Significance
Pair bonds are universal in human societies and distinguish us from our closest living relatives. They characteristically involve men’s proprietary claims over women—mate guarding—which in animals generally is both predicted and observed to be more frequent when sex ratios in the fertile ages are male-biased. A marked male bias in the fertile ages evolved in our lineage as longevity increased without an extension of female fertility. We compare the sex-ratio shift in simulations of the grandmother hypothesis to demographic data from chimpanzees and human hunter–gatherers then connect the expanded proportions of older men to benefits for mate guarding, the evolution of pair bonds, and the long recognized importance of male alliances in human social life.
Keywords: grandmother hypothesis, human life history, human evolution, mate guarding, mating sex ratios
Abstract
The evolution of distinctively human life history and social organization is generally attributed to paternal provisioning based on pair bonds. Here we develop an alternative argument that connects the evolution of human pair bonds to the male-biased mating sex ratios that accompanied the evolution of human life history. We simulate an agent-based model of the grandmother hypothesis, compare simulated sex ratios to data on great apes and human hunter–gatherers, and note associations between a preponderance of males and mate guarding across taxa. Then we explore a recent model that highlights the importance of mating sex ratios for differences between birds and mammals and conclude that lessons for human evolution cannot ignore mammalian reproductive constraints. In contradiction to our claim that male-biased sex ratios are characteristically human, female-biased ratios are reported in some populations. We consider the likelihood that fertile men are undercounted and conclude that the mate-guarding hypothesis for human pair bonds gains strength from explicit links with our grandmothering life history.
We call attention to evidence that connects the evolution of human pair bonds to the male-biased sex ratios in fertile ages that characterize human populations. As in mammals generally, age-specific mortality is higher in males than in females (e.g., refs. 1–3). However, this difference is overshadowed by a distinctive feature of human life history: Oldest ages at parturition are about the same in humans as in other living hominids, the great apes (4, 5), whereas longevity is substantially greater and male fertility continues to older ages (6). Exceptional longevity with a distinctive postmenopausal life stage (7–9) may have evolved in our lineage when grandmothers’ subsidies for weaned dependents allowed mothers to have next babies sooner. According to this grandmother hypothesis (10–16), longevity increased as longer-lived grandmothers could help more and so left more longer-lived descendants of both sexes. Women’s postfertile life stage (7) produces a bias in the sex ratio of fertile adults with repercussions for male strategies. As longevity increased, older-aged males expanded the pool of competitors for the still-fertile females. With more competitors for each paternity, males’ average success in finding new mates inevitably declined until defending a current mate became the better option. Our distinctive life history thus supplies previously unrecognized support for a mate-guarding hypothesis for the evolution of human pair bonds.
Here we simulate hominid mating sex ratios with an agent-based model of the evolution of human longevity via grandmothering (13, 15). We then compare simulated sex ratios to demographic data from both great apes and human hunter–gatherers. Having identified the human bias, we connect it to increased male payoffs for mate guarding, noting some broad patterns in humans, the tradeoffs observed in other taxa, and a history of modeling in which increased guarding is the likely outcome of more competing males.
We then consider a recent model (17) of the evolution of sex roles built to show that anisogamy, the primary sex difference of large (female) and small (male) gametes (18), is insufficient by itself to explain why females care for offspring more often whereas males more often compete for mates. Mating sex ratios are decisive for mating strategies in this model. Although it does not include guarding as an option, it does highlight a connection between mating sex ratios and broad differences between mammals and birds. We elaborate key phylogenetic constraints, consider recent work on birds, and underline features of mammalian offspring production that temper direct application of the model to mammals, including hominids.
An important challenge to our claim that human life history entails male-biased mating sex ratios comes from reports of female bias in some human populations. We identify common measurement problems and link men’s age-specific fertilities to status hierarchies, concluding that the mate-guarding hypothesis remains both promising and directly relevant to explaining the long-recognized importance of male alliances in human societies.
Grandmothering Simulations
We track mating sex ratios through simulations of an agent-based model first built to investigate the evolution of human longevity via grandmothering (13, 15). For reasons elaborated below, we follow both the adult sex ratio (ASR), defined as the ratio of males to females in the fertile ages, and the operational sex ratio (OSR), which counts only the subset of adults currently capable of a conception (19) (see Supporting Information for model parameters and definitions).
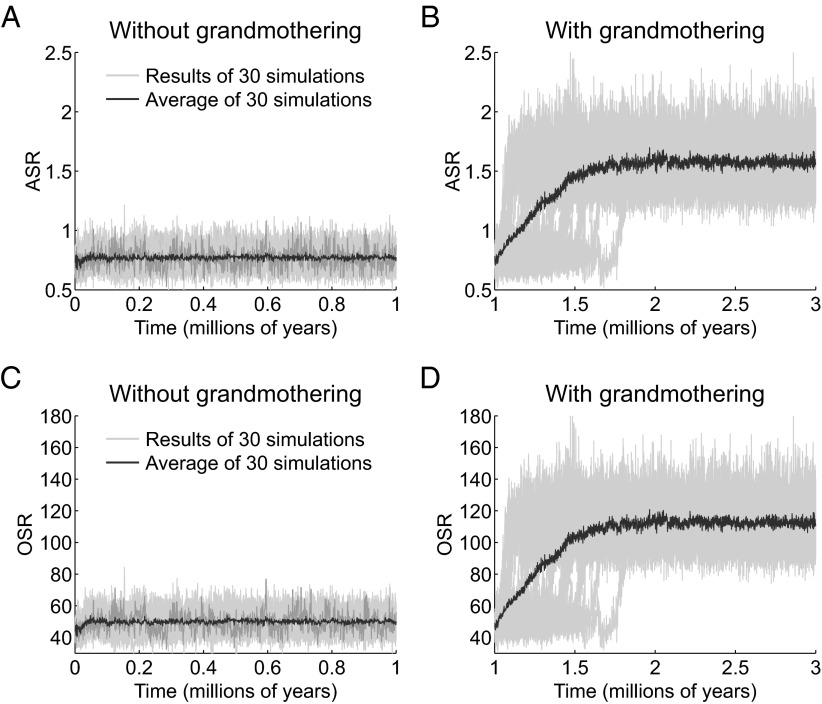
Elsewhere we have shown that simulations of this model result in two life history equilibria: a great ape-like one with no grandmothering and a human hunter–gatherer-like one when grandmothers’ subsidies allow mothers to have next babies sooner (15). Not surprisingly, each equilibrium is associated with distinct, characteristic sex ratios in the fertile ages (Fig. 1). Of 30 simulations without grandmothering run over a million years, the ASR (Fig. 1A) fluctuates around an average of 0.77 males for every female whereas OSR (Fig. 1C) remains at about 50.
