Significance
Fibrosis is involved in 30–45% of deaths in the U.S. The disease may involve a runaway positive feedback loop between fibroblasts and monocyte-derived fibrocytes. The signals from fibrocytes to fibroblasts include inflammatory cytokines such as TNF-α, but the signals from TNF-α-stimulated fibroblasts to fibrocytes are unknown. We find that one of these signals is the protein lumican, and show that lumican levels are increased in fibrotic lesions. The identification of lumican as a signal in fibrotic tissues that promotes fibrocyte differentiation represents a major advance in our understanding of the regulation of the innate immune system, supports the positive feedback loop model of fibrosis, and indicates that lumican-inhibiting drugs may be beneficial in regulating fibrosis.
Keywords: fibrocyte, lumican, fibrosis, inflammation, decorin
Abstract
In healing wounds and fibrotic lesions, fibroblasts and monocyte-derived fibroblast-like cells called fibrocytes help to form scar tissue. Although fibrocytes promote collagen production by fibroblasts, little is known about signaling from fibroblasts to fibrocytes. In this report, we show that fibroblasts stimulated with the fibrocyte-secreted inflammatory signal tumor necrosis factor-α secrete the small leucine-rich proteoglycan lumican, and that lumican, but not the related proteoglycan decorin, promotes human fibrocyte differentiation. Lumican competes with the serum fibrocyte differentiation inhibitor serum amyloid P, but dominates over the fibroblast-secreted fibrocyte inhibitor Slit2. Lumican acts directly on monocytes, and unlike other factors that affect fibrocyte differentiation, lumican has no detectable effect on macrophage differentiation or polarization. α2β1, αMβ2, and αXβ2 integrins are needed for lumican-induced fibrocyte differentiation. In lung tissue from pulmonary fibrosis patients with relatively normal lung function, lumican is present at low levels throughout the tissue, whereas patients with advanced disease have pronounced lumican expression in the fibrotic lesions. These data may explain why fibrocytes are increased in fibrotic tissues, suggest that the levels of lumican in tissues may have a significant effect on the decision of monocytes to differentiate into fibrocytes, and indicate that modulating lumican signaling may be useful as a therapeutic for fibrosis.
During wound healing, monocytes leave the circulation, enter the tissue, and differentiate into fibroblast-like cells called fibrocytes (1–6). Fibrocytes are also found in the scar tissue-like lesions associated with fibrotic diseases such as pulmonary fibrosis, congestive heart failure, cirrhosis of the liver, and nephrogenic systemic fibrosis (3, 7–11). Fibrocytes express markers of both hematopoietic cells (CD34, CD45, FcγR, LSP-1, MHC class II) and stromal cells (collagens, fibronectin, and matrix metalloproteases) (2, 3, 12–14). Fibrocytes also promote angiogenesis by secreting VEGF, bFGF, IL-8, and PDGF (15). A key question about fibrocyte differentiation and fibrosis is why fibrocytes are readily observed in fibrotic lesions, but are rarely observed in healthy tissues (3, 10, 16–19).
Fibrosis is a dynamic process involving many cells besides fibrocytes (20, 21). In fibrotic lesions, tissue-resident fibroblasts proliferate and produce excessive amounts of extracellular matrix (ECM) that distorts tissue architecture, leading to tissue destruction (21, 22). Fibrocytes secrete a variety of cytokines including IL-13, TGF-β, CTGF, and TNF-α that promote the proliferation, migration, and extracellular matrix production by the local fibroblasts (15, 23–26). Conversely, fibroblasts secrete a variety of factors that promote leukocyte entry, survival, and retention during inflammation (27–30). An intriguing possibility is that a runaway positive feedback loop involving unknown signals from fibrocyte-activated fibroblasts back to fibrocytes may lead to the persistence of fibrotic lesions.
In this report, we show that fibroblasts stimulated with TNF-α secrete the small leucine-rich proteoglycan lumican, and that lumican promotes fibrocyte differentiation. In addition, we show that in a mouse pulmonary fibrosis model as well as human patients with pulmonary fibrosis, there appears to be an increase in lumican levels in the lungs, suggesting that pulmonary fibrosis may be in part due to elevated lumican levels. These data suggest that lumican may be one of the unknown signals from fibroblasts to fibrocytes that mediates part of a fibroblast-fibrocyte feedback loop that potentiates fibrosis.
Results
TNF-α–Stimulated Fibroblasts Promote Fibrocyte Differentiation.
To test the hypothesis that activated fibroblasts might secrete soluble factors that promote fibrocyte differentiation, we added conditioned medium from human fibroblasts incubated with TNF-α (FCM+TNF-α) to human peripheral blood mononuclear cells (PBMC) in conditions where some of the monocytes in the PBMC would normally differentiate into fibrocytes. We used TNF-α as the stimulus, as other profibrotic cytokines such as IL-4, IL-13, and TGF-β act directly on monocytes to regulate fibrocyte differentiation (31). TNF-α is produced by monocytes, macrophages, and fibrocytes, serum TNF-α levels are increased in fibrosis patients, and TNF-α induces fibrosis in animal models (32–36).
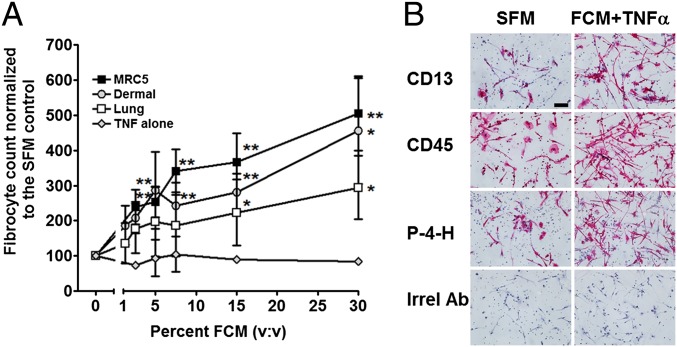
In the absence of fibroblast-conditioned medium, we observed 160–1,500 fibrocytes per 105 PBMC from the different donors, similar to what we and others have previously observed (12, 13, 37, 38). We and others have previously shown that ∼1–2% of PBMC or 10–20% of monocytes can readily differentiate into fibrocytes (12, 13, 39). Because of this variability, for each donor, fibrocyte numbers were normalized to serum-free controls. For all donors, compared with the control with no added FCM+TNF-α, 7.5% and above FCM+TNF-α led to a significant increase in fibrocyte differentiation (Fig. 1A). As previously observed (31), the presence of TNF-α alone did not significantly affect fibrocyte differentiation (Fig. 1A).
Fig. 1.
TNF-α–stimulated fibroblasts secrete factors that potentiate fibrocyte differentiation. (A) Human normal adult lung, dermal, and MRC-5 fetal lung fibroblasts were incubated with 20 ng/mL TNF-α for 2 d, and the conditioned medium (FCM+TNF-α) was then collected. Human peripheral blood mononuclear cells (PBMC) were then cultured in the presence or absence of FCM+TNF-α for 5 d. As a control, PBMC were incubated with dilutions of SFM containing 20 ng/mL TNF-α. Values are mean ± SEM (n = 5). *P < 0.05; **P < 0.01 compared with the no-FCM control (t test). (B) PBMC were cultured for 5 d in SFM or SFM with 30% MRC-5 FCM+TNF-α. After 5 d, PBMC were air-dried, fixed, and stained with antibodies against the fibrocyte and macrophage marker CD13, the pan leukocyte marker CD45, prolyl-4-hyroxylase, a key enzyme in collagen synthesis, and mouse IgG1 antibodies (red staining). Cells were then counterstained with hematoxylin to identify nuclei (blue). Photomicrographs are representative results from five different donors. The spindle-shaped elongated cells are fibrocytes. (Scale bar: 20 μm.)
To verify that the FCM+TNF-α was affecting the number of fibrocytes, and to determine whether FCM+TNF-α altered the phenotype of fibrocytes, we stained PBMC after 5 d of culture with or without FCM+TNF-α for fibrocyte markers (Fig. 1B). In the absence of FCM+TNF-α, the elongated cells were positive for markers expressed by fibrocytes including CD13, CD45, and prolyl-4-hydroxylase (14). In the presence of 10% (vol/vol) FCM+TNF-α, the number of fibrocytes was significantly increased, although we did not detect any observable differences in the expression level per cell of CD13, CD45, and prolyl-4-hyrdoxylase (Fig. 1B).
Fibroblasts Secrete Lumican, which Promotes Fibrocyte Differentiation.
