Abstract
OBJECTIVE:
Compare effectiveness of maternal vitamin D3 supplementation with 6400 IU per day alone to maternal and infant supplementation with 400 IU per day.
METHODS:
Exclusively lactating women living in Charleston, SC, or Rochester, NY, at 4 to 6 weeks postpartum were randomized to either 400, 2400, or 6400 IU vitamin D3/day for 6 months. Breastfeeding infants in 400 IU group received oral 400 IU vitamin D3/day; infants in 2400 and 6400 IU groups received 0 IU/day (placebo). Vitamin D deficiency was defined as 25-hydroxy-vitamin D (25(OH)D) <50 nmol/L. 2400 IU group ended in 2009 as greater infant deficiency occurred. Maternal serum vitamin D, 25(OH)D, calcium, and phosphorus concentrations and urinary calcium/creatinine ratios were measured at baseline then monthly, and infant blood parameters were measured at baseline and months 4 and 7.
RESULTS:
Of the 334 mother-infant pairs in 400 IU and 6400 IU groups at enrollment, 216 (64.7%) were still breastfeeding at visit 1; 148 (44.3%) continued full breastfeeding to 4 months and 95 (28.4%) to 7 months. Vitamin D deficiency in breastfeeding infants was greatly affected by race. Compared with 400 IU vitamin D3 per day, 6400 IU/day safely and significantly increased maternal vitamin D and 25(OH)D from baseline (P < .0001). Compared with breastfeeding infant 25(OH)D in the 400 IU group receiving supplement, infants in the 6400 IU group whose mothers only received supplement did not differ.
CONCLUSIONS:
Maternal vitamin D supplementation with 6400 IU/day safely supplies breast milk with adequate vitamin D to satisfy her nursing infant’s requirement and offers an alternate strategy to direct infant supplementation.
What’s Known on This Subject:
The vitamin D concentration in breast milk of women taking 400 IU vitamin D per day is relatively low, leading to vitamin D deficiency in breastfeeding infants. As a result, the American Academy of Pediatrics recommends breastfeeding infant vitamin D supplementation within days after birth.
What This Study Adds:
Maternal vitamin D supplementation alone with 6400 IU/day safely supplies breast milk with adequate vitamin D to satisfy the requirement of her nursing infant and offers an alternate strategy to direct infant supplementation.
Breast milk has long been held as the “perfect” food for the human neonate with one caveat: it contains insufficient vitamin D for nursing neonates to maintain minimal circulating levels of the precursor hormone 25-hydroxy-vitamin D (25(OH)D; calcidiol), and thus skeletal integrity.1 In fact, when compared with formula-fed infants, solely breastfed infants are at increased risk of developing rickets.2,3 This is especially true in African American breastfed infants.4 Vitamin D activity in “normal” lactating women’s milk is known to be in the range of 5 to 80 IU/L depending on the method of assay1,5,6; however, the vitamin D content of human milk can be greatly increased by maternal oral vitamin D supplementation and/or increasing solar exposure of the mother.7–9 Infants solely breastfed by women with vitamin D intakes of 400 IU/day typically attain a circulating 25(OH)D concentration in the marginally sufficient to severely deficient (<12.5 nmol/L) range.10 Therefore, to address this risk of deficiency, supplementation of all breastfeeding infants beginning within a few days of birth has been recommended by both the American Academy of Pediatrics (AAP)11 and the Institute of Medicine (IOM).1 Although this has been the recommendation for decades, it is rarely followed for various reasons, with low compliance ranging from 2% to 19%,12–15 leaving the nursing infant at significant risk for vitamin D deficiency.
The amount of vitamin D required by a lactating woman to normalize her own vitamin D status and ensure adequate vitamin D concentrations in her milk for her breastfeeding infant is predicted by known pharmacokinetics about vitamin D transfer into human milk.7,8,16–18 Early studies demonstrated some effectiveness of maternal vitamin D supplementation on increasing circulating 25(OH)D levels in nursing infants.7,8,16,19,20 Our research group performed an interventional study providing 6400 IU vitamin D3 per day to lactating mothers for a 6-month period that produced dramatic increases in both milk vitamin D and infant circulating 25(OH)D concentrations.8 The results of that pilot study became the basis for this larger National Institute of Child Health and Human Development, 2-site randomized clinical trial (RCT) using 3 maternal doses of oral vitamin D3 in a diverse group of women for a 6-month period starting at 1 month postpartum. Baseline characteristics of the lactating mother and infant cohort have been published previously.21
Our study was designed to test the primary hypothesis that the lactating woman requires substantially more dietary vitamin D than the amount received from maternal supplementation with 400 IU/day.1,11 We based our maternal supplementation dosing on previous studies: for every 1000 IU per day vitamin D3, milk antirachitic activity would increase by ∼80 IU/L in a way that would sustain the nursing infant.8,22 Thus, if successful, our strategy could offer an alternative to the largely failed direct infant supplementation strategy.1,11,14,15 The findings of this supplementation trial are presented here.
Methods
Design
This was a randomized, double-blind, comparative effectiveness trial of 3 doses of vitamin D supplementation in lactating mothers and their breastfeeding infants (November 2005–August 2012). The study was conducted at the Medical University of South Carolina (MUSC) and the University of Rochester (U of R). Approval was granted by (1) MUSC’s Institutional Review Board for Human Subjects HR 16536 and Clinical and Translational Research Center (CTRC; Protocol 752); and (2) U of R’s Institutional Review Board (14460) and CTRC (Protocol 1129), and registered via ClinicalTrials.gov NCT00412074.
