Significance
Mechanotransductive release of ATP from RBCs participates in the regulation of microvascular tone and plays essential roles in vascular physiopathology. The mechanism responsible for the release of ATP, however, is poorly understood. We show for the first time, to our knowledge, that Piezo1, the recently identified mechanically activated cation channel, regulates the mechanotransductive release of ATP from RBCs. In particular, we uncover the link between Piezo1, calcium influx, and ATP release and propose a previously unidentified mechanotransductive pathway that modulates the release of ATP from RBCs. The outcome of this study will allow the development of much needed approaches to regulate the release of ATP from RBCs and thus, impact significantly the fields of red cell research, cellular mechanosensing, and Piezo1 channels.
Keywords: Pizeo1, mechanosensing, ATP release, calcium flux, RBCs
Abstract
Piezo proteins (Piezo1 and Piezo2) are recently identified mechanically activated cation channels in eukaryotic cells and associated with physiological responses to touch, pressure, and stretch. In particular, human RBCs express Piezo1 on their membranes, and mutations of Piezo1 have been linked to hereditary xerocytosis. To date, however, physiological functions of Piezo1 on normal RBCs remain poorly understood. Here, we show that Piezo1 regulates mechanotransductive release of ATP from human RBCs by controlling the shear-induced calcium (Ca2+) influx. We find that, in human RBCs treated with Piezo1 inhibitors or having mutant Piezo1 channels, the amounts of shear-induced ATP release and Ca2+ influx decrease significantly. Remarkably, a critical extracellular Ca2+ concentration is required to trigger significant ATP release, but membrane-associated ATP pools in RBCs also contribute to the release of ATP. Our results show how Piezo1 channels are likely to function in normal RBCs and suggest a previously unidentified mechanotransductive pathway in ATP release. Thus, we anticipate that the study will impact broadly on the research of red cells, cellular mechanosensing, and clinical studies related to red cell disorders and vascular disease.
Mechanical stress-induced deformation of human red blood cells (RBCs) plays important physiopathological roles in oxygen delivery, blood rheology, transfusion, and malaria (1–4). Recent studies show that, in response to shear-induced stretch, RBCs release adenosine triphosphate (ATP) (5–9), suggesting the existence of mechanotransductive pathways in RBCs. Most importantly, RBCs participate in vascular signaling through the mechanotransductive release of ATP and contribute to the control of microvascular tone (10, 11). The released ATP from RBCs, for example, binds and activates the purinergic G protein-coupled receptors (P2Y receptors) on vascular endothelial cells and induces the synthesis and release of nitric oxide (12, 13), a well-known vasodilator. Moreover, impaired release of ATP from RBCs has been linked to diseases, such as type II diabetes and cystic fibrosis (14, 15). Given that RBCs experience shear stresses continuously during the circulation cycle and that the released ATP plays a central role in vascular pathophysiology, understanding of the mechanotransductive release of ATP from RBCs will provide not only fundamental insights to the roles of RBCs in vascular homeostasis but also, potential therapeutic strategies for red cell dysfunction and vascular disease.
Previous studies have shown that the addition of chemicals that stiffen RBC membranes decreases the amount of ATP released (9, 16), indicating that deformation of the cell membrane is a necessary trigger. In addition, biological mediators, such as cystic fibrosis transmembrane conductance regulator (CFTR) and pannexin-1 hemichannels, are involved in the release pathways of mechanotransductive ATP release from RBCs (9, 14, 17, 18). Inhibition of CFTR leads to an impaired ATP release from deformed RBCs (14). Recent studies, including our previous findings, suggest that interactions between membrane-associated actin and CFTR play important roles in the mechanotransductive ATP release from RBCs (9, 17). Pannexin-1, however, is a channel-forming protein and has been suggested as a mechanosensing ATP release channel (18). Under osmotic stress, for example, ATP released from RBCs was attenuated by carbenoxolone, a highly effective pannexin channel blocker, suggesting that pannexin-1 might be one of the conductance channels responsible for the mechanotransductive release of ATP (18). Although progress has been made in understanding mechanotransductive ATP release from RBCs, many questions remain about the signal transduction pathways. For example, how does mechanical force transduce signals to ATP release channels? Are there any stretch-activated ion channels on RBCs that may sense mechanical forces and activate ATP release? If so, are there any secondary messengers that could be generated by mechanical stimuli and regulate ATP release?
Piezo proteins (Piezo1 and Pizeo2) are recently identified mechanically activated cation channels in mammals (19, 20) and can be fully activated without involvement of additional proteins (20, 21). Piezo-induced cationic currents were first observed in the Neuro2A mouse cell line, but subsequent studies have shown that Piezo proteins are able to mediate mechanically activated cationic currents in a variety of cell types, including endothelial cells (22, 23) and neuronal stem cells (24). In particular, mature RBCs and erythroid progenitor cells express Piezo1 on their membranes (25), and mutations in the Piezo1 channels on mature RBCs are associated with hereditary xerocytosis (HX) (26, 27), a disease that is characterized by RBC dehydration and hemolytic anemia. To date, however, the physiological roles of Piezo1 in healthy RBCs remain poorly understood (27), and whether Piezo1 participates in the mechanotransductive release of ATP from RBCs is completely unknown. We hypothesize that Piezo1 controls shear-induced Ca2+ influx in RBCs and participates in the regulation of mechanotransductive release of ATP from RBCs. To test the hypothesis, we have implemented a microfluidic approach to control the shear-induced deformation of RBCs in flow and identify the regulatory roles of Piezo1 in shear-induced ATP release and Ca2+ influx in RBCs. Additionally, we show the correlation between stretch-evoked Ca2+ influx and ATP release from RBCs and reveal a threshold concentration of extracellular Ca2+ necessary for triggering shear-induced ATP release. Lastly, functional roles of membrane-associated ATP pools and potential ATP release channels in the shear-induced ATP release are investigated, and a model of mechanotransductive ATP release from RBCs is proposed.
Results
Inhibition of Piezo1 Impairs Shear-Induced ATP Release and Ca2+ Influx in Human RBCs.
