Significance
Protein palmitoylation has been proposed to mediate the recruitment of signaling proteins into lipid rafts. However, a significant shortcoming of this hypothesis is that the proposed kinetics of protein palmitoylation/depalmitoylation heretofore have been too slow to mediate very rapid signaling events. Using a combination of a highly sensitive palmitoylation assay with pulsed metabolic labeling, we were able to detect a population of the Src kinase Lck undergoing extremely rapid palmitoylation and depalmitoylation subsequent to Fas receptor stimulation. The rapid rise and fall in Lck palmitoylation was highly synchronized with other signaling events occurring during the first minutes after activation of the Fas pathway, suggesting the existence of a previously undescribed signal transduction mechanism.
Keywords: apoptosis, calcium, Fas, Lck, protein palmitoylation
Abstract
Palmitoylation is the posttranslational modification of proteins with a 16-carbon fatty acid chain through a labile thioester bond. The reversibility of protein palmitoylation and its profound effect on protein function suggest that this modification could play an important role as an intracellular signaling mechanism. Evidence that palmitoylation of proteins occurs with the kinetics required for signal transduction is not clear, however. Here we show that engagement of the Fas receptor by its ligand leads to an extremely rapid and transient increase in palmitoylation levels of the tyrosine kinase Lck. Lck palmitoylation kinetics are consistent with the activation of downstream signaling proteins, such as Zap70 and PLC-γ1. Inhibiting Lck palmitoylation not only disrupts proximal Fas signaling events, but also renders cells resistant to Fas-mediated apoptosis. Knockdown of the palmitoyl acyl transferase DHHC21 eliminates activation of Lck and downstream signaling after Fas receptor stimulation. Our findings demonstrate highly dynamic Lck palmitoylation kinetics that are essential for signaling downstream of the Fas receptor.
Palmitoylation of cysteine residues is a common posttranslational protein modification that affects a variety of protein functions by regulating their intracellular localization, trafficking, and stability (1). Compared with other lipid modifications such as myristoylation and prenylation, palmitoylation is the only reversible modification. Furthermore, palmitoylated proteins can undergo multiple cycles of acylation and deacylation (2). The enzymatic control of protein palmitoylation in mammalian cells is mediated by a family of more than 20 protein acyltransferases (PATs) sharing a common DHHC (Asp-His-His-Cys) motif within the catalytic center (2–4). Traditionally, protein palmitoylation is thought to be catalyzed in the Golgi apparatus, and indeed systematic characterization of human DHHC PAT intracellular distribution has confirmed that the majority of these enzymes localized to the Golgi compartment (5); however, several enzymes, including DHHC5, 20, and 21, could be localized at the plasma membrane.
The depalmitoylation step is likely mediated by the acyl protein thioesterases APT1 and APT2, the only two enzymes known to reverse palmitoylation of cytosolic proteins in mammalian cells. APT1 was initially identified as a rat liver lysophospholipase widely spread among different tissues (6, 7). Later, its substrate specificity was expanded to a number of palmitoylated proteins, including Ras, heterotrimeric G protein α subunit, RGS4, SNAP-23, and eNOS (8). APT1 was the sole known cytosolic thioesterase until cloning of its close homolog, APT2 (9), which recently was reported to be involved in depalmitoylation of growth-associated protein 43 (10).
A number of signaling proteins that play critical roles in the regulation of T-cell function are known to be palmitoylated, including coreceptors CD4 and CD8, kinases Lck and Fyn, and adaptor proteins LAT and Cbp/PAG (11–13). In many cases, palmitoylation of these proteins is essential for their signaling function. Lck, a member of the Src family of tyrosine kinases, is cotranslationally myristoylated at glycine residue 2 and then posttranslationally palmitoylated at cysteine residues 3 and 5 (14, 15). These cysteines are dispensable for the catalytic activity of Lck, and mutagenesis of either site does not prevent palmitoylation or membrane binding (16–18). Simultaneous mutation of cysteine 3 and cysteine 5 produces a palmitoylation-deficient mutant of Lck that is unable to propagate T-cell receptor signaling, demonstrating a critical role for palmitoylation in regulating Lck function (16, 17, 19). The regulatory mechanisms controlling Lck palmitoylation remain largely unknown, however.
We have previously demonstrated that Lck is an essential component of the Fas signaling pathway (20). Lck is required for the most proximal Fas signaling events, including PLC-γ1 activation, proapoptotic calcium release, and cell death in T cells (20). Using a pulsed metabolic labeling and detection strategy, here we show that Lck palmitate turnover is very rapid even in resting T cells. Stimulation of T cells with Fas ligand results in a rapid increase and decrease in Lck palmitoylation that closely correlates with the activation state of downstream signaling molecules such as PLC-γ1. Finally, we identify the plasma membrane PAT DHHC21 as the enzyme responsible for Lck palmitoylation and downstream apoptotic calcium release. Our results indicate that agonist-induced protein palmitoylation and depalmitoylation occur with unprecedented kinetics, and suggest that lipidation of signaling proteins may be widely involved in the activation of second messenger signaling cascades.
Results
Palmitoylation of Lck Is Essential for Apoptotic Calcium Release.