Following written informed consent, mothers were randomized to 1 of 3 vitamin D supplementation regimens: Group 1: 400 IU vitamin D3 per day (0 IU vitamin D3: placebo and 1 prenatal vitamin containing 400 IU vitamin D3); Group 2: 2400 IU (2000 vitamin D3 per day and 1 prenatal containing 400 IU vitamin D3); and Group 3: 6400 IU vitamin D3 per day (6000 IU vitamin D3 and 1 prenatal vitamin containing 400 IU vitamin D3). Breastfeeding infants also were given 1 drop per day of a liquid suspension vitamin D supplement (Bio-D-Mulsion, Biotics Research, Rosenberg, TX) as follows: those infants in Group 1 received 400 IU vitamin D3 as previously described,23 and infants in Groups 2 and 3 received a placebo emulsion containing 0 IU vitamin D3 for the 6-month study period.
Mothers and infants were evaluated monthly with maternal blood samples and urine samples obtained at those visits. Infant urine samples were obtained monthly but blood samples were drawn only at baseline (4–6 weeks postpartum; V1), month 4 (V4), and month 7 (V7).
Participants
Exclusively breastfeeding mothers and their singleton infants receiving no other form of nutrition other than human milk at the time of study entry24,25 within 4 to 6 weeks postpartum were eligible for inclusion in the study if they planned to continue exclusive/full breastfeeding for the next 6 months.24,26 Infants had to be ≥35 weeks’ gestation and in good general health at the time of enrollment. Exclusion criteria are summarized in the Methods section of the Supplemental information.
Outcome Measures
Laboratory Measurements
Maternal and infant baseline serum calcium and phosphorus were measured using standard methodology and laboratory normative data by MUSC’s and U of R’s Clinical Chemistry Laboratories. Cross-validation between laboratories was performed for 5% of the samples (interassay variation 5.4%).
Circulating 25(OH)D and vitamin D (parent compound) were measured using high performance liquid chromatography and radioimmunoassay techniques as previously described.27–30 On the basis of clinical laboratory classifications31,32 and the work of Heaney et al33 and Vieth et al,35,36 deficiency was defined a priori as total circulating 25(OH)D <50 nmol/L (<20 ng/mL).16,30,32,36 The inter- and intraassay coefficient of variation was ≤10% (see Methods in the Supplemental Information).
Maternal and Infant Circulating intact PTH Concentrations were measured by immunoradiometric assay (Diasorin, Stillwater, MN).37
Statistical Methods
Sample Size and Power Considerations
One hundred and eighty-nine participants were to be randomized into 3 treatment arms, with 63 per supplementation arm substratified by race/ethnicity. With stopping of the 2400 IU arm (see Methods in the Supplemental Information for details), there were to be 126 mother/infant pairs enrolled in the two remaining arms. The loss of the 2400 IU group (see Methods in the Supplemental Information) did not alter the capability of the other arms in assessing their effectiveness because each can be viewed as an independent trial.
Of the 564 women who consented to participate in the study, 175 women/infant pairs met exclusion criteria (Fig 1). Of the remaining women, 389 women met the criterion for exclusive breastfeeding at entrance into the study and had baseline 25(OH)D concentrations measured; 55 were in the 2400 IU group and excluded from final analysis, with 334 mother/infant pairs randomized to the 400 IU and 6400 IU groups. Allocation, interim analysis, follow-up, and final analysis as well as reasons for exclusion at V4 and V7 are included in Fig 1 and in the Results section of the Supplemental Information.
FIGURE 1.
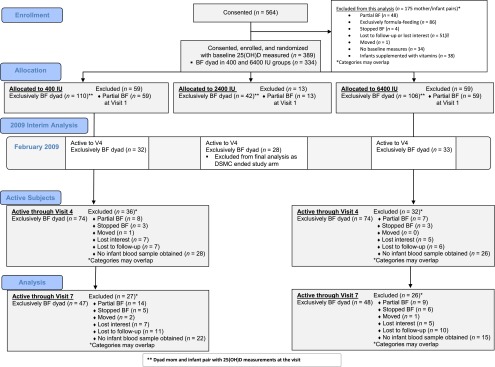
CONSORT Flow Diagram of Study Participants throughout the Trial. CONSORT, Consolidated Standards of Reporting Trials.
Statistical Analyses
The primary outcome was change from baseline maternal and infant total circulating 25(OH)D concentrations at 4 and 7 months postpartum in exclusively/fully lactating pairs by treatment group, and the secondary outcome was the percent of women and infants by treatment group with 25(OH)D <50 nmol/L at baseline, 4 and 7 months’ postpartum (V4 and V7).
The analysis was undertaken as intention-to-treat in which all exclusively/fully breastfeeding mothers randomized to 1 group were considered to be within that group throughout the analysis.38 Comparison of the 3 treatment groups at entrance into the study was performed to detect potential differences with regard to sociodemographic and baseline clinical characteristics (no statistically significant differences; data not shown). After stopping the 2400 IU arm due to safety concerns for the infants (see Methods in the Supplemental Information), time-point measures were restricted to the 2 remaining 400 IU and 6400 IU treatment groups.
Statistical analyses were performed by using SAS version 9.3 (SAS, Cary, NC). Descriptive statistics were used to characterize and compare the groups at baseline. χ2 and analysis of variance were used to test for differences in categorical data. Student’s t test analyses were used to test for differences in normally distributed variables. The Wilcoxon rank-sum test was used for analyses involving nonparametric variables. Regression methods (multivariate and logistic) included variables that were significant in bivariate analysis to model 25(OH)D status. Correlation analysis was performed by Spearman’s correlation. Significance was set a priori as P < .05.