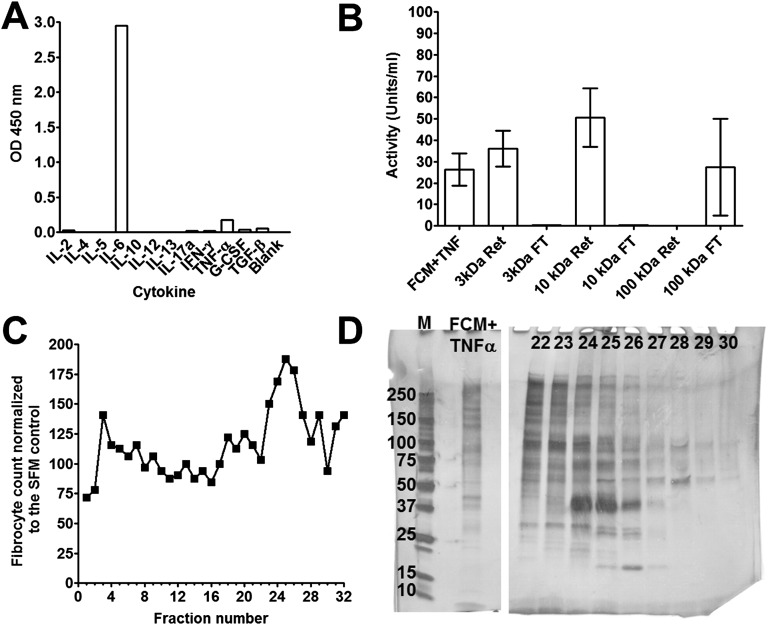
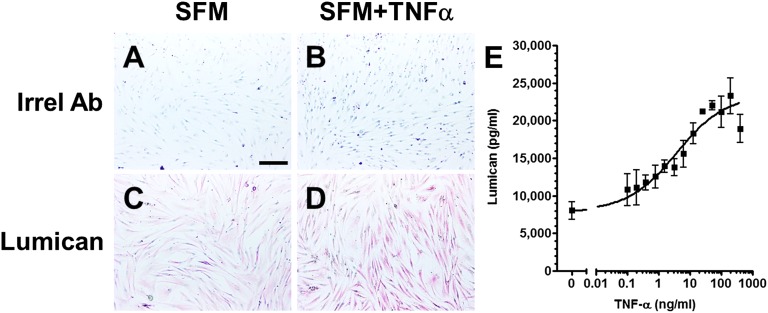
The factor (or factors) secreted by the fibroblasts were stable when stored for 2 wk at 4 °C or frozen at −20 °C or −80 °C. We tested the FCM+TNF-α for cytokines known to regulate fibrocyte differentiation or involvement in fibrotic responses. We did not detect any cytokines known to regulate fibrocyte differentiation, such as IL-4, IL-10, IL-12, IL-13, or IFN-γ (Fig. S1A). We did detect TNF-α and IL-6, but we have previously shown that these cytokines do not significantly affect fibrocyte differentiation (31). Fractionation with centrifugal filters indicated that the activity secreted by fibroblasts was a factor greater than 10 kDa but smaller than 100 kDa (Fig. S1B). Concentrates of FCM+TNF-α that passed through 100-kDa filters but were retained by 10-kDa filters were fractionated by ion exchange chromatography (Fig. S1 C and D). The factor(s) eluted from an anion exchange column as a single peak between fractions 23 and 26 (Fig. S1 C and D). Using mass spectrometry of tryptic digests from fraction 25, we identified several components of the active peak (Table S1). Of these, only albumin, the proteoglycan lumican, and the neuronal factor Slit2 are known to be extracellular proteins (40, 41). We previously observed that albumin does not regulate fibrocyte differentiation (37), and that Slit2 inhibits fibrocyte differentiation (41), suggesting that lumican may be the fibrocyte-inducing factor in FCM+TNF-α. To determine whether TNF-α increased lumican levels in human lung fibroblasts, cells were incubated in the presence or absence of TNF-α for 2 d. Compared with cells cultured in the absence of TNF-α, cells cultured in the presence of TNF-α had increased lumican staining and increased lumican levels in the conditioned medium (Fig. S2).
Fig. S1.
Fractionation of FCM+TNF-α and identification of lumican. (A) FCM+TNF-α was collected and analyzed for cytokines. (B) FCM+TNF-α was fractionated by centrifugation using 100-, 10-, and 3-kDa cutoff filters. PBMC were cultured for 5 d in the presence or absence of FCM+TNF-α, retentate (Ret), or flow through (FT). After 5 d, the number of fibrocytes was counted. Values are mean ± SEM, n = 3. (C) Fractions (10% v:v) from an ion exchange column were incubated with PBMC for 5 d, and fibrocytes were counted. (D) The size-fractionated FCM+TNFα loaded onto, and fractions from, the ion exchange chromatography were analyzed by PAGE on a 10% reducing gel. M is molecular mass markers with masses in kDa indicated at left. Numbers indicate fractions from C. The cell counts and gel are representative of three independent experiments.
Table S1.
Identification of tryptic peptides from FCM+TNF-α
| GenBank ID | Peptide sequences detected | Protein |
| 28592 | LVTDLTK | albumin |
| ATKEQLK | ||
| AEFAEVSK | ||
| QTALVELVK | ||
| LVAASQAALGL | ||
| YTKKVPQVSTPTLVEVSR | ||
| 642534 | ILGPLSYSK | lumican |
| FNALQYLR | ||
| NNQIDHIDEK | ||
| ISNIPDEYFK | ||
| SLEYLDLSFNQIAR | ||
| NIPTVNENLENYYLEVNQLEK | ||
| 190784 | TIAQYAR | glycogen phosphorylase |
| NNVVNTMR | ||
| VIFLENYR | ||
| GYNAQEYYDRIPELR | ||
| VIPAADLSEQISTAGTEASGTGNMK | ||
| 4151205 | LEQNTIK | slit2 |
| SLNSLVLYGNK | ||
| LNNNEFTVLEATGIFK | ||
| 178027 | EITALAPSTMK | alpha-actin |
| SYELPDGQVITIGNER | ||
| VAPEEHPTLLTEAPLNPK | ||
| 306820 | ITQSNAILR | glutathione transferase M3 |
| 2282013 | LTGMAFR | GAPDH-2 like |
| AGIALNDNFVK | ||
| 46276863 | SVEAAAELSAK | parathymosin |
| 30130 | LYGPSSVSFADDFVR | heat shock protein 47 (colligin) |
Fig. S2.
TNF-α potentiates lumican accumulation in fibroblasts. Human fibroblasts were cultured in the absence (A and C) or the presence (B and D) of 50 ng/mL TNF-α for 2 d. Cells were then air-dried, fixed, and stained with rabbit irrelevant antibodies (A and B) or anti-lumican antibodies (C and D). Positive staining was identified by red staining, and nuclei are counterstained blue. (Scale bar: 200 μm.) Images are representative of three independent experiments. (E) FCM+TNF-α was collected and analyzed by ELISA for lumican. All values are mean ± SEM, n = 3. Data were fit to a sigmoidal dose–response curve with a variable Hill coefficient.
Lumican is a small leucine-rich proteoglycan related to fibromodulin, decorin, and biglycan (42). Lumican has a protein core of ∼40 kDa, but is usually secreted as a higher molecular weight proteoglycan, with varying amounts keratan sulfate glycosaminoglycan (43, 44). Lumican is vital for the correct formation of collagen fibrils and promotes cell adhesion and migration (40, 45–47). Lumican knockout mice have multiple defects, including corneal opacity, skin and tendon fragility, and defective leukocyte migration (46, 48–51).
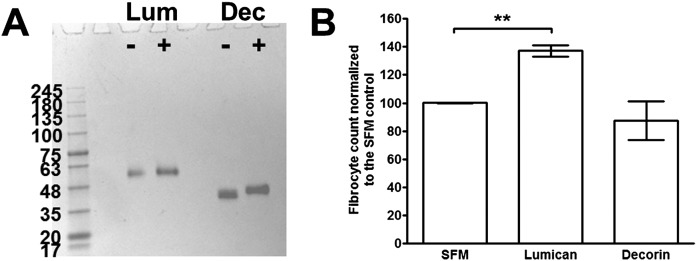
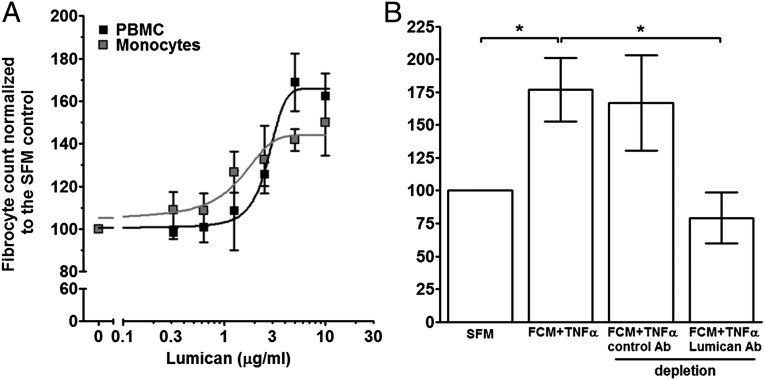
Recombinant human lumican, which is composed of the core 40-kDa protein and appears to be decorated with additional proteoglycan residues to give a mass of ∼60 kDa (Fig. S3A), was added to PBMC to determine whether it can affect fibrocyte differentiation. Compared with PBMC cultured in SFM, cells cultured in the presence of lumican had significantly increased numbers of fibrocytes (Fig. 2A). The related SLRP decorin had no effect on fibrocyte differentiation (Fig. S3B). The lumican EC50 for promoting human fibrocyte differentiation was 2.9 ± 1.2 μg/mL, with a Hill coefficient of 7.4 ± 4.3 (mean ± SEM, n = 3). To determine whether lumican altered the phenotype of fibrocytes, we stained PBMC after 5 d of culture with or without lumican for collagen-I. In the absence of lumican, 88.7 ± 2.3% (mean ± SEM, n = 3) of fibrocytes were collagen-I positive, whereas in the presence of lumican 93.1 ± 1.4% were collagen-I positive, suggesting that lumican does not alter the expression of collagen-I. To determine whether the potentiation of fibrocyte differentiation by lumican is a direct effect on monocytes, or due to an indirect effect mediated by the B cells, dendritic cells, NK cells, or T cells present in the PBMC preparation, we incubated purified human monocytes with lumican (Fig. 2A). For all donors, lumican significantly promoted fibrocyte differentiation from isolated monocytes with an EC50 of 1.8 ± 0.9 μg/mL and a Hill coefficient of 2.9 ± 0.9 (mean ± SEM, n = 3). The EC50 and Hill coefficient for monocytes were not significantly different from those of PBMC (t tests). These data suggest that lumican acts directly on monocytes to potentiate fibrocyte differentiation.