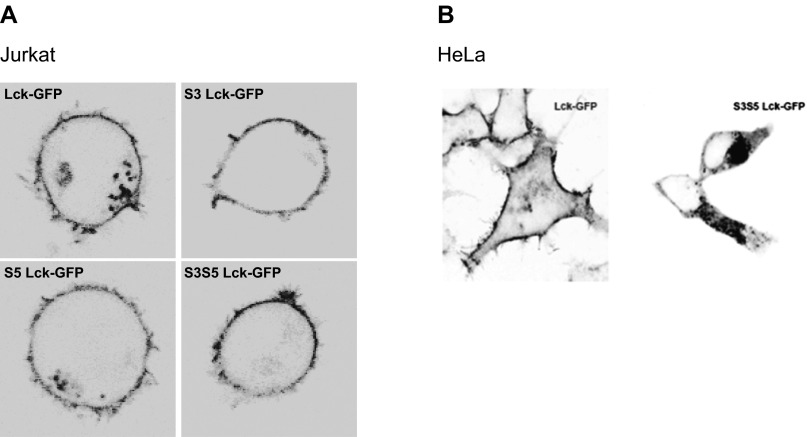
To determine whether palmitoylation of Lck is required for Fas signaling, we mutated the Lck palmitoylation sites cysteine 3 to serine (S3), cysteine 5 to serine (S5), or both cysteines to serines (S3S5). Palmitoylation-deficient mutants of Lck disrupted plasma membrane localization of Lck in HeLa cells, but not in Jurkat cells (Fig. S1 A and B). Thus, plasma membrane localization of Lck in T cells does not depend on its palmitoylation status. All palmitoylation mutants of Lck were incapable of rescuing Fas-mediated calcium release in Lck-deficient Jurkat cells (Fig. 1A), however, indicating that palmitoylation of both cysteine residues is essential for initiation of Fas signaling.
Fig. S1.
Intracellular localization of palmitoylated Lck. Confocal images of (A) JCam1.6 (Lck null) Jurkat cells and (B) HeLa cells transiently transfected with Lck-GFP, S3 Lck-GFP, S5 Lck-GFP, and S3S5 Lck-GFP expression vectors.
Fig. 1.
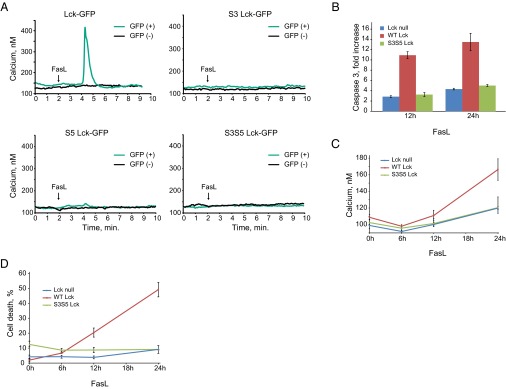
Palmitoylation of Lck is required for Fas-mediated apoptosis. (A) Fura-2 calcium imaging of Lck-deficient JCam1.6 Jurkat cells transiently transfected with Lck-GFP, S3 Lck-GFP, S5 Lck-GFP, and S3S5 Lck-GFP expression vectors. Black line, Lck-GFP negative; green line, Lck-GFP positive. (B) Caspase 3 activity in JCam1.6 cells stably transfected with WT and S3S5 Lck-GFP expression vectors at 12 and 24 h after Fas ligand stimulation, relative to untreated cells. (C) Time-dependent increases in cytosolic calcium concentration in JCam1.6 cells stably transfected with WT and S3S5 Lck-GFP expression vectors after Fas ligand treatment. (D) Cell death (propidium iodide-positive cells as a percentage of the total) in JCam1.6 cells stably transfected with WT and S3S5 Lck-GFP expression vectors at 12 and 24 h after Fas ligand stimulation. Data in B–D are presented as mean ± SEM from three separate determinations.
Loss of Lck Palmitoylation Impairs Fas-Mediated Apoptosis.
Fas-mediated cell death is characterized by two phases of calcium release, an initial transient increase mediated by PLC-γ1 and a late increase mediated by cytochrome c binding to the IP3R calcium channel (21). The second phase is associated with chronic calcium elevation, permeabilization of mitochondria, effector caspase activation, and ultimately cell death (21). To determine whether palmitoylation of Lck is required for the second phase of Fas-mediated apoptosis, we monitored the late elevation in cytoplasmic calcium, activation of caspase 3, and cell death in Lck-deficient Jurkat cells stably expressing either wild type (WT) or palmitoylation-deficient Lck. As shown in Fig. 1 B–D, WT Lck, but not the S3S5 Lck mutant, could rescue apoptotic calcium release, caspase 3 activation, and cell death in Lck null cells. Thus, our observations indicate that Lck palmitoylation is essential for all phases of Fas-mediated apoptotic cell death.
Lck Association with Lipid Rafts Is Required for Fas Signaling.
The attachment of fatty acids to proteins results in increased affinity toward liquid-ordered membrane fractions (lipid rafts) (22). We hypothesized that an increase in Lck palmitoylation would provide a molecular basis for stimulus-dependent partitioning into lipid rafts. To investigate whether Lck translocation into lipid rafts has a functional significance for Fas receptor signaling, we disrupted rafts using a brief (30 min) treatment with methyl-β-cyclodextrin (MβCD), a cholesterol-depleting agent (23). We found that preincubation with 5 mM MβCD inhibited activation of the Lck downstream targets Zap70 and PLC-γ1 in response to Fas ligand stimulation (Fig. S2A). A higher dose of MβCD (10 mM) completely suppressed activation of Lck and resulted in diminished calcium release on Fas receptor activation (Fig. S2 A and B), suggesting that lipid rafts are necessary for Lck-mediated activation of the Fas signaling pathway.