Fig. 1.
Time evolutions of ASRs and OSRs with and without grandmothering. (A) ASRs of 30 simulations over 1 million y without grandmothering. Each simulation is shown in light gray. The average of the 30 simulations is shown in black and ends at an ASR of 0.77. The ending point of the simulation shown in medium gray serves as the starting point for the 30 new simulations with grandmothering shown in B. (B) ASRs of 30 simulations over 2 million y with grandmothering. Each simulation is shown in gray. The average of the 30 simulations, in black, ends at an ASR of 1.56. (C) OSRs of 30 simulations over 1 million y without grandmothering. Colors as in A. The average of the 30 simulations ends at an OSR of 50. (D) OSRs of 30 simulations over 2 million y with grandmothering. Colors as in B. The average ends at an OSR of 111.
To evaluate the effect of grandmothering on the mating sex ratios, we use the end point of a simulation chosen at random to start 30 new simulations with grandmothering. All 30 simulations evolve away from the previously stable ape-like mating sex ratios toward values roughly two times higher. ASR (Fig. 1B), initially female-biased, increases with grandmothering to about 1.56 fertile males for every female in the fertile ages, whereas OSR (Fig. 1D) rises to ∼111 available males for every female ready to conceive.
Life Tables
To see whether the simulated values are empirically plausible we compare them to the same sex ratios calculated using life tables for living populations. Our empirical comparison for the simulated ape-like equilibrium is limited to chimpanzees because they are the only apes for which life tables based on multiple, long-studied populations have been published. Bonobo population dynamics are still poorly known. We expect, based on arguments here, ASRs to be higher in mate-guarding gorillas than in chimpanzees. Demography of the Virunga population (20) indicates that, unlike chimpanzees, mortality is not higher in males until the oldest ages. Sexes are not always distinguished in more recent gorilla censuses (21, 22), so we await more data. For orangutans (23), relatively low male mortality is also indicated, but it is difficult to know which males to include among the competitors for mates.
We estimate chimpanzee mating sex ratios using demographic data from the five-study site synthesis of Hill et al. (24) and also include Muller and Wrangham’s (25) more recent (though very small) sample for one of those sites where overall mortality is lowest.
For humans, age structure data come from four well-known hunter–gatherer groups: Howell’s (26, 27) life tables for Dobe !Kung, Hill and Hurtado’s (28) for forest Ache, Hill et al.’s (29) for Hiwi, and Blurton Jones’s (30) for Hadza. We chose these groups because of their reliance on wild foods and the quality of demographic data collected among them. Although contemporary foragers are not living fossils, their mortality regimes make demographic patterns observed among them the best representation of human demography during our evolutionary past.
Table 1 shows that the empirical values computed from chimpanzee sources (ASRs of 0.47 and 0.70; OSRs of 41 and 61) are in general accord with the simulated values without grandmothering. Among the hunter–gatherers (Table 2) ASRs average 1.64, whereas OSRs average 83 (range 69–92), mirroring the equilibrium ASR and OSR values reached in our simulations with grandmothering. Two attributes of these results are especially important. First, every empirical human ASR and OSR is encompassed, or nearly so, in every simulation. Second, in both the real world and simulations with grandmothering the human ratios are distinctly higher than those of the great apes.
Table 1.
Demographic parameters for chimpanzees
| Population | Males age 15+ years | Females age 10–45 years | Birth interval, year | Male paternity, days/year | Females fecundable days per cycle | Cycles to conception | ASR M/F | OSR M/F |
| Synthesis of five (24) | 0.321 | 0.679 | 5.72 | 365 | 6 | 4 | 0.47 | 40.13 |
| Kanyawara (25) | 0.411 | 0.589 | 5.72 | 365 | 6 | 4 | 0.70 | 60.70 |
Assuming stationary populations, the mortality curve mirrors the age structure. To model age structures we used probability of survival to each age in the published life tables, summing the calculated number of survivors for males and females to each of the fertile ages, then dividing the sum for each sex by their combined total to get the fraction fertile adults by sex (columns 2 and 3). We included males older than 15 years and females between ages 10 and 45 years (31). Data on chimpanzee birth intervals come from averaging reports in Knott (32).
In this equation, m and f are the fraction of fertile adults that are male and female, respectively; B is the average birth interval; and 365 is the days per year that males can compete for a paternity. The summation in the denominator is the fecundable days per birth interval for fertile females. It depends on the number of conception risk days per estrous cycle (s) and the number of cycles per conception (n). As in the simulations we use observations from humans (34, 35) and fix s = 6 and n = 4.
Table 2.
Demographic parameters for human hunter–gatherers
| Population | Males age 20–65 years | Females age 20–40 years | Birth interval, year | Male paternity, days/year | Female fecundable days per cycle | Cycles to conception | ASR M/F | OSR M/F |
| Dobe !Kung (26, 27) | 0.593 | 0.407 | 4.17 | 365 | 6 | 4 | 1.46 | 92.40 |
| Ache forest (28) | 0.652 | 0.348 | 2.44 | 365 | 6 | 4 | 1.87 | 69.52 |
| Hiwi (29) | 0.618 | 0.382 | 3.70 | 365 | 6 | 4 | 1.62 | 91.04 |
| Hadza (30) | 0.616 | 0.384 | 3.23 | 365 | 6 | 4 | 1.60 | 78.80 |
Assuming stationary populations, the mortality curve mirrors the age structure. To model age structures we used probability of survival to each age in the published life tables, summing the calculated number of survivors for men and women to each of the fertile ages, then dividing the sum for each sex by their combined total to get the fraction fertile adults by sex (columns 2 and 3). We included men from 20 and 65 years based on reported age ranges of fertilities from the ethnographers and those reported by Tuljapurkar et al. (36). Women from 20 to 40 years are included based on average ages of first and last birth (4). Since populations are younger when increasing, and the growth rates for the human populations range from 0.26% to 2.5%, our figures overestimate the ASRs by 2–12%.
We computed birth intervals by dividing years of female fertility by the total fertility rate reported in the ethnographic sources. OSR was calculated as in Table 1.
Mating Sex Ratios and Mating Strategies
Marlowe and Berbesque (37) pointed to the effects of a strongly male-biased OSR on male competition and marriage resulting from women’s long postfertile life stage. In general, male responses to such a situation should depend on the costs and benefits of available alternatives. Parker (38) was the first to suggest a positive relationship between the relative number of males in a population and the fitness benefits of defending females, or mate guarding. Predictions about the evolution of mate guarding have been elaborated by a number of subsequent treatments (e.g., refs. 39–44). The hypothesized relationship between biased sex ratios and mate guarding has also been supported by observational and experimental studies conducted across a range of species (45–50).