We have developed a microfluidic strategy to investigate the effects of Piezo1 on shear-induced ATP release and Ca2+ influx in human RBCs. The principle of the strategy is similar to our previously shown approach (9). Briefly, a microfluidic channel with a constriction (lc = 800 µm; wc = 20 µm) is used to control the magnitude and duration of increased shear stress in flow, and the dynamics of shear-induced ATP release from human RBCs are studied with millisecond resolution. In particular, healthy human RBCs treated with Piezo1 inhibitors [gadolinium (Gd3+) (19), ruthenium red (19), or the peptide Grammostola spatulata mechanotoxin 4 (GsMTx4) (21, 28)] or loaded with a Ca2+-sensitive fluorescence dye (Fluo-4) were injected into the microfluidic channel at a constant flow rate (3 µL/min) (Fig. 1A). The flow rate is chosen such that RBCs flowing inside the constriction channel experience a physiological level of shear stress comparable with that in arterioles (29, 30) (i.e., calculated average shear stress is ∼4 Pa in the constriction channel) (Table S1). In addition, the flow velocity in the channel does not change with time, and thus, the flow in the channel is steady. Because space and time are interchangeable for a steady flow, we are able to apply increased shear and measure the release of ATP and Ca2+ influx with millisecond resolution. The average flow velocity in the constriction channel (vc), for example, is ∼0.083 m s−1; the duration (tc) of increased shear is, thus, ∼9.6 ms (tc = lc/vc). To measure the amount of released ATP, we used the Luciferase-ATP bioluminescent reaction and detected photon emission rate by using a photon-counting photomultiplier tube (PMT). Average concentration of released ATP was obtained based on an independently measured calibration curve (Fig. S1). The magnitude of shear-induced Ca2+ influx (i.e., the fluorescence intensity of Fluo-4) was measured by using the PMT or a fluorescence camera. In addition, we used a high-speed camera to track the deformation of individual cells.
Fig. 1.
Inhibition of Piezo1 impairs shear-induced ATP release from human RBCs. (A) Schematic of the microfluidic setup for measuring shear-induced ATP release from RBCs (not to scale). Note that x = 0 indicates the onset of increased shear. (B) Representative measurements of the photon emission rate resulting from the reaction between luciferase/luciferin and ATP for healthy control and GsMTx4-treated RBCs; t = 0 ms corresponds to the position of x = 0 in A. The approximate average ATP concentration converted from the calibration curve (Fig. S1) is shown on the right axis. Note that GsMTx4 is a peptide that has been used to inhibit mechanically activated Piezo1 channels. The error bars are reported as the SDs of the mean (n = 11 and 4 for control and treated RBCs, respectively). (C) Average concentration of released ATP from control and Piezo1 inhibitor-treated RBCs. **P < 0.01. (D) Superimposed series of time-lapse images showing the deformation of an individual RBC passing through a short constriction (lc = 100 µm; wc = 20 µm). (Scale bar: 20 µm.) (E) Normalized change of RBC length (measured in the flow direction) for cells passing through the short constriction shown in D (). Data were averaged from more than 30 cells for each sample. NS, not significant; RR, ruthenium red.
Table S1.
Calculation of average shear stress in the constriction channel
| Calculating parameter | Value |
| Viscosity (µ) | 1 × 10−3 Pa⋅s |
| Volume flow rate (Q) | 3 × 10−9 m3/min |
| Width of the constriction channel (wc) | 20 × 10−6 m |
| Height of the constriction channel (hc) | 30 × 10−6 m |
| Approximate average shear stress in the constriction channel (τc) | 4.2 Pa |
Note that τc is calculated based on the equation .
Fig. S1.
Calibration curve of photons per second as a function of ATP concentration in microfluidic channels.
We showed that the amount of released ATP from GsMTx4-treated RBCs (10% vol/vol) was approximately twofold lower than that from untreated, healthy control RBCs (Fig. 1B). In addition, control RBCs showed a peak of maximum ATP release between 125 and 150 ms after the constriction, whereas treated RBCs had no such releasing pattern. Decreased ATP release was also observed when RBCs were treated with other Piezo1 inhibitors (i.e., Gd3+ and ruthenium red), strongly suggesting that Piezo1 channels are involved in the regulation of shear-induced ATP release from RBCs (Fig. 1C). To rule out the possibility that decreased ATP release might arise from the impaired deformability of RBCs on the treatment of Piezo1 inhibitors, we performed a cell deformability study by flowing control and Piezo1 inhibitor-treated RBCs through a short constriction channel (lc = 100 µm; wc = 20 µm) (Fig. 1D). By measuring the change of RBC length using a high-speed camera, we found that there was no significant difference between control RBCs and treated cells (Fig. 1E), indicating that treatment with Piezo1 inhibitors does not affect RBC deformability.
Inhibition of Piezo1 channels reduces shear-induced Ca2+ influx in RBCs at both the single-cell and population levels. Images presented in Fig. 2A show typical responses of single RBCs to a flow-induced stretch in terms of Ca2+ influx. In the experiment, RBCs were loaded with Fluo-4 and immobilized at the bottom surface of the microfluidic channel, where an average wall shear stress was estimated as 3.4 Pa. Shear-induced changes in fluorescence caused by Ca2+ influx were recorded using a fluorescence camera. The fluorescence intensity of control RBCs subjected to flow was significantly higher than that of RBCs treated with Piezo1 inhibitors (Fig. 2B). At the population level, we flowed Fluo-4–loaded RBCs (10% vol/vol) through the microfluidic constriction channel (lc = 800 µm; wc = 20 µm) and examined the fluorescence intensity of RBCs before and after the constriction using a photon-counting PMT. Again, a significant increase of fluorescence intensity was observed only when control RBCs were flowing through the constriction channel (Fig. 2C). In addition, the increase of Ca2+ influx occurs right after the constriction and continuously increases along the channel (Fig. S2), which is unlike the pattern that we observed for shear-induced ATP release, in which the maximum release of ATP occurs a period after the constriction.
Fig. 2.
Inhibition of Piezo1 reduces shear-induced Ca2+ influx in human RBCs. (A) Fluorescence images of Fluo-4–loaded control and Piezo1 inhibitor-treated RBCs stretched by shear in a microfluidic device. Calculated average shear stress is ∼3.4 Pa. (Scale bar: 20 µm.) (B) Average fluorescence intensity of control and Piezo1 inhibitor-treated single RBCs. The error bars are reported as the SDs of the mean (n = 3). ***P < 0.001. (C) Average fluorescence intensity of control and Piezo1 inhibitor-treated RBCs (10% vol/vol) flowing before and after the constriction channel. The error bars are reported as the SDs of the mean (n = 8 and 3 for normal RBCs and treated RBCs, respectively). NS, not significant; RR, ruthenium red. *P < 0.05.
Fig. S2.