Fig. S2.
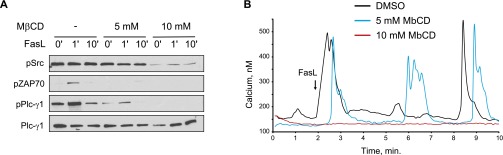
MβCD inhibits Fas-mediated PLC-γ1 activation and calcium release. (A) Jurkat cells preincubated with 5 mM and 10 mM MβCD for 30 min and treated with Fas ligand for 0, 1, and 10 min. Total cell lysates were analyzed by Western blot analysis. (B) Jurkat cells pretreated for 5 min with MβCD. Intracellular calcium release was determined with Fura-2 imaging.
Lck Has a High Palmitate Turnover Rate in Resting Cells.
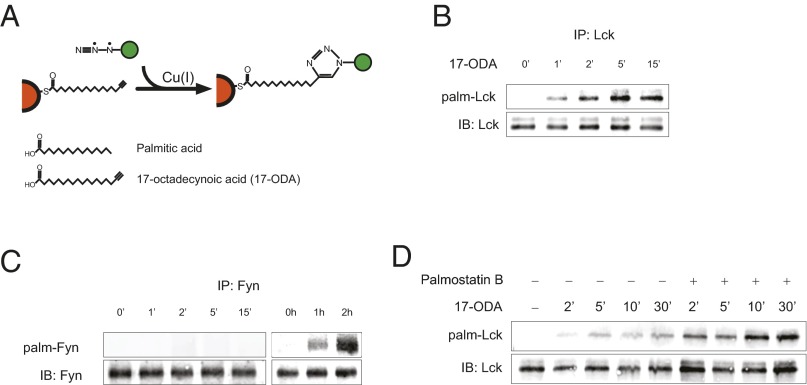
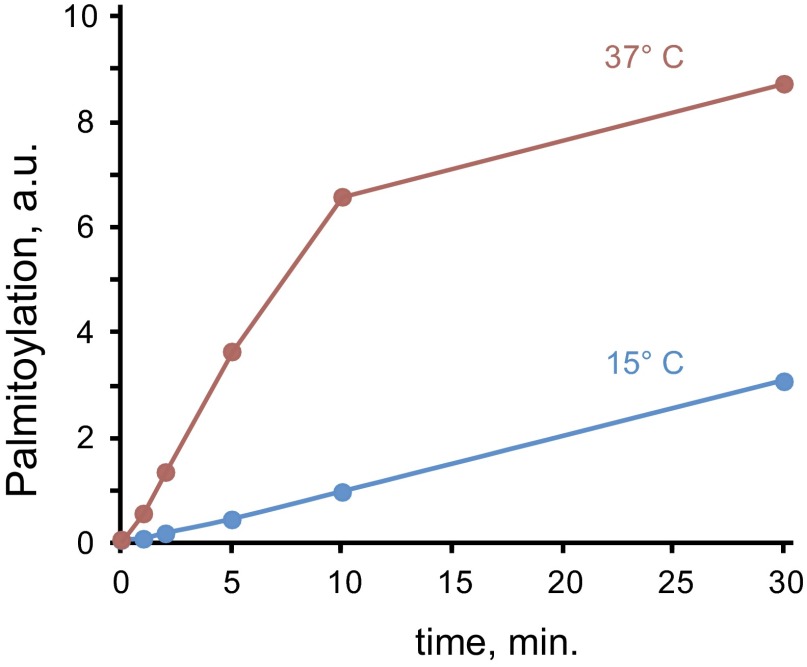
We next directly assessed the Lck palmitate turnover rate in unstimulated Jurkat T cells using bioorthogonal labeling with the palmitic acid analog 17-octadecynoic acid (17-ODA), followed by coupling to a fluorescent azide-reporter tag (24) (Fig. 2A). We found that incubation of Jurkat cells with 1 µM 17-ODA resulted in robust and selective labeling of palmitoylated Lck within minutes, indicating a remarkably high turnover rate of Lck palmitate even in the absence of extracellular stimulation (Fig. 2B). Quantitative analysis of Lck palmitate turnover kinetics revealed strong temperature dependence, suggesting that palmitoylation of Lck is an enzyme-facilitated reaction (Fig. S3). Interestingly, the palmitate turnover rate of the Lck paralog Fyn was markedly slower, implying distinct palmitoylation regulation of Lck and Fyn despite strong similarities in protein structure and intracellular localization (25) (Fig. 2C).
Fig. 2.
Rapid turnover of Lck palmitate in unstimulated cells. (A) Schematic of 17-ODA metabolic labeling and detection of palmitoylated proteins using the click chemistry reaction. (B) Lck palmitate turnover kinetics. Jurkat cells were incubated with 1 μM 17-ODA for the indicated times. Palm-Lck, palmitoylated Lck detected by infrared imaging of labeled 17-ODA; IB, immunoblot of total Lck. (C) Fyn palmitate turnover kinetics. (Left) No labeling was noted after 15 min of 17-ODA incubation at room temperature. (Right) Labeling became evident after 1 h of incubation at 37 °C. (D) Lck palmitate turnover kinetics in the presence of APT1 inhibitor palmostatin B. Jurkat cells were pretreated with 10 μM palmostatin B or DMSO for 30 min before the addition of 1 μM 17-ODA.