Fig. S3.
Lumican but not decorin potentiates human fibrocyte differentiation. (A) Recombinant human lumican (Lum) and decorin (Dec) were analyzed by PAGE, in the absence (-) or presence (+) of reducing agents. Proteins were stained with silver stain. (B) Human PBMC were cultured in the presence or absence of recombinant lumican or decorin for 5 d. Cells were then air-dried, fixed, stained, and fibrocytes were counted. Values are mean ± SEM (n = 3). **P < 0.01 (one-way ANOVA, Tukey’s test).
Fig. 2.
Lumican potentiates human fibrocyte differentiation. (A) Human PBMC and isolated monocytes were cultured in the presence or absence of recombinant lumican for 5 d. Cells were then air-dried, fixed, stained, and fibrocytes were counted. Values are mean ± SEM (n = 3). Lumican concentrations at 3 μg/mL and above significantly inhibited fibrocyte differentiation (t test). Lines are fits to sigmoidal dose–response curves with variable Hill coefficients. (B) MRC-5 FCM+TNF-α was incubated with beads labeled with anti-lumican antibodies or goat IgG (a control IgG). Human PBMC were cultured in the presence or absence of 10% v:v antibody-depleted FCM+TNF-α for 5 d. Values are mean ± SEM (n = 4). *P < 0.05 (one-way ANOVA, Tukey's test).
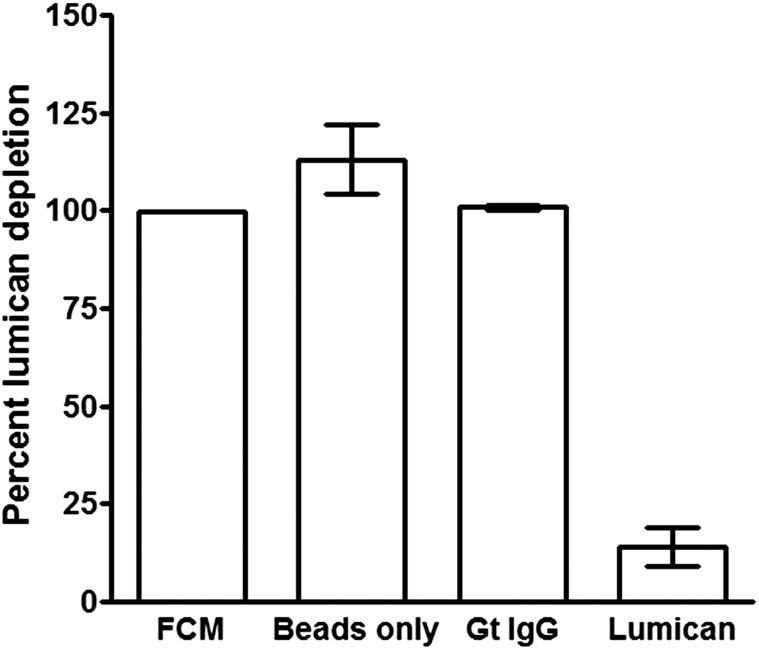
To further test the hypothesis that lumican is a key factor in FCM+TNF-α that potentiates fibrocyte differentiation, lumican was immunodepleted from FCM+TNF-α. Compared with control IgG, immunodepletion with lumican antibodies reduced lumican levels by ∼82% (Fig. S4). Immunodepletion with control IgG did not significantly alter the effect of fibrocyte differentiation by FCM+TNF-α (Fig. 2B). Compared with untreated FCM+TNF-α, immunodepletion of lumican from FCM+TNF-α led to a significant reduction in the ability of FCM+TNF-α to promote human fibrocyte differentiation (Fig. 2B). Together, these data suggest that lumican is the active factor in FCM+TNF-α that inhibits fibrocyte differentiation.
Fig. S4.
Antibodies immunodeplete lumican. Following immunodepletion of FCM+TNF-α with either beads alone, control goat IgG, or anti-lumican antibodies, samples were analyzed by ELISA for lumican. Values are mean ± SEM (n = 3).
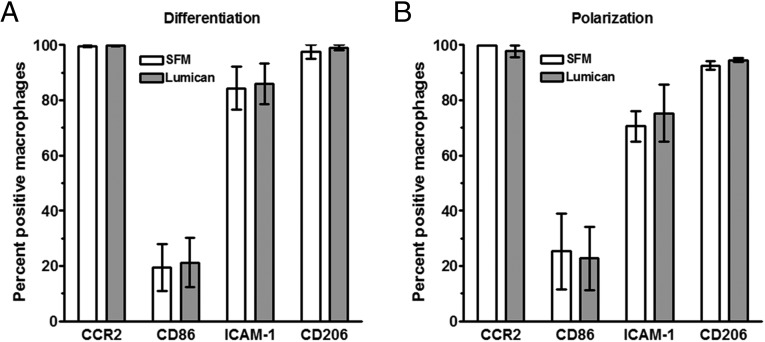
To determine whether lumican alters the differentiation of monocytes, or the polarization of macrophages, PBMC were cultured for 6 d with or without lumican, or PBMC were differentiated into macrophages for 6 d, and then incubated for 3 d in the presence or absence of lumican. Cells were then stained with antibodies to the M1 markers CCR2, ICAM-1 (CD54), or CD86, or the M2 marker CD206 (Fig. S5). We did not detect any observable differences in the expression levels of these receptors, suggesting that lumican regulates monocyte to fibrocyte differentiation, rather than monocyte or macrophage polarization.
Fig. S5.
The effect of lumican on monocyte differentiation and macrophage polarization. (A) Human PBMC were cultured in the presence or absence of recombinant lumican for 6 d. (B) Human PBMC were cultured for 6 d, the medium was then replaced with medium in the presence or absence of recombinant lumican for 3 d. Cells were then air-dried, fixed, and stained, and the percentage of macrophages stained for the indicated marker was counted. Values are mean ± SEM (n = 4).
SAP Competes with Lumican to Regulate Fibrocyte Differentiation.
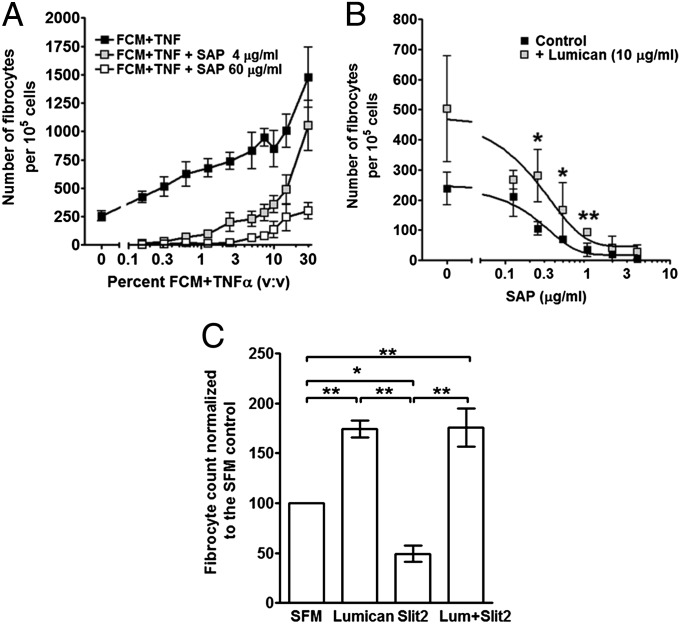
As fibrotic environments contain a wide variety of pro- and anti-fibrocyte inducing factors, we examined how SAP, a potent inhibitor of fibrocyte differentiation (13, 16, 38, 52), and FCM+TNF-α might compete to regulate fibrocyte differentiation. PBMC were cultured with increasing concentrations of FCM+TNF-α in the presence or absence of SAP. SAP inhibits human fibrocyte differentiation with an IC50 of ∼0.3 μg/mL (3 nM), and completely inhibits fibrocyte differentiation at 2 μg/mL (13, 38, 53). In the presence of increasing concentrations of FCM+TNF-α we observed increased fibrocyte differentiation (Fig. 3A). When SAP was added to PBMC in SFM at either 4 μg/mL (double the amount needed to inhibit fibrocyte differentiation (13), or 60 μg/mL (double the average human plasma level of SAP; ref. 54), fibrocyte differentiation was reduced compared with cells cultured in FCM+TNF-α alone, but fibrocyte differentiation was not inhibited at the higher concentrations of FCM+TNF-α (Fig. 3A). These data suggest that the fibrocyte-potentiating factor in FCM+TNF-α competes with SAP to regulate fibrocyte differentiation.