Although mate guarding is not synonymous with pair bonding, similar tradeoffs are involved. Guarding a mate (or potential mate) is beneficial for members of the more numerous sex when it leads to greater reproductive success (RS) than continually seeking out new mates (38). The logic underlying pair bonding is the same: individuals of the more prevalent sex should guard continuously (i.e., form pairs) when doing so leads to greater RS than the alternatives (44).
Parker and Stuart’s (39) model assumed that males acquire females through either guarding a current mate (G) or searching for another one (S). Clutton-Brock and Parker (51) retained this S vs. G notation, but they posed the alternatives differently (ref. 51, p. 447), defining “time in” the mating pool (S) to include both searching and guarding, with guarding also part of “time out” (G).
Of special relevance here, Kokko and Jennions (17) used Clutton-Brock and Parker’s time in vs. time out framework to build new models for the evolution of mating behavior. Revisiting critiques of Trivers’ (1) influential arguments about the role of parental investment (52–54), they constructed a model to show “from first principles” why anisogamy alone cannot explain sex-role divergence. Then they faced the “more fundamental question: what factors create the asymmetry that biases females towards caring for offspring and males towards competing for mates?” (ref. 17, p. 920). Their answer followed Queller’s (53) earlier analysis that identified multiple mating by females, which lowers average male parentage and so favors less parental care from males, and sexual selection, which makes expected mating success higher than average for some males.
The particular importance of ASR (54–57) was further emphasized by Kokko and Jennions (17). Their model did not include a mate-guarding option but it has influenced subsequent discussion of the effects of human mating sex ratios on male strategies. In their model individuals can enter the mating pool or stay out to gain benefits in offspring survival from continued parental care. Because the sex that is more numerous must have a lower average mating rate, that sex does better to stay out longer. As they do, bias in OSR declines toward parity and sex roles diverge less. How even the OSR can get depends on the ASR because of the Fisher condition (55–58); because everyone has one mother and one father, the number of offspring produced by one sex must be the same as the number produced by the other. This model has been read by some (59) as undermining OSR as a predictor of mating patterns, warranting focus on ASR instead.
If the tradeoffs for hominid males were the ones assumed in Kokko and Jennions’ (17) basic model, the evolution of increasingly male-biased OSRs would be unstable. Where OSR is male-biased, males take longer to find another mate and so do better to choose an alternative to searching for them. While emphasizing this important effect, the only alternative considered in their basic model is to supply parental care, with each additional unit of care (from either sex) earning equivalent marginal gains in offspring survival. Given those assumptions, male bias in the mating pool must decline as males stay out to parent longer.
However, strongly male-biased OSRs persist across the entire class of mammals where male parental care is rare. In our simulations and reference populations the OSRs are male-biased throughout, as is typical of mammals. Even though birth intervals are shorter in humans, the addition of older males makes the bias twice as strong in humans as in chimpanzees. However, the nonhuman ASR is female-biased (the common mammal pattern), while—again because of so many old men—the human ASR is male-biased—a pattern not typical of mammals but of birds. Kokko and Jennions (17) refer to this broad taxonomic difference in ASRs and relate it to the sex roles generally recognized as typical of these two classes of vertebrates. Do the “bird-like” human ASRs indicate useful parallels with class Aves (19, 60–62)?
Key Constraints
In birds either males or females can brood and guard eggs, as well as guard and feed nestlings. OSR depends on how much each sex is occupied with care. High frequency of biparental care (60) implies that sex-role convergence is common. Especially striking evidence of this near sexual equivalence comes from penduline tits (Remiz pendulinus), where either the male or the female always deserts to mate again and nearly a third of nests fail from desertion by both parents (63). Liker et al. (64) found that in shore birds (where sex-role reversal is common), the variation in sex roles correlates with ASRs as predicted (17, 56) and proposed (ref. 64, p. 3) “that the evolutionary flexibility of both sexes to provide full care on their own and variation in ASR among species are among the key factors that facilitate the evolution of diverse sex roles.” Extending the analysis, they found ASRs predict how much males care among a wider range of wild bird populations (65).
Even in birds, however, the sexes cannot make equivalent contributions to offspring production (66). The difference is much larger in mammals. That difference, as Kokko and Jennions (ref. 17, p. 940) note, “might be the result of simple constraint (e.g. that the evolution of lactation is difficult for male mammals).” Once internal gestation and lactation have evolved, care is much less interchangeable between the sexes. Fundamental mammalian constraints apply to the primate radiation and are directly relevant to expectations we develop about male-biased OSRs and ASRs in our lineage.
Schacht and colleagues (ref. 59, p. 215) downplay this issue to argue that emphasis on OSR as a predictor of mating strategies has been misleading because, “contrary to the intuitions drawn from Emlen and Oring 1977, a male-biased OSR only accurately predicts intense sexual selection among males under a limited set of circumstances….” For this they cite Kokko et al. (67), who note that although Emlen and Oring’s (19) emphasis on OSR as an index of mating behavior seems borne out empirically, the ties between OSR and sexual selection depend on “conditions that make selection favour traits that reduce the time it takes to acquire another mating” (ref. 67, p. 1341). When time out is a necessarily large fraction of a fertile lifespan, higher mating rates make little difference to lifetime RS (ref. 67, p. 1349). This is precisely the case for female mammals and especially for female primates, who are committed by gestation and long lactation to extended times out.
In contrast, mating rates do make a difference to lifetime RS for mammalian males. When too many males lower the expected success rate for seeking a new mate, doing something else may be a better option. Harts and Kokko (44) investigated mate guarding as the alternative and showed “in line with earlier work more male competitors select for more guarding” (ref. 44, p. 2842). Among primates with multilevel social systems (68–70), including humans (71), a male’s RS usually depends on his success at claiming and retaining mates, which depends on other males deferring to his claims.
Measuring Human Sex Ratios: ASRs or Elder Advantage?
In our simulations mating sex ratios become increasingly male-biased with a growing fraction of elders as a human-like life history evolves from a great ape-like one. This reflects the approximate doubling of longevity while female fertility maintains its ancestral decline to near zero by 45. Although mortality is generally higher in men than in women (2, 3), the increased number of older men who are not yet frail makes human ASRs inevitably male-biased.
How, then, can some explorations of human mating sex ratios report so many to be female-biased? Using variance in RS reported by Brown et al. (72), Kokko and Jennions (ref. 73, pp. 113–114) used the Fisher condition and the RSs reported to infer ASRs for 18 human populations, estimating them to be about even for the societies classified as monogamous or serially monogamous and significantly female-biased for the groups classified as polygynous. For nine cases Brown et al. (72) classified as polygynous, the mean RS for males averages 31% higher than the female mean—possibly due, as Kokko and Jennions (73) conclude, to female-biased ASRs. But Brown et al. (ref. 72, p. 300) note another possibility: missing men who were less successful in the mating competition.