Results
Of the 334 women randomized into the 400 and 6400 IU arms of the study who had baseline 25(OH)D concentration measured, 118 women stopped exclusively breastfeeding after randomization. Of the remaining 216-exclusively/fully breastfeeding mother-infant pairs enrolled and randomized into the 400 and 6400 IU arms of the study with baseline 25(OH)D values, 148 (64.7%) continued to exclusively/fully breastfeed and completed the study to V4; 95 (28.4%) completed the study through visit 7. As shown in Fig 1, the main reason for subject attrition was change of breastfeeding status. Table 1 summarizes baseline sociodemographic and clinical characteristics data in the 400 vs 6400 IU groups of mothers who were exclusively/fully breastfeeding at the time of enrollment. The average maternal dietary vitamin D intake (IU/day) was ∼200 IU/day.
TABLE 1.
Exclusively/Fully Breastfeeding Maternal and Infant Sociodemographic and Clinical Characteristics by Vitamin D Supplementation Group at V1
| Characteristic | 400 IU Group (n = 110) n (%) or Median (Range, n) | 6400 IU Group (n = 106) n (%) or Median (Range, n) | P |
|---|---|---|---|
| Maternal race/ethnicity | .5 | ||
| Black | 26 (23.6) | 23 (21.7) | |
| Hispanic | 32 (29.1) | 25 (23.6) | |
| White | 52 (47.3) | 58 (54.7) | |
| Education | .7 | ||
| Less than high school education | 16 (14.6) | 14 (13.2) | |
| High school graduate | 22 (20.0) | 17 (16.0) | |
| College or more | 72 (65.5) | 75 (70.8) | |
| Employed full-time at study entrance | 72 (65.5) | 75 (70.8) | .4 |
| Insurance | .2 | ||
| Commercial | 49 (44.6) | 57 (53.8) | |
| Medicaid/none | 61 (55.5) | 49 (46.2) | |
| BMI >30 | 28 (25.5) | 21 (19.8) | .3 |
| Season at study entry | .5 | ||
| April–September | 57 (51.8) | 60 (56.6) | |
| October–March | 53 (48.2) | 46 (43.4) | |
| Interpregnancy interval (mo) | 24.0 (1.0–132.0, 83) | 24.0 (1.0–156.0, 81) | .4 |
| Parity | 2.0 (0.0–6.0, 110) | 2.0 (0.0–5.0, 106) | .4 |
| Maternal Health Rating Scale | 9.5 (0.0–10.0, 110) | 9.00 (0.0–10.0, 106) | .9 |
| Maternal age (y) | 28.7 ± 6.5 (18.0–48.0, 110) | 29.0 ± 5.8 (18.0–42.0, 106) | .8 |
| Maternal wt (lb) | 156.5 ± 35.3 (90.6–266.1, 110) | 161.2 ± 30.4 (95.9–266.5, 105) | .3 |
| Maternal BMI | 27.8 ± 5.5 (19.4–46.7, 85) | 27.4 ± 4.3 (19.5–40.8, 85) | .6 |
| Days postpartum | 37.5 ± 8.6 (3.0–68.0, 110) | 36.0 ± 7.2 (7.0–64.0, 104) | .2 |
| Maternal smart probe forearm | 54.1 ± 9.5 (31.7–69.2, 110) | 55.0 ± 9.5 (32.7–68.4, 106) | .5 |
| Maternal vitamin D dietary intake (IU) | 234.8 ± 147.4 (29.3–562.9, 49) | 201.5 ± 119.1 (28.7–593.7, 59) | .2 |
| Maternal Kcal intake | 2378.4 ± 919.0 (873.7–5220.6, 49) | 2274.7 ± 883.7 (806.5–5145.9, 59) | .6 |
| Maternal calcium intake, mg/day | 1236.2 ± 522.5 (332.5–2641.5, 49) | 1201.4 ± 500.2 (418.2–2486.1, 59) | .7 |
| Maternal baseline total circulating 25(OH)D (nmol/L) | 82.1 ± 31.8 (14.5–230.3, 110) | 90.7 ± 34.6 (20.8–191.0, 106) | .06 |
| Maternal baseline total circulating vitamin D3 (nmol/L) | 9.5 ± 21.5 (1.5–159.5, 76) | 6.4 ± 10.0 (1.5–60.5, 71) | .2 |
| Infant birth wt (g) | 3345.6 ± 475.7 (2133.0–4443.0, 110) | 3460.3 ± 481.1 (2370.0–4840.0, 106) | .08 |
| Infant gestational age (wk) | 39.3 ± 1.4 (34.0–42.0, 110) | 39.4 ± 1.0 (36.2–41.6, 104) | .5 |
| Infant fontanelle area (cm) | 9.5 ± 5.9 (0.8–30.0, 108) | 9.8 ± 6.1 (0.4–40.0, 104) | .7 |
| Infant birth head circumference (cm) | 37.8 ± 1.3 (34.5–41.5, 110) | 37.7 ± 1.5 (34.0–42.0, 105) | .6 |
| Infant length (cm) | 54.7 ± 2.4 (49.0–63.0, 110) | 54.5 ± 2.6 (47.0–59.5, 106) | .6 |
| Infant baseline total circulating 25(OH)D (nmol/L) | 33.7 ± 23.5 (2.5–106.5, 110) | 37.9 ± 23.3 (2.5–113.8, 106) | .2 |
Vitamin D status at baseline for mother and infant by race are found in Table 2. African American mothers and infants had substantially lower circulating 25(OH)D levels than did white subjects with several minority infants exhibiting severe vitamin D deficiency (2.5 nmol/L 25(OH)D) after 1 month of breastfeeding.