Fig. S3.
Temperature dependence of Lck palmitoylation. Palmitoylation was determined as in Fig. 2 at 37 °C or 15 °C, and quantified as the percentage of total Lck. Shown is a representative experiment of three separate determinations.
To further examine enzymatic control of Lck depalmitoylation, we took advantage of the recently described selective inhibitor of APT1, palmostatin B (26). We found that a 30-min preincubation of Jurkat cells with 10 µM palmostatin B resulted in significantly increased rates of de novo Lck palmitoylation, suggesting that APT1 directly participates in the regulation of Lck palmitate turnover (Fig. 2D). Thus, our data demonstrate that highly dynamic palmitoylation of Lck is selectively supported by a balancing act of palmitoylating and depalmitoylating enzymes, and identify Lck as a possible physiological target of the thioesterase APT1.
Fas Receptor Stimulation Leads to a Rapid and Transient Increase in Lck Palmitoylation.
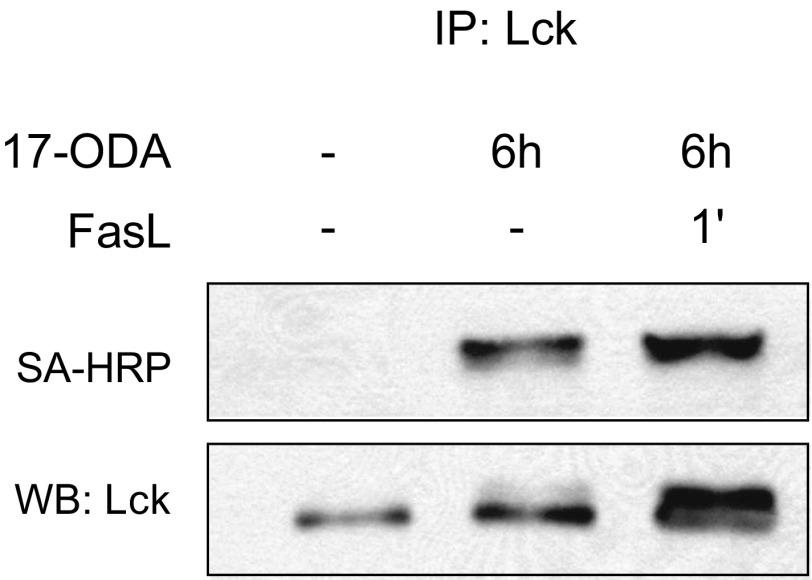
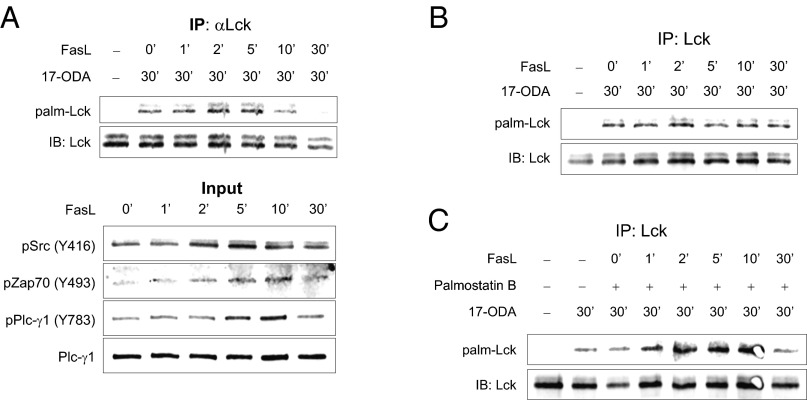
We next wished to determine whether Lck palmitoylation was regulated by Fas receptor stimulation. The fast palmitoylation turnover of Lck (Fig. 2) suggests that 17-ODA metabolic labeling could rapidly saturate the entire Lck pool within hours, thereby masking any stimulus-dependent changes in Lck. Indeed, Fas receptor stimulation of Jurkat cells preincubated with 17-ODA for 6 h or longer did not result in a detectable increase in Lck palmitoylation (Fig. S4). Thus, we hypothesized that short-term exposure of cells to 17-ODA would allow us to selectively detect a pool of Lck proteins palmitoylated in response to Fas receptor activation. We limited the total incubation time of Jurkat cells with 1 μM 17-ODA to 30 min in the presence or absence of Fas receptor stimulation. As shown in Fig. 3A, stimulation of Jurkat cells with Fas ligand resulted in a rapid increase in de novo palmitoylation of Lck detectable within 2 min of Fas receptor engagement. Surprisingly, we found that longer stimulation (>10 min) of the Fas receptor was associated with a rapid decrease in palmitoylated Lck to levels even lower than those seen in unstimulated cells.
Fig. S4.
Metabolic labeling of Jurkat cells for 6 h with 17-ODA masks Fas ligand-dependent palmitoylation of Lck. In contrast to the pulse-labeling protocol, extended incubations with 17-ODA saturates the Lck pool and masks FasL-dependent increases in Lck palmitoylation. Palmitoylated Lck was detected by conjugation of 17-ODA with biotin-azide and detection with streptavidin conjugated to horseradish peroxidase (SA-HRP).
Fig. 3.