Fig. 3.
The competition of lumican with SAP or Slit2. (A) PBMC were incubated with increasing concentrations of FCM+TNF-α in the presence of 0, 4. or 60 μg/mL SAP. Values are mean ± SEM (n = 4). (B) PBMC were incubated with increasing concentrations of SAP in the presence or absence of 10 μg/mL lumican. After 5 d, cells were air-dried, fixed, and stained, and the number of fibrocytes was counted. Values are mean ± SEM (n = 3). **P < 0.01 (t test). Lines are fits to sigmoidal dose–response curves with variable Hill coefficients. (C) PBMC were incubated in the presence or absence of lumican (10 μg/mL), Slit2 (500 pg/mL), or lumican and Slit2 combined. After 5 d, cells were air-dried, fixed, and stained, and the number of fibrocytes was counted. Values are mean ± SEM (n = 5). *P < 0.05, **P < 0.01 (one-way ANOVA, Tukey’s test).
To determine whether lumican also competes with SAP to regulate fibrocyte differentiation, PBMC were cultured in SFM with increasing concentrations of SAP in the absence or presence of 10 μg/mL lumican (Fig. 3B). As observed previously (13, 38, 53), SAP inhibited fibrocyte differentiation with an IC50 of 0.29 ± 0.16 μg/mL In the presence of lumican, the inhibitory activity of SAP was reduced, with significantly more fibrocytes in cultures of SAP at 0.25, 0.5, and 1 μg/mL Although the IC50 of SAP was shifted to 0.52 ± 0.09 μg/mL in the presence of lumican, this was not significant (Fig. 3B; t test). These data indicate that lumican reduces the ability of SAP to inhibit fibrocyte differentiation.
Slit2 Does Not Inhibit Lumican-Induced Fibrocyte Differentiation.
We previously found that fibroblasts secrete the neuronal guidance protein Slit2, and that Slit2 inhibits fibrocyte differentiation (41). To determine how lumican and Slit2 might compete to regulate fibrocyte differentiation, PBMC were cultured in SFM in the absence or presence of 10 μg/mL lumican and 500 pg/mL Slit2. Slit2 inhibited fibrocyte differentiation, lumican potentiated fibrocyte differentiation, and the addition of Slit2 was unable to block this effect of lumican on fibrocyte differentiation (Fig. 3C). These data indicate that the fibrocyte-potentiating effect of lumican is dominant over the effect of Slit2.
Integrin-Blocking Antibodies Inhibit Lumican-Induced Fibrocyte Differentiation.
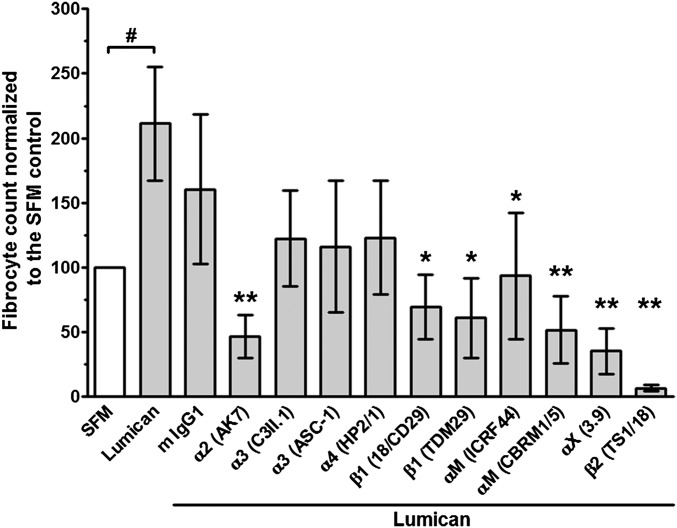
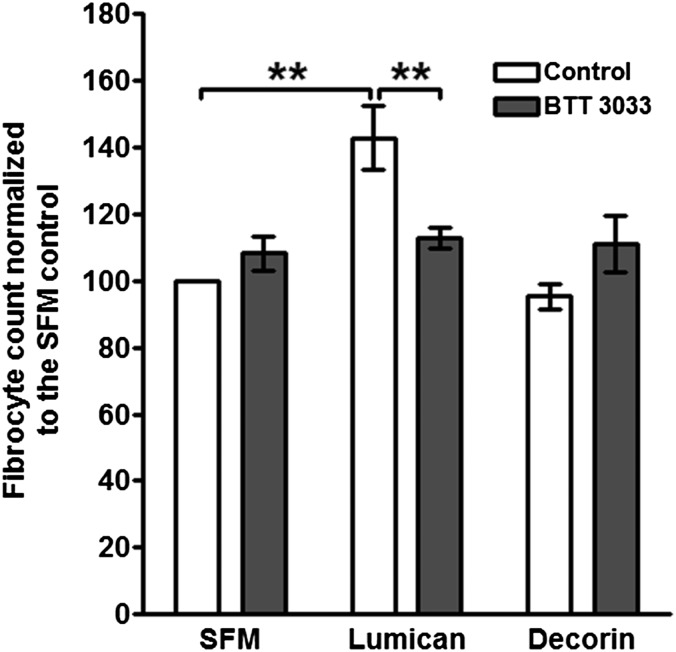
Monocyte-derived fibrocytes express a wide variety of receptors that bind extracellular matrix proteins, including many β1 and β2 integrins (3, 4, 14). Lumican regulates fibroblast activation and migration via α2β1 (CD49b/CD29) integrins (55, 56), and antibodies against αM (CD11b), β2 (CD18), and β1 (CD29) integrins inhibit neutrophil migration on lumican (57). To determine if these integrins are necessary for lumican potentiation of fibrocyte differentiation, we incubated PBMC with anti-integrin antibodies and then cultured the PBMC in the presence or absence of lumican. Antibodies to α2 (AK7), β1 (18/CD29 and TDM29), αΜ (ICRF44 and CBRM1/5), αX (3.9), and β2 (TS1/18) integrins inhibited lumican-induced fibrocyte differentiation, whereas antibodies to α3 (C3II.1 and ASC-1) and α4 (HP2/1) integrins had no significant effect (Fig. 4). As an alternative strategy to inhibit integrin-lumican binding, PBMC were incubated with the α2 integrin small molecule inhibitor BTT 3033 (58). At 500 nM, BTT3033 did not significantly regulate fibrocyte differentiation in the presence or absence of decorin, but did significantly inhibit lumican-induced fibrocyte differentiation (Fig. S6). Because α2 binds to β1, and αM and αX bind to β2 (59), these data suggest that α2β1, αMβ2, and αXβ2 integrins are important for lumican potentiation of fibrocyte differentiation.
Fig. 4.
Some anti-integrin antibodies inhibit lumican-induced fibrocyte differentiation. PBMC were incubated with 5 μg/mL of mouse IgG1 or the indicated anti-integrin antibodies (specific integrin Ab in parenthesis), and then cultured in the presence or absence of 10 μg/mL lumican. After 5 d, cells were air-dried, fixed, and stained, and the number of fibrocytes was counted. Values are mean ± SEM (n = 6). # P < 0.05 compared with the SFM control (t test), *P < 0.05, **P < 0.01 compared with lumican control (one-way ANOVA, Dunnett's test).
Fig. S6.
α2 integrin small molecule inhibitor BTT 3033 inhibits lumican-induced fibrocyte differentiation. PBMC were incubated in the presence or absence of 500 nM BTT3033 for 30 min, and then cultured in the presence or absence of 10 μg/mL lumican or decorin. After 5 d, cells were air-dried, fixed, and stained, and the number of fibrocytes was counted. Values are mean ± SEM (n = 3). *P < 0.01, compared with lumican control (one-way ANOVA, Tukey’s test).
Lung Lumican Levels Increase in a Mouse Model of Pulmonary Fibrosis.