TABLE 2.
Baseline 25(OH)D (nmol/L)a at 1 Month Postpartum in Exclusively Breastfeeding Mothers and Infants by Race/Ethnicity Who Participated Through V4
| Race/Ethnicity | 25(OH)D (nmol/L), Mean ± SD (Range) |
|---|---|
| Mother | |
| Black/African American, n = 28 | 69.8 ± 27.7 (26.5–132.5) |
| Hispanic, n = 32 | 77.2 ± 24.5 (14.5–133.3) |
| White, n = 88 | 105.4 ± 32.7 (47.8–230.3) |
| Infant | |
| Black/African American, n = 28 | 24.1 ± 23.1, (≤2.5–113.8)b |
| Hispanic, n = 32 | 29.4 ± 20.8, (≤2.5–89.5)b |
| White, n = 88 | 43.4 ± 22.9, (10.5–106.5) |
Profound deficiency by the IOM’s Guidelines is defined as a 25(OH)D concentration <25 nmol/L (10 ng/mL) for both adults and children (including neonates and young infants).1
The level of detection of the assay for 25(OH)D is 2.5 nmol/L.
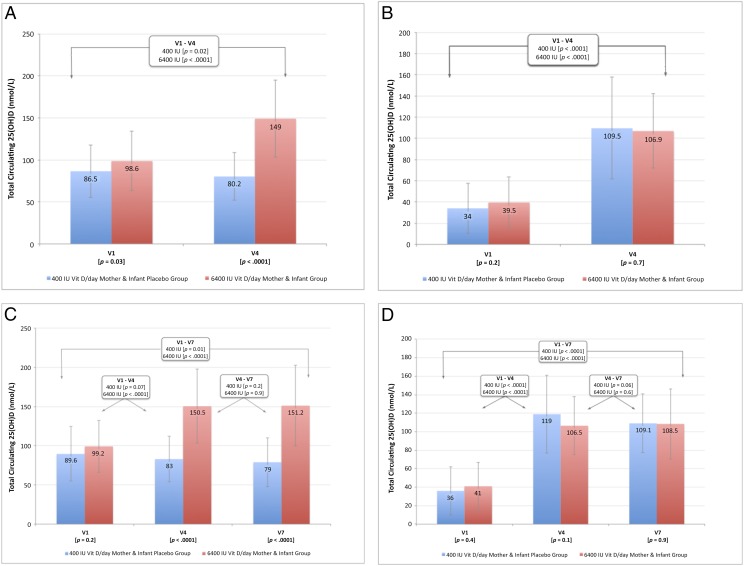
Comparison of maternal and infant laboratory parameters in the 400 IU and 6400 IU groups are found in Fig 2A, 2B, 2C, and 2D (see also Supplemental Tables 3, 4, 5, and 6). Whereas women who were exclusively/fully breastfeeding through V4 differed on baseline 25(OH)D by treatment group with a slightly higher initial concentration in the 6400 IU group, this difference was not seen in the group of women who were exclusively/fully breastfeeding through V7. There were similar numbers of women in both treatment groups who met the IOM definition of vitamin D deficiency (25(OH)D <50 nmol/L) at baseline. The other vitamin D–related laboratory values did not differ at baseline between the treatment groups.
FIGURE 2.
Total circulating 25(OH)D concentration (nmol/L) by treatment (400 IU vs 6400 IU groups) of breastfeeding mothers: A, through V4; B, through V7; and of breastfeeding infants: C, through V4; D, through V7.
By V4 there was a difference in maternal 25(OH)D (Fig 2A) and the parent compound vitamin D but not in other parameters measured. Compared with 6400 IU group, there was a trend with the 400 IU group at V4 being more likely to have 25(OH)D concentration <50 nmol/L. In those women who continued to fully breastfeed through V7, significant differences were noted by treatment group at V4 and V7 with 25(OH)D (Fig 2B) and vitamin D, and additionally at V7 only with iPTH and serum phosphorus being lower in the 6400 IU group. Within group comparisons of the mothers over time revealed the following: exclusively/fully lactating women in the 400 IU group had –6.5 nmol/L decline in 25(OH)D between V1 and V4 and –10.5 nmol/L decline in 25(OH)D between V1 and V7 (P = .02) compared with +51.3 nmol/L in the 6400 IU group between V1 and V4 that was sustained through V7 (P < .0001). In a model predicting maternal 25(OH)D that included race/ethnicity, treatment, and maternal BMI, treatment with 6400 IU was the strongest predictor (parameter estimate 67.2 ± 5.8 nmol/L; P < .0001).
Focusing on the infants in the study, those infants fully breastfed through V4 did not differ by treatment group on any of the parameters measured at either baseline or at V4 (see also Supplemental Tables 5 and 6). Of note, >70% of those babies at 1 month (V1) met the IOM definition of vitamin D deficiency (25(OH)D concentration <50 nmol/L). Those infants who were fully breastfed through V7 did not differ by treatment group at baseline, V4 or V7 on any of the parameters measured but there was deficiency at baseline in >75% of the infants at V1. By V4, there was marked improvement that was sustained to V7 in both treatment groups. Thus, infants whose only source of vitamin D was maternal (6400 IU group) did not differ from those infants who received oral supplementation of 400 IU/day (400 IU Group) on any of the laboratory parameters tested. Mean 25(OH)D (SD) by treatment group of exclusively/fully breastfeeding infants through V4 is depicted in Fig 2B, and through V7 is depicted in Fig 2D. Across the visits, there were no differences in infant serum calcium, creatinine, phosphorus, or urinary calcium/creatinine ratios.