Rapid and transient Fas-mediated palmitoylation of Lck. (A) Palmitoylation of Lck in the presence of Fas ligand (Upper) and input representing 5% of total protein extracts (Lower). Jurkat cells were incubated with 1 µM 17-ODA or DMSO at room temperature for 30 min. Fas ligand was added during incubation with 17-ODA for the indicated times. (B) Fas-mediated palmitoylation of Lck in J.gamma1 (PLC-γ1 null) Jurkat cells. (C) Fas-mediated palmitoylation of Lck in the presence of APT1 inhibitor palmostatin B. Jurkat cells were pretreated with 10 µM palmostatin B or DMSO for 30 min before the addition of 1 µM 17-ODA and Fas ligand.
Importantly, the rapid but transient increase in palmitoylation of Lck closely matched the activation kinetics of signaling proteins involved in the initiation of Fas receptor signaling (Fig. 3A, input), indicating that the enzymatic mechanisms controlling stimulus-dependent protein palmitoylation and depalmitoylation likely are directly activated by components of the Fas signaling pathway. We confirmed our findings in two additional human leukemic T-cell lines, Molt-4 and CCRF-CEM. Similar to Jurkat cells, stimulation of Molt-4 and CCRF-CEM cells with Fas ligand resulted in a rapid and transient increase in Lck palmitoylation, confirming the generality of the observed phenomenon (Fig. S5).
Fig. S5.
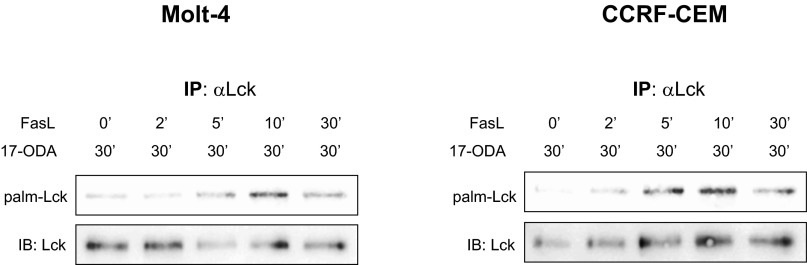
Fas-mediated palmitoylation of Lck in human leukemic T-cell lines. Molt-4 (Left) and CCRF-CEM (Right) cells were incubated with 1 µM 17-ODA at room temperature for 30 min. Fas ligand and 17-ODA were added during incubation for the indicated times. These results are essentially identical to those obtained in Jurkat cells (Fig. 3A).
Fas-Mediated Palmitoylation of Lck Is Regulated by PLC-γ1.
We previously demonstrated that PLC-γ1–mediated calcium release from endoplasmic reticulum stores is required for Fas signaling (20, 21). To determine whether rapid and transient palmitoylation of Lck depends on Fas-mediated elevations in cytoplasmic calcium, we used J.gamma1 (PLC-γ1 null) Jurkat cells deficient in Fas-mediated calcium release (20, 21). We found that basal Lck palmitoylation levels in J.gamma1 cells remained unchanged, but Fas-mediated changes in Lck palmitoylation were completely absent in this cell line (Fig. 3B). This finding indicates that both agonist-induced Lck palmitoylation and depalmitoylation processes likely are regulated directly or indirectly by calcium and/or diacylglycerol.
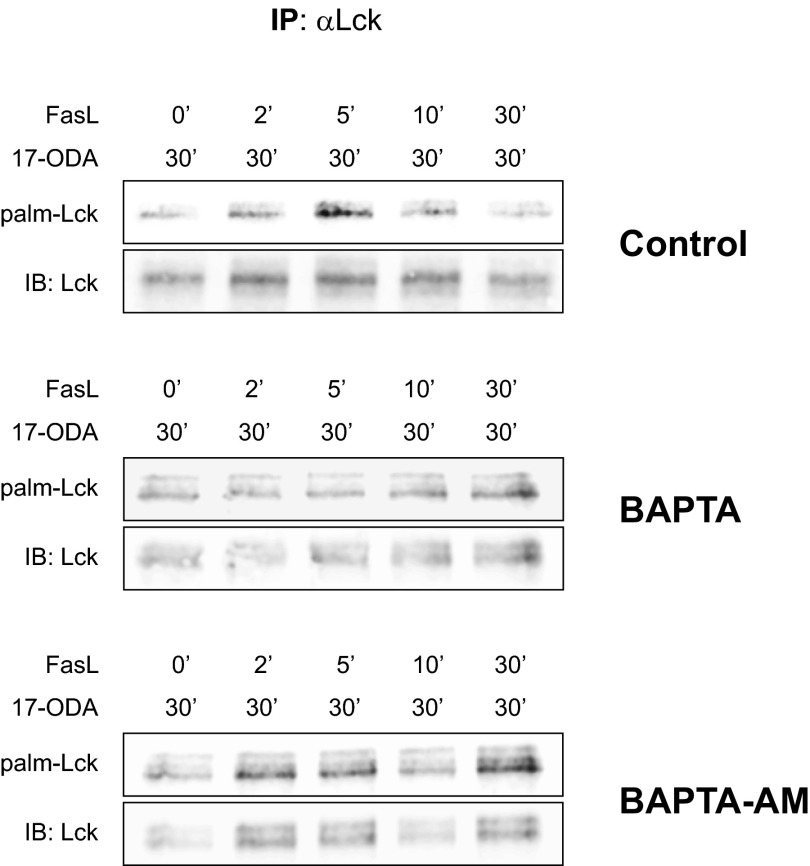
To determine whether Lck palmitoylation is regulated by increases in cytosolic calcium, we chelated extracellular calcium with BAPTA or intracellular calcium by loading with BAPTA-AM. As shown in Fig. S6, a 30-min preincubation of Jurkat cells with either 5 mM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) or 10 µM BAPTA-acetoxymethyl ester (BAPTA-AM) before the addition of Fas ligand prevented the agonist-induced increase in Lck palmitoylation. This result, combined with the requirement for PLC-γ1, indicates that elevations in cytosolic calcium originating from both intracellular and extracellular stores are required for efficient Lck palmitoylation.