(A) Shear-induced Ca2+ influx (Fluo-4 intensity) and (B) ATP release (photons per second) vs. time. (C) Normalized increase of Ca2+ influx and ATP release vs. time. Ia is the intensity after the constriction, and Ib is the average intensity before the constriction; t = 0 ms indicates the onset of increased shear. Note that, to emphasize the trend in Ca entry and ATP release, the error bar (±0.05) is not shown in C.
Shear-Induced ATP Release from Human RBCs Depends on Ca2+ Influx and Membrane-Associated ATP Pools.
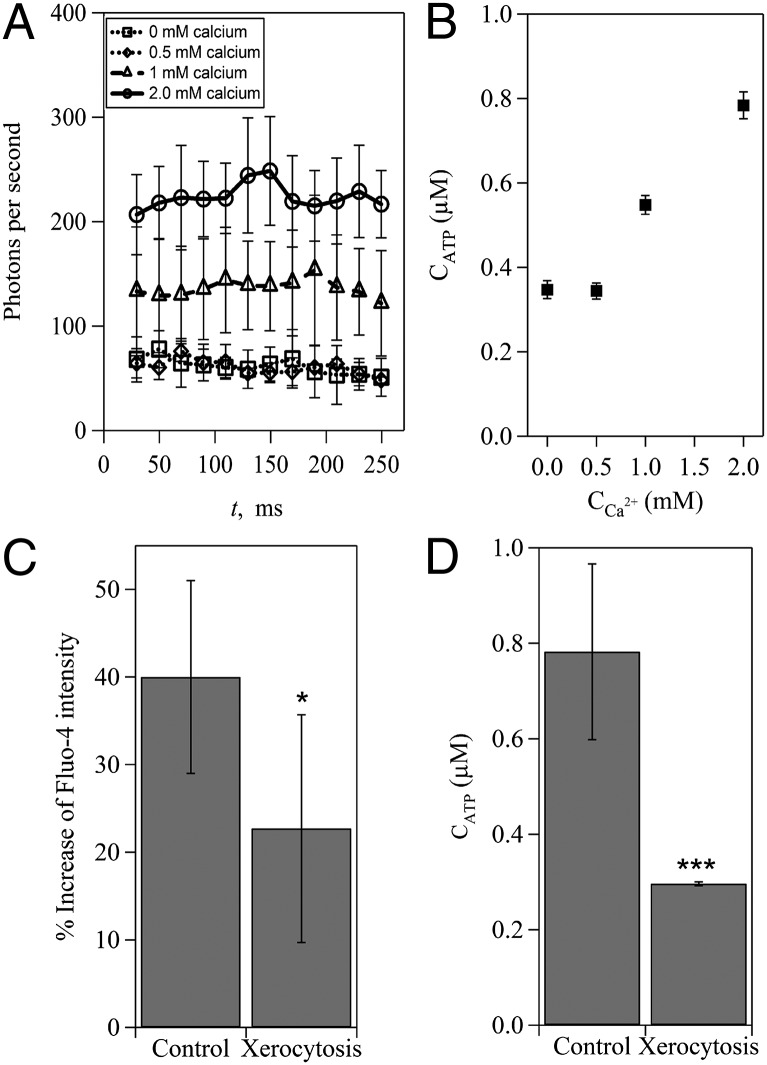
To identify whether Ca2+ influx in RBCs is necessary for shear-induced ATP release, we compared the amount of released ATP from RBCs that were prepared in different concentrations of extracellular Ca2+. Tests on cells labeled with Fluo-4 revealed that the influx of calcium increased in proportion to the extracellular calcium concentration (Fig. S3). In the absence of external Ca2+, ATP release remained at baseline (Fig. 3A). The average amount of released ATP remained almost unchanged (∼0.34 µM), even when the extracellular Ca2+ concentration increased from 0 to 0.5 mM (Fig. 3B). When the extracellular Ca2+ concentration was above 0.5 mM, however, the average amount of released ATP increased significantly and in proportion to the extracellular Ca2+ concentration (Fig. 3B). This observation indicates the existence of a threshold of extracellular Ca2+ concentration required for shear-induced ATP release. We further examined the correlation between shear-induced ATP release and Ca2+ influx by using RBCs from patients with HX, which is known to be associated with mutations of Piezo1 channels on RBCs (25–27). Data in Fig. 3 C and D show that both the amount of released ATP and Ca2+ influx from HX RBCs decreased significantly, suggesting that mutant Piezo1 channels impair Ca2+ influx and consequently, reduce ATP release.
Fig. S3.
Effect of extracellular Ca2+ concentration on shear-induced Ca2+ influx in RBCs. The error bars are reported as the SDs of the mean (n = 6, 4, 3, and 8 for 0, 0.5, 1, and 2 mM, respectively).
Fig. 3.
Ca2+ influx regulates shear-induced ATP release from human RBCs. (A) Measurements of the photon emission rate caused by shear-induced ATP release in solutions with different concentrations of Ca2+. (B) Dependence of shear-induced ATP release on extracellular Ca2+ concentrations. (C) Ca2+ influx-induced increase of Fluo-4 intensity and (D) shear-induced ATP release from healthy control RBCs and RBCs from patients with xerocytosis. The error bars are reported as the SDs of the mean (n = 11 and 4 for control RBCs and RBCs from patients with xerocytosis, respectively). *P < 0.05; ***P < 0.001.