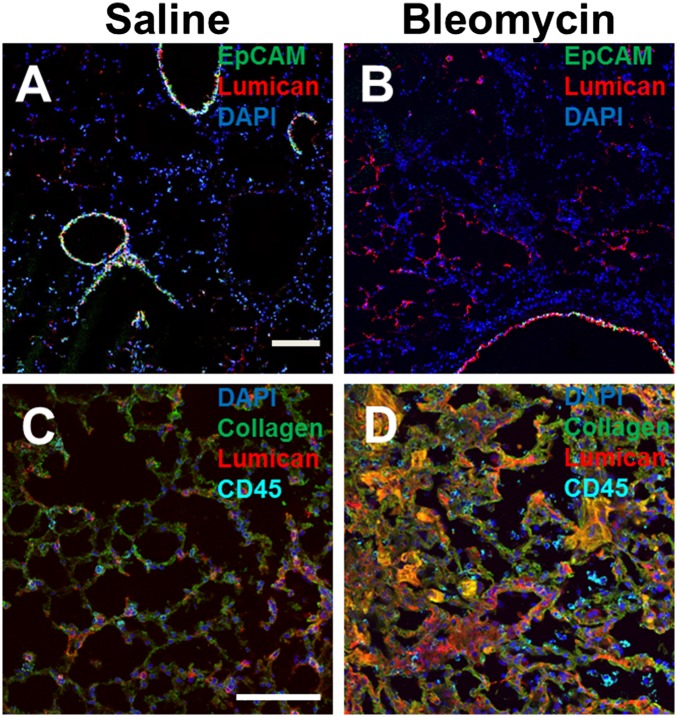
Lumican is present in human and murine lungs, and serum lumican levels are increased following lung inflammation and asthma (50, 60, 61). To determine whether lung lumican levels are up-regulated in pulmonary fibrosis, we stained lung tissue from mice that aspirated bleomycin or saline. At 21 d after saline exposure, lumican localized to alveolar walls and EpCAM-positive airway epithelial cells (Fig. 5). At 21 d, compared with mice that received saline, mice that aspirated bleomycin had more lumican staining, especially in the walls of alveoli (Fig. 5). In addition, the CD45-positive, collagen-I–positive cells were closely associated with areas of lumican (Fig. 5 and Fig. S7). These data suggest that in a mouse model, pulmonary fibrosis is associated with increased local accumulation of lumican in the lungs.
Fig. 5.
Distribution of lumican in mouse lungs following bleomycin aspiration. Cryosections of mouse lungs 21 d after saline (A and C) or bleomycin (B and D) aspiration were incubated with antibodies against EpCAM (A and B, green) and rabbit anti-lumican (red), collagen (C and D, green), goat anti-lumican, (red), and CD45 (cyan). Nuclei were counterstained with DAPI (blue). (Scale bar: 100 μm.) Images are representative of three independent experiments.
Fig. S7.
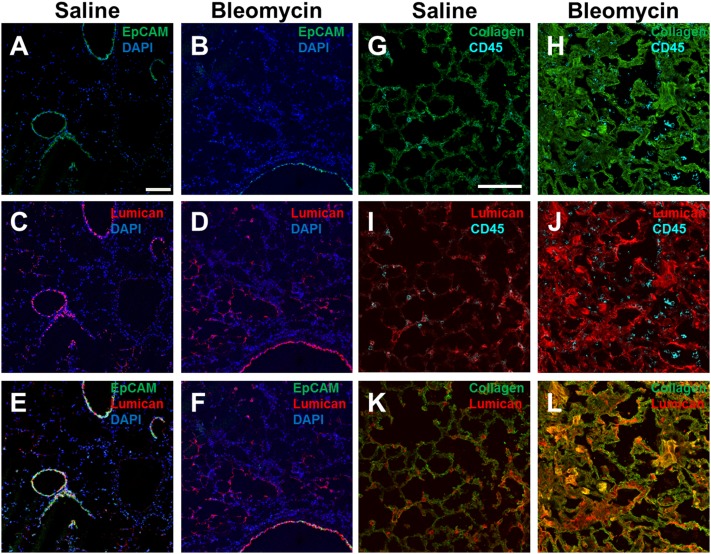
Distribution of lumican in mouse lungs following bleomycin aspiration. Cryosections of mouse lungs 21 d after saline or bleomycin aspiration were incubated with antibodies against EpCAM (A and B, green), rabbit anti-lumican (C and D, red), or EpCAM and rabbit anti-lumican (E and F). Nuclei were counterstained with DAPI (blue). (Scale bar: 0.1 mm.) Sections were also incubated with antibodies against collagen (G and H; green) and CD45 (cyan), goat anti-lumican (I and J; red), and CD45 (cyan) collagen (green) and goat anti-lumican (red) (K and L). E and F are overlays of one section and K and L are overlays of a different section, and are representative of three independent experiments.
Lumican Levels Are Abnormally High in Human Pulmonary Fibrosis Lesions.
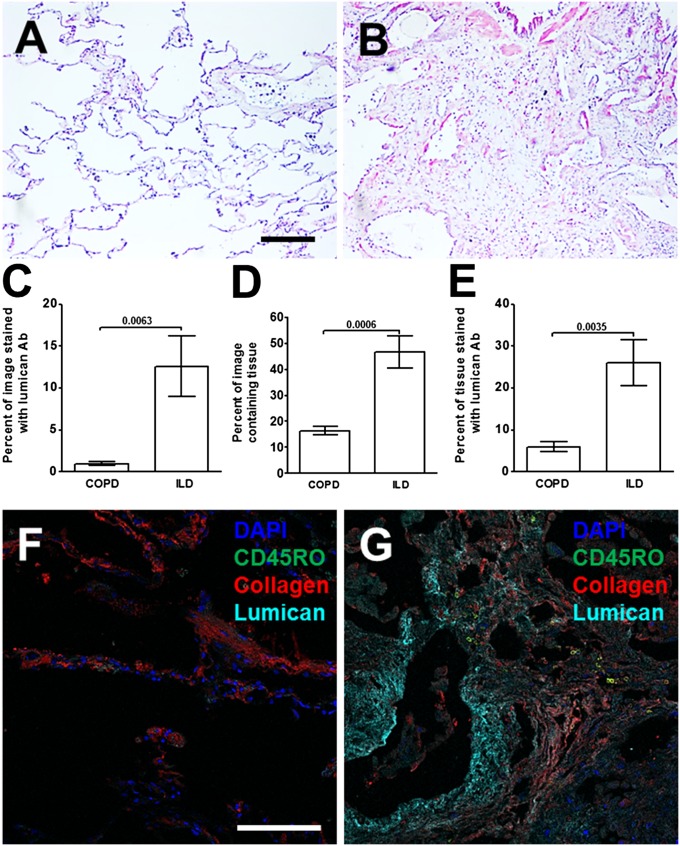
To determine if lumican is associated with human lung fibrosis, we examined the distribution of lumican in lung tissue from chronic obstructive pulmonary disease (COPD) patients with relatively normal lungs (>80% forced vital capacity; FVC) and pulmonary fibrosis patients with advanced disease (<50% FVC) (Table S2). Lung tissue from patients with an FVC of >80% showed limited lumican staining (Fig. 6A). In lung tissue from pulmonary fibrosis patients with advanced disease, lumican distribution was significantly increased, and was especially pronounced in areas adjacent to epithelial layers (Fig. 6B). The pulmonary fibrosis lungs had a greater area of tissue (and thus less airspace) and a greater area of lumican staining than the COPD lungs (Fig. 6 C and D). Ratios of these two values indicated that the fibrotic lungs had a greater percentage of the tissue showing positive staining for lumican than the COPD lungs (Fig. 6E). As seen in the mouse model, compared with COPD patients, in the fibrotic lungs CD45RO-positive, collagen-I–positive cells were closely associated with areas of lumican (Fig. 6 F and G and Fig. S8). These data suggest that human pulmonary fibrosis may involve an increase in the levels of lumican, and that these areas may promote fibrocyte differentiation.
Table S2.
Clinical data
| Group | FEV1, mean ± SD | Sex | Age in years, mean ± SD | Clinical details |
| ILD <50% | 33.40 ± 5.941 | 3 male | 45.80 ± 9.04 | n = 2 NSIP |
| 2 female | n = 3 fibrosis | |||
| COPD >80% | 89.00 ± 7.572 | 3 male | 71.00 ± 10.85 | n = 5 Emphysema with carcinoma |
| 4 female | n = 2 emphysema | |||
| Significance (Mann–Whitney) | P < 0.0001 | ns | P = 0.0025 |
Clinical data from the National Heart Lung and Blood Institute-sponsored Lung Tissue Research Consortium (LTRC) sections used in Fig. 6. Pulmonary function test: Forced expiratory volume at 1 second (FEV1) Clinical diagnosis: chronic obstructive pulmonary disease (COPD); fibrosis indicates uncharacterized interstitial lung disease (ILD); NSIP, nonspecific interstitial pneumonia.
Fig. 6.
Increased lumican in human lungs with pulmonary fibrosis. Lung tissue sections from COPD or pulmonary fibrosis patients were stained with anti-lumican antibodies. (A) Section from COPD patient with FVC >80%. (B) Section from IPF patient with FVC <50%. Bar is 0.2 mm. (C) The percentage area of the image stained by lumican antibodies. (D) The percentage of total area of image containing lung tissue. (E) the percentage of lung tissue stained by lumican antibodies. Values are mean ± SEM, n = 5–7 patients per group. (t test). Lung sections from a COPD patient with FVC >80% (F) or an IPF patient with FVC <50% were incubated with antibodies against CD45RO (green), collagen (red), and lumican (cyan) (G). In F and G, nuclei were counterstained with DAPI (blue). (Scale bar: 0.1 mm.) Images are representative of three different patients.
Fig. S8.