Fig. S6.
Effect of extracellular (BAPTA) and intracellular (BAPTA-AM) calcium chelators on Fas-mediated Lck palmitoylation. Control Jurkat cells (Top) or Jurkat cells preincubated with 5 mM BAPTA (Middle) or 10 µM BAPTA-AM (Bottom) for 30 min at room temperature were stimulated with Fas ligand in the presence of 17-ODA. Both BAPTA and BAPTA-AM inhibited FasL-dependent palmitoylation.
To determine whether APT1 is the palmitoyl thioesterase mediating Lck depalmitoylation after Fas receptor engagement, we treated Jurkat cells with APT1 inhibitor palmostatin B before Fas receptor stimulation. Palmostatin B failed to block Lck depalmitoylation in response to Fas stimulation (Fig. 3C), indicating that stimulus-dependent depalmitoylation of Lck could be mediated by an as-yet unidentified protein thioesterase. Thus, our observations suggest that constitutively active APT1 regulates Lck palmitoylation in resting cells (Fig. 2D), whereas stimulus-dependent Lck depalmitoylation is supported by an inducible enzyme.
Palmitoyl Acyltransferase DHHC21 Is Required for Fas Signaling.
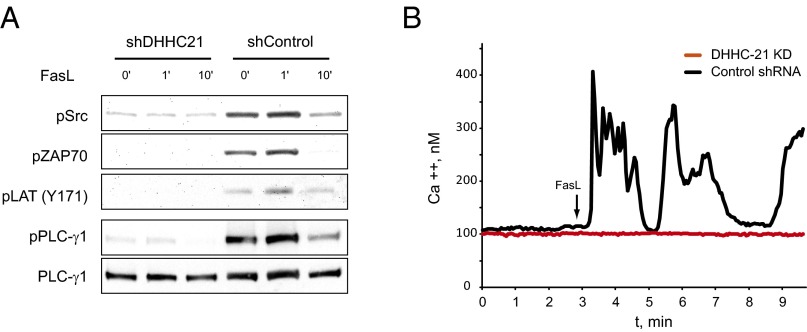
Coexpression studies have suggested that DHHC21, a member of the human DHHC PAT family, has a specific substrate preference toward Lck (27). Furthermore, DHHC21 has been identified as one of only a few plasma membrane-localized PATs (5), suggesting that this enzyme could modulate rapid activation of Lck upon stimulation of the Fas receptor. To examine whether endogenously expressed DHHC21 in T cells mediates Fas receptor signaling, we transduced Jurkat cells with lentivirus carrying either DHHC21-targeting or nontargeting control shRNA vectors, and found that down-regulation of endogenous DHHC21 significantly suppressed both basal and Fas-mediated phosphorylation of Lck, Zap70, LAT, and PLC-γ1 (Fig. 4A). Consistent with these findings, knockdown of DHHC21 abolished calcium release from IP3R channels in response to Fas receptor stimulation (Fig. 4B). Thus, DHHC21 is the PAT regulating Lck activation after Fas receptor engagement.
Fig. 4.
Palmitoyl acyltransferase DHHC21 mediates activation of the Fas signaling pathway. (A) Activation of the Fas signaling pathway in Jurkat cells stimulated with Fas ligand in cells transduced with DHHC21-specific (shDHHC21) or control (shControl) shRNA. (B) Fas-dependent calcium release in Jurkat cells expressing DHHC21-specific (shDHHC21) or control (shControl) shRNA. Shown are single-cell responses representative of hundreds of determinations.
Discussion
Our results show that engagement of the Fas receptor results in very rapid changes in Lck palmitoylation levels that are consistent with the transient activation kinetics of proximal T-cell signaling proteins. Rapid increases in Lck palmitoylation levels on Fas receptor stimulation followed by depalmitoylation (Fig. 3A) suggest a highly synchronized sequence of events modulating activation of palmitoyl acyltransferases and protein thioesterases. This observation is supported by our experiments showing that shRNA-mediated down-regulation of DHHC21 compromised the activation of proximal Fas signaling events (Fig. 4). Although future studies are needed to fully characterize the enzymes involved in the direct regulation of Lck palmitoylation status in response to extracellular stimulation, our findings allow us to propose a model in which rapid agonist-induced changes in palmitoylation levels of regulatory proteins are essential for mediating signal transduction events. In particular, rapid changes in protein palmitoylation could provide a molecular basis for activation of plasma membrane-localized signaling proteins by targeting them into specific plasma membrane subdomains. As such, we propose that protein palmitoylation is analogous to other transient posttranslational modifications that regulate signaling, such as phosphorylation. Finally, the discovery of a novel class of regulatory enzymes not previously implicated in T-cell signaling would greatly expand a range of potential therapeutic targets for diseases associated with altered T-cell homeostasis.