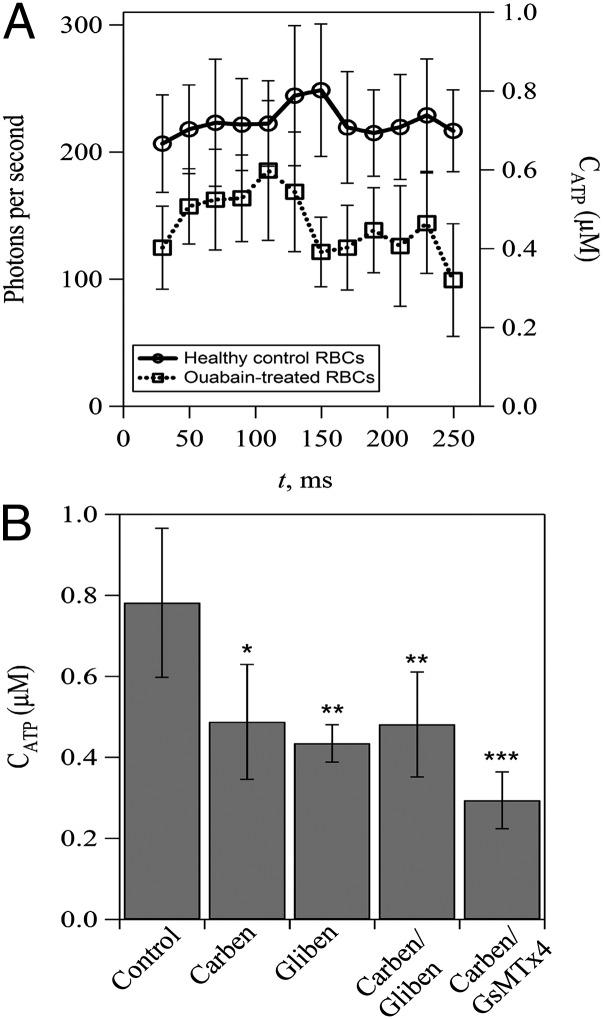
Lastly, we investigated the roles of membrane-associated ATP pools and ATP-releasing channels in shear-induced ATP release from RBCs. Membrane-associated ATP pools in RBCs have been shown to be able to fuel RBC membrane cation pumps, including the Ca2+ pumps (31–33). In particular, it has been suggested that membrane-associated ATP pools supply hypoxia-induced ATP release from RBCs (31). In our experiment, we measured the shear-induced ATP release from RBCs treated with ouabain, which is known to prevent bulk ATP from entering the ATP pools in RBC ghosts (32). The results showed that ouabain-treated RBCs have a decreased amount of released ATP compared with control RBCs (Fig. 4A), implying that limited access to bulk ATP reduces shear-induced ATP release. Meanwhile, the release of ATP from ouabain-treated RBCs reached its maximum at ∼100 ms after the onset of increased shear, which is ∼50 ms earlier than that of control RBCs. In addition, we performed experiments with the pannexin-1 inhibitor carbenoxolone and the CFTR inhibitor glibenclamide to explore their roles in shear-induced ATP release. The results showed that the amount of released ATP decreases significantly (approximately twofold) in treated RBCs (Fig. 4B). Notably, the amount of released ATP from RBCs treated with both carbenoxolone and glibenclamide was not significantly different from RBCs that were treated with either carbenoxolone or glibenclamide alone. These findings indicate that carbenoxolone and glibenclamide do not distinguish between different potential ATP transport pathways. Furthermore, to determine the role of pannexin-1 relative to Piezo1 in terms of ATP release, we treated RBCs with both carbenoxolone and Piezo1 inhibitor GsMTx4 and measured ATP release. The results showed an additional reduced release of ATP (0.29 ± 0.07 µM) (Fig. 4B) compared with RBCs treated with either carbenoxolone (0.49 ± 0.14 µM) (Fig. 4B) or GsMTx4 (0.45 ± 0.05 µM) (Fig. 1C) alone.
Fig. 4.
Effects of membrane-associated ATP pools and potential ATP-releasing channels on shear-induced ATP release from human RBCs. (A) Effect of ouabain treatment on shear-induced ATP release. Note that ouabain treatment is used to prevent bulk ATP from entering the membrane-associated ATP pools in RBCs. (B) Effect of inhibition of CFTR and/or pannexin-1 and Piezo1 channels on shear-induced ATP release. Carbenoxolone (Carben) and glibenclamide (Gliben) are used to inhibit pannexin-1 and CFTR, respectively. GsMTx4 is used to inhibit Piezo1 channels. The error bars are reported as the SDs of the mean (n = 4 for Carben and Carben/Gliben-treated RBCs; n = 3 for Gliben-treated RBCs; and n = 4 for Carben/GsMTx4-treated RBCs). *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The main finding presented here is that the mechanosensing cation channel Piezo1 on RBCs regulates Ca2+ influx and participates in shear-induced ATP release. This finding is shown by measuring reduced ATP release and Ca2+ influx in RBCs that are treated with Piezo1 inhibitors. In addition, our data show that significant ATP release can be activated only when the extracellular Ca2+ concentration is above a threshold, suggesting a regulatory role of Ca2+ influx in ATP release. Thus, it is likely that shear-induced stretch of RBCs activates the mechanosensing cation channel Piezo1, which allows Ca2+ influx and consequently, induces ATP release from RBCs. A paper published after the initial submission of this report shows a role for Piezo1 in red cell volume regulation (34), but to the best of our knowledge, our study is the first to show a role of Piezo1 in the response of normal human RBCs to physiologically relevant fluid forces that could have relevance to the regulation of vascular tone.
We previously identified two distinct timescales associated with the mechanotransductive release of ATP from normal human RBCs (9) and showed that these were consistent with the physical processes described in a model of the process involving CFTR and actin (17). In addition, we investigated the links between single-RBC dynamics and ATP release and concluded, based on the effects of different inhibitors, that CFTR and pannexin-1 respond differently to shear (35). Carbenoxolone is a pannexin-specific blocker and has been used to block specifically pannexin activity in a wide range of cell lines, including RBCs (18, 36–39). In studies that appeared subsequent to our prior report, however, it was shown that glibenclamide can attenuate pannexin-1 channel currents (40, 41) and thus, is not specific to CFTR (42). As a result, the decreased ATP release from RBCs treated with both glibenclamide and carbenoxolone may reflect the reduced activity of pannexin-1 only. The role of CFTR in the process of ATP release is unclear (43, 44). Early reports (14) implicated CFTR as a contributor to ATP release from RBCs based on inhibitor activity and different behaviors of red cells from cystic fibrosis patients. Subsequently, a direct role for CFTR in the release of ATP from RBCs was called into question for several reasons: first, the inability to detect CFTR in RBC membranes (45); second, whether CFTR itself transports ATP remains controversial (46); and third, the fact that inhibitors originally thought to act on CFTR preferentially also block ATP transport through pannexin-1 (40). The most direct explanation for our results using carbenoxolone and glibenclamide is that ATP transport occurs primarily through pannexin-1. The different ATP transport behavior in cystic fibrosis patients could be explained by a modulatory effect of CFTR on transport through pannexin-1.
