Abstract
BACKGROUND:
A substudy of the Management of Myelomeningocele Study evaluating urological outcomes was conducted.
METHODS:
Pregnant women diagnosed with fetal myelomeningocele were randomly assigned to either prenatal or standard postnatal surgical repair. The substudy included patients randomly assigned after April 18, 2005. The primary outcome was defined in their children as death or the need for clean intermittent catheterization (CIC) by 30 months of age characterized by prespecified criteria. Secondary outcomes included bladder and kidney abnormalities observed by urodynamics and renal/bladder ultrasound at 12 and 30 months, which were analyzed as repeated measures.
RESULTS:
Of the 115 women enrolled in the substudy, the primary outcome occurred in 52% of children in the prenatal surgery group and 66% in the postnatal surgery group (relative risk [RR]: 0.78; 95% confidence interval [CI]: 0.57–1.07). Actual rates of CIC use were 38% and 51% in the prenatal and postnatal surgery groups, respectively (RR: 0.74; 95% CI: 0.48–1.12). Prenatal surgery resulted in less trabeculation (RR: 0.39; 95% CI: 0.19–0.79) and fewer cases of open bladder neck on urodynamics (RR: 0.61; 95% CI: 0.40–0.92) after adjustment by child’s gender and lesion level. The difference in trabeculation was confirmed by ultrasound.
CONCLUSIONS:
Prenatal surgery did not significantly reduce the need for CIC by 30 months of age but was associated with less bladder trabeculation and open bladder neck. The implications of these findings are unclear now, but support the need for long-term urologic follow-up of patients with myelomeningocele regardless of type of surgical repair.
What’s Known on This Subject:
Urologic outcomes of prenatal myelomeningocele closure have previously been reported. This study, however, represents a large, prospectively followed cohort of these patients and presents detailed findings of urologic outcomes. To our knowledge, this is the largest study of this type.
What This Study Adds:
Our study is the only trial to compare urologic outcomes in children with myelomeningocele having undergone prenatal closure with those who had postnatal repair in a prospective and systematic manner. We report our findings at 12 and 30 months.
Nowhere is the complex nature of spina bifida more evident than in the bladder. Only a small proportion of children with spina bifida are born with a normal innervation of their bladder and sphincter muscles and few will void spontaneously without the need for either medical or surgical management. Even patients who are ambulatory, with minimal orthopedic defects, are not guaranteed normal bladder function. The long term sequelae of urinary tract infection, incontinence, and renal insufficiency in children born with a myelomeningocele are well known.1,2 The majority of children will ultimately require clean intermittent catheterization (CIC) along with anticholinergic therapy to manage their neuropathic bladders.3,4
In utero repair of myelomeningocele, first performed in humans in 1997, was initially shown to be associated with a significant reduction in hindbrain herniation5 and possible decreased need for a ventriculoperitoneal shunt and improved lower extremity function. Theoretically, in utero repair of myelomeningocele could be beneficial with respect to recovery of urologic deficits because experimental data suggest that the neonatal nervous system has great plasticity. However, observational studies of children who underwent fetal surgery revealed no significant differences in bladder function.6–8 Limitations of these studies included selection or treatment bias, migratory study populations being managed by different providers, and parental perception of disease status with compliance being a potential issue. In another study, timing of postnatal repair was a factor, noting that those infants repaired within the first day of life had more favorable bladder pressures and bladder capacity as compared with infants repaired after 72 hours.9 Finally, a group from Sao Paolo showed that at mean follow-up of 5.4 months after in utero repair, a considerable proportion of infants had the usual adverse urologic outcomes of spina bifida: one-third of patients had hydronephrosis, one-quarter showed vesicoureteral reflux, pyelonephritis was noted in half, and abnormal bladder dynamics was documented in approximately half of patients.10
From 2003 to 2011, we conducted the Management of Myelomeningocele Study (MOMS), a randomized controlled trial to compare the safety and efficacy of prenatal repair of myelomeningocele with that of standard postnatal repair. Recruitment was stopped early for benefit according to predefined stopping rules, and the results of the trial at that point were reported quickly according to the recommendations of the Data and Safety Monitoring Committee.11 In summary, prenatal surgery was associated with less need for cerebrospinal fluid shunt at 12 months and a better composite score for mental development and motor function at 30 months. Prenatal surgery also revealed benefit in several key secondary outcomes including hindbrain herniation, ability to walk unaided, and a better score on the Bayley II Psychomotor Development Index. These results were tempered by an increase in preterm birth and the risk of uterine dehiscence in the prenatal surgery group. Urologic outcomes were not reported because these data were comparatively incomplete at the time of publication; however, we did follow the children prospectively in a standardized manner. Herein, we report our findings on the urologic outcomes at 12 and 30 months after prenatal or postnatal repair of myelomeningocele.