Materials and Methods
Antibodies and Reagents.
Antibodies against phospho-Src, Lck, Fyn, LAT, phospho-ZAP70 (Tyr319), PLC-γ1, and phospho-PLC-γ1 were obtained from Cell Signaling Technology and Millipore. Secondary antibodies were purchased from Cell Signaling Technology and LI-COR Biosciences. Fas ligand microvesicles were either purchased from Millipore or purified from culture supernatants of NIH 3T3 cells overexpressing Fas ligand, as described previously (28). Other reagents included palmostatin B (Millipore), EDTA-free complete protease inhibitor mixture tablets (Roche Applied Science), 17-ODA (Cayman Chemical), IRDye 800CW Azide Infrared Dye (LI-COR Biosciences), biotin-azide (Invitrogen), and z-DEVD-R110 (American Peptide). The following chemicals were obtained from Sigma-Aldrich: phosphatase inhibitor mixture 2, n-dodecyl β-d-maltoside, poly-l-lysine, Tris-(2-carboxyethyl)phosphine hydrochloride (TCEP), Tris-(benzyltriazolylmethyl)amine (TBTA), CuSO4, t-butanol, methyl-β-cyclodextrin, and puromycin. BAPTA and BAPTA-AM were purchased from Invitrogen.
Cell Culture.
The following cell lines were obtained and cultured according to the guidelines of the American Type Culture Collection: human embryonic kidney HEK 293T cells, Molt-4 and CCRF-CEM acute lymphoblastic leukemia cells, Jurkat T-cell leukemia cells (clone E6-1), and Jurkat derivatives J.CaM1.6 (Lck null) and J.gamma1 (PLC-γ1 null).
Calcium Imaging.
Calcium measurements and stimulation with Fas ligand microvesicles were performed exactly as described previously (20, 21).
Caspase Activity.
Caspase activity was determined fluorometrically as described previously (29) using z-DEVD-R110 as the protease substrate at a final concentration of 50 µM (American Peptide Company). Each experiment was repeated a minimum three times, and data are presented as the mean ± SEM of three experiments.
Cell Death.
Cell death was quantified as described previously (21, 30) using propidium iodide staining. Each experiment was repeated a minimum three times, and data are presented as the mean ± SEM of three experiments.
Protein Palmitoylation Assay.
Metabolic labeling of Jurkat cells was performed as described previously (31). In brief, cells were incubated with 1 μM 17-ODA in Dulbecco's PBS (DPBS) at room temperature or in serum-free media at 37 °C for the indicated times. Cells were lysed in 1% n-dodecyl β-d-maltoside in DPBS, supplemented with a protease inhibitor mixture, phosphatase inhibitor mixture 2, and 10 µM palmostatin B. After overnight incubation with anti-Lck or anti-Fyn antibody, proteins were collected using protein A beads. The protein A beads were then washed three times with ice-cold DPBS, after which the click chemistry reaction was performed on the beads by incubating them for 1 h at room temperature with 1 µM IRDye 800CW Azide Infrared Dye, 5 mM CuSO4, 1 mM TCEP, and 0.17 mM TBTA. The reaction was quenched by the addition of nonreducing Laemmli buffer, followed by a 30-min incubation at 80 °C. The labeled palmitoylated proteins were analyzed after SDS/PAGE and transfer to nitrocellulose using an Odyssey infrared imaging system (LI-COR Biosciences).
Plasmids and Mutagenesis.
The vector driving the expression of Lck with a C-terminal fusion of GFP was purchased from OriGene Technologies. To produce the cysteine to serine mutants of palmitoylation sites, we performed site-directed mutagenesis using appropriate oligonucleotides (sequences available on request).
RNA Interference.
For DHHC21 knockdown, a specific short hairpin RNA (shRNA) construct set was obtained from Sigma-Aldrich, and lentivirus production plasmids psPAX2 and pMD2.G were obtained from Addgene. Screening of shRNA constructs identified that clones TRCN0000143485 and TRCN0000140807 resulted in a significant reduction of DHHC21 mRNA levels. Lentivirus production and shRNA knockdown were performed according to protocols from Addgene (www.addgene.org/tools/protocols/plko/).
Acknowledgments
We thank Brent Martin (University of Michigan, Ann Arbor), Ilya Levental (University of Texas Health Science Center at Houston), and José M. Barral (University of Texas Medical Branch) for helpful suggestions. This work was supported by National Institutes of Health Grant GM081685 and by startup funds provided by the University of Texas Health Science Center at Houston (to D.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509929112/-/DCSupplemental.