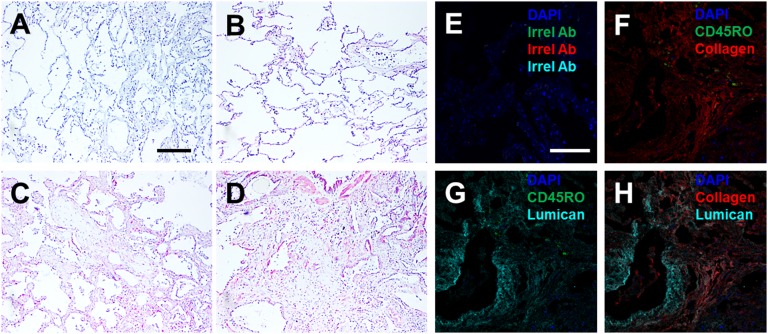
Increased lumican in human lungs with pulmonary fibrosis. Lung tissue sections from COPD or pulmonary fibrosis patients were stained with rabbit anti-lumican antibodies. (A and B) Sections from COPD patients with FVC >80%. (C and D) Sections from IPF patients with FVC <50%. (A) Section stained with control rabbit antibodies. (B–D) Sections stained with rabbit anti-lumican antibodies. (E) A lung section from an IPF patient with FVC <50% was incubated with irrelevant mouse, rabbit, and goat IgG. In F-H the same section of a IPF patient lung with FVC <50% was stained with antibodies against CD45RO (green) and collagen (red) (F), CD45RO (green) and goat anti-lumican (cyan) (G), collagen (red) and goat anti-lumican (cyan) (H). In E-H nuclei were counterstained with DAPI (blue). Images in E–H are overlays of the same section and are representative of three different patients. (Scale bars: 0.2 mm in A and E.)
Discussion
Tissue lumican levels are increased in cardiac and liver fibrosis (47, 62, 63), and we observed that lumican levels are increased in pulmonary fibrosis. Fibrocytes are rare in normal heart, lung, and liver, whereas fibrocytes are readily detected in fibrotic lesions in these tissues (3, 10, 16–19). Fibrocytes play a key role in wound healing and fibrosis, and in agreement with our observation that lumican promotes fibrocyte differentiation, lumican knockout mice have poor wound healing and are resistant to carbon tetrachloride-induced liver fibrosis (3, 47, 64). Poor wound healing and reduced fibrosis of lumican knockout mice are thought to be related to reduced collagen tensile strength and disorganized collagen fibrils, suggesting that lumican promotes extracellular matrix stiffness (46, 65). In fibrotic lesions, the extracellular matrix has increased stiffness, leading to enhanced fibroblast proliferation, myofibroblast formation, and increased extracellular matrix deposition (66, 67). Combined, these observations suggest that elevated lumican, by increasing tissue stiffness, may promote fibrosis.
Fibroblasts bind to lumican using β1 integrins, whereas hematopoietic cells use β2 integrins (55–57, 68). Monocytes, macrophages, and monocyte-derived fibrocytes express both β1 and β2 integrins (14), and our results indicate that both β1 and β2 integrins are involved in lumican-induced fibrocyte differentiation. The Hill coefficient of 7.4 ± 4.3 (mean ± SEM) in the lumican dose–response curves indicates a cooperativity in lumican binding or the lumican signal transduction pathway. The ability of α2 integrin blocking antibodies and the small molecule inhibitor BTT3033, to inhibit lumican-induced fibrocyte differentiation mirrors the inhibition of cell migration observed with fibroblasts and melanoma cells (55, 56), and the ability of αM and αX antibodies to inhibit lumican-induced fibrocyte differentiation mirrors the inhibition of cell migration observed for leukocytes (57).
The ECM proteins collagen-IV, fibronectin, and vitronectin, which bind to integrin receptors (59), have no significant effect on fibrocyte differentiation (69). However, collagen-I has a modest inhibitory effect on fibrocyte differentiation (69). As both collagen-I and lumican bind to α2β1 integrin (70, 71), additional receptors, such as αMβ2 and αXβ2, may differentially regulate the effects of lumican and collagen-I on fibrocyte differentiation.
We detected slit2 in the conditioned media from TNF-α-stimulated fibroblasts, and observed that the fibrocyte-potentiating activity of lumican appears to dominate over the fibrocyte-inhibiting activity of slit2. These data suggest a model where fibroblasts constitutively secrete slit2 to inhibit fibrocyte differentiation, but when there is tissue damage, inflammatory signals induce fibroblasts to up-regulate lumican production to drive fibrocyte differentiation. That the inflammatory signals use fibroblasts as an intermediate, rather than signaling directly to the fibrocytes, suggests that additional information obtained by fibroblasts might allow fibroblasts to modulate the lumican signal to fibrocytes. The observations that the fibrocyte potentiating signal from TNF-α–stimulated fibroblasts can be removed with anti-lumican antibodies, that high concentrations of SAP can strongly inhibit fibrocyte differentiation in the presence of high concentrations of lumican, and that high concentrations of conditioned media from TNF-α–stimulated fibroblasts can potentiate fibrocyte differentiation in the presence of high concentrations of SAP suggest that stimulated fibroblasts secrete factors that act in conjunction with lumican to counteract the effect of SAP. Together, our results support a model where a positive feedback loop between fibroblasts and fibrocytes, involving multiple signals, potentiates fibrosis. Lumican appears to be a major signal in this loop, suggesting that lumican-inhibiting drugs may be beneficial in regulating fibrosis.
Materials and Methods
All animals were used in accordance with National Institutes of Health guidelines and with a protocol approved by the Texas A&M University Institutional Animal Care and Use Committee. Human blood was obtained with written consent and with specific approval from the Texas A&M University human subjects Institutional Review Board. Human PBMC, monocytes, and fibroblasts were isolated, cultured, and incubated with antibodies, as described (37, 41). Fibrocytes were identified as described (13, 37). Fibroblast conditioned medium was fractionated, and the immunodepletion of lumican was performed, as described (13, 41). Pulmonary fibrosis in mice was induced by bleomycin instillation, as described previously (52, 72, 73). Lung sections were prepared, fixed, and stained, as described (16, 52). Detailed information about mice, experimental procedures, and statistical analyses can be found in SI Materials and Methods.
SI Materials and Methods
Cell Isolation.
Human peripheral blood was collected from healthy adult volunteers who gave written consent and with specific approval from the Texas A&M University human subjects Institutional Review Board. Peripheral blood mononuclear cells (PBMC) were isolated from blood using Ficoll-Paque Plus (GE Healthcare Biosciences), as described (37). Monocytes were enriched from PBMC using EasySep monocyte enrichment kits (StemCell Technologies), as described (41, 74).
Fibroblasts.
Human MRC5 lung fibroblasts (ATCC) and primary human dermal and lung fibroblasts (Lonza) were grown in RPMI-1640 (Sigma-Aldrich), as described (41). To make fibroblast conditioned medium (FCM+TNF-α), ∼80% confluent fibroblast cultures in 75 cm2 flasks (BD Bioscience) were rinsed three times with FibroLife basal medium (LifeLine Cell Technology), and then incubated in FibroLife basal medium for 2–3 h to remove exogenous serum products. The medium was removed, the cells were rinsed three times with basal medium, and fibroblasts were then cultured in FibroLife protein-free medium (PFM), containing FibroLife basal medium supplemented with 10 mM Hepes, 1x nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin (all from Sigma-Aldrich), and 2 mM glutamine (Invitrogen), and 20 ng/mL recombinant human TNF-α (Peprotech). 20 ng/mL additional TNF-α was added every 24 h. After 3 d, the medium was collected, clarified by centrifugation at 500 × g for 10 min, and stored at 4 °C. Clarified conditioned medium was used within 2 d. Conditioned media were analyzed for cytokines using an ELISArray kit (SABiosciences). Human lung bronchial epithelial cells (PromoCell) were cultured following the vendor’s protocol.
Purification and Characterization of Lumican.
FCM+TNF-α was fractionated using 3-, 10-, and 100-kDa cutoff centrifugal filters (Amicon Ultra, Millipore) as described (16, 41). Activity units were defined as the dilution of conditioned medium that promoted fibrocyte differentiation to 150% of the SFM control value. Chromatography was performed using an AKTA system (GE Healthcare Bio-Sciences). 300 mL of FCM+TNF-α was fractionated with a 100 kDa cutoff centrifugal filter (Amicon). The flow through was concentrated with a 10 kDa cutoff centrifugal filter (Amicon), and then washed three times with 10 mL of 10 mM sodium phosphate buffer, pH 7.4 (SB). The retained material was resuspended in 1 mL of SB and loaded onto a 1 mL anion exchange column (HiTrap Q, GE Healthcare) and the column was washed with 10 mM SB, and verifying that the absorbance at 280 nm returned to baseline. Bound material was eluted using 50 mL SB, with a 0–1,000 mM gradient of NaCl. The fractions that promoted fibrocyte differentiation were pooled, concentrated, and desalted using 10 kDa cutoff centrifugal filters and SB, as described previously (41). Fractions that promoted fibrocyte differentiation were digested with trypsin and analyzed at the Mass Spectrometry and Proteomics Core Facility, University of Utah, Salt Lake City.