An earlier study by Ember (74) also found an association between polygyny and female-biased sex ratios using data from the Human Relations Area Files. While high enough male mortality could make ASRs female-biased, Ember’s sex ratios were not ASRs but sex ratios of whole populations—immatures and postfertile women included. Schacht et al. (ref. 59, p. 218) note this difficulty with “which sex ratio.” They review social science research into sex-ratio effects listing 20 studies in their first table; none of them used fertile ages.
Figure 2 in Schacht et al. (ref. 59, p. 217) is the most serious challenge to our characterization of human mating sex ratios as male-biased. It plots results from the authors’ queries to ethnographers about 15 traditional populations. Here, they say, “Sex ratio is determined from the ethnographers’ data on the number of individuals of mating age in their population, hence it approximates ASR.” Eight of these societies—more than half—have female-biased ASRs, a pattern that our model and arguments suggest is “not human.”
At least three factors could lead ethnographers to underestimate sex ratios in the fertile ages. First, physiologically fertile young men may be excluded because local conventions class them as not yet of mating age. Székely et al. (ref. 75, p. 1501), reviewing broader cross-species issues of ASR and OSR associations with breeding systems, define an adult as “an animal (male or female) that is physiologically capable of producing offspring.”
Second, counting unmarried men is difficult if they move more often than others, a common pattern among hunter–gatherers. Such high mobility gave Hawkes et al. (ref. 76, p. 683) an insufficient sample of unmarried Hadza men to compare their time allocation patterns with those of married men. Székely et al. (ref. 75, p. 1501) note similar ascertainment biases with other animals, because “nonbreeding adults (e.g. floaters) often…remain unnoticed.”
A third, closely related possibility is that ethnographers chose not to include unmarried men. For example, polygynous Kipsigis have a notably low ASR. Reporting that Kipsigis men have much higher average RS compared with women (which, given the Fisher condition, would require extremely high male mortality), Borgerhoff Mulder (ref. 77, p. 433) said that her tabulation excluded “poor men, who leave the community to become plantation laborers and marry late in life, if at all.”
Marlowe and Berbesque (37) showed the advantage that elder men can have in ethnographically known hunter–gatherers by plotting the relationship between age of first marriage and the extent of polygyny across 130 hunter–gatherer societies. Their figure 1 (ref. 37, p. 836) shows that men’s age at first marriage rises from an average near 20 y to more than 30 y as the fraction of polygynously married men rises from few to more than 50%.
We tentatively conclude that apparent female bias in some human ASRs may actually index the shape of the male status hierarchy. Exclusion of some men, young and old, from paternities occurs not because they are infertile but because they are outcompeted by other men. As Darwin said in developing his theory of sexual selection, it favors features that “serve only to give one male an advantage over another male, for the less well endowed males, if time were allowed them, would succeed in pairing with the females…” (ref. 78, p. 257).
Unmarried men are not only a part of the ASR, their relationships with married men have long been reported by cultural anthropologists to shape much of community life (71, 79). Estimates of fertile ages for men confront entirely different measurement challenges than do estimates for women. As Vinicius and colleagues (ref. 80, pp. 4–5) concluded, noting variation in paternities attributed to older men across a sample of traditional societies, “Since there is no evidence of widespread male mid- or late-life sterility, variation in late-life reproduction in men must therefore reflect differences in opportunities to reproduce at old age.” This and the likelihood of undercounting men less successful in the competition provide grounds for skepticism about the reported female biases. Age structures themselves support, if indirectly, our characterization of human mating sex ratios as characteristically male-biased.
Conclusion
Our hypothesis is that human pair bonds evolved with increasing payoffs for mate guarding, which resulted from the evolution of our grandmothering life history. This mate-guarding hypothesis is an alternative to long-favored arguments that pairing evolved in our lineage as a consequence of the benefits of cooperative parenting (e.g., refs. 81–86). We are far from the first to connect human pair bonds with mate guarding. Wilson and Daly (87) assembled a wide range of cross-cultural evidence and argument nearly 25 y ago. Nor is the proposal that human pair bonds more likely began with mate guarding than with paternal care a novel suggestion (e.g., refs. 88–90). As Chapais (90) notes, the group living patterns of humans differ from the territorial monogamy of other pair-living mammals, but where parental cooperation did evolve in mammals or more narrowly primates, it followed the prior establishment of pair bonds (91–93).
We have focused on changes in the mating sex ratio that accompany the evolution of our grandmothering life history and raise the net benefits to males for mate guarding and pair bonding—patterns that distinguish humans from our closest living relatives. This emphasizes an aspect of human pair bonds ignored by models that assume the only reproductive options are parental care or competition for another mate. An early attempt to distinguish mate guarding as an allocation of reproductive effort that trades off with other kinds of mating competition—as well as with offspring care—found unexpectedly wide conditions under which guarding displaced the other options even without varying mating sex ratios (41). Although all observed human societies feature pair bonds, their character and stability is notably variable. Across our hunter–gatherer sample pair-bond stability is greater where mating sex ratios are more male-biased (94). Guttentag and Secord (95) showed the same correlation over time and across socioeconomic classes in state societies. Ethnographic explorations of the tension between a man’s conjugal bonds and his alliances with other men have a rich history in anthropology (71, 96–98). The argument here begins to link that tension to the evolution of our grandmothering life history.
The Agent-Based Model of Life History Evolution
Our simulated data come from a two-sex, stochastic, agent-based model. To determine the effect of grandmothering on OSR and ASR, we ran 30 simulations for 1 million y without grandmothering, then, from that equilibrium, 30 more 2-million-y simulations with the addition of grandmothering. Because details of the model are reported in-depth elsewhere (13, 15), we provide a brief summary here (see Table S1 for model parameters). Expected adult lifespan, or L, is the only heritable trait and offspring inherit the geometric mean of their parents’ longevities, with a 2% probability of a mutation. Mutations result in a proportional shift governed by a lognormal distribution with mean 0 and an SD of 2% (i.e., a 2% chance of a shift in longevity based on a lognormal distribution with SD 2%). As shown in Table S1, we assume that many aspects of life history scale with L, whereas other components remain fixed. Our justifications for these assumptions come from theory (99, 100) and empirical evidence [e.g., infant survival depends on mothers in both chimpanzees and humans until age 2 y (101, 102) and oldest parturitions are in the forties for all living hominids (4, 5)]. For further justification of model assumptions, see Kim et al. (13, 15).
Table S1.