Methods
MOMS was conducted by 3 established maternal–fetal surgery centers, The Children’s Hospital of Philadelphia, Vanderbilt University, and the University of California, San Francisco, an independent data coordinating center at the George Washington University, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Details of the trial design and procedures have been previously published.11 Briefly, pregnant women between 19 and 25 weeks of gestation with a fetus diagnosed with myelomeningocele were randomly assigned to either prenatal surgery or postnatal surgery. For those randomly assigned to prenatal surgery, the participants underwent hysterotomy to repair the fetal myelomeningocele and stayed at the maternal–fetal surgery center with close monitoring and follow-up until delivery. For those randomly assigned to postnatal surgery, the women went home and were asked to return to 1 of the 3 maternal–fetal surgery centers for delivery and repair of the myelomeningocele, which was performed within 48 hours of birth. All infants were delivered via cesarean delivery, scheduled at 37 weeks of gestation if not already delivered. Families returned to the maternal–fetal surgery centers for follow-up of the children at 12 and 30 months of age.
After the MOMS trial had started, supplementary funding was obtained to add a standardized postnatal urologic protocol and evaluation of the children. This protocol was followed for the infants of all women recruited on or after April 18, 2005. At birth, urinalysis was obtained within the first 48 hours of life and before administration of antibiotics if possible. In addition, a renal bladder ultrasound was obtained before postnatal repair surgery if possible or within the first 48 hours of life. Video urodynamic studies were conducted before discharge. By protocol, CIC was instituted for 48 hours after repair for the postnatal group or for 48 hours after birth for the prenatal group unless the infant was <1500 g or <30 weeks’ postmenstrual age. For all women, including those who delivered at their community of origin, the study urologist contacted the local urologist, to communicate the protocol with respect to the renal ultrasound and video urodynamic studies, as well as indications for CIC. At the 12- and 30-month visits, a renal bladder ultrasound was obtained and video urodynamic studies were performed. In addition, medical records were obtained from the children’s local care providers.
The primary outcome for the supplementary urology study was death or the need for CIC through 30 months of age. Death was included in the primary outcome because it is a competing risk. The necessity for CIC was defined as meeting 1 or more of the following criteria: leak point pressure greater than 40 cm of water on urodynamic study that correlates with other urodynamic and radiographic data, progression of hydronephrosis that is grade II or greater, or grade III at birth (Society for Fetal Urology [SFU] grading system), development or worsening of vesicoureteral reflux, flaccidity of the bladder with nonemptying (defined as greater than 2 times the predicted bladder capacity for age or less than 50% bladder volume emptying), or recurrent urinary tract infection (2 or more distinct urinary tract infections over a 6-month period and for a child on CIC, fever with accompanying symptoms for children 12 months and older). Temporary initiation of CIC during the initial period after myelomeningocele repair (spinal shock) did not qualify.
An independent review committee that comprised 3 pediatric urologists and a radiologist, blinded to the treatment assignment, reviewed the clinical and radiologic data for each child. The urologists determined whether CIC criteria were met on the basis of patient records. If so, the child was deemed to have the primary outcome, regardless of whether CIC was actually initiated. The radiologist interpreted all of the renal/bladder ultrasounds, and recorded kidney size, SFU grade of hydronephrosis if present (grade I = renal pelvis, II = few calices, III = all calices, and IV = parenchymal thinning), other kidney abnormalities, grade of ureteral dilation if present, distention of the bladder and if not empty, presence of trabeculation and diverticula. Other secondary outcomes included actual initiation of CIC by 30 months, surgical procedures (vesicostomy, urethral dilation), hydroureteronephrosis, vesicoureteral reflux, and kidney and bladder abnormalities noted during the video urodynamic procedures, evaluated at both 12 and 30 months.