Our results here also show that ATP release correlates with Ca2+ influx and that the amount of released ATP and Ca2+ influx in HX RBCs is significantly lower than that in healthy control RBCs (Fig. 3). HX is linked to mutations of Piezo1 channels on RBCs, and such mutations alter the kinetics of Piezo1 and lead to RBC dehydration and hemolytic anemia (26, 27). The mutations result in two important differences in the kinetic behavior of the Piezo1 channels in HX cells: the rate of inactivation is slower (∼200 ms), and the latency to activation is longer (26, 27). The slower rate of inactivation would be expected to extend the open time of the channels and lead to larger Ca2+ influx, contrary to experimental findings. Thus, the more important effect is most likely the longer latency period before activation, because this latency should lead to lower Ca2+ influx, consistent with experimental findings. In healthy RBCs, activation of Piezo1 is fast (i.e., within a few milliseconds) (26). In our experiment, the duration of increased shear (tc) is ∼9.6 ms, which is long enough to activate Piezo1 in healthy RBCs but much shorter than the latency for activation of mutant Piezo1 channels in HX RBCs. As a result, shear-induced Ca2+ influx in HX RBCs is significantly lower than that in healthy control RBCs in our experimental setup.
We also consider the possible contributions of membrane-associated ATP pools in RBCs to the shear-induced ATP release. An estimate of the number of ATP molecules released per cell in our experiments indicates that the membrane pool of ATP is not large enough to account for all of the ATP released. The approximate number of ATP molecules released from single RBCs calculated from our data is ∼466,000 (Table S2). This value is about 20 times more than the pool ATP (e.g., ∼27,000 molecules of ATP for a typical RBC) (33). Thus, it is unlikely that all of the released ATP comes from the ATP pools, unless there is a much more rapid refilling of the pool from bulk ATP than has been previously established. If it is true, as Chu et al. (31) suggest, that the released ATP comes from the membrane pool, then constant, facile access to bulk ATP (possibly because of membrane deformation) is presumably required to maintain the pool ATP for release. This scenario is consistent with our observations of the inhibitory effects of ouabain that we have observed. Experiments on RBC ghosts have led to the conclusion that ouabain acts to prevent replenishment of the membrane pools from bulk ATP. This limited access of membrane-associated ATP pools to the bulk ATP because of ouabain treatment would result in a reduced amount of releasable ATP in the pools and consequently, a decreased ATP release, which we have observed. It should be noted, however, that ATP compartmentation in human RBCs was discovered in RBC ghosts, and direct evidence of the presence of ATP pools in intact RBCs remains elusive, although indirect arguments in favor of the presence of pools in intact cells have been made (32, 47).
Table S2.
Calculation of ATP release from single RBCs
| Calculating parameter | Value |
| Concentration of RBCs (10% vol/vol) in a PSS (MRBC) | 1 × 1015/m3 |
| Detecting volume for ATP release in microfluidics (V) | 1.02 × 10−12 m3 |
| Number of RBCs in the detection volume (NRBC = MRBC × V) | 1.02 × 103 |
| Average released ATP from the detection volume (MATP) | 0.78 µM |
| Molecules of released ATP in the detection volume (NATP) | 4.75 × 108 |
| Molecules of released ATP from single RBCs (nATP = NATP/NRBC) | 4.66 × 105 |
Our results, however, raise the question of how Ca2+ influx in RBCs relates to ATP release. The dependence of ATP release on intracellular Ca2+ has been shown in various cell lines, including urothelial and endothelial cells (48, 49). Indeed, functional roles of Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures have been shown recently (48). In these cell types, however, a Ca2+-regulated vesicular exocytosis is commonly involved in the process of ATP release. RBCs are lacking organelles, such as the Golgi complex, to form vesicles, and consequently, the Ca2+-regulated vesicular exocytosis is not applicable to the observed relation between Ca2+ flux and ATP release from RBCs. However, it has been shown that locally increased Ca2+ levels near the RBC plasma membrane reduce the interactions between 4.1R and the spectrin-actin network (50) and increase the depolymerization of actin filaments (51). Given that CFTR can be activated by membrane-associated actin molecules and induces ATP release (14, 17), it is possible that Ca2+ participates in the process of ATP release by modulating the interactions between actin and CFTR. Furthermore, because pannexin-1 channels can be activated by cytoplasmic Ca2+ (52), it is also possible that shear-induced Ca2+ influx activates pannexin-1 directly and triggers ATP release. However, Piezo1 inhibitors do not block completely Ca2+ influx (Fig. 2B), and thus, a basal ATP release is still observed (Fig. 1C). This basal ATP release can be further reduced when both Piezo1 and ATP-releasing channel pannexin-1 are inhibited (Fig. 4B, carbenoxolone and GsMTx4 treatment). In this case, the amount of released ATP (0.29 ± 0.07 µM) (Fig. 4B) is comparable with that of ATP released in 0 mM extracellular Ca2+ solution (0.34 ± 0.02 µM) (Fig. 3B), emphasizing the regulatory role of Ca2+ influx in ATP release. The fraction of released ATP inhibited by carbenoxolone (0.49 ± 0.14 µM) (Fig. 4B), however, is approximately the same as that inhibited by Piezo1 inhibitor GsMTx4 (0.45 ± 0.05 µM) (Fig. 1C).
Shear-induced Ca2+ influx can activate plasma membrane Ca2+ ATPase (PMCA) (53–55) that pumps extra Ca2+ out of the cell rapidly. As a result, intracellular Ca2+ decreases quickly, which may down-regulate the ATP release. Indeed, considering the kinetics of PMCA activity and the timeframe of ATP release, this mechanism seems feasible. The turnover rate of PMCA is 50–300 per second (56, 57), the number of pump units per cell is 400–700 (57), and the increased intracellular Ca2+ levels in RBCs caused by the mechanical deformation are 12–24 nM (55). Combining these numbers, we estimate a timescale for PMCA to remove the extra Ca2+ of 30–100 ms, which is comparable with the duration of ATP release (∼100 ms). Therefore, it seems likely that the parallel activities of PMCA and ATP release compete in Ca2+ use inside the cell and that the dynamic concentration of intracellular Ca2+ determines the patterns of ATP release. When a slight increase of intracellular Ca2+ occurs because of shear-induced Ca2+ influx, for example, PMCA pumps the extra Ca2+ out of the cell, rapidly leaving a negligible amount of Ca2+ to trigger ATP release. When intracellular Ca2+ increases significantly, however, it will take a period for the PMCA to pump the extra Ca2+ out of the cell, and consequently, there will be a sufficient amount of intracellular Ca2+ to trigger ATP release. After extra intracellular Ca2+ ions are pumped out completely, ATP release stops. This process explains the existence of a threshold of extracellular Ca2+ concentration that triggers ATP release and the transient ATP release patterns that we observed in this study and previous studies. Collectively, we propose a model to account for the observed mechanotransductive release of ATP from RBCs (Fig. S4). It is important to note that our data indicate that Ca2+ influx is important for mechanically induced ATP release, but whether this observation results from modification of cytoskeletal stability or whether the release might be modulated by either PMCA activity or Ca2+ acting directly on ATP release channels remains to be determined. In addition, our data show that neither inhibitors nor mutation can block ATP release or Ca2+ entry completely. Instead, the decrease in ATP release and Ca2+ entry brought about by inhibitors or mutation is about one-half of the normal amount. Furthermore, basal ATP release can still occur when extracellular Ca2+ is 0 mM (Fig. 3B) or both Piezo1 and pannexin-1 channels are inhibited (Fig. 4B). These results, thus, suggest that (i) Ca2+ influx is necessary only when a significant amount of ATP release is required and (ii) pathways other than Piezo1-regulated Ca2+ influx also participate in shear-induced Ca2+ entry and ATP release.
