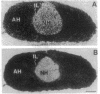
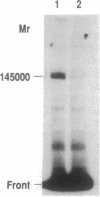
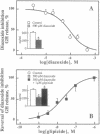
Abstract
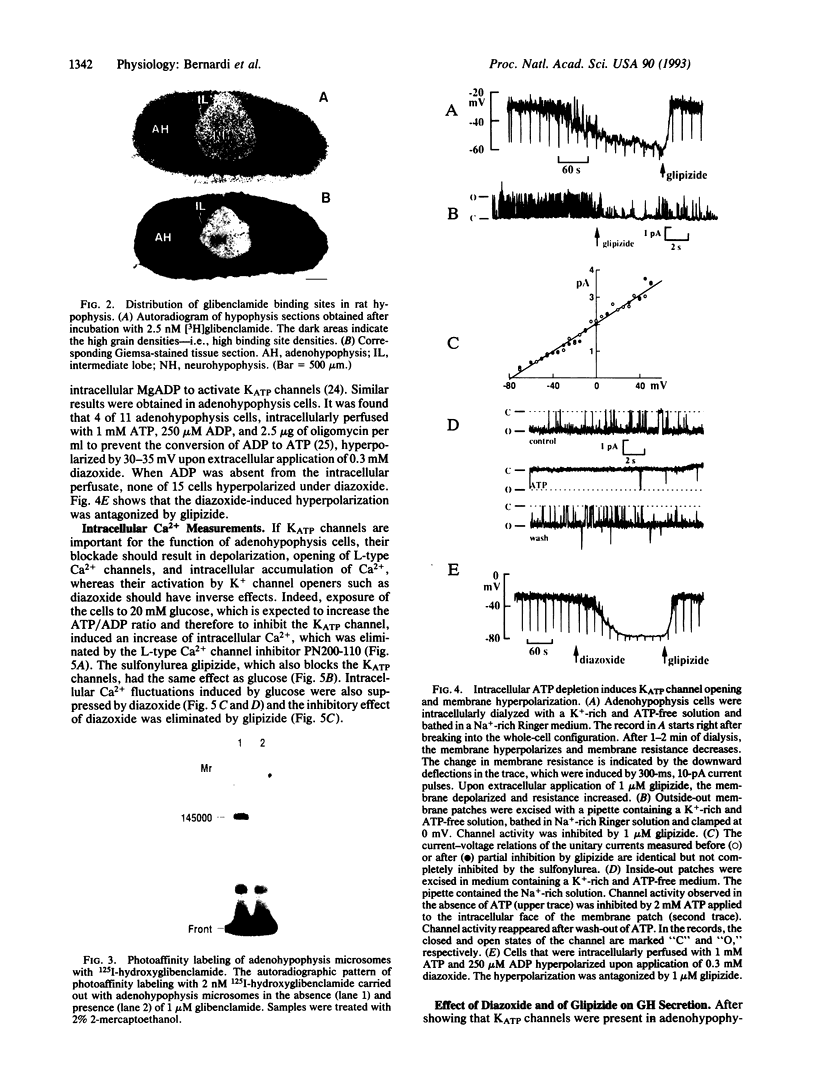
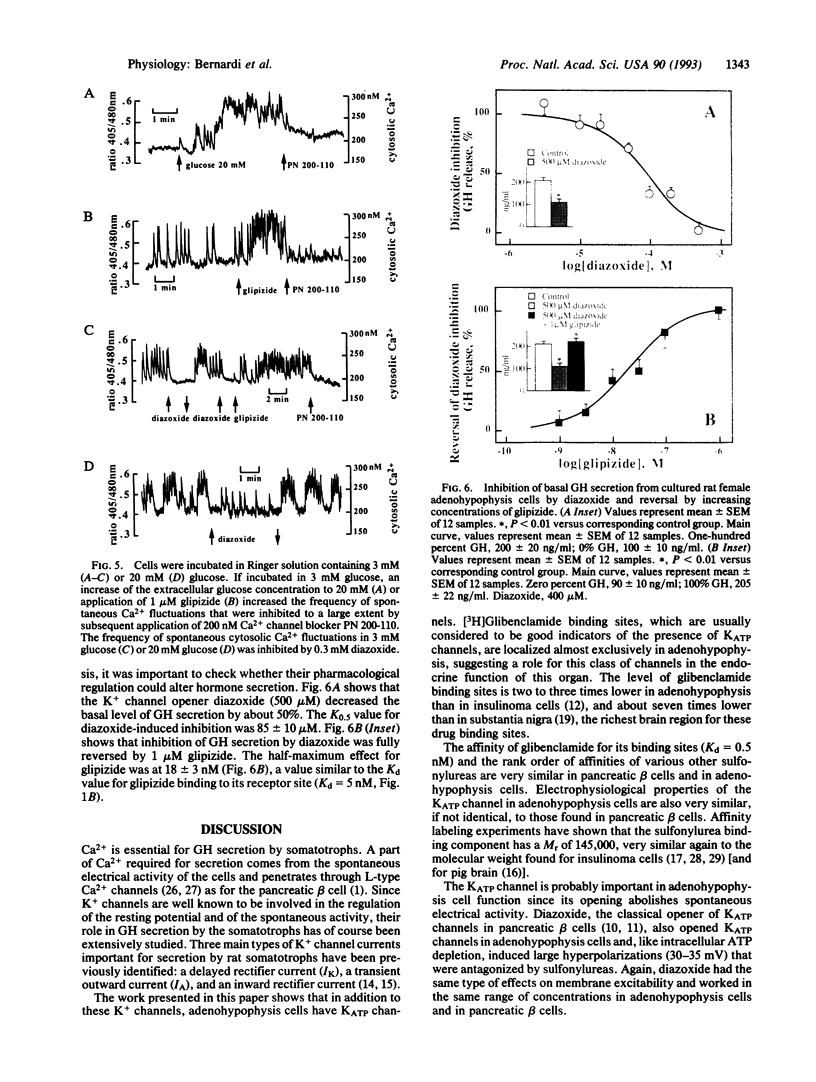
The adenohypophysis contains high-affinity binding sites for antidiabetic sulfonylureas that are specific blockers of ATP-sensitive K+ channels. The binding protein has a M(r) of 145,000 +/- 5000. The presence of ATP-sensitive K+ channels (26 pS) has been demonstrated by electrophysiological techniques. Intracellular perfusion of adenohypophysis cells with an ATP-free medium to activate ATP-sensitive K+ channels induces a large hyperpolarization (approximately 30 mV) that is antagonized by antidiabetic sulfonylureas. Diazoxide opens ATP-sensitive K+ channels in adenohypophysis cells as it does in pancreatic beta cells and also induces a hyperpolarization (approximately 30 mV) that is also suppressed by antidiabetic sulfonylureas. As in pancreatic beta cells, glucose and antidiabetic sulfonylureas depolarize the adenohypophysis cells and thereby indirectly increase Ca2+ influx through L-type Ca2+ channels. The K+ channel opener diazoxide has an opposite effect. Opening ATP-sensitive K+ channels inhibits growth hormone secretion and this inhibition is eliminated by antidiabetic sulfonylureas.
Full text
PDF
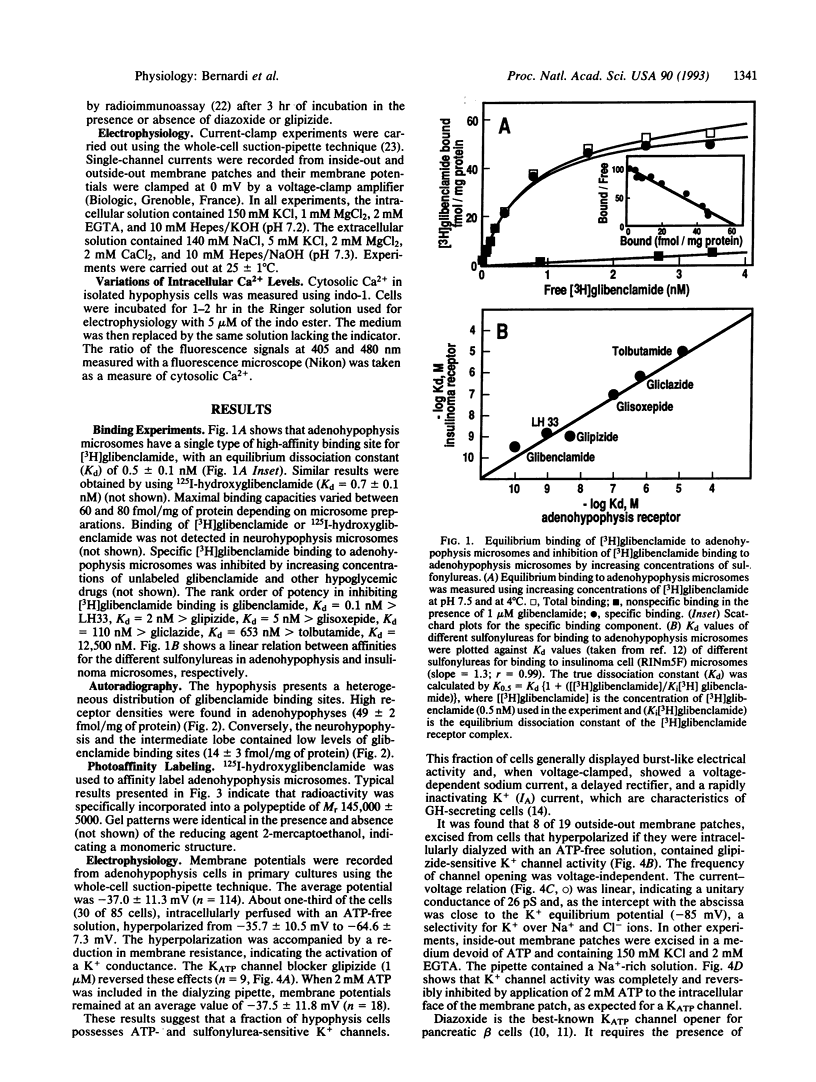

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Bryan L., Nelson D. A., Vu Q. A., Humphrey M. B., Boyd A. E., 3rd Photoaffinity labeling and partial purification of the beta cell sulfonylurea receptor using a novel, biologically active glyburide analog. J Biol Chem. 1990 May 15;265(14):8218–8224. [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Bernardi H., Fosset M., Lazdunski M. Characterization, purification, and affinity labeling of the brain [3H]glibenclamide-binding protein, a putative neuronal ATP-regulated K+ channel. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9816–9820. doi: 10.1073/pnas.85.24.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhang J., Vincent J. D., Israel J. M. Somatostatin increases voltage-dependent potassium currents in rat somatotrophs. Am J Physiol. 1990 Dec;259(6 Pt 1):C854–C861. doi: 10.1152/ajpcell.1990.259.6.C854. [DOI] [PubMed] [Google Scholar]