Before beginning the urology protocol, a sample size of 120 (60 in each group) was estimated to yield over 80% power to detect a 50% reduction in the primary outcome rate in the prenatal surgery group, with type I error 5% 2-sided, assuming that the incidence of the primary outcome in the postnatal surgery group would be ∼50%. Analyses were performed according to the intention-to-treat principle. In univariable analysis of the primary outcome and related categorical outcomes, analysis was by χ2 or Fisher’s exact test as appropriate. Relative risks (RRs) and 95% confidence intervals (CIs) were calculated. Secondary outcomes obtained at 12 and 30 months were analyzed together because they are repeated measures. We did not include neonatal data in the repeated measures because the renal/bladder ultrasounds were performed before postnatal surgical repair, and because of the possibility of temporary spinal shock. We used generalized linear models for continuous measures and generalized estimating equations for categorical variables to account for the correlation between time points. A log binomial model was used to calculate RRs and corresponding 95% CIs. A multivariable model adjusting for baseline characteristics that differed by treatment group was also implemented. For all outcomes a nominal P value of <.05 was considered to indicate statistical significance. No adjustment was made for multiple comparisons.
Results
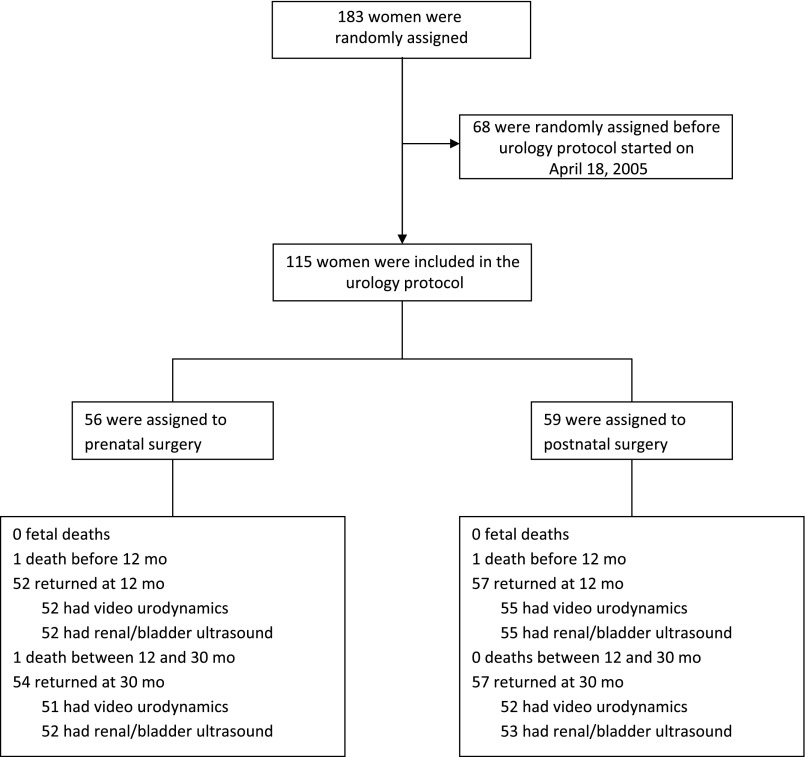
A total of 115 patients (56 in the prenatal surgery group and 59 in the postnatal surgery group) were recruited into the trial from April 18, 2005, to December 7, 2010, when the study was stopped (Fig 1). The baseline characteristics are presented in Table 1 and are not different between the surgery groups except for spina bifida lesion level L3 or lower (64% in the prenatal surgery group, 81% in the postnatal surgery group; P = .04) and gender (41% of patients were girls in the prenatal surgery group, 63% in the postnatal surgery group; P = .02). These characteristics also differed between groups in the overall trial cohort.
FIGURE 1.
Flow diagram.
TABLE 1.
Baseline Characteristics
| Prenatal Surgery (N = 56) | Postnatal Surgery (N = 59) | |
|---|---|---|
| Gestational age at randomization, wk | 23.9 (1.2) | 24.0 (1.2) |
| Maternal age at screening, ya | 28.8 (5.0) | 28.5 (5.1) |
| Race/ethnicity | ||
| White Non-Hispanic | 52 (93) | 56 (95) |
| Black Non-Hispanic | 1 (2) | 0 (0) |
| Hispanic | 3 (5) | 3 (5) |
| Married | 52 (93) | 57 (97) |
| Years of schoolinga | 14.9 (1.7) | 14.9 (1.8) |
| BMI at screeninga | 26.0 (3.7) | 26.6 (4.0) |
| Currently smoking | 3 (5) | 3 (5) |
| Nulliparous | 22 (39) | 19 (32) |
| Lesion level L3 or lower | 36 (64) | 48 (81) |
| Fetal gender female | 23 (41) | 37 (63) |
Data are presented as n (%) except, a presented as mean (SD).