Fig. S4.
Schematic of the proposed mechanotransductive release of ATP from RBCs. (A) Shear-induced stretch of RBCs activates Piezo1 channels and induces an increased Ca2+ influx, which (B) activates Ca2+ pumps to remove extra Ca2+ rapidly. (C) Meanwhile, increased Ca2+ influx activates pannexin-1 channels (Px1) directly and triggers ATP release from the membrane-associated ATP pools. (D) It is also possible, although controversial, that increased Ca2+ influx depolymerizes actin filaments (brown spheres), which activates CFTR and induces the release of ATP. Regardless of the precise mechanism, our results show clearly that a critical concentration of intracellular Ca2+ caused by shear-induced Ca2+ influx is required for the mechanotransductive release of ATP.
In summary, we show for the first time, to our knowledge, a regulatory role of Piezo1 in shear-induced ATP release from human RBCs and provide evidence for a Ca2+-triggered ATP release model. Our studies represent a substantial step forward in understanding the mechanotransductive release of ATP from RBCs, which will be critical to the development of improved therapeutic strategies for red cell dysfunction and vascular disease. After such strategies become available, for example, in diseases that are associated with impaired ATP release from RBCs, pharmacologic regulation of the calcium influx in RBCs could be used to achieve enhanced ATP release. Conversely, when less ATP release is needed (for example, to store RBCs or in the case of handling blood using mechanical devices), the calcium influx could be decreased. Decreasing activity of Piezo1 is likely to be sufficient for this purpose. Thus, important advances in the therapy of diseases and complications that are associated with the release of ATP from RBCs are expected. In addition, because of the high spatiotemporal resolution of the developed microfluidic approach, we anticipate that the experimental procedure developed here will be useful for elucidating the dynamics of Piezo1 channels at the whole-cell level, which has been known to be critical and much needed for extrapolation of patch recordings results to functionality (58).
Materials and Methods
ATP Measurement.
We used a microfluidic channel with a constriction for shear-induced ATP measurement. The width of the channel before and after the constriction is 100 µm; the constriction is 20-µm wide (wc) and 800-µm long (lc). The height of the channel is uniformly 30 µm. Bioluminescent light was measured at different positions along the microfluidic channel by using a 63× oil objective on a microscope (Leica DMI 6000B; Leica). [This objective has lower magnification than the one that we used in previous studies, resulting in a larger field of view. This large field of view has the consequence that sensitivity is increased slightly, because we are gathering light from a larger area, but temporal resolution is decreased, because cells remain in the field of view for longer periods of time. This lower temporal resolution results in an ATP release profile that is slightly different in appearance than that reported in previous studies (9).] The emitted light is passed to the photomultiplier detector, which is connected to the microscope by a C mount as a part of the separate photo detection system (RatioMaster; Photon International Technology). The time of release of ATP is then obtained by taking advantage of the space–time equivalence in time-invariant flows in microfluidic channels. All experiments were performed at room temperature (25 °C) and inside a dark room. Microfluidic device fabrication, RBC preparation, cell deformability measurements, and statistical analysis are described in SI Text. This research was approved by the Institutional Review Boards at the Rochester Institute of Technology and the University of Rochester School of Medicine.
Ca2+ Measurement.
The same microfluidic device used to measure ATP release was used to measure shear-induced Ca2+ influx. RBCs at 10% (vol/vol) were loaded with Fluo-4 by incubating the cells at 37 °C for 30 min in 5.0 µM Fluro-4:00 AM Dye (Life Technologies). Cells were then washed three times using a physiological salt solution and injected into the microfluidic device. Cells were excited at 488 nm with a monochromator light source (RatioMaster; Photon International Technology), and the emitted light intensity was measured with the photon detection system (RatioMaster; Photon International Technology) using a GFP filter. Images of cells stretched by fluid shear were taken with a digital charge-coupled device (CCD) (Hamamatsu ORCA-R2; Hamamatsu). A 75-W Xenon Lamp (Leica) was used to excite the cells, and software exposure settings were kept the same throughout each experiment.
SI Text
Microfluidic Fabrication and Experimental Setup.
Microfluidic devices were fabricated out of polydimethylsiloxane (PDMS) (Slygard 184) using the standard soft lithography technique. Three-inch silicon wafers (University Wafers) were patterned using epoxy-based negative photoresist (SU-8) (Microchem) photoresist, and then, PDMS (10:1 curing ratio) was cast on the wafer molds. For each experiment, 1.0 mL 10% (vol/vol) RBCs suspension was loaded into a syringe (Hamilton), which is connected to a polyethylene (PE 20) tube by a syringe needle (27 gauge). The other end of the tube was inserted into the inlet of microfluidic devices. The syringe was placed onto a syringe pump (Harvard Apparatus Phd Ultra; Harvard Apparatus), and suspension was flowed at a rate of 3.0 µL/min.
RBC Preparation.
Human RBCs were drawn from healthy donors and used on the same day of experiments. To treat RBCs with Piezo1 inhibitors, 10% (vol/vol) RBCs were incubated with 10 µM GsMTx4 (Peptides International, Inc.), 30 µM Gd3+ (Sigma), or ruthenium red (Sigma) in physiological salt solution (PSS) at 37 °C for 30 min. To inhibit CFTR and/or pannexin-1 channels on RBCs, 10% (vol/vol) RBCs were incubated with 50 µM glibenclamide and/or carbenoxolone at 37 °C for 30 min. Glibenclamide (Sigma) was prepared by following the protocol in ref. 14 and diluted from a 10 mM stock solution to 50 µM in PSS. Carbenoxolone was diluted from 1.0 mM stock solution to 50 µM in PSS. All treated cells were washed with a PBS solution three times and resuspended into the luciferase-luciferin (LL) solution for ATP measurements. RBCs with FAM38A mutations of Piezo1 channels were obtained from patients with HX at the University of Rochester Medical Center under a material transfer agreement.