Parameters of the agent-based model
| Parameter | Description | Estimate |
| Average expected adult life span, y | Variable | |
| Age of weaning, y | 2 | |
| Age of independence, y | ||
| Age of female sexual maturity, y | ||
| Age female fertility ends, y | 45 | |
| Age of female frailty (i.e., ineligibility to adopt), y | ||
| Age male eligibility starts, y | 15 | |
| Age male eligibility ends, y | ||
| Rate of female conception and delivery, 1/y | 1 | |
| Male weighting factor for mating | Decreasing function of | |
| Probability of mutation in at birth, % | 2 | |
| SD of mutations, % | 2 | |
| Population carrying capacity | 1,000 |
Agents progress through a number of sex-specific life stages, starting with conception and birth. After birth, individuals are dependent on their mothers for the first 2 y of life; if their mother dies, so do they. They are still dependent at age 2 y and, in simulations without grandmothering, they remain with their mothers until independence. In simulations with grandmothering, they become eligible for adoption. We assume that grandmothers are just as good as mothers at keeping dependents alive. If a grandmother adopts a weaned dependent, its mother conceives and delivers her next offspring within a year. Mothers or grandmothers care for juveniles until the age of independence.
Females then go through a period of immature independence until fertility begins at and lasts until age 45. In simulations with grandmothering, females older than 45 y may then adopt weaned dependents. They are eligible to adopt until they reach frailty at age 75 y or 2L, whichever comes first. We include a frail period to guard against improbably old grandmothers—a consequence of constant mortality (1/L).
Males also live through a period of immature independence before first reproduction. Unlike females, males become eligible to compete for paternities once they mature at age 15 y. They remain eligible until , at which point they become frail and no longer eligible, a limit on improbably old fathers. In contrast to constant female fertility, males compete for paternities with variable competitive competence, , that decreases with expected adult life span. The likelihood of winning a paternity depends upon a male’s own competence and those of the other males in the population.
Our model specifications make adult sex ratios simple to compute (fertile males, m, are those between and ; fertile females, f, are between and ):
| [S1] |
The OSR is a subset of fertile adults: only those currently capable of a conception. To calculate it, we rely on the formula for nonseasonal breeders derived by Mitani et al. (33), where
| [S2] |
In Eq. S2, m and f are the numbers of fertile males and females, respectively; B is the average birth interval; and 365 is the days per year that males can compete for a paternity. The summation in the denominator is the fecundable days per birth interval for fertile females. It depends on the number of conception risk days per estrous cycle (s) and the number of cycles per conception (n).
As noted by Marlowe and Berbesque (37), estimates of OSR can vary widely depending on which individuals are counted as adults. For our simulations, that part of the equation is simply the ASR, Eq. S1: m = males between and , f = females between and . The model also computes the mean birth interval in the population. For female risk days per cycle (s), our assumptions differ from those of Emlen and Oring (19) and the formulations of both Mitani et al. (33) and Marlowe and Berbesque (37) by quantifying fecundability, not receptivity, because our concern is actual conceptions. For number of days a female can conceive during each cycle we use observations from humans (34) and fix s = 6. The number of cycles to conception, n, varies widely in great apes (32), so we use human data (35) to set n = 4.
Mutation Rates and the Time Course of Life History Shifts
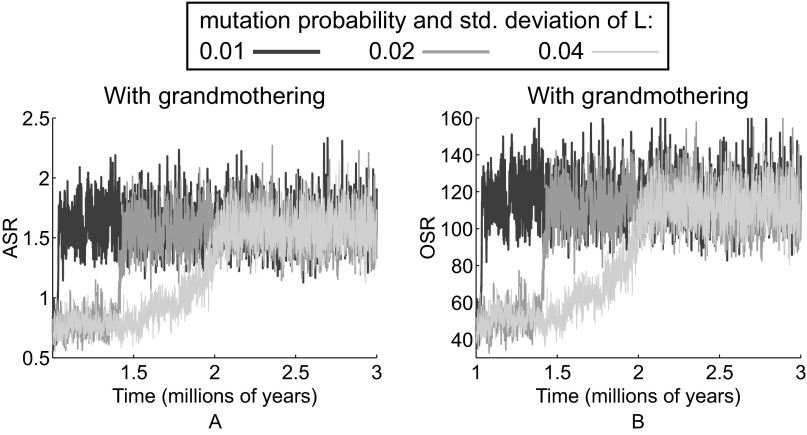
Because the life history parameter values are set by real-world data, the time scale in Fig. 1 in the main text is real years. However, the mutation rate strongly affects the time it takes these simulations to escape the basin of attraction surrounding ape-like longevities and sex ratios. The mutation rate also determines how long it takes to move to the human-like equilibrium. In Fig. 1 the mutation rate is 2%. In Fig. S1 we show example trajectories for the evolution of the ASR and the OSR with the mutation probability and SD of L set to 1, 2, and 4%, respectively.
Fig. S1.
Evolution time depends on the mutation parameter. Example time evolutions of (A) ASRs and (B) OSRs with grandmothering for cases when the mutation probability and SD of L are set to 1% (light gray), 2% (medium gray, used in the text and Fig. 1), and 4% (black).
Trajectories can, however, vary widely from simulation to simulation. Although not shown, we ran 30 simulations for each of the three cases and measured the first time in each that L reached 25 y and then 35 y, representing, respectively, our thresholds for escape from the previous, chimpanzee-like equilibrium and arrival at the new, human-like longevity.
Of the 30 simulations corresponding to a mutation parameter of 1%, only 8 reached the human-like threshold within the 2 million y of simulation. Of these eight, the mean ± SD transition time from the first to the second threshold was 372,000 ± 91,700 y. When the mutation parameter was set at 2%, all 30 simulations reached the human-like equilibrium within 2 million y, crossing the first threshold in 187,000 ± 147,000 y and rising from there to the human-like threshold in 134,000 ± 147,000 y. With a mutation parameter of 4% all 30 simulations crossed the first threshold in only 23,500 ± 11,500 y and reached the second after 9,770 ± 4,770 y more.
Acknowledgments
We thank Nick Blurton Jones, Sarah Hrdy, Jim O’Connell, Ryan Schacht, Michael Weight, Monique Borgerhoff Mulder, Martin Daly, and Bobbi Low for useful comments. P.S.K. was funded by Australian Research Council Discovery Early Career Research Award DE120101113.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1599993112/-/DCSupplemental.