The primary outcome of death or meeting criteria for CIC at 30 months was assessed for all 115 patients. There was no significant difference between the groups: 29 of 56 (52%) had the primary outcome in the prenatal group and 39 of 59 (66%) in the postnatal group (RR: 0.78; 95% CI: 0.57–1.07). Overall, 44% of boys compared with 73% of girls met criteria for CIC (RR: 0.57; 95% CI: 0.34–0.94, adjusted for surgery group, data not shown). Lesion level was not related to outcome, and there was no interaction between fetal surgery group and either gender or lesion level.
The most frequent criterion met was flaccid bladder with nonemptying, which was present in 15 (27%) children in the prenatal surgery group and 22 (37%) in the postnatal surgery group (P = .25). There was a significant difference in the proportion of children who met the criterion of vesicoureteral reflux: 4 (7%) in the prenatal surgery group and 12 (20%) in the postnatal surgery group (P = .04). Overall, 21 (38%) of children in the prenatal surgery group and 30 (51%) in the postnatal surgery group were started on CIC by 30 months of age (RR: 0.74; 95% CI: 0.48–1.12; Table 2). However, of those who met criteria, only 14 (50%) in the prenatal surgery group and 23 (62%) in the postnatal surgery group were actually placed on CIC.
TABLE 2.
Primary Outcome and Need for CIC by Group
| Prenatal Surgery (N = 56) | Postnatal Surgery (N = 59) | RR (95% CI) | |
|---|---|---|---|
| Primary outcomea | 29 (52) | 39 (66) | 0.78 (0.57–1.07) |
| Criteria metb | |||
| Death | 1 | 1 | |
| Leak point pressure >40 mm H2O | 6 | 3 | |
| Hydronephrosis ≥ grade II (or grade III at birth) | 5 | 3 | |
| Vesicoureteral reflux | 4 | 12 | |
| Flaccid bladder with nonemptying | 15 | 22 | |
| Recurrent urinary tract infection | 4 | 9 | |
| On CIC by 30 moa | 21 (38) | 30 (51) | 0.74 (0.48–1.12) |
Data presented as n (%).
Fifteen children met more than 1 criterion.
Video urodynamics was performed before neonatal discharge on 53 children in the prenatal surgery group and 55 in the postnatal surgery group. The neonatal renal bladder ultrasound was obtained for 55 and 59 children in the 2 groups, respectively. There was no significant difference between the 2 groups for any of the secondary outcomes evaluated shortly after birth, although 16 (37%) children in the postnatal group and 9 (21%) in the prenatal group had a bladder shape noted to be not smooth and round on video urodynamics (RR: 0.55; 95% CI: 0.27–1.11; Table 3).
TABLE 3.
Neonatal Urology Evaluations by Group
| Prenatal Surgery | Postnatal Surgery | RR (95% CI) | |
|---|---|---|---|
| Video urodynamics | |||
| Any grade reflux | 4/53 (8) | 3/55 (6) | 1.38 (0.33–5.89) |
| Bladder not round and smooth | 9/44 (21) | 16/43 (37) | 0.55 (0.27–1.11) |
| Trabeculation | 4/45 (9) | 0/44 (0) | — |
| Diverticula | 1/44 (2) | 0/44 (0) | — |
| Open bladder neck | 11/44 (25) | 8/43 (19) | 1.34 (0.60–3.01) |
| Renal/bladder ultrasound | |||
| Hydronephrosis SFU grade (worst side) | |||
| None | 33/55 (60) | 44/59 (75) | |
| Grade I | 15/55 (27) | 8/59 (14) | |
| Grade II | 6/55 (11) | 5/59 (9) | |
| Grade III | 1/55 (2) | 2/59 (3) | |
| Grade IV | 0/55 (0) | 0/59 (0) | |
| Any hydronephrosis | 22/55 (40) | 15/59 (25) | 1.57 (0.91–2.71) |
| Any ureteral dilatation | 1/55 (2) | 1/59 (2) | 1.07 (0.07–16.74) |
| Trabeculation | 3/30 (10) | 3/31 (10) | 1.03 (0.23–4.72) |
Data are presented as n/N (%). —, RR not calculated due to zero cell.