References
- 1.Linder ME, Deschenes RJ. Palmitoylation: Policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8(1):74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 2.Baekkeskov S, Kanaani J. Palmitoylation cycles and regulation of protein function (review) Mol Membr Biol. 2009;26(1):42–54. doi: 10.1080/09687680802680108. [DOI] [PubMed] [Google Scholar]
- 3.Ladygina N, Martin BR, Altman A. Dynamic palmitoylation and the role of DHHC proteins in T cell activation and anergy. Adv Immunol. 2011;109:1–44. doi: 10.1016/B978-0-12-387664-5.00001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Draper JM, Smith CD. Palmitoyl acyltransferase assays and inhibitors (review) Mol Membr Biol. 2009;26(1):5–13. doi: 10.1080/09687680802683839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 2006;1761(4):474–483. doi: 10.1016/j.bbalip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto H, Hayashi H, Yamashita S. Purification, cDNA cloning, and regulation of lysophospholipase from rat liver. J Biol Chem. 1996;271(13):7705–7711. doi: 10.1074/jbc.271.13.7705. [DOI] [PubMed] [Google Scholar]
- 7.Wang A, Yang HC, Friedman P, Johnson CA, Dennis EA. A specific human lysophospholipase: cDNA cloning, tissue distribution and kinetic characterization. Biochim Biophys Acta. 1999;1437(2):157–169. doi: 10.1016/s1388-1981(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 8.Zeidman R, Jackson CS, Magee AI. Protein acyl thioesterases (review) Mol Membr Biol. 2009;26(1):32–41. doi: 10.1080/09687680802629329. [DOI] [PubMed] [Google Scholar]
- 9.Toyoda T, Sugimoto H, Yamashita S. Sequence, expression in Escherichia coli, and characterization of lysophospholipase II. Biochim Biophys Acta. 1999;1437(2):182–193. doi: 10.1016/s1388-1981(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 10.Tomatis VM, Trenchi A, Gomez GA, Daniotti JL. Acyl-protein thioesterase 2 catalyzes the deacylation of peripheral membrane-associated GAP-43. PLoS One. 2010;5(11):e15045. doi: 10.1371/journal.pone.0015045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bijlmakers M-J. Protein acylation and localization in T cell signaling (review) Mol Membr Biol. 2009;26(1):93–103. doi: 10.1080/09687680802650481. [DOI] [PubMed] [Google Scholar]
- 12.Hundt M, et al. Palmitoylation-dependent plasma membrane transport but lipid raft-independent signaling by linker for activation of T cells. J Immunol. 2009;183(3):1685–1694. doi: 10.4049/jimmunol.0803921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem. 2000;275(1):261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 14.Abraham N, Veillette A. Activation of p56lck through mutation of a regulatory carboxy-terminal tyrosine residue requires intact sites of autophosphorylation and myristylation. Mol Cell Biol. 1990;10(10):5197–5206. doi: 10.1128/mcb.10.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paige LA, Nadler MJ, Harrison ML, Cassady JM, Geahlen RL. Reversible palmitoylation of the protein-tyrosine kinase p56lck. J Biol Chem. 1993;268(12):8669–8674. [PubMed] [Google Scholar]
- 16.Kosugi A, et al. A pivotal role of cysteine 3 of Lck tyrosine kinase for localization to glycolipid-enriched microdomains and T cell activation. Immunol Lett. 2001;76(2):133–138. doi: 10.1016/s0165-2478(01)00174-2. [DOI] [PubMed] [Google Scholar]
- 17.Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 1997;16(16):4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda K, et al. Serine 6 of Lck tyrosine kinase: A critical site for Lck myristoylation, membrane localization, and function in T lymphocytes. J Immunol. 2000;165(6):3226–3231. doi: 10.4049/jimmunol.165.6.3226. [DOI] [PubMed] [Google Scholar]
- 19.Yurchak LK, Sefton BM. Palmitoylation of either Cys-3 or Cys-5 is required for the biological activity of the Lck tyrosine protein kinase. Mol Cell Biol. 1995;15(12):6914–6922. doi: 10.1128/mcb.15.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akimzhanov AM, Wang X, Sun J, Boehning D. T-cell receptor complex is essential for Fas signal transduction. Proc Natl Acad Sci USA. 2010;107(34):15105–15110. doi: 10.1073/pnas.1005419107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wozniak AL, et al. Requirement of biphasic calcium release from the endoplasmic reticulum for Fas-mediated apoptosis. J Cell Biol. 2006;175(5):709–714. doi: 10.1083/jcb.200608035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006(359):14–28. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 23.Scheel-Toellner D, et al. The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem Biophys Res Commun. 2002;297(4):876–879. doi: 10.1016/s0006-291x(02)02311-2. [DOI] [PubMed] [Google Scholar]
- 24.Martin BR, Cravatt BF. Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods. 2009;6(2):135–138. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilangumaran S, Briol A, Hoessli DC. CD44 selectively associates with active Src family protein tyrosine kinases Lck and Fyn in glycosphingolipid-rich plasma membrane domains of human peripheral blood lymphocytes. Blood. 1998;91(10):3901–3908. [PubMed] [Google Scholar]
- 26.Dekker FJ, et al. 2010. Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat Chem Biol 6(6):449–456.
- 27.Tsutsumi R, et al. Identification of G protein alpha subunit-palmitoylating enzyme. Mol Cell Biol. 2009;29(2):435–447. doi: 10.1128/MCB.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jodo S, et al. Apoptosis-inducing membrane vesicles: A novel agent with unique properties. J Biol Chem. 2001;276(43):39938–39944. doi: 10.1074/jbc.M107005200. [DOI] [PubMed] [Google Scholar]
- 29.Boehning D, et al. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5(12):1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 30.Boehning D, van Rossum DB, Patterson RL, Snyder SH. A peptide inhibitor of cytochrome c/inositol 1,4,5-trisphosphate receptor binding blocks intrinsic and extrinsic cell death pathways. Proc Natl Acad Sci USA. 2005;102(5):1466–1471. doi: 10.1073/pnas.0409650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin BR, Cravatt BF. Large-scale profiling of protein palmitoylation in mammalian cells. Mat Methods. 2009;6(2):135–138. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]