References
- 1.Trivers R. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man 1871–1971. Aldine; Chicago: 1972. pp. 139–179. [Google Scholar]
- 2.Kruger DJ, Nesse RM. An evolutionary life-history framework for understanding sex differences in human mortality rates. Hum Nat. 2006;17(1):74–97. doi: 10.1007/s12110-006-1021-z. [DOI] [PubMed] [Google Scholar]
- 3.Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: Good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. 2008;20(2):91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robson SL, van Schaik CP, Hawkes K. The derived features of human life history. In: Hawkes K, Paine R, editors. The Evolution of Human Life History. SAR; Santa Fe, NM: 2006. pp. 17–44. [Google Scholar]
- 5.Robbins AM, Robbins MM, Gerald-Steklis N, Steklis HD. Age-related patterns of reproductive success among female mountain gorillas. Am J Phys Anthropol. 2006;131(4):511–521. doi: 10.1002/ajpa.20474. [DOI] [PubMed] [Google Scholar]
- 6.Bribiescas RG, Ellison PT, Gray PB. Male life history, reproductive effort, and the evolution of the genus Homo: New directions and perspectives. Curr Anthropol. 2012;53(S6):S424–S435. [Google Scholar]
- 7.Levitis DA, Burger O, Lackey LB. The human post-fertile lifespan in comparative evolutionary context. Evol Anthropol. 2013;22(2):66–79. doi: 10.1002/evan.21332. [DOI] [PubMed] [Google Scholar]
- 8.Alberts SC, et al. Reproductive aging patterns in primates reveal that humans are distinct. Proc Natl Acad Sci USA. 2013;110(33):13440–13445. doi: 10.1073/pnas.1311857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croft DP, Brent LJN, Franks DW, Cant MA. The evolution of prolonged life after reproduction. Trends Ecol Evol. 2015;30(7):407–416. doi: 10.1016/j.tree.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Hawkes K, O’Connell JF, Blurton Jones NG, Alvarez H, Charnov EL. Grandmothering, menopause, and the evolution of human life histories. Proc Natl Acad Sci USA. 1998;95(3):1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connell JF, Hawkes K, Blurton Jones NG. Grandmothering and the evolution of Homo erectus. J Hum Evol. 1999;36(5):461–485. doi: 10.1006/jhev.1998.0285. [DOI] [PubMed] [Google Scholar]
- 12.Hawkes K. Grandmothers and the evolution of human longevity. Am J Hum Biol. 2003;15(3):380–400. doi: 10.1002/ajhb.10156. [DOI] [PubMed] [Google Scholar]
- 13.Kim PS, Coxworth JE, Hawkes K. Increased longevity evolves from grandmothering. Proc Biol Sci. 2012;279(1749):4880–4884. doi: 10.1098/rspb.2012.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkes K. Primate sociality to human cooperation. Why us and not them? Hum Nat. 2014;25(1):28–48. doi: 10.1007/s12110-013-9184-x. [DOI] [PubMed] [Google Scholar]
- 15.Kim PS, McQueen JS, Coxworth JE, Hawkes K. Grandmothering drives the evolution of longevity in a probabilistic model. J Theor Biol. 2014;353:84–94. doi: 10.1016/j.jtbi.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Hawkes K, Coxworth JE. Grandmothers and the evolution of human longevity: A review of findings and future directions. Evol Anthropol. 2013;22(6):294–302. doi: 10.1002/evan.21382. [DOI] [PubMed] [Google Scholar]
- 17.Kokko H, Jennions MD. Parental investment, sexual selection and sex ratios. J Evol Biol. 2008;21(4):919–948. doi: 10.1111/j.1420-9101.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 18.Parker GA, Baker RR, Smith VGF. The origin and evolution of gamete dimorphism and the male-female phenomenon. J Theor Biol. 1972;36(3):529–553. doi: 10.1016/0022-5193(72)90007-0. [DOI] [PubMed] [Google Scholar]
- 19.Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197(4300):215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- 20.Gerald CN. 1995. Demography of the Virunga mountain gorilla (Gorilla gorilla beringei). Master’s thesis (Princeton Univ, Princeton)
- 21.Robbins MM, Robbins AM. Simulation of the population dynamics and social structure of the Virunga mountain gorillas. Am J Primatol. 2004;63(4):201–223. doi: 10.1002/ajp.20052. [DOI] [PubMed] [Google Scholar]
- 22.McNeilage A, et al. Census of the mountain gorilla Gorilla beringei beringei population in Bwindi Impenetrable National Park, Uganda. Oryx. 2006;40(4):419–427. [Google Scholar]
- 23.Wich SA, et al. Life history of wild Sumatran orangutans (Pongo abelii) J Hum Evol. 2004;47(6):385–398. doi: 10.1016/j.jhevol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Hill K, et al. Mortality rates among wild chimpanzees. J Hum Evol. 2001;40(5):437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- 25.Muller MN, Wrangham RW. Mortality rates among Kanyawara chimpanzees. J Hum Evol. 2014;66:107–114. doi: 10.1016/j.jhevol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Howell N. Demography of the Dobe !Kung. Academic; New York: 1979. [Google Scholar]
- 27.Coale AJ, Demeny P. Regional Model Life Tables and Stable Populations. Princeton Univ Press; Princeton: 1966. [Google Scholar]
- 28.Hill K, Hurtado AM. Ache Life History: The Ecology and Demography of a Foraging People. Aldine de Gruyter; New York: 1996. [Google Scholar]
- 29.Hill K, Hurtado AM, Walker RS. High adult mortality among Hiwi hunter-gatherers: Implications for human evolution. J Hum Evol. 2007;52(4):443–454. doi: 10.1016/j.jhevol.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Blurton Jones NG. Demography and Evolutionary Ecology of Hadza Hunter-Gatherers. Cambridge Univ Press; Cambridge, MA: 2015. [Google Scholar]
- 31.Emery Thompson M, et al. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol. 2007;17(24):2150–2156. doi: 10.1016/j.cub.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knott C. Female reproductive ecology of the apes: Implications for human evolution. In: Ellison PT, editor. Reproductive Ecology and Human Evolution. Aldine de Gruyter; New York: 2001. pp. 429–463. [Google Scholar]
- 33.Mitani JC, Gros-Louis J, Richards AF. Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. Am Nat. 1996;147:966–980. [Google Scholar]
- 34.Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333(23):1517–1521. doi: 10.1056/NEJM199512073332301. [DOI] [PubMed] [Google Scholar]