At follow-up, prenatal surgery was associated with a significant reduction in abnormal bladder shape (not round and smooth) as observed by video urodynamics (RR: 0.59; 95% CI: 0.36–0.97; Table 4). However, after adjustment for gender and lesion level, the 2 baseline characteristics that differed significantly by surgery group, the effect was no longer significant (adjusted RR: 0.64; 95% CI: 0.37–1.09). In the model, female gender was associated with an increased risk of abnormal bladder shape (P = .02). The presence of open bladder neck was not significantly different in the unadjusted model (Table 4) but after adjustment for gender and lesion level, there was a significant reduction in open bladder neck in the prenatal surgery group (adjusted RR: 0.61; 95% CI: 0.40–0.92). In this model, female gender was associated with a decreased risk of open bladder neck (P = .02). Similarly, the presence of diverticula was not significantly different in the unadjusted model (Table 4), but after adjusting for gender and lesion level, there was a significant reduction in diverticula in the prenatal surgery group (adjusted RR: 0.37; 95% CI: 0.15–0.91). There was a significant difference in trabeculation on video urodynamics (RR: 0.39; 95% CI: 0.19–0.79; Table 4), which was very similar in the adjusted model. Examining change in trabeculation over time revealed that only 1 of the children repaired prenatally who did not have trabeculation at 12 months was noted to have trabeculation at 30 months compared with 10 in the postnatal group (P = .01). Trabeculation as assessed by the centrally read bladder ultrasounds was also significantly different between the surgery groups (RR: 0.24; 95% CI: 0.07–0.87; Table 5).
TABLE 4.
Secondary Outcomes: Video Urodynamics
| 12 moa | 30 moa | RR (95% CI)b | |||
|---|---|---|---|---|---|
| Prenatal Surgery | Postnatal Surgery | Prenatal Surgery | Postnatal Surgery | ||
| Any grade reflux | 5/52 (10) | 9/55 (16) | 3/51 (6) | 9/52 (17) | 0.47 (0.17–1.30) |
| Bladder not round and smooth | 13/52 (25) | 22/53 (42) | 10/51 (20) | 17/50 (34) | 0.59 (0.36–0.97) |
| Trabeculation | 8/52 (15) | 15/54 (28) | 4/51 (8) | 17/52 (33) | 0.39 (0.19–0.79) |
| Diverticula | 2/52 (4) | 4/54 (7) | 3/51 (6) | 8/52 (15) | 0.42 (0.16–1.16) |
| Open bladder neck | 17/52 (33) | 21/54 (39) | 13/51 (26) | 23/52 (44) | 0.71 (0.46–1.09) |
Data are presented as n/N (%).
Based on infants with assessments at both 12 and 30 mo.
TABLE 5.
Secondary Outcomes From the Bladder/Renal Ultrasounds
| 12 moa | 30 moa | RR (95% CI)b | |||
|---|---|---|---|---|---|
| Prenatal Surgery (N = 52) | Postnatal Surgery (N = 55) | Prenatal Surgery (N = 52) | Postnatal Surgery (N = 53) | ||
| Left kidney, cm | 6.11 (0.70) | 5.91 (0.59) | 6.83 (0.70) | 6.68 (0.67) | |
| Right kidney, cm | 6.05 (0.47) | 5.90 (0.64) | 6.70 (0.65) | 6.66 (0.54) | |
| Hydronephrosis SFU grade (worst side) | |||||
| None | 40 (77) | 40 (73) | 43 (83) | 44 (83) | |
| Grade I | 5 (10) | 11 (20) | 6 (12) | 5 (9) | |
| Grade II | 4 (8) | 2 (4) | 1 (2) | 1 (2) | |
| Grade III | 2 (4) | 1 (2) | 2 (4) | 2 (4) | |
| Grade IV | 1 (2) | 1 (2) | 0 (0) | 1 (2) | |
| Any hydronephrosis | 12 (23) | 15 (27) | 9 (17) | 9 (17) | 0.90 (0.47–1.71) |
| Ureteral dilatation (worst side), mm | NA | ||||
| None | 49 (94) | 49 (89) | 48 (92) | 48 (91) | |
| <7 | 1 (2) | 4 (7) | 1 (2) | 3 (6) | |
| 7–10 | 1 (2) | 1 (2) | 2 (4) | 1 (2) | |
| >10 | 1 (2) | 1 (2) | 1 (2) | 1 (2) | |
| Any ureteral dilatation | 3 (6) | 6 (11) | 4 (8) | 5 (9) | 0.67 (0.20–2.20) |
| Trabeculation | 3/48 (6) | 7/47 (15) | 1/45 (2) | 9/45 (20) | 0.24 (0.07–0.87) |
| Diverticula | 1/48 (2) | 1/47 (2) | 0/45 (0) | 1/45 (2) | — |
NA, not applicable; —, RR not calculated due to zero cell.