Immunodepletion of lumican was performed as described (13, 41). Briefly, 0.5 mL of FCM+ TNF-α was incubated overnight at 4 °C with 0.1 mL packed volume of protein G magnetic beads (Life Technologies), coated with 100 μg of goat anti-lumican antibodies (R&D Systems), goat IgG (Jackson ImmunoResearch) as a control, or unlabeled beads. Beads were then removed by magnetic separation, and the immunodepleted FCM+ TNF-α was assayed as described below. Fibroblast conditioned media and immunodepleted samples were analyzed for lumican using an ELISA kit (R&D Systems) following the vendor’s protocol.
Fibrocyte and Macrophage Differentiation Assays.
PBMC were cultured in FibroLife SFM, as described (37), in the presence or absence of FCM+TNF-α conditioned medium, SAP (EMD Millipore), recombinant Slit2 (R&D Systems), recombinant lumican (R&D Systems or Novoprotein), or recombinant decorin (R&D Systems). After 5 d, plates were air-dried, fixed, and stained, and fibrocytes were identified as described (13, 37). To assess the effect of inhibiting integrin receptor binding, PBMC were incubated on ice for 60 min in SFM containing 5 μg/mL mouse IgG1 antibodies to CD11b (ICRF44, BioLegend), CD11b (CBRM1/5, BioLegend), CD11c (3.9, BioLegend), CD18 (TS1/18, BioLegend), CD29 (18/CD29, BD Bioscience), CD29 (TDM29, Southern Biotech, Birmingham, AL), CD49b (AK7, EMD Millipore), CD49c (C3II.1, BD Bioscience), CD49c (ASC-1, BioLegend), or CD49d (HP2/1, EMD Millipore). Mouse IgG1 (MG1-45, BioLegend) antibodies were used as controls. In addition, PBMC were incubated with the α2 integrin small molecule inhibitor BTT 3033 (58) (Tocris/Bio-Techne, Minneapolis, MN). After 60 min, 5 × 104 cells in 50 μL were added to 96 well plate wells containing 50 μL lumican (10 μg/mL) in SFM, and fibrocytes were cultured and counted as above. Human monocytes were differentiated into macrophages in RPMI-1640 (Lonza) containing 10% FCS (Seradigm) for 6 d in 8 well slides (EMD Millipore) in the presence or absence of 10 µg/mL lumican, as described (14). Macrophages that had not been exposed to lumican were polarized by replacing the medium at 6 d with fresh serum-free RPMI-1640, and culturing the cells in the presence or absence of 10 µg/mL lumican for an additional 3 d. Slides were then air-dried, fixed, and stained with antibodies to CCR2 (48607, R&D Systems), CD54/ICAM-1 (HCD54, BioLegend), CD86 (IT2.2, BioLegend), or CD206 (15-2, BioLegend), as described (13, 37).
Bleomycin-Induced Lung Inflammation.
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Texas A&M University Animal Use and Care Committee. All procedures were performed under anesthesia, and all efforts were made to minimize suffering. To induce fibrosis, 4- to 6-wk-old C57BL/6 mice (Jackson Laboratory) were treated with an oropharyngeal aspiration of 50 μL of 3 U/kg bleomycin (EMD Millipore) in 0.9% saline or saline alone, as described previously (52, 72, 73, 75). Mice were euthanized 21 d after bleomycin aspiration, lungs were inflated with and embedded in optimal cutting temperature (OCT) compound (VWR), frozen, and stored as described previously (16, 52). Sections of these lungs were used as controls in a previous study (41).
Immunohistochemistry and Western Blotting.
PBMC, fibroblasts, or lung epithelial cells were cultured for 5 d on eight-well glass microscope slides (Millipore), and then fixed and stained as described previously (14, 41). The PBMC were stained with mouse monoclonal antibodies to CD13 (clone WM15, BD-Biosciences) to detect monocyte-derived cells and fibroblasts, CD45 (clone HI30, BioLegend) to detect all leukocytes, prolyl-4-hydroxylase (clone 3–2B12, Millipore, Temecula, CA) as a marker of collagen synthesis, or isotype-matched irrelevant mouse monoclonal antibodies (BioLegend). Human lung tissue sections were obtained from the National Heart Lung and Blood Institute-sponsored Lung Tissue Research Consortium (LTRC) (Table S2). Fibroblasts, lung epithelial cells, or lung tissue sections were prepared and stained with rabbit monoclonal antibody to lumican (EPR8898-2, Abcam), as described previously (14). Isotype-matched rabbit anti-chicken IgY antibodies (Bethyl Laboratories) were used as controls. Secondary F(ab’)2 biotin-conjugated donkey anti-mouse or donkey anti-rabbit antibodies were from Jackson ImmunoResearch. Lung tissue sections stained with lumican antibodies were analyzed with ImageJ software (Rasband, W. S., ImageJ, US National Institutes of Health, Bethesda, MD). The percentage area of lung tissue stained with lumican antibodies was quantified as a percentage of the total area of the lung tissue, as described (16, 41, 52). Murine lung sections were also stained with combinations of antibodies to CD45 (Rat IgG2b, clone 30-F11, BioLegend), EpCAM (rat IgG2a, clone G8.8, BioLegend), collagen-I (rabbit IgG, Abcam), lumican (rabbit IgG, Abcam), lumican (goat IgG, R&D Systems), rabbit anti chicken IgY (Bethyl), or goat IgG (R&D Systems), as described (52). Staining was revealed with combinations of DyLight488-conjugated goat anti-rat antibodies (Novus), Alexa-488-conjugated donkey anti-rabbit (Jackson ImmunoResearch), Alexa-594-conjugated donkey anti-rat (Jackson ImmunoResearch), biotin-conjugated donkey anti-goat (Jackson ImmunoResearch), or Alexa-647-conjugated donkey anti-rabbit antibodies (Life Technologies). Biotinylated antibodies were revealed with streptavidin-conjugated Alexa-647 (Life Technologies). Coverslips were mounted with fluorescent mounting medium containing DAPI (Vector Laboratories). Immunofluorescence images were captured on an Olympus FV1000 confocal microscope, and analyzed using Olympus Fluoview software, as described previously (52, 76, 77).
Statistical Analysis.
Statistical analysis was performed using Prism (GraphPad Software). Statistical significance between two groups was determined by t tests or Mann–Whitney tests, or between multiple groups using ANOVA. Significance was defined as P < 0.05.
Acknowledgments
We thank the staff at Beutel Student Health Services for the phlebotomy work, and the volunteers for donating blood. This work was supported by National Institutes of Health Grant R01 HL118507.
Footnotes
Conflict of interest statement: D.P. and R.H.G. are inventors on patents for the use of SAP as a therapeutic for fibrosing diseases, and patents for the use of SAP-depleting materials to enhance wound healing. D.P. and R.H.G. are members of the Science Advisory Board of, and have stock options from, Promedior, a start-up company that is developing SAP as a therapeutic for fibrosing diseases, and receive a share of milestone payments made by Promedior to Rice University.
This article is a PNAS Direct Submission. E.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507387112/-/DCSupplemental.