LL Solution Preparation.
PSS was prepared with 4.7 mM KCl, 2.0 mM CaCl2, 1.2 mM MgSO4, 140.5 mM NaCl, 21 mM Tris(hydroxymethyl)aminomethane, 11.1 mM dextrose, and 1 mg/mL BSA. The pH of the solution was adjusted to 7.4. d-luciferin and firefly luciferase were purchased from Sigma. LL solution was prepared by adding 1.7 mg d-luciferin and 100 µL firefly luciferase (1.0 mg/mL in PSS) into 5.0 mL PSS. PSS with different Ca2+ concentrations was prepared by dissolving a proper amount of CaCl2. The resulting solution was then titrated using 100 mM EDTA. Calcium concentration at the equivalence point was calculated using the Ca2+-EDTA binding constant Kd = 1010.65 assuming 1:1 binding of Ca2+:EDTA (Y4−). The fraction of EDTA (Y4−) is used as 3.8 × 10−4 at pH 7. Calcium-free buffer was obtained by preparing PSS without adding CaCl2 and including 1.5 mM EDTA. A Ca2+-free firefly luciferase solution (1.0 mg/mL) was prepared by using calcium-free PSS. ATP-sodium salt solution was prepared in 100 µM stock solution using deionized (DI) water, and then, the stock was diluted into PSS to generate specific concentrations for calibration experiments. Both LL and ATP-sodium salt solutions were prepared on the day of use. All chemicals, proteins, and enzymes were purchased from Sigma without additional purification.
RBC Deformability Measurements.
To measure the deformability of RBCs, we diluted RBCs at ∼1.0% (vol/vol) in PSS and flowed the solution through another microfluidic constriction channel. The constriction channel dimensions used for cell deformability were 20 µm in width, 100 µm in length, and 30 µm in height. The cells were injected at a flow rate of 3.0 µL/min, and high-speed videos are captured with a high-speed camera (Phantom M120; Vision Research). The videos were recorded at 1,900 frames per second with a resolution of 1,216 × 700. The videos were analyzed using Image J to determine the lengths of cells in pixels.
Statistical Analysis.
A two-tailed Student’s t test was performed for ATP and Ca2+ measurements; values were considered statistically significant at P value < 0.05. All experiments were performed in at least three biological replicates for statistical analysis.
Acknowledgments
We thank Joseph F. Hoffman for constructive suggestions and generous advice. We also thank Missy Maschoff for blood drawn and Brian D. Smith for providing blood samples from patients with xerocytosis. We acknowledge support from Rochester Institute of Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507309112/-/DCSupplemental.
References
- 1.Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: When is it not safe? Crit Care Med. 2003;31(12 Suppl):S687–S697. doi: 10.1097/01.CCM.0000099349.17094.A3. [DOI] [PubMed] [Google Scholar]
- 2.Schmid-Schöenbein H, Wells R. Fluid drop-like transition of erythrocytes under shear. Science. 1969;165(3890):288–291. doi: 10.1126/science.165.3890.288. [DOI] [PubMed] [Google Scholar]
- 3.Vague P, Juhan I. Red cell deformability, platelet aggregation, and insulin action. Diabetes. 1983;32(Suppl 2):88–91. doi: 10.2337/diab.32.2.s88. [DOI] [PubMed] [Google Scholar]
- 4.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: Sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006;22(11):503–508. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Fischer DJ, Torrence NJ, Sprung RJ, Spence DM. Determination of erythrocyte deformability and its correlation to cellular ATP release using microbore tubing with diameters that approximate resistance vessels in vivo. Analyst (Lond) 2003;128(9):1163–1168. doi: 10.1039/b308225n. [DOI] [PubMed] [Google Scholar]
- 6.Price AK, Fischer DJ, Martin RS, Spence DM. Deformation-induced release of ATP from erythrocytes in a poly(dimethylsiloxane)-based microchip with channels that mimic resistance vessels. Anal Chem. 2004;76(16):4849–4855. doi: 10.1021/ac0495992. [DOI] [PubMed] [Google Scholar]
- 7.Price AK, Martin RS, Spence DM. Monitoring erythrocytes in a microchip channel that narrows uniformly: Towards an improved microfluidic-based mimic of the microcirculation. J Chromatogr A. 2006;1111(2):220–227. doi: 10.1016/j.chroma.2005.07.083. [DOI] [PubMed] [Google Scholar]
- 8.Sprung R, Sprague R, Spence D. Determination of ATP release from erythrocytes using microbore tubing as a model of resistance vessels in vivo. Anal Chem. 2002;74(10):2274–2278. doi: 10.1021/ac011144e. [DOI] [PubMed] [Google Scholar]
- 9.Wan J, Ristenpart WD, Stone HA. Dynamics of shear-induced ATP release from red blood cells. Proc Natl Acad Sci USA. 2008;105(43):16432–16437. doi: 10.1073/pnas.0805779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269(6 Pt 2):H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 11.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: The red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol. 1996;271(6 Pt 2):H2717–H2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 12.Janigro D, Nguyen TS, Gordon EL, Winn HR. Physiological properties of ATP-activated cation channels in rat brain microvascular endothelial cells. Am J Physiol. 1996;270(4 Pt 2):H1423–H1434. doi: 10.1152/ajpheart.1996.270.4.H1423. [DOI] [PubMed] [Google Scholar]
- 13.Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: Signaling in and from erythrocytes. Trends Endocrinol Metab. 2007;18(9):350–355. doi: 10.1016/j.tem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol. 1998;275(5 Pt 2):H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 15.Subasinghe W, Spence DM. Simultaneous determination of cell aging and ATP release from erythrocytes and its implications in type 2 diabetes. Anal Chim Acta. 2008;618(2):227–233. doi: 10.1016/j.aca.2008.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moehlenbrock MJ, Price AK, Martin RS. Use of microchip-based hydrodynamic focusing to measure the deformation-induced release of ATP from erythrocytes. Analyst (Lond) 2006;131(8):930–937. doi: 10.1039/b605136g. [DOI] [PubMed] [Google Scholar]