Data are presented as n (%) or mean (SD).
Based on infants with assessments at both 12 and 30 mo.
There was no difference in reflux assessed at the time of video urodynamics (Table 4), or in the size of either kidney, presence of hydronephrosis, or ureteral dilatation between the groups (Table 5) on centrally read ultrasound. Diverticula read by ultrasound occurred too rarely to assess. No patient in either group underwent a vesicostomy. Three patients in the postnatal group and none in the prenatal surgery group underwent urethral dilation, but the difference between the groups was insignificant (P = .24).
Discussion
In our trial, the primary urologic outcome was defined by death or meeting prespecified criteria for the necessity of CIC by 30 months of age or death. Meeting criteria only resulted in actually receiving CIC in a little more than half of the cases, which is likely due to differing local practices or regional bias. The criteria nevertheless represented an objective composite outcome of bladder status in infancy and early childhood in this population of children with spina bifida. We did not find a significant difference between the groups. Although the outcome rate in the prenatal surgery group was higher than assumed in the power analysis, the trial was powered for a large effect size (ie, a 50% reduction in the primary outcome rate in the prenatal surgery compared with the postnatal surgery group). Thus, the trial was underpowered for a more modest but possibly still meaningful reduction.
We found some differences in secondary outcomes potentially favoring prenatal surgery. Perhaps the most pertinent and significant finding was that of less bladder trabeculation in the prenatal surgery group. This finding was confirmed both on video urodynamics and on an independently read ultrasound. One study of patients with neurogenic bladder dysfunction attempted to correlate deformity of bladder shape, which in the study implied bladder trabeculation, to worsening upper tract deterioration. Upper tract deterioration was defined as hydronephrosis and/or reflux. Over half of the patients with high-grade deformity as compared with 2% to 8% of those with low-grade deformity had upper tract deterioration, whereas most patients with decreased compliance also had a high-grade bladder deformity.12 Other studies of patients with spina bifida have attempted to correlate bladder wall thickness with findings on video urodynamics. One study revealed that ultrasound measurement of dorsal bladder wall thickness was significantly correlated with higher leak point pressures and maximum detrusor pressures. However, the bladder volume at which these measurements took place was not well-defined.13 When bladder volumes were more accurately defined, another study revealed that bladder wall thickness measured at 50% maximum cystometric capacity was significantly increased in patients with bladder trabeculation. In contrast, bladder wall thickness failed to correlate with any of the other unfavorable video urodynamic findings as defined by decreased bladder compliance, leak point pressure >40 cm of H2O, detrusor overactivity, or vesicoureteral reflux.14 Therefore, the assumption that increased trabeculation should directly correlate with increasing bladder pressures or elevated leak point pressure is not well supported and in the current study did not appear to be the case. There was a significant reduction in the presence of an open bladder neck in girls in the prenatal closure group, the significance of which is unknown at this time. One may assume that the presence of an open bladder neck may be associated with an increased propensity for incontinence, as well as decreased leak point pressures. However, leak point pressure (LPP) was not statistically different between the 2 groups and longer follow-up is needed to determine how an open bladder neck affects overall management in terms of bladder/sphincter dynamics, as well as interventions to become continent.
Our study had several major strengths. First is the randomized design: ours is the only trial to compare urologic outcomes between children with myelomeningocele who have undergone prenatal closure with those who had postnatal repair in a prospective and systematic manner. The study was conducted by using a standardized protocol, and data were collected by trained research staff. Our follow-up rate was excellent; only 1 of the surviving children did not return for the 30-month follow-up visit, and because we were able to obtain the medical records for all of the children, the primary outcome was obtained on the whole cohort. To eliminate ascertainment bias, the primary outcome and the renal bladder ultrasound results were determined by independent experts blinded to surgery group.