References
- 1.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27(1):669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 2.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 3.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: Emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11(6):427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87(9):858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 5.Peng H, Herzog EL. Fibrocytes: Emerging effector cells in chronic inflammation. Curr Opin Pharmacol. 2012;12(4):491–496. doi: 10.1016/j.coph.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox N, Pilling D, Gomer RH. Serum amyloid P: A systemic regulator of the innate immune response. J Leukoc Biol. 2014;96(5):739–743. doi: 10.1189/jlb.1MR0114-068R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips RJ, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haudek SB, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103(48):18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kisseleva T, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45(3):429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Mehrad B, et al. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353(1):104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 11.Sakai N, et al. Fibrocytes are involved in the pathogenesis of human chronic kidney disease. Hum Pathol. 2010;41(5):672–678. doi: 10.1016/j.humpath.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol. 2001;166(12):7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 13.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171(10):5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4(10):e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartlapp I, et al. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15(12):2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- 16.Pilling D, et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179(6):4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson-Sjöland A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40(10):2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171(1):380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 19.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304(1):81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8(1):241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 23.Wang JF, et al. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15(1):113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 24.Kleaveland KR, Moore BB, Kim KK. Paracrine functions of fibrocytes to promote lung fibrosis. Expert Rev Respir Med. 2014;8(2):163–172. doi: 10.1586/17476348.2014.862154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore BB, et al. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35(2):175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi H, et al. IL-33 enhanced the proliferation and constitutive production of IL-13 and IL-5 by fibrocytes. BioMed Res Int. 2014;2014:738625. doi: 10.1155/2014/738625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley CD, et al. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22(4):199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto T, Okamoto H, Toyama Y, Momohara S. Molecular aspects of rheumatoid arthritis: Chemokines in the joints of patients. FEBS J. 2008;275(18):4448–4455. doi: 10.1111/j.1742-4658.2008.06580.x. [DOI] [PubMed] [Google Scholar]
- 29.McGettrick HM, Butler LM, Buckley CD, Rainger GE, Nash GB. Tissue stroma as a regulator of leukocyte recruitment in inflammation. J Leukoc Biol. 2012;91(3):385–400. doi: 10.1189/jlb.0911458. [DOI] [PubMed] [Google Scholar]
- 30.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10(10):712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 31.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83(6):1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160(1):419–425. [PubMed] [Google Scholar]
- 33.Mathai SK, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 2010;90(6):812–823. doi: 10.1038/labinvest.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith RE, Strieter RM, Phan SH, Lukacs N, Kunkel SL. TNF and IL-6 mediate MIP-1alpha expression in bleomycin-induced lung injury. J Leukoc Biol. 1998;64(4):528–536. [PubMed] [Google Scholar]
- 35.Thomson EM, Williams A, Yauk CL, Vincent R. Overexpression of tumor necrosis factor-α in the lungs alters immune response, matrix remodeling, and repair and maintenance pathways. Am J Pathol. 2012;180(4):1413–1430. doi: 10.1016/j.ajpath.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki Y, et al. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96(1):250–259. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilling D, Vakil V, Gomer RH. Improved serum-free culture conditions for the differentiation of human and murine fibrocytes. J Immunol Methods. 2009;351(1-2):62–70. doi: 10.1016/j.jim.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox N, Pilling D, Gomer RH. Distinct Fcγ receptors mediate the effect of serum amyloid p on neutrophil adhesion and fibrocyte differentiation. J Immunol. 2014;193(4):1701–1708. doi: 10.4049/jimmunol.1400281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox N, Pilling D, Gomer RH. NaCl potentiates human fibrocyte differentiation. PLoS One. 2012;7(9):e45674. doi: 10.1371/journal.pone.0045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikitovic D, Papoutsidakis A, Karamanos NK, Tzanakakis GN. Lumican affects tumor cell functions, tumor-ECM interactions, angiogenesis and inflammatory response. Matrix Biol. 2014;35(0):206–214. doi: 10.1016/j.matbio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Pilling D, Zheng Z, Vakil V, Gomer RH. Fibroblasts secrete Slit2 to inhibit fibrocyte differentiation and fibrosis. Proc Natl Acad Sci USA. 2014;111(51):18291–18296. doi: 10.1073/pnas.1417426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikitovic D, Katonis P, Tsatsakis A, Karamanos NK, Tzanakakis GN. Lumican, a small leucine-rich proteoglycan. IUBMB Life. 2008;60(12):818–823. doi: 10.1002/iub.131. [DOI] [PubMed] [Google Scholar]
- 44.Melching LI, Roughley PJ. Modulation of keratan sulfate synthesis on lumican by the action of cytokines on human articular chondrocytes. Matrix Biol. 1999;18(4):381–390. doi: 10.1016/s0945-053x(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 45.de Medeiros Matsushita M, et al. Airway proteoglycans are differentially altered in fatal asthma. J Pathol. 2005;207(1):102–110. doi: 10.1002/path.1818. [DOI] [PubMed] [Google Scholar]
- 46.Chakravarti S, et al. Lumican regulates collagen fibril assembly: Skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141(5):1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnan A, et al. Lumican, an extracellular matrix proteoglycan, is a novel requisite for hepatic fibrosis. Lab Invest. 2012;92(12):1712–1725. doi: 10.1038/labinvest.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeh JT, et al. Impaired skin wound healing in lumican-null mice. Br J Dermatol. 2010;163(6):1174–1180. doi: 10.1111/j.1365-2133.2010.10008.x. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi Y, et al. Lumican is required for neutrophil extravasation following corneal injury and wound healing. J Cell Sci. 2010;123(Pt 17):2987–2995. doi: 10.1242/jcs.068221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shao H, et al. Extracellular matrix lumican promotes bacterial phagocytosis, and Lum-/- mice show increased Pseudomonas aeruginosa lung infection severity. J Biol Chem. 2012;287(43):35860–35872. doi: 10.1074/jbc.M112.380550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lohr K, et al. Extracellular matrix protein lumican regulates inflammation in a mouse model of colitis. Inflamm Bowel Dis. 2012;18(1):143–151. doi: 10.1002/ibd.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pilling D, Gomer RH. Persistent lung inflammation and fibrosis in serum amyloid P component (APCs-/-) knockout mice. PLoS One. 2014;9(4):e93730. doi: 10.1371/journal.pone.0093730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crawford JR, Pilling D, Gomer RH. FcγRI mediates serum amyloid P inhibition of fibrocyte differentiation. J Leukoc Biol. 2012;92(4):699–711. doi: 10.1189/jlb.0112033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15(2):81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 55.Liu X-J, et al. Lumican Accelerates Wound Healing by Enhancing α2β1 Integrin-Mediated Fibroblast Contractility. PLoS One. 2013;8(6):e67124. doi: 10.1371/journal.pone.0067124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeltz C, et al. Lumican inhibits cell migration through α2β1 integrin. Exp Cell Res. 2010;316(17):2922–2931. doi: 10.1016/j.yexcr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Lee S, Bowrin K, Hamad AR, Chakravarti S. Extracellular matrix lumican deposited on the surface of neutrophils promotes migration by binding to β2 integrin. J Biol Chem. 2009;284(35):23662–23669. doi: 10.1074/jbc.M109.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nissinen L, et al. Novel α2β1 integrin inhibitors reveal that integrin binding to collagen under shear stress conditions does not require receptor preactivation. J Biol Chem. 2012;287(53):44694–44702. doi: 10.1074/jbc.M111.309450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo B-H, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25(1):619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J, Olivenstein R, Taha R, Hamid Q, Ludwig M. Enhanced proteoglycan deposition in the airway wall of atopic asthmatics. Am J Respir Crit Care Med. 1999;160(2):725–729. doi: 10.1164/ajrccm.160.2.9809040. [DOI] [PubMed] [Google Scholar]
- 61.Frey H, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013;280(10):2165–2179. doi: 10.1111/febs.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waehre A, et al. Chemokines regulate small leucine-rich proteoglycans in the extracellular matrix of the pressure-overloaded right ventricle. J Appl Physiol. 2012;112(8):1372–1382. doi: 10.1152/japplphysiol.01350.2011. [DOI] [PubMed] [Google Scholar]
- 63.Engebretsen KVT, et al. Lumican is increased in experimental and clinical heart failure, and its production by cardiac fibroblasts is induced by mechanical and proinflammatory stimuli. FEBS J. 2013;280(10):2382–2398. doi: 10.1111/febs.12235. [DOI] [PubMed] [Google Scholar]
- 64.Reich B, et al. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int. 2013;84(1):78–89. doi: 10.1038/ki.2013.84. [DOI] [PubMed] [Google Scholar]
- 65.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151(4):779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: Nearing the starting line. Sci Transl Med. 2013;5(167):167sr1. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 67.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Funderburgh JL, et al. Macrophage receptors for lumican. A corneal keratan sulfate proteoglycan. Invest Ophthalmol Vis Sci. 1997;38(6):1159–1167. [PubMed] [Google Scholar]
- 69.Pilling D, Gomer RH. Regulatory pathways for fibrocyte differentiation. In: Bucala R, editor. Fibrocytes-New Insights into Tissue Repair and Systemic Fibroses. World Scientific Publishing Co.; Singapore: 2007. pp. 37–60. [Google Scholar]
- 70.Eckes B, et al. Mechanical tension and integrin alpha 2 beta 1 regulate fibroblast functions. J Investig Dermatol Symp Proc. 2006;11(1):66–72. doi: 10.1038/sj.jidsymp.5650003. [DOI] [PubMed] [Google Scholar]
- 71.Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27(1):265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 72.Maharjan AS, Roife D, Brazill D, Gomer RH. Serum amyloid P inhibits granulocyte adhesion. Fibrogenesis Tissue Repair. 2013;6(1):2. doi: 10.1186/1755-1536-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herlihy SE, Pilling D, Maharjan AS, Gomer RH. Dipeptidyl peptidase IV is a human and murine neutrophil chemorepellent. J Immunol. 2013;190(12):6468–6477. doi: 10.4049/jimmunol.1202583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White MJV, Glenn M, Gomer RH. Trypsin potentiates human fibrocyte differentiation. PLoS One. 2013;8(8):e70795. doi: 10.1371/journal.pone.0070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walters DM, Kleeberger SR. Mouse Models of Bleomycin-Induced Pulmonary Fibrosis. John Wiley; New York: 2008. [DOI] [PubMed] [Google Scholar]
- 76.Pilling D, Cox N, Vakil V, Verbeek JS, Gomer RH. The long pentraxin PTX3 promotes fibrocyte differentiation. PLoS One. 2015;10(3):e0119709. doi: 10.1371/journal.pone.0119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cox N, Pilling D, Gomer RH. DC-SIGN activation mediates the differential effects of SAP and CRP on the innate immune system and inhibits fibrosis in mice. Proc Natl Acad Sci USA. 2015;112(27):8385–8390. doi: 10.1073/pnas.1500956112. [DOI] [PMC free article] [PubMed] [Google Scholar]