When analyzed by treatment group, there were no differences in infant weight, length, and head circumference at any of the visits, which persisted even after controlling for race/ethnicity (data not shown). Baseline anterior fontanelle area (AFA) did not differ by treatment group (see Table 1). Maternal and infant 25(OH)D concentration at V1 correlated with AFA only in Hispanic infants (P < .05). At V4, there were significant differences between treatment groups: AFA 7.0 ± 4.8 cm2 in the 400 IU group infants versus 3.7 ± 3.7 cm2 in the 6400 IU group (P = .037). This difference was not seen in the subcohort of infants who continued to breastfeed through V7.
The number of adverse events and serious adverse events did not differ by treatment group. There were 7 adverse events among the breastfeeding mothers/infants equally distributed by treatment group. The Data and Safety Monitoring Committee (DSMC) deemed these events as not being related to treatment dose.
Discussion
In this study of 3 dosing schedules in lactating women and their exclusively/fully breastfeeding infants, maternal supplementation with 6400 IU vitamin D3/day was superior to either 2400 IU or 400 IU/day in safely achieving robust maternal vitamin D sufficiency that allowed sufficient vitamin D transfer in the breast milk for infant vitamin D sufficiency for the 6-month study period. Thus, when compared with infants receiving a daily oral vitamin D supplement of 400 IU/day, infants whose mothers were taking 6400 IU vitamin D daily (as their sole source of vitamin D) achieved equivalent vitamin D status. With appropriate vitamin D intake, the lactating mother can fully transfer from her blood to her milk the vitamin D required to sustain optimal vitamin D nutrition in the nursing infant with no additional supplementation required for the infant.8 Furthermore, the safety profiles of women in each treatment group were equivalent. As viewed by the DSMC, there were no instances of adverse events attributable to vitamin D supplementation.
When this study was initiated, the IOM upper limit for vitamin D was 2000 IU per day.39 An Investigational New Drug application to the US Food and Drug Administration was mandated to conduct both the current study and our pregnancy vitamin D supplementation trials.40,41 Since that time, the IOM has increased the upper limit to 4000 IU per day,1 and the Endocrine Society set the upper limit at 10 000 IU/day.42 During the past decade several studies, including our own, were performed using our original Food and Drug Administration Investigational New Drug application involving several thousand patients. To our knowledge, not a single adverse event has been attributed to vitamin D supplementation at the doses ranging from 2000 to 6400 IU/day.
It is universally accepted that vitamin D toxicity is associated with hypercalciuria, hypercalcemia, and risk of renal stones.1 In 2006, Jackson et al43 published the Women’s Health Initiative study that claimed an adjusted vitamin D intake of 280 IU per day resulted in an increase in renal stone incidence. These findings are in marked contrast to the results of a recent report involving several thousand subjects consuming up to 10 000 IU vitamin D per day for 1.5 years that demonstrated no relationship with renal stones.44 In our studies, we have never observed an event of hypercalciuria associated with vitamin D intake or circulating levels of 25(OH)D.7,8,40,41 Concern remains about vitamin D toxicity as it relates to mortality.45 A recent meta-analysis by Garland et al on the subject, however, clearly demonstrated increased all-cause mortality at low circulating levels with no such relationship at higher levels.467 Finally, the levels of circulating 25(OH)D we report here are robust and consistent with levels achieved in various populations involving only solar exposure with no dietary supplementation.47–49
Human milk has long been known to supply inadequate amounts of vitamin D to nutritionally support the solely breastfed infant.1,3,11 Over the decades, we and others have reported the vitamin D content of human milk and thus its antirachitic activity.8,22,28,50–52 These studies have provided valuable information. Universally, the antirachitic activity of human milk is quite low, 5 to 80 IU/L, unless the lactating mother is ingesting a significant amount of vitamin D daily or getting significant total body UV exposure.7,8,18 It is the parent compound, vitamin D itself, which overwhelmingly gets transferred into human milk from the maternal circulation.8,17,22,52,53 This is an important yet almost universally misunderstood fact. Although circulating vitamin D readily gains access to human milk, circulating 25(OH)D does not, and this transfer relationship occurs over a massive range of vitamin D intakes and/or circulating levels.8,22,52,53 Thus, one cannot assume that because a lactating mother’s circulating 25(OH)D level is adequate, her milk vitamin D activity will be. This is confirmed in our baseline data (Table 1) in which mothers had been breastfeeding their infants for 1 month. Maternal baseline circulating 25(OH)D levels were quite good, ∼80 to 90 nmol/L; however, infant circulating 25(OH)D levels were in the very low range, ∼35 nmol/L, with many exhibiting dire deficiency, <2.5 nmol/L. This is because circulating vitamin D3 in the mothers was low, and, in many cases, undetectable (<4 nmol/L), making mother’s milk a poor source of vitamin D activity. Why? Because the circulating half-life of 25(OH)D is 3 to 4 weeks, and that of vitamin D is ∼12 to 24 hours, reflecting their binding affinity to vitamin D binding protein.17 This reduced affinity of vitamin D3 allows the unbound vitamin D3 to diffuse across cell membranes from blood into the milk. This concept is discussed in depth elsewhere.17 Thus, a daily dose of vitamin D is required to sustain both circulating and milk levels of vitamin D in the lactating woman.