- Chen C., Zhang J., Vincent J. D., Israel J. M. Two types of voltage-dependent calcium current in rat somatotrophs are reduced by somatostatin. J Physiol. 1990 Jun;425:29–42. doi: 10.1113/jphysiol.1990.sp018090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. W., Standen N. B., Stanfield P. R. ATP-dependent potassium channels of muscle cells: their properties, regulation, and possible functions. J Bioenerg Biomembr. 1991 Aug;23(4):509–535. doi: 10.1007/BF00785809. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Aspinall R. J., Petersen O. H. The effects of cromakalim on ATP-sensitive potassium channels in insulin-secreting cells. Br J Pharmacol. 1990 Jan;99(1):169–175. doi: 10.1111/j.1476-5381.1990.tb14672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M. J., Bullett M. J., Li G. D., Wollheim C. B., Petersen O. H. Galanin activates nucleotide-dependent K+ channels in insulin-secreting cells via a pertussis toxin-sensitive G-protein. EMBO J. 1989 Feb;8(2):413–420. doi: 10.1002/j.1460-2075.1989.tb03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M. J., Illot M. C., Peterson O. H. Interaction of diazoxide, tolbutamide and ATP4- on nucleotide-dependent K+ channels in an insulin-secreting cell line. J Membr Biol. 1987;99(3):215–224. doi: 10.1007/BF01995702. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Potassium selective ion channels in insulin-secreting cells: physiology, pharmacology and their role in stimulus-secretion coupling. Biochim Biophys Acta. 1991 Mar 7;1071(1):67–82. doi: 10.1016/0304-4157(91)90012-l. [DOI] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Structure-activity relationships of K+ channel openers. Trends Pharmacol Sci. 1990 Oct;11(10):417–422. doi: 10.1016/0165-6147(90)90149-3. [DOI] [PubMed] [Google Scholar]