- 35.Gnoth C, Godehardt D, Godehardt E, Frank-Herrmann P, Freundl G. Time to pregnancy: Results of the German prospective study and impact on the management of infertility. Hum Reprod. 2003;18(9):1959–1966. doi: 10.1093/humrep/deg366. [DOI] [PubMed] [Google Scholar]
- 36.Tuljapurkar SD, Puleston CO, Gurven MD. Why men matter: Mating patterns drive evolution of human lifespan. PLoS One. 2007;2(8):e785. doi: 10.1371/journal.pone.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marlowe FW, Berbesque JC. The human operational sex ratio: Effects of marriage, concealed ovulation, and menopause on mate competition. J Hum Evol. 2012;63(6):834–842. doi: 10.1016/j.jhevol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Parker GA. Courtship persistence and female-guarding as male time investment strategies. Behaviour. 1974;48(1-2):157–184. [Google Scholar]
- 39.Parker GA, Stuart RA. Animal behavior as a strategy optimizer: Evolution of resource assessment strategies and optimal emigration thresholds. Am Nat. 1976;110(976):1055–1076. [Google Scholar]
- 40.Grafen A, Ridley M. A model of mate guarding. J Theor Biol. 1983;102(4):549–567. [Google Scholar]
- 41.Hawkes K, Rogers AR, Charnov EL. The male’s dilemma: Increased offspring production is more paternity to steal. Evol Ecol. 1995;9:662–677. [Google Scholar]
- 42.Yamamura N, Tsuji N. Postcopulatory guarding strategy in a finite mating period. Theor Popul Biol. 1989;35:36–50. [Google Scholar]
- 43.Fromhage L, Elgar MA, Schneider JM. Faithful without care: The evolution of monogyny. Evolution. 2005;59(7):1400–1405. [PubMed] [Google Scholar]
- 44.Harts AMF, Kokko H. Understanding promiscuity: When is seeking additional mates better than guarding an already found one? Evolution. 2013;67(10):2838–2848. doi: 10.1111/evo.12163. [DOI] [PubMed] [Google Scholar]
- 45.Møller AP. Extent and duration of mate guarding in swallows Hirundo rustica. Ornis Scand. 1987;18(2):95–100. [Google Scholar]
- 46.Rondeau A, Sainte-Marie B. Variable mate-guarding time and sperm allocation by male snow crabs (Chionoecetes opilio) in response to sexual competition, and their impact on the mating success of females. Biol Bull. 2001;201(2):204–217. doi: 10.2307/1543335. [DOI] [PubMed] [Google Scholar]
- 47.Sherman PW. Mate guarding as paternity insurance in Idaho ground squirrels. Nature. 1989;338(6214):418–420. doi: 10.1038/338418a0. [DOI] [PubMed] [Google Scholar]
- 48.Alcock J. Postinsemination associations between males and females in insects: the mate-guarding hypothesis. Annu Rev Entomol. 1994;39:1–21. [Google Scholar]
- 49.Jormalainen V. Precopulatory mate guarding in crustaceans: Male competitive strategy and intersexual conflict. Q Rev Biol. 1998;73(3):275–304. [Google Scholar]
- 50.Weir LK, Grant JWA, Hutchings JA. The influence of operational sex ratio on the intensity of competition for mates. Am Nat. 2011;177(2):167–176. doi: 10.1086/657918. [DOI] [PubMed] [Google Scholar]
- 51.Clutton-Brock TH, Parker GA. Potential reproductive rates and the operation of sexual selection. Q Rev Biol. 1992;67(4):437–456. [Google Scholar]
- 52.Dawkins R, Carlisle TR. Parental investment, mate desertion and a fallacy. Nature. 1976;262:131–133. [Google Scholar]
- 53.Queller DC. Why do females care more than males? Proc Biol Sci. 1997;264:1555–1557. [Google Scholar]
- 54.Wade MJ, Shuster SM. The evolution of parental care in the context of sexual selection: A critical reassessment of parental investment theory. Am Nat. 2002;160(3):285–292. doi: 10.1086/341520. [DOI] [PubMed] [Google Scholar]
- 55.Houston AI, McNamara JM. A self-consistent approach to paternity and parental effort. Philos Trans R Soc Lond B Biol Sci. 2002;357(1419):351–362. doi: 10.1098/rstb.2001.0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mcnamara JM, Székely T, Webb JN, Houston AI. A dynamic game-theoretic model of parental care. J Theor Biol. 2000;205(4):605–623. doi: 10.1006/jtbi.2000.2093. [DOI] [PubMed] [Google Scholar]
- 57.Székely T, Webb JN, Cuthill IC. Mating patterns, sexual selection and parental care: An integrative approach. In: Apollonio M, Festa-Bianchet M, Mainardi D, editors. Vertebrate Mating Systems. World Science; London: 2000. pp. 194–223. [Google Scholar]
- 58.Fisher RA. The Genetical Theory of Natural Selection. Oxford Univ Press; Oxford: 1930. [Google Scholar]
- 59.Schacht R, Rauch KL, Borgerhoff Mulder M. Too many men: The violence problem? Trends Ecol Evol. 2014;29(4):214–222. doi: 10.1016/j.tree.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Lack D. Ecological Adaptations for Breeding in Birds. Methuen; London: 1968. [Google Scholar]
- 61.Birkhead T. Sperm competition in birds. Trends Ecol Evol. 1987;2(9):268–272. doi: 10.1016/0169-5347(87)90033-4. [DOI] [PubMed] [Google Scholar]
- 62.Westneat DF, Stewart IRK. Extra-pair paternity in birds: Causes, correlates, and conflict. Annu Rev Ecol Evol Syst. 2003;34:365–396. [Google Scholar]
- 63.Szentirmai I, Székely T, Komdeur J. Sexual conflict over care: Antagonistic effects of clutch desertion on reproductive success of male and female penduline tits. J Evol Biol. 2007;20(5):1739–1744. doi: 10.1111/j.1420-9101.2007.01392.x. [DOI] [PubMed] [Google Scholar]
- 64.Liker A, Freckleton RP, Székely T. The evolution of sex roles in birds is related to adult sex ratio. Nat Commun. 2013;4:1587. doi: 10.1038/ncomms2600. [DOI] [PubMed] [Google Scholar]
- 65.Liker A, Freckleton RP, Székely T. Divorce and infidelity are associated with skewed adult sex ratios in birds. Curr Biol. 2014;24(8):880–884. doi: 10.1016/j.cub.2014.02.059. [DOI] [PubMed] [Google Scholar]
- 66.Sibly RM, et al. Energetics, lifestyle, and reproduction in birds. Proc Natl Acad Sci USA. 2012;109(27):10937–10941. doi: 10.1073/pnas.1206512109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kokko H, Klug H, Jennions MD. Unifying cornerstones of sexual selection: operational sex ratio, Bateman gradient and the scope for competitive investment. Ecol Lett. 2012;15(11):1340–1351. doi: 10.1111/j.1461-0248.2012.01859.x. [DOI] [PubMed] [Google Scholar]
- 68.Kummer H. Social Organization of Hamadryas Baboons: A Field Study. Univ of Chicago Press; Chicago: 1968. [Google Scholar]