From the standpoint of nature, low vitamin D content in breast milk is an odd circumstance. Would nature allow so little vitamin D in breast milk that the nursing infant would develop rickets from ingesting it?1,3,11 We did not believe so. Our belief was that breast milk was deficient in vitamin D due solely to lack of solar exposure and dietary recommendations for vitamin D put forth in recent decades. The current IOM recommendation for vitamin D intake during lactation is 400 to 600 IU/d, yet historical data suggest that this level of maternal supplementation does nothing to increase the vitamin D content of her milk8,17,53 and/or support adequate nutritional vitamin D status in her nursing infant.7,8,19,52 This fact is precisely why the AAP recommends every nursing infant receive a daily supplement of 400 IU vitamin D.11 However, this last recommendation treats only the infant and does not address the core problem of why breast milk has such low concentrations of vitamin D. Also, the AAP recommendation11 is rarely followed as evidenced by our baseline entry data for breastfed infants (Table 1). In our study infants, only 12% were being given supplements at baseline, which concurs with previous reports.12–15 This is reflected by the base circulating 25(OH)D levels in nonsupplemented infants of ∼35 nmol/L following the first month of breastfeeding, which was less than half that of the supplemented breastfeeding infants (data not shown). This fact alone highlights how the AAP recommendation is ignored to the detriment of the infant.
The strengths of this 2-site study are that it was conducted at 2 distinct latitudes with strong racial/ethnic diversity such that the results can be applied to a wide-range of breastfeeding mothers and their infants. Additional strengths of the study are that it was conducted as an RCT to assess the comparative effectiveness of 3 treatments. Maternal and infant laboratory measures further ensured the safety of the higher dose treatment groups. Limitations of this study, however, are that of the original enrolled women, 64.7% at V1 and 44.3% at V4 were still exclusively breastfeeding. That rate continued to decrease in the ensuing months, with only 28.4% at 7 months still fully breastfeeding (with the addition of complementary foods at 6 months). The rates of breastfeeding decline in the study mirrored what has been reported nationally by the Centers for Disease Control and Prevention.54 This attrition rate had been taken into account in the original study design, and the number of subjects available for analysis at 7 months was according to the sample size and power calculations. Another limitation is that although it was not possible to measure the vitamin D moieties in the breast milk samples in this study because of cost, we had previously demonstrated how the parent compound vitamin D (cholecalciferol and ergocalciferol) is transferred from the mother to her milk and to her recipient infant.7,8 With that being said, however, the most important factor is the amount of vitamin D in mother’s milk that will support the vitamin D status of her nursing infant, which was shown to be the case in this study.
Vitamin D deficiency is almost universal among solely breastfed infants not receiving oral vitamin D supplementation. This problem is especially acute in the black population.4 This issue is depicted in Table 2 in which one can see that several minority infants exhibited dire vitamin D deficiency, ≤2.5 nmol/L circulating 25(OH)D, after 1 month of being solely breastfed. The newborn human infant who is solely breastfed can only acquire vitamin D through direct dietary supplementation, direct sun exposure, and/or ingestion of breast milk. Direct supplementation is not adhered to12,13 and direct infant sun exposure is contrary to the AAP’s recommendations of no direct sun exposure during the first 6 months of life.11,55 That leaves breast milk as the only alternative.
The medical community has accepted the fact that low concentrations of vitamin D are an inherent defect in human milk that has prompted the recommendation of vitamin D supplementation for breastfeeding infants starting within the first few days after birth.1,11 The current study clearly refutes this misconception. The inherent flaw is not the design of human milk but in the dietary vitamin D recommendation with respect to the lactating mother. The current recommendation of 400 IU per day to these individuals does little to sustain blood concentrations of the parent vitamin D compound, the form that crosses from the maternal circulation into human milk; thus, minimal vitamin D is transferred into human milk. The result: dire vitamin D deficiency in the breastfeeding infant, especially darker-pigmented infants. Our study clearly demonstrates that with appropriate vitamin D intake, the lactating mother can fully transfer from her blood to her milk the vitamin D required to sustain optimal vitamin D nutrition in the nursing infant with no additional supplementation required for the infant.
Acknowledgments
We thank the hundreds of women and their infants who participated in this clinical trial sponsored by National Institute of Child Health and Human Development/National Institutes of Health, without whom this study would not have been possible. We also thank the dedication and hard work of the research and medical staff of the CTSA-sponsored Clinical Research Centers at the MUSC and the U of R. Lastly, we acknowledge Biotics Research Corp, Rosenberg, Texas, for providing Bio-D-Mulsion vitamin D drops and Mead Johnson, Inc, Ohio (Mead Johnson, Evansville, IN), for providing vitamin D–free formula for the infants enrolled in the study.