- Epelbaum J., Enjalbert A., Krantic S., Musset F., Bertrand P., Rasolonjanahary R., Shu C., Kordon C. Somatostatin receptors on pituitary somatotrophs, thyrotrophs, and lactotrophs: pharmacological evidence for loose coupling to adenylate cyclase. Endocrinology. 1987 Dec;121(6):2177–2185. doi: 10.1210/endo-121-6-2177. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Farquhar M. G. Hormone secretion by cells dissociated from rat anterior pituitaries. J Cell Biol. 1973 Nov;59(2 Pt 1):276–303. doi: 10.1083/jcb.59.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N., Hayafuji C., Konagaya H., Takahashi K. 17 beta-estradiol induces somatostatin (SRIF) inhibition of prolactin release and regulates SRIF receptors in rat anterior pituitary cells. Endocrinology. 1986 Sep;119(3):1028–1036. doi: 10.1210/endo-119-3-1028. [DOI] [PubMed] [Google Scholar]
- Kramer W., Oekonomopulos R., Pünter J., Summ H. D. Direct photoaffinity labeling of the putative sulfonylurea receptor in rat beta-cell tumor membranes by [3H]glibenclamide. FEBS Lett. 1988 Mar 14;229(2):355–359. doi: 10.1016/0014-5793(88)81155-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lussier B. T., French M. B., Moore B. C., Kraicer J. Free intracellular Ca2+ concentration ([Ca2+]i) and growth hormone release from purified rat somatotrophs. I. GH-releasing factor-induced Ca2+ influx raises [Ca2+]i. Endocrinology. 1991 Jan;128(1):570–582. doi: 10.1210/endo-128-1-570. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Glucose-regulated potassium channels are sweet news for neurobiologists. Trends Neurosci. 1990 Jun;13(6):197–199. doi: 10.1016/0166-2236(90)90158-7. [DOI] [PubMed] [Google Scholar]
- Mourre C., Widmann C., Lazdunski M. Sulfonylurea binding sites associated with ATP-regulated K+ channels in the central nervous system: autoradiographic analysis of their distribution and ontogenesis, and of their localization in mutant mice cerebellum. Brain Res. 1990 Jun 11;519(1-2):29–43. doi: 10.1016/0006-8993(90)90057-i. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Renier G., Serri O. Effects of acute and prolonged glucose excess on growth hormone release by cultured rat anterior pituitary cells. Neuroendocrinology. 1991 Nov;54(5):521–525. doi: 10.1159/000125947. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., De Weille J., Fosset M., Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987 Nov 25;262(33):15840–15844. [PubMed] [Google Scholar]
