.M., Suren P., Gunnes N., Alsaker E.R., Bresnahan M., Hirtz D., Hornig M., Lie K.K., Lipkin W.I., Reichborn-Kjennerud T., et al. Analysis of self-selection bias in a population-based cohort study of autism spectrum disorders. Paediatr. Perinat. Epidemiol. 2013;27:553–563. doi: 10.1111/ppe.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilder D., Pinborough-Zimmerman J., Miller J., McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics. 2009;123:1293–1300. doi: 10.1542/peds.2008-0927. [DOI] [PubMed] [Google Scholar]
- 20.Burstyn I., Sithole F., Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis. Canada. 2010;30:125–134. [PubMed] [Google Scholar]
- 21.Dodds L., Fell D.B., Shea S., Armson B.A., Allen A.C., Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J. Autism Dev. Disord. 2011;41:891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- 22.Haglund N.G., Kallen K.B. Risk factors for autism and Asperger syndrome. Perinatal factors and migration. Autism. 2011;15:163–183. doi: 10.1177/1362361309353614. [DOI] [PubMed] [Google Scholar]
- 23.Hultman C.M., Sparen P., Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology (Cambridge Mass.) 2002;13:417–423. doi: 10.1097/00001648-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Kalkbrenner A.E., Braun J.M., Durkin M.S., Maenner M.J., Cunniff C., Lee L.C., Pettygrove S., Nicholas J.S., Daniels J.L. Maternal smoking during pregnancy and the prevalence of autism spectrum disorders, using data from the autism and developmental disabilities monitoring network. Environ. Health Perspect. 2012;120:1042–1048. doi: 10.1289/ehp.1104556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson H.J., Eaton W.W., Madsen K.M., Vestergaard M., Olesen A.V., Agerbo E., Schendel D., Thorsen P., Mortensen P.B. Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. Am. J. Epidemiol. 2005;161:916–925; discussion 926–928. doi: 10.1093/aje/kwi123. [DOI] [PubMed] [Google Scholar]
- 26.Larsson M., Weiss B., Janson S., Sundell J., Bornehag C.G. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. Neurotoxicology. 2009;30:822–831. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee B.K., Gardner R.M., Dal H., Svensson A., Galanti M.R., Rai D., Dalman C., Magnusson C. Brief report: Maternal smoking during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2012;42:2000–2005. doi: 10.1007/s10803-011-1425-4. [DOI] [PubMed] [Google Scholar]
- 28.Maimburg R.D., Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatr. Scand. 2006;114:257–264. doi: 10.1111/j.1600-0447.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 29.Mrozek-Budzyn D., Majewska R., Kieltyka A. Prenatal, perinatal and neonatal risk factors for autism—Study in Poland. Cent. Eur. J. Med. 2013;8:424–430. doi: 10.2478/s11536-013-0174-5. [DOI] [Google Scholar]
- 30.Xiang A.H., Wang X., Martinez M.P., Walthall J.C., Curry E.S., Page K., Buchanan T.A., Coleman K.J., Getahun D. Association of maternal diabetes with autism in offspring. JAMA. 2015;313:1425–1434. doi: 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- 31.Tiesler C.M., Heinrich J. Prenatal nicotine exposure and child behavioural problems. Eur. Child Adolesc. Psychiatry. 2014;23:913–929. doi: 10.1007/s00787-014-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dwyer J.B., McQuown S.C., Leslie F.M. The dynamic effects of nicotine on the developing brain. Pharmacol. Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekblad M., Gissler M., Korkeila J., Lehtonen L. Trends and risk groups for smoking during pregnancy in Finland and other Nordic countries. Eur. J. Public Health. 2014;24:544–551. doi: 10.1093/eurpub/ckt128. [DOI] [PubMed] [Google Scholar]
- 34.Raisanen S., Kramer M.R., Gissler M., Saari J., Hakulinen-Viitanen T., Heinonen S. Smoking during pregnancy was up to 70% more common in the most deprived municipalities—A multilevel analysis of all singleton births during 2005–2010 in Finland. Prev. Med. 2014;67:6–11. doi: 10.1016/j.ypmed.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Rai D., Lewis G., Lundberg M., Araya R., Svensson A., Dalman C., Carpenter P., Magnusson C. Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population-based study. J. Am. Acad. Child Adolesc. Psychiat. 2012;51:467–476. doi: 10.1016/j.jaac.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal A., Scherrer J.F., Grant J.D., Sartor C.E., Pergadia M.L., Duncan A.E., Madden P.A., Haber J.R., Jacob T., Bucholz K.K., et al. The effects of maternal smoking during pregnancy on offspring outcomes. Prev. Med. 2010;50:13–18. doi: 10.1016/j.ypmed.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James W.H. Potential explanation of the reported association between maternal smoking and autism. Environ. Health Perspect. 2013;121 doi: 10.1289/ehp.1206268. [DOI] [PMC free article] [PubMed] [Google Scholar]