Glossary
- AAP
American Academy of Pediatrics
- AFA
anterior fontanelle area
- CTRC
Clinical and Translational Research Center
- DSMC
Data and Safety Monitoring Committee
- IOM
Institute of Medicine
- MUSC
Medical University of South Carolina
- RCT
randomized controlled trial
- U of R
University of Rochester
- V1
Visit 1 at 1 month postpartum
- V4
Visit 4 at 4 months postpartum
- V7
Visit 7 at 7 months postpartum
Footnotes
Dr Hollis, as the principal investigator (PI) of the project, worked with Dr Wagner in the conception of the project, study design, implementation of the study, laboratory analyses, data analyses, and writing of the manuscript; Dr, Wagner as clinical PI of the study, worked with Drs Hollis and Howard, site PI at the University of Rochester (U of R), and all other coinvestigators in the conception of the project, study design, implementation of the study, review of clinical and laboratory data, subject safety, data analyses, and writing of the manuscript; Dr Howard as clinical site PI at the U of R worked directly with Dr Wagner; she was involved in the conception of the project, study design, implementation of the study, laboratory analyses, data analyses, and writing of the manuscript; Ms Ebeling, as Data Manager and part of the biostatistics team, was involved in all aspects of study design, data analyses, and interpretation of the data, writing of the manuscript; Ms Shary, as project manager, was involved in study design, data collection, and data analyses, as well as interpretation of the data and writing of the manuscript; Ms. Smith, as research associate, was involved in study design, data collection, and data analyses, as well as interpretation of the data and writing of the manuscript; Dr Taylor, as a clinical coinvestigator at Medical University of South Carolina, was involved in the conception of the project, study design, implementation of the study, laboratory analyses, data analyses, and writing of the manuscript; Ms Morella as a biostatistician as part of the biostatistics team was involved in data analyses, interpretation of the data, and writing of the manuscript; Dr Lawrence as a clinical coinvestigator at the U of R was involved in the conception of the project, study design, implementation of the study, laboratory analyses, data analyses, and writing of the manuscript; Dr Hulsey, as senior coinvestigator, was involved in the conception of the project, study design, implementation of the study, laboratory analyses, data analyses, and writing of the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00412074); FDA Investigational New Drug approval 66,346.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded in part by National Institutes of Health (NIH) 5R01HD043921, NIH RR01070, Medical University of South Carolina Department of Pediatrics, and by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/National Center for Advancing Translational Sciences grant UL1 TR000062.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found on page 763 and online at www.pediatrics.org/cgi/doi/10.1542/peds.2015-2312.
References
- 1.Food and Nutrition Board Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Vitamin D and Calcium. Washington, DC: National Academy Press; 2010 [Google Scholar]
- 2.Girish M, Subramaniam G. Rickets in exclusively breast fed babies. Indian J Pediatr. 2008;75(6):641–643 [DOI] [PubMed] [Google Scholar]
- 3.Gartner LM, Greer FR, Section on Breastfeeding and Committee on Nutrition. American Academy of Pediatrics . Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111(4 pt 1):908–910 [DOI] [PubMed] [Google Scholar]
- 4.Kreiter SR, Schwartz RP, Kirkman HN, Jr, Charlton PA, Calikoglu AS, Davenport ML. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137(2):153–157 [DOI] [PubMed] [Google Scholar]
- 5.Greer FR. Issues in establishing vitamin D recommendations for infants and children. Am J Clin Nutr. 2004;80(Suppl 6):1759S–1762S [DOI] [PubMed] [Google Scholar]
- 6.Specker BL, Tsang RC, Hollis BW. Effect of race and diet on human-milk vitamin D and 25-hydroxyvitamin D. Am J Dis Child. 1985;139(11):1134–1137 [DOI] [PubMed] [Google Scholar]
- 7.Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80(Suppl 6):1752S–1758S [DOI] [PubMed] [Google Scholar]
- 8.Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1(2):59–70 [DOI] [PubMed] [Google Scholar]
- 9.Widdowson EM. Food intake and growth in the newly-born. Proc Nutr Soc. 1971;30(2):127–135 [DOI] [PubMed] [Google Scholar]
- 10.Ziegler EE, Hollis BW, Nelson SE, Jeter JM. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics. 2006;118(2):603–610 [DOI] [PubMed] [Google Scholar]
- 11.Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding. American Academy of Pediatrics Committee on Nutrition . Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152 [DOI] [PubMed] [Google Scholar]
- 12.Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin d among infants breastfed for prolonged periods. Pediatrics. 2010;125(1):105–111 [DOI] [PubMed] [Google Scholar]
- 13.Gordon CM, Feldman HA, Sinclair L, et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162(6):505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrine CG, Sharma AJ, Jefferds ME, Serdula MK, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics. 2010;125(4):627–632 [DOI] [PubMed] [Google Scholar]
- 15.Ahrens KA, Rossen LM, Simon AE. Adherence to Vitamin D Recommendations Among US Infants Aged 0 to 11 Months, NHANES, 2009 to 2012. Clin Pediatr (Phila). 2015;0009922815589916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79(5):717–726 [DOI] [PubMed] [Google Scholar]
- 17.Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98(12):4619–4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer FR, Hollis BW, Cripps DJ, Tsang RC. Effects of maternal ultraviolet B irradiation on vitamin D content of human milk. J Pediatr. 1984;105(3):431–433 [DOI] [PubMed] [Google Scholar]