- Sims S. M., Lussier B. T., Kraicer J. Somatostatin activates an inwardly rectifying K+ conductance in freshly dispersed rat somatotrophs. J Physiol. 1991 Sep;441:615–637. doi: 10.1113/jphysiol.1991.sp018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum G. S., Martin J. B., Colle E. Ultradian growth hormone rhythm in the rat: effects of feeding, hyperglycemia, and insulin-induced hypoglycemia. Endocrinology. 1976 Sep;99(3):720–727. doi: 10.1210/endo-99-3-720. [DOI] [PubMed] [Google Scholar]
- Trube G., Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984 Jun;401(2):178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- Welsh J. B., Szabo M. Impaired suppression of growth hormone release by somatostatin in cultured adenohypophyseal cells of spontaneously diabetic BB/W rats. Endocrinology. 1988 Nov;123(5):2230–2234. doi: 10.1210/endo-123-5-2230. [DOI] [PubMed] [Google Scholar]
- de Weille J. R., Fosset M., Mourre C., Schmid-Antomarchi H., Bernardi H., Lazdunski M. Pharmacology and regulation of ATP-sensitive K+ channels. Pflugers Arch. 1989;414 (Suppl 1):S80–S87. doi: 10.1007/BF00582253. [DOI] [PubMed] [Google Scholar]
- de Weille J. R., Lazdunski M. ATP-sensitive K+ channels reveal the effects of intracellular chloride variations on cytoplasmic ATP concentrations and mitochondrial function. Biochem Biophys Res Commun. 1990 May 16;168(3):1137–1142. doi: 10.1016/0006-291x(90)91147-k. [DOI] [PubMed] [Google Scholar]
- de Weille J. R., Schmid-Antomarchi H., Fosset M., Lazdunski M. Regulation of ATP-sensitive K+ channels in insulinoma cells: activation by somatostatin and protein kinase C and the role of cAMP. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2971–2975. doi: 10.1073/pnas.86.8.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weille J., Schmid-Antomarchi H., Fosset M., Lazdunski M. ATP-sensitive K+ channels that are blocked by hypoglycemia-inducing sulfonylureas in insulin-secreting cells are activated by galanin, a hyperglycemia-inducing hormone. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1312–1316. doi: 10.1073/pnas.85.4.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]