Conclusions
Although prenatal surgery has made a tremendous impact on the neurosurgical outcomes in these children, at the current time, the significance of these urologic results remains somewhat unclear. It is possible that decreased trabeculation and more normal bladder shape that we have seen by 30 months will result in better bladder control and a lower prevalence of interventions at a later age. From a urologic standpoint, the ultimate benefit of prenatal repair may be to delay augmentation cystoplasty. We are currently conducting a follow-up study of these children at school age. Until we analyze long-term outcome data from patients in the MOMS trial and are able to address this question, it is imperative to continue to monitor all children with myelomeningocele from birth no matter how the spinal defect was closed.
Acknowledgments
Other participants in the urologic substudy of the MOMS trial were as follows: The Children’s Hospital of Philadelphia (Philadelphia, PA): Monica Moran, RN, MEd, Jamie Koh, RN, MSN; University of California, San Francisco (San Francisco, CA): Angie Champeau, PNP, Anne Arnhym, PNP, Tamara Ryan, RN, Rachel Perry, RN; Vanderbilt University Medical Center (Nashville, TN): Lisa Trusler, RN, MSN, Tracy Perry; The Biostatistics Center, George Washington University (Washington, DC): Erin Greenbaum Musok, MA, Kristen Holloway, MA; The Eunice Kennedy Shriver National Institute of Child Health and Human Development (Bethesda, MD): Catherine Y. Spong, MD, Rosemary Higgins, MD.
The authors thank Dr Catherine Y. Spong for protocol development and oversight.
Glossary
- CI
confidence interval
- CIC
clean intermittent catheterization
- MOMS
Management of Myelomeningocele Study
- RR
relative risk
- SFU
Society for Fetal Urology
Footnotes
Dr Brock participated in the conception of the study design and protocol development, and contributed to critical aspects of the conduct of this research including monitoring study implementation, progress, and data quality; promotion of patient recruitment; acquisition of data; supervision of clinical centers; and data analysis. Dr Brock provided significant intellectual contribution to the drafting and revision of this manuscript with regard to scientific content and form; Drs Carr and Adzick participated in study design and protocol development, and contributed to critical aspects of the conduct of this research including assessment of patient recruitment; monitoring center performance; oversight of data quality; and evaluation and analysis of data. Dr Carr provided significant intellectual contribution to the drafting and revision of this manuscript with regard to scientific content and form; Ms Burrows participated in conception of the study design and protocol development, and contributed to critical aspects of the conduct of this research including monitoring of recruitment and study progress; data quality evaluation and interpretation; provision of administrative support; and statistical analysis. Ms Burrows provided significant intellectual contribution to the drafting and revision of this manuscript with regard to scientific content and form; Dr Thomas contributed to critical aspects of the conduct of this research including assessment of patient recruitment; monitoring center performance; oversight of data quality; and evaluation and analysis of data. Dr Thomas provided significant intellectual contribution to the drafting and revision of this manuscript with regard to scientific content and form; Dr Thom participated in conception of the study design and protocol development, and contributed to critical aspects of the conduct of this research including monitoring of recruitment and study progress; data quality evaluation and interpretation; provision of administrative support; and statistical analysis. Dr Thom provided significant intellectual contribution to the drafting and revision of this manuscript with regard to scientific content and form; Ms Howell and Ms Dabrowiak participated in conception of the study design and protocol development, enrollment of patients, acquisition of data, and monitoring of data quality, evaluation, and analysis. Ms Howell and Ms Dabrowiak provided significant intellectual contribution to the drafting and revision of this manuscript with regard to scientific content and form; Ms Farrell participated in conception of the study design and protocol development, enrollment of patients, acquisition of data, and monitoring of data quality, evaluation, and analysis. Ms Farrell provided significant intellectual contribution to the drafting and revision of this manuscript with regard to scientific content and form; Dr Farmer contributed to critical aspects of the conduct of this research including assessment of patient recruitment; monitoring center performance; oversight of data quality; and evaluation and analysis of data. Dr Farmer provided significant intellectual contribution to the drafting and revision of this manuscript with regard to scientific content and form; Drs Cheng, Kropp, Caldemone, and Bulas participated as members of the independent committee of pediatric urologists and contributed to critical aspects of the conduct of this research particularly related to the blinded central reviews of medical records and imaging studies, and provided significant intellectual contribution to the drafting and revision of this manuscript with regard to scientific laboratory analyses; Ms Tolivaisa made substantial contributions to acquisition of the data for this manuscript and contributed to critical aspects of the conduct of this research including overall supervision of clinical centers; assessment of recruitment and study progress; critically reviewed it for important content; and provided administrative and technical support; Dr Baskin contributed to critical aspects of the conduct of this research including assessment of patient recruitment; monitoring center performance; oversight of data quality; and evaluation and analysis of data. Dr Baskin provided significant intellectual contribution to the drafting and revision of this manuscript with regard to scientific content and form. All authors approved the final manuscript as submitted.