- 19.Ala-Houhala M. 25-Hydroxyvitamin D levels during breast-feeding with or without maternal or infantile supplementation of vitamin D. J Pediatr Gastroenterol Nutr. 1985;4(2):220–226 [DOI] [PubMed] [Google Scholar]
- 20.Ala-Houhala M, Koskinen T, Terho A, Koivula T, Visakorpi J. Maternal compared with infant vitamin D supplementation. Arch Dis Child. 1986;61(12):1159–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner CL, Howard CR, Hulsey TC, et al. Maternal and infant vitamin D status during lactation: is latitude important? Health. 2013;5(12):2004 [Google Scholar]
- 22.Greer FR, Hollis BW, Napoli JL. High concentrations of vitamin D2 in human milk associated with pharmacologic doses of vitamin D2. J Pediatr. 1984;105(1):61–64 [DOI] [PubMed] [Google Scholar]
- 23.Wagner CL, Howard C, Hulsey TC, et al. Circulating 25-hydroxyvitamin d levels in fully breastfed infants on oral vitamin d supplementation. Int J Endocrinol. 2010;2010:235035 [DOI] [PMC free article] [PubMed]
- 24.Labbok MH, Belsey M, Coffin CJ. A call for consistency in defining breast-feeding. Am J Public Health. 1997;87(6):1060–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labbok M, Krasovec K. Toward consistency in breastfeeding definitions. Stud Fam Plann. 1990;21(4):226–230 [PubMed] [Google Scholar]
- 26.Coffin CJ, Labbok MH, Belsey M. Breastfeeding definitions. Contraception. 1997;55(6):323–325 [DOI] [PubMed] [Google Scholar]
- 27.Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem. 1984;21(1):81–86 [DOI] [PubMed] [Google Scholar]
- 28.Hollis BW. Individual quantitation of vitamin D2, vitamin D3, 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in human milk. Anal Biochem. 1983;131:211–219 [DOI] [PubMed] [Google Scholar]
- 29.Hollis BW, Pittard WB, III. Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59(4):652–657 [DOI] [PubMed] [Google Scholar]
- 30.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39(3):529–533 [PubMed] [Google Scholar]
- 31.Laboratories MC. Laboratory Reference Data. Rochester, MN: Mayo Clinic; 2004 [Google Scholar]
- 32.Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352:515–516 [DOI] [PubMed]
- 33.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204–210 [DOI] [PubMed] [Google Scholar]
- 34.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69(5):842–856 [DOI] [PubMed] [Google Scholar]
- 35.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73(2):288–294 [DOI] [PubMed] [Google Scholar]
- 36.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135(2):317–322 [DOI] [PubMed] [Google Scholar]
- 37.Vieth R, Ladak Y, Walfish P. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinal Metab. 2003;88(1):185–191 [DOI] [PubMed]
- 38.Appelgren KE, Nietert PJ, Hulsey TC, Hollis BW, Wagner CL. Analyzing adherence to prenatal supplement: does pill count measure up? Int J Endocrinol. 2010;2010:631971 [DOI] [PMC free article] [PubMed]
- 39.Food and Nutrition Board Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1997 [PubMed] [Google Scholar]
- 40.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26(10):2341–2357 [DOI] [PMC free article] [PubMed]
- 41.Wagner CL, McNeil R, Hamilton SA, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208(2):137.e1–e13 [DOI] [PMC free article] [PubMed]
- 42.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97(4):1153–1158 [DOI] [PubMed] [Google Scholar]
- 43.Jackson RD, LaCroix AZ, Gass M, et al. Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683 [DOI] [PubMed] [Google Scholar]
- 44.Nguyen S, Baggerly L, French C, Heaney RP, Gorham ED, Garland CF. 25-Hydroxyvitamin D in the range of 20 to 100 ng/mL and incidence of kidney stones. Am J Public Health. 2014;104(9):1783–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durup D, Jørgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD study. J Clin Endocrinol Metab. 2012;97(8):2644–2652 [DOI] [PubMed] [Google Scholar]
- 46.Garland CF, Kim JJ, Mohr SB, et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am J Public Health. 2014;104(8):e43–e50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luxwolda MF, Kuipers RS, Kema IP, van der Veer E, Dijck-Brouwer DA, Muskiet FA. Vitamin D status indicators in indigenous populations in East Africa. Eur J Nutr. 2013;52(3):1115–1125 [DOI] [PubMed] [Google Scholar]
- 48.Luxwolda MF, Kuipers RS, Kema IP, Dijck-Brouwer DA, Muskiet FA. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr. 2012;108(9):1557–1561 [DOI] [PubMed] [Google Scholar]
- 49.Halliday TM, Peterson NJ, Thomas JJ, Kleppinger K, Hollis BW, Larson-Meyer DE. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med Sci Sports Exerc. 2011;43(2):335–343 [DOI] [PubMed] [Google Scholar]
- 50.Hollis BW, Roos BA, Draper HH, Lambert PW. Occurrence of vitamin D sulfate in human milk whey. J Nutr. 1981;111(2):384–390 [DOI] [PubMed] [Google Scholar]
- 51.Reeve LE, Chesney RW, DeLuca HF. Vitamin D of human milk: identification of biologically active forms. Am J Clin Nutr. 1982;36(1):122–126 [DOI] [PubMed] [Google Scholar]
- 52.Oberhelman SS, Meekins ME, Fischer PR, et al. Maternal vitamin D supplementation to improve the vitamin D status of breast-fed infants: a randomized controlled trial. Mayo Clin Proc. 2013;88(12):1378–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollis BW, Pittard WB, III, Reinhardt TA. Relationships among vitamin D, 25-hydroxyvitamin D, and vitamin D-binding protein concentrations in the plasma and milk of human subjects. J Clin Endocrinol Metab. 1986;62(1):41–44 [DOI] [PubMed] [Google Scholar]
- 54.Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. Breastfeeding Among U.S. Children Born 2001–2011, CDC National Immunization Survey. 2015. Available at: http://www.cdc.gov/breastfeeding/data. Accessed August 6, 2015
- 55.American Academy of Pediatrics. Committee on Environmental Health . Ultraviolet light: a hazard to children. Pediatrics. 1999;104(2 pt 1):328–333 [PubMed] [Google Scholar]