- 69.Swedell L, Leedom L, Saunders J, Pines M. Sexual conflict in a polygynous primate: Costs and benefits of a male-imposed mating system. Behav Ecol Sociobiol. 2013;68:263–273. [Google Scholar]
- 70.Grueter CC, Chapais B, Zinner D. Evolution of multilevel social systems in nonhuman primates and humans. Int J Primatol. 2012;33(5):1002–1037. doi: 10.1007/s10764-012-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodseth L. From bachelor threat to fraternal security: Male associations and modular organization in human societies. Int J Primatol. 2013;33:1194–1214. [Google Scholar]
- 72.Brown GR, Laland KN, Borgerhoff Mulder M. Bateman’s principles and human sex roles. Trends Ecol Evol. 2009;24(6):297–304. doi: 10.1016/j.tree.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kokko H, Jennions MD. Sex differences in parental care. In: Royle NJ, Smiseth PT, Kolliker M, editors. The Evolution of Parental Care. Oxford Univ Press; Oxford: 2012. pp. 110–116. [Google Scholar]
- 74.Ember M. Warfare, sex ratio, and polygyny. Ethnology. 1974;13(2):197–206. [Google Scholar]
- 75.Székely T, Weissing FJ, Komdeur J. Adult sex ratio variation: Implications for breeding system evolution. J Evol Biol. 2014;27(8):1500–1512. doi: 10.1111/jeb.12415. [DOI] [PubMed] [Google Scholar]
- 76.Hawkes K, O’Connell JF, Blurton Jones NG. Hadza women’s time allocation, offspring provisioning and the evolution of post-menopausal lifespans. Curr Anthropol. 1997;38(4):551–577. [Google Scholar]
- 77.Borgerhoff Mulder M. Reproductive success in three Kipsigis cohorts. In: Clutton-Brock TH, editor. Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems. Univ of Chicago Press; Chicago: 1988. pp. 419–435. [Google Scholar]
- 78.Darwin C. 1871. The Descent of Man, and Selection in Relation to Sex (Murray, London); reprinted (1981) (Princeton Univ Press, Princeton)
- 79.Collier JF, Rosaldo MZ. Politics and gender in simple societies. In: Ortner SB, Whitehead H, editors. Sexual Meanings: The Cultural Construction of Gender and Sexuality. Cambridge Univ Press; Cambridge, UK: 1981. pp. 275–329. [Google Scholar]
- 80.Vinicius L, Mace R, Migliano A. Variation in male reproductive longevity across traditional societies. PLoS One. 2014;9(11):e112236. doi: 10.1371/journal.pone.0112236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Washburn SL, Lancaster CS. The evolution of hunting. In: Lee RB, DeVore I, editors. Man the Hunter. Aldine; New York: 1968. pp. 293–303. [Google Scholar]
- 82.Isaac G. The food-sharing behavior of protohuman hominids. Sci Am. 1978;238(4):90–108. doi: 10.1038/scientificamerican0478-90. [DOI] [PubMed] [Google Scholar]
- 83.Lovejoy CO. The origin of man. Science. 1981;211(4480):341–350. doi: 10.1126/science.211.4480.341. [DOI] [PubMed] [Google Scholar]
- 84.Fisher H. The Anatomy of Love. Norton; New York: 1992. [Google Scholar]
- 85.Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: Diet, intelligence, and longevity. Evol Anthropol. 2000;9:156–185. [Google Scholar]
- 86.Kaplan H, Gurven M, Winking J, Hooper PL, Stieglitz J. Learning, menopause, and the human adaptive complex. Ann N Y Acad Sci. 2010;1204:30–42. doi: 10.1111/j.1749-6632.2010.05528.x. [DOI] [PubMed] [Google Scholar]
- 87.Wilson M, Daly M. The man who mistook his wife for a chattle. In: Barkow JH, Cosmides L, Tooby J, editors. The Adapted Mind: Evolutionary Psychology and the Generation of Culture. Oxford Univ Press; New York: 1992. pp. 289–322. [Google Scholar]
- 88.Hawkes K. 2004. Mating, parenting and the evolution of human pair bonds. Kinship and Behavior in Primates, eds Chapais B, Berman C (Oxford Univ Press, Oxford), pp 443–473.
- 89.Chapais B. Primeval Kinship: How Pairbonding Gave Birth to Human Society. Harvard Univ Press; Cambridge, MA: 2008. [Google Scholar]
- 90.Chapais B. The deep structure of human society: Primate origins and evolution. In: Kappeler PM, Silk JB, editors. Mind the Gap: Tracing the Origins of Human Universals. Springer; Berlin: 2010. pp. 19–51. [Google Scholar]
- 91.Komers PE, Brotherton PN. Female space use is the best predictor of monogamy in mammals. Proc Biol Sci. 1997;264(1386):1261–1270. doi: 10.1098/rspb.1997.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Shaik CP, Kappeler PM. The evolution of social monogamy in mammals. In: Reichard UH, Boesch C, editors. Monogamy: Mating Strategies and Partnerships in Birds, Humans, and Other Mammals. Cambridge Univ Press; Cambridge, UK: 2003. pp. 59–80. [Google Scholar]
- 93.Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341(6145):526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- 94.Blurton Jones NG, Marlowe FW, Hawkes K, O’Connell JF. Paternal investment and hunter-gatherer divorce rates. In: Cronk L, Chagnon N, Irons W, editors. Adaptation and Human Behavior: An Anthropological Perspective. Aldine de Gruyter; New York: 2000. pp. 31–55. [Google Scholar]
- 95.Guttentag M, Secord PF. Too Many Women? The Sex Ratio Question. Sage; Beverly Hills, CA: 1983. [Google Scholar]
- 96.Langness LL. Sexual antagonism in the New Guinea Highlands: A Bena Bena example. Oceania. 1967;37(3):161–177. [Google Scholar]
- 97.Whiting JWM, Whiting BB. Aloofness and intimacy of husbands and wives: A cross-cultural study. Ethos. 1975;3(2):183–207. [Google Scholar]
- 98.Smuts BB. Male aggression against women: An evolutionary perspective. Hum Nat. 1992;3(1):1–44. doi: 10.1007/BF02692265. [DOI] [PubMed] [Google Scholar]
- 99.Charnov EL. Evolution of life history variation among female mammals. Proc Natl Acad Sci USA. 1991;88(4):1134–1137. doi: 10.1073/pnas.88.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Charnov EL. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology. Oxford Univ Press; Oxford: 1993. [Google Scholar]
- 101.Boesch C, Bolé C, Eckhardt N, Boesch H. Altruism in forest chimpanzees: The case of adoption. PLoS One. 2010;5(1):e8901. doi: 10.1371/journal.pone.0008901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sear R, Mace R. Who keeps children alive? A review of the effects of kin on child survival. Evol Hum Behav. 2008;29(1):1–18. [Google Scholar]