Comments and views of the authors do not necessarily represent views of the National Institutes of Health.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00060606).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10-HD041669, U10-HD041667, U10-041666, and U01-HD041665) and the National Institute of Diabetes and Digestive and Kidney Diseases. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bauer SB, Hallett M, Khoshbin S, et al. Predictive value of urodynamic evaluation in newborns with myelodysplasia. JAMA. 1984;252(5):650–652 [PubMed] [Google Scholar]
- 2.Veenboer PW, Bosch JL, van Asbeck FW, de Kort LM. Upper and lower urinary tract outcomes in adult myelomeningocele patients: a systematic review. PLoS One. 2012;7(10):e48399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu HY, Baskin LS, Kogan BA. Neurogenic bladder dysfunction due to myelomeningocele: neonatal versus childhood treatment. J Urol. 1997;157(6):2295–2297 [PubMed] [Google Scholar]
- 4.Kaefer M, Pabby A, Kelly M, Darbey M, Bauer SB. Improved bladder function after prophylactic treatment of the high risk neurogenic bladder in newborns with myelomentingocele. J Urol. 1999;162(3 pt 2):1068–1071 [DOI] [PubMed] [Google Scholar]
- 5.Sutton LN, Adzick NS, Bilaniuk LT, Johnson MP, Crombleholme TM, Flake AW. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA. 1999;282(19):1826–1831 [DOI] [PubMed] [Google Scholar]
- 6.Clayton DB, Tanaka ST, Trusler L, et al. Long-term urological impact of fetal myelomeningocele closure. J Urol. 2011;186(suppl 4):1581–1585 [DOI] [PubMed] [Google Scholar]
- 7.Holmes NM, Nguyen HT, Harrison MR, Farmer DL, Baskin LS. Fetal intervention for myelomeningocele: effect on postnatal bladder function. J Urol. 2001;166(6):2383–2386 [DOI] [PubMed] [Google Scholar]
- 8.Lee NG, Gomez P, Uberoi V, et al. In utero closure of myelomeningocele does not improve lower urinary tract function. J Urol. 2012;188(suppl 4):1567–1571 [DOI] [PubMed] [Google Scholar]
- 9.Tarcan T, Onol FF, Ilker Y, Alpay H, Simşek F, Ozek M. The timing of primary neurosurgical repair significantly affects neurogenic bladder prognosis in children with myelomeningocele. J Urol. 2006;176(3):1161–1165 [DOI] [PubMed] [Google Scholar]
- 10.Macedo A, Jr, Leal M, Rondon A, Ortiz V, Moron AF, Cavalheiro S. Urological evaluation of patients that had undergone in utero myelomeningocele closure: A prospective assessment at first presentation and early follow-up. Do their bladder benefit from it? Neurourol Urodyn. 2015;34(5):461–464 [DOI] [PubMed] [Google Scholar]
- 11.Adzick NS, Thom EA, Spong CY, et al. MOMS Investigators . A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa T. Bladder deformities in patients with neurogenic bladder dysfunction. Urol Int. 1991;47(suppl 1):59–62 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, Matsuda M, Moriya K, Mitsui T, Kitta T, Nonomura K. Ultrasonographic measurement of bladder wall thickness as a risk factor for upper urinary tract deterioration in children with myelodysplasia. J Urol. 2008;180(1):312–316, discussion 316 [DOI] [PubMed] [Google Scholar]
- 14.Kim WJ, Shiroyanagi Y, Yamazaki Y. Can Bladder Wall Thickness Predict Videourodynamic Findings in Children with Spina Bifida? J Urol. 2015;194(1):180–183 [DOI] [PubMed] [Google Scholar]