Significance
Cellular respiration in humans depends on the correct assembly of a copper site (CuA) in cytochrome oxidases. Because copper is toxic at high levels, cells require specific mechanisms to regulate copper transport and delivery. Two proteins from the Sco family are involved in this mechanism in human mitochondria, and mutations on any of these proteins jeopardize the assembly of the CuA site, leading to lethal pathologies in newborns. We show that Sco1 is a protein responsible for delivering the copper ions to the oxidase, with a high degree of selectivity. Sco2, instead, provides electrons to reduce the nonmetallated site of the oxidase. This evidence allow us to propose a mechanism for assembly of the CuA site in eukaryotes.
Keywords: cytochrome oxidase, CuA site, metal site assembly, metallochaperones, Sco proteins
Abstract
Maturation of cytochrome oxidases is a complex process requiring assembly of several subunits and adequate uptake of the metal cofactors. Two orthologous Sco proteins (Sco1 and Sco2) are essential for the correct assembly of the dicopper CuA site in the human oxidase, but their function is not fully understood. Here, we report an in vitro biochemical study that shows that Sco1 is a metallochaperone that selectively transfers Cu(I) ions based on loop recognition, whereas Sco2 is a copper-dependent thiol reductase of the cysteine ligands in the oxidase. Copper binding to Sco2 is essential to elicit its redox function and as a guardian of the reduced state of its own cysteine residues in the oxidizing environment of the mitochondrial intermembrane space (IMS). These results provide a detailed molecular mechanism for CuA assembly, suggesting that copper and redox homeostasis are intimately linked in the mitochondrion.
Copper is an essential transition metal in cells, where it binds to biomolecules with high affinity and plays a myriad of catalytic and signaling roles, all of them stemming from its redox capabilities. At the same time, these features make free copper ions toxic by outcompeting other metal ions in binding the target metalloproteins and as a source of reactive oxygen species (1). Living organisms thus resort to different strategies aimed to transport copper ions and regulate their levels in the cell, such as the use of metallochaperones, which bind copper ions and deliver them specifically to the target proteins (2–6).
Copper is an essential cofactor of cytochrome c oxidase (COX), the terminal oxidase of the respiratory chain in most organisms. COX I and COX II are the two copper-containing subunits harboring the CuB and CuA sites, respectively, conserved in heme-copper oxidases (7–9). Assembly of the oxidase is a complex process involving the synthesis and folding of the individual subunits and the incorporation of the metal cofactors (10). For the human oxidase, at least seven genes encoding for different proteins are required for maturation of these two copper sites (11–17).
CuA is a binuclear copper center that provides the electron entry port of the oxidase in subunit II, located in the intermembrane space (IMS) of mitochondria or in the bacterial periplasm (18). Proteins Cox17, Sco1, and Sco2 are involved in the assembly of the CuA site in eukaryotes. All these proteins (including COX II) bind copper through two Cys ligands whose redox status is tightly linked to their function (19, 20). Cox17 is a soluble metallochaperone that transfers Cu(I) ions to both Sco1 and Sco2 (21, 22). Sco1 and Sco2 are homologous proteins, anchored to the inner mitochondrial membrane through a single transmembrane helix that locates their soluble, copper-binding domain in the IMS (15, 23). Both proteins share a thioredoxin fold and bind copper ions through a functional CX3C motif complemented by a histidine residue located in a β-hairpin absent in thioredoxins (24–27). This CX3CXnH motif allows Sco proteins either to bind Cu(I) with high affinity or to act as thiol-oxidoreductases (27–29). Both Sco1 and Sco2 are essential for CuA biogenesis in humans, but their specific roles are unknown, and a detailed mechanism for CuA assembly in mammalian oxidases is still lacking. The fact that metal site assembly requires the involvement of two orthologous, essential proteins, remains puzzling. So far, the lack of a soluble variant of human COX II has precluded a biochemical study of the function of these proteins.
Here, we report the design of a chimeric COX II variant that enables a biochemical and biophysical study. Based on these experiments, we posit a mechanism of assembly of the CuA site in human COX II that allows us to propose specific, complementary roles for Sco1 and Sco2. We show that (i) Sco1 is the metallochaperone that delivers the two Cu(I) equivalents to COX II; (ii) Sco1 and COX II have evolved specific protein–protein recognition based on the sequences of the loops containing the copper ligands; and (iii) Sco2 is responsible of reducing the Cys residues of COX II, but it performs this redox function in its Cu(I)-bound form rather than through a simple disulfide exchange reaction as observed in bacteria. This model reveals the versatility of the Sco scaffold in fulfilling different copper-dependent functions and, at the same time, providing high selectivity in the metal homeostasis network.
Results
Engineering a Soluble Human COX II.
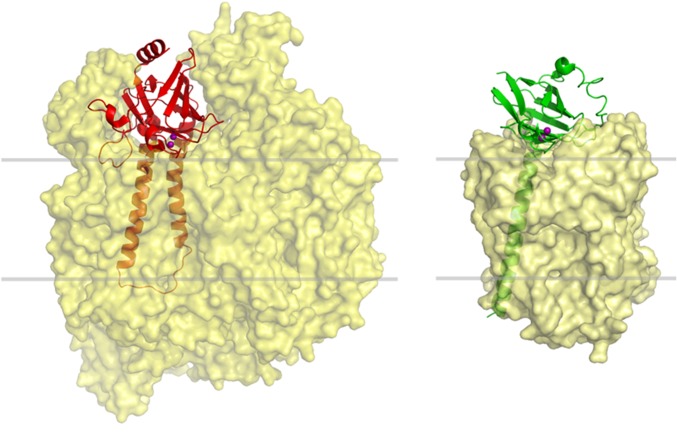
COX IIs from different organisms present a characteristic copper-binding site defined by loops protruding from a conserved β-barrel fold (known as the cupredoxin fold) anchored to the membrane by one or two long N-terminal transmembrane helices (Fig. 1 and Fig. S1). Removal of this transmembrane stretch has allowed the expression of soluble COX II domains from several bacterial oxidases (30, 31). Among them, the soluble fragment from Thermus thermophilus COX II (Tt COX II hereafter) is the only one stable for long periods of time and under different conditions (32, 33). Instead, no eukaryotic COX II subunit has been expressed in a stable, soluble form to date. This may be due to the fact that COX II from eukaryotic oxidases displays many hydrophobic interactions with neighboring subunits, whereas the smaller bacterial proteins contain a periplasmic COX II subunit with no additional interactions (Fig. S1). We attempted expression of the human COX II (containing only the cupredoxin domain), but we were unable to obtain a soluble and properly folded protein. Different constructs were considered: (i) replacing hydrophobic residues in the protein surface by polar residues to improve the solubility, (ii) removing different insertions present in the eukaryotic oxidases that allow interaction with other COX subunits, and (iii) different truncation levels. None of these constructs provided a soluble human COX II variant to allow in vitro biochemical studies. We then resorted to a different strategy.
Fig. 1.
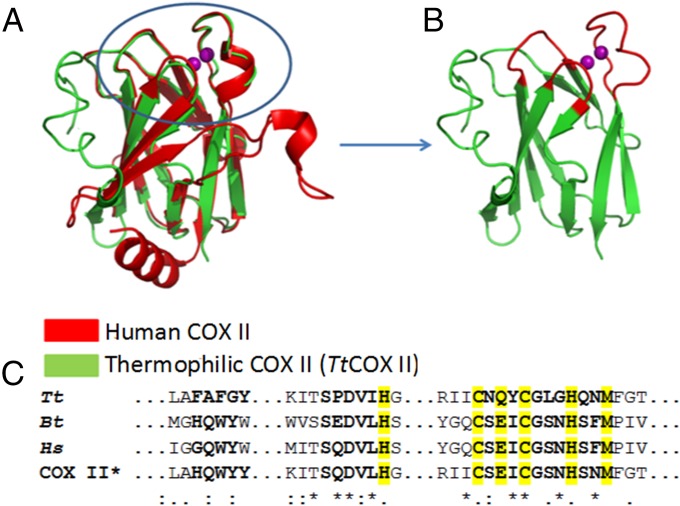
Design of the chimeric COX II* protein. (A) Comparison of the structures of the soluble COX II domains from Thermus thermophilus ba3 oxidase [Protein Data Bank (PDB) ID code 2CUA; green] and human cytochrome c oxidase (red). The latter was modeled based on the structure of the bovine oxidase (PDB ID code 1V54) (46). (B) Model of the chimeric COX II* colored in green in the region coming from the Thermus oxidase and in red in the loops taken from the human protein. (C) Sequence alignment of the loops corresponding to the COX II subunits from Thermus thermophilus (Tt), bovine (Bt), human (Hs), and the chimeric COX II*.
Fig. S1.
Arrangement of the COX II subunit within the structure of different oxidases. Eukaryotic COX II (Left; PDB ID code 1OCC for bovine COX) (9) displays several interactions with other subunits of the complex, whereas prokaryotic COX II (Right; PDB ID code 1EHK for Tt COX) (47) displays a small region of interaction with the rest of the complex. Gray lines define approximate limits of the lipid membrane surrounding the complexes.
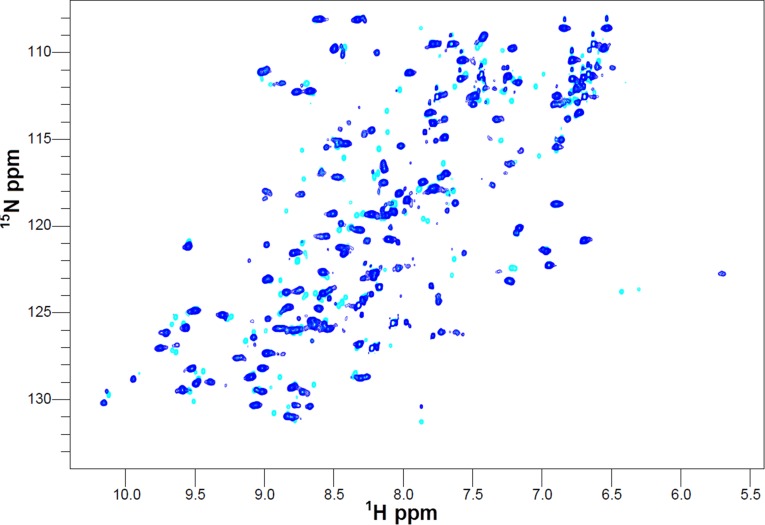
The metal-binding region of COX II comprises a long loop containing five of the six CuA ligands in a conserved CX(E/Q/W)XCX3HX2M motif (Fig. 1) (34). We have proposed that this region, together with two other neighboring loops, could be involved in the interaction with the metallochaperone (33). Based on this hypothesis, we have designed a chimeric protein by grafting the three loops of the human protein into the stable scaffold of the Thermus protein (Fig. 1). The chimeric protein (COX II* hereafter) binds two copper ions and, in its oxidized form, displays the typical spectroscopic signatures of mixed-valence CuA sites (35). The heteronuclear single-quantum correlation (HSQC) spectra of 15N apo-COX II*2SH (where 2SH indicates that the Cys ligands are in the reduced state) show upon copper(I) titration (i) chemical shift perturbations of resonances corresponding to residues close to the metal-binding region, and (ii) the appearance of several cross peaks corresponding to residues in the engineered loops (Fig. S2) that were not observed in the spectrum of the metal-free protein, most likely broadened because of conformational exchange processes occurring in the intermediate regime (33). These differences in the NMR spectra of apo and di-Cu(I) forms of COX II* can be exploited to follow copper uptake from the potential protein donors. Furthermore, the oxidation state of the cysteine thiols in apo-COX II* can also be monitored through the HSQC spectra, as revealed by chemical shift changes and signal broadening in the reduced form (apo-COX II*2SH) with respect to the oxidized one (apo-COX II*S-S) (Fig. S3). We can thus use NMR spectroscopy to follow the two essential events required for formation of the CuA site, namely reduction of the oxidized cysteines and metal binding. In addition, we conclude that the chimeric soluble protein is a good model to study the interactions with the putative human copper chaperones Sco1 and Sco2 and to unravel their role in copper incorporation into COX II.
Fig. S2.
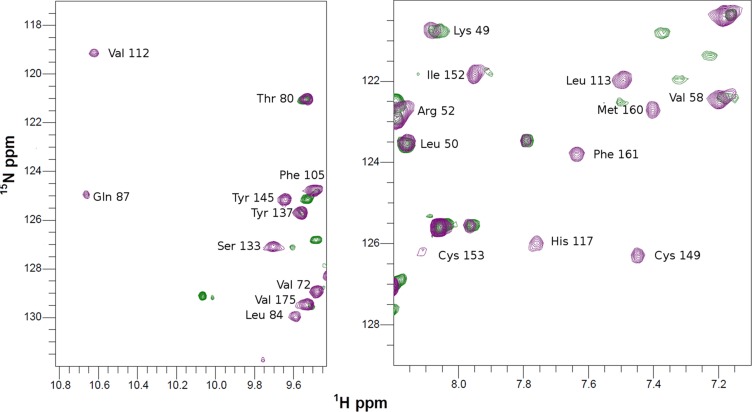
Cu(I) uptake of Apo-COX II* followed by NMR. 1H-15N HSQC spectra of apo-COX II*2HS (green) and upon addition of two equivalents of Cu(I) (purple). The spectrum of the metallated form has been assigned, showing that the main difference is the appearance of cross peaks belonging to the ligands loops (Right) or to the two other loops surrounding the metal site (Left).
Fig. S3.
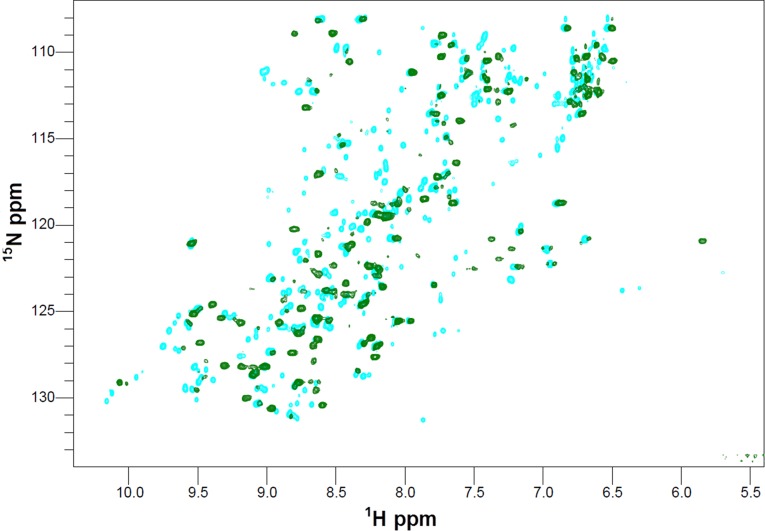
Reduction of Apo-COX II*S-S to Apo-COX II*2SH followed by NMR. 1H-15N HSQC spectra of Apo-COX II*S-S (cyan) and Apo-COX II*2SH (green).
Sco1 Is a Specific Copper Chaperone for Human COX II.
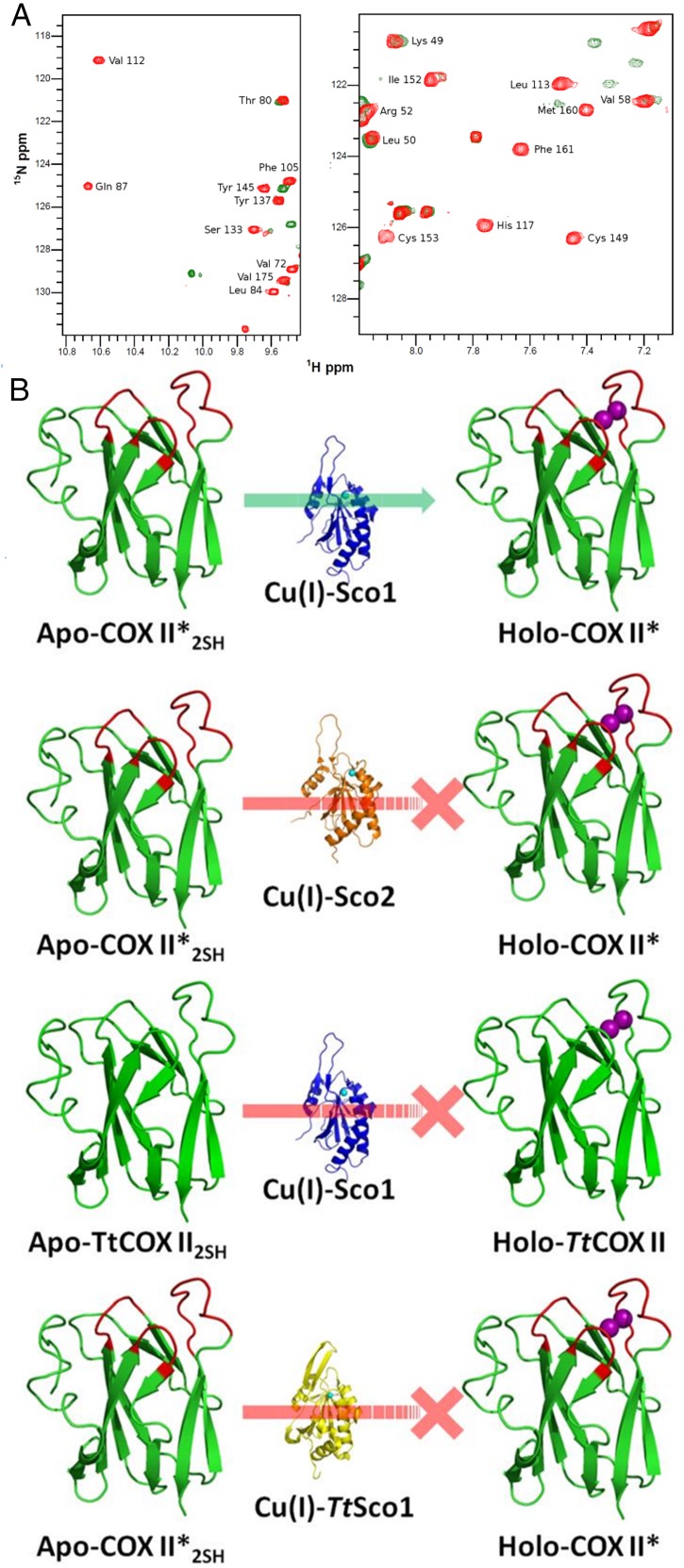
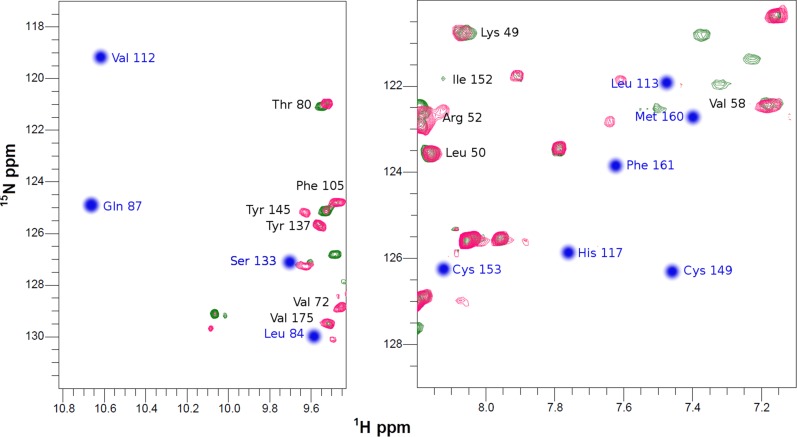
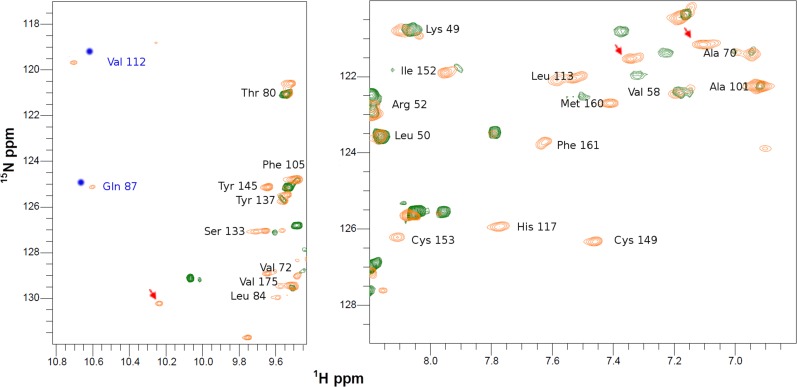
When 15N apo-COX II*2SH was reacted with two equivalents of unlabeled human Cu(I)-Sco1, we observed formation of the colorless di-Cu(I) adduct, identical, based on an NMR spectrum, to that obtained