- 17.Gov NS, Safran SA. Red blood cell membrane fluctuations and shape controlled by ATP-induced cytoskeletal defects. Biophys J. 2005;88(3):1859–1874. doi: 10.1529/biophysj.104.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: Function without a gap. Proc Natl Acad Sci USA. 2006;103(20):7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coste B, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coste B, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottlieb PA, Sachs F. Piezo1: Properties of a cation selective mechanical channel. Channels (Austin) 2012;6(4):214–219. doi: 10.4161/chan.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranade SS, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA. 2014;111(28):10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515(7526):279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathak MM, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA. 2014;111(45):16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarychanski R, et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120(9):1908–1915. doi: 10.1182/blood-2012-04-422253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci USA. 2013;110(12):E1162–E1168. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albuisson J, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. doi: 10.1038/ncomms2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50(29):6295–6300. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tangelder GJ, Slaaf DW, Arts T, Reneman RS. Wall shear rate in arterioles in vivo: Least estimates from platelet velocity profiles. Am J Physiol. 1988;254(6 Pt 2):H1059–H1064. doi: 10.1152/ajpheart.1988.254.6.H1059. [DOI] [PubMed] [Google Scholar]
- 30.Pries AR, Secomb TW. In: Handbook of Physiology: Microcirculation. 2nd Ed. Tuma RF, et al., editors. Elsevier; Oxford: 2008. p. 17. [Google Scholar]
- 31.Chu H, et al. Identification of cytoskeletal elements enclosing the ATP pools that fuel human red blood cell membrane cation pumps. Proc Natl Acad Sci USA. 2012;109(31):12794–12799. doi: 10.1073/pnas.1209014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman JF, Dodson A, Proverbio F. On the functional use of the membrane compartmentalized pool of ATP by the Na+ and Ca++ pumps in human red blood cell ghosts. J Gen Physiol. 2009;134(4):351–361. doi: 10.1085/jgp.200910270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proverbio F, Hoffman JF. Membrane compartmentalized ATP and its preferential use by the Na,K-ATPase of human red cell ghosts. J Gen Physiol. 1977;69(5):605–632. doi: 10.1085/jgp.69.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cahalan SM, et al. Piezo1 links mechanical forces to red blood cell volume. eLife. 2015;4:e07370. doi: 10.7554/eLife.07370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forsyth AM, Wan J, Owrutsky PD, Abkarian M, Stone HA. Multiscale approach to link red blood cell dynamics, shear viscosity, and ATP release. Proc Natl Acad Sci USA. 2011;108(27):10986–10991. doi: 10.1073/pnas.1101315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silverman WR, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284(27):18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JE, Kang TC. The P2X7 receptor-pannexin-1 complex decreases muscarinic acetylcholine receptor-mediated seizure susceptibility in mice. J Clin Invest. 2011;121(5):2037–2047. doi: 10.1172/JCI44818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poornima V, et al. P2X7 receptor-pannexin 1 hemichannel association: Effect of extracellular calcium on membrane permeabilization. J Mol Neurosci. 2012;46(3):585–594. doi: 10.1007/s12031-011-9646-8. [DOI] [PubMed] [Google Scholar]
- 39.Chekeni FB, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467(7317):863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahl G, Keane RW. Pannexin: From discovery to bedside in 11±4 years? Brain Res. 2012;1487:150–159. doi: 10.1016/j.brainres.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu F, Wang J, Spray DC, Scemes E, Dahl G. Two non-vesicular ATP release pathways in the mouse erythrocyte membrane. FEBS Lett. 2011;585(21):3430–3435. doi: 10.1016/j.febslet.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabe A, Disser J, Frömter E. Cl- channel inhibition by glibenclamide is not specific for the CFTR-type Cl- channel. Pflugers Arch. 1995;429(5):659–662. doi: 10.1007/BF00373986. [DOI] [PubMed] [Google Scholar]
- 43.Schwiebert EM. ABC transporter-facilitated ATP conductive transport. Am J Physiol. 1999;276(1 Pt 1):C1–C8. doi: 10.1152/ajpcell.1999.276.1.C1. [DOI] [PubMed] [Google Scholar]
- 44.Wan J, Forsyth AM, Stone HA. Red blood cell dynamics: From cell deformation to ATP release. Integr Biol (Camb) 2011;3(10):972–981. doi: 10.1039/c1ib00044f. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman JF, Dodson A, Wickrema A, Dib-Hajj SD. Tetrodotoxin-sensitive Na+ channels and muscarinic and purinergic receptors identified in human erythroid progenitor cells and red blood cell ghosts. Proc Natl Acad Sci USA. 2004;101(33):12370–12374. doi: 10.1073/pnas.0404228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abraham EH, et al. Cystic fibrosis transmembrane conductance regulator and adenosine triphosphate. Science. 1997;275(5304):1324–1326. doi: 10.1126/science.275.5304.1324. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman JF. ATP compartmentation in human erythrocytes. Curr Opin Hematol. 1997;4(2):112–115. doi: 10.1097/00062752-199704020-00006. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto T, et al. Functional role for Piezo1 in stretch-evoked Ca²⁺ influx and ATP release in urothelial cell cultures. J Biol Chem. 2014;289(23):16565–16575. doi: 10.1074/jbc.M113.528638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto K, et al. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci. 2011;124(Pt 20):3477–3483. doi: 10.1242/jcs.087221. [DOI] [PubMed] [Google Scholar]
- 50.Bogdanova A, Makhro A, Wang J, Lipp P, Kaestner L. Calcium in red blood cells-a perilous balance. Int J Mol Sci. 2013;14(5):9848–9872. doi: 10.3390/ijms14059848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuhlman PA, Hughes CA, Bennett V, Fowler VM. A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J Biol Chem. 1996;271(14):7986–7991. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- 52.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580(1):239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Schatzmann HJ. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson RM. Membrane stress increases cation permeability in red cells. Biophys J. 1994;67(5):1876–1881. doi: 10.1016/S0006-3495(94)80669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larsen FL, Katz S, Roufogalis BD, Brooks DE. Physiological shear stresses enhance the Ca2+ permeability of human erythrocytes. Nature. 1981;294(5842):667–668. doi: 10.1038/294667a0. [DOI] [PubMed] [Google Scholar]
- 56.Romero PJ, Romero EA. Differences in Ca2+ pumping activity between sub-populations of human red cells. Cell Calcium. 1997;21(5):353–358. doi: 10.1016/s0143-4160(97)90028-2. [DOI] [PubMed] [Google Scholar]
- 57.Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- 58.Gottlieb PA, Bae C, Sachs F. Gating the mechanical channel Piezo1: A comparison between whole-cell and patch recording. Channels (Austin) 2012;6(4):282–289. doi: 10.4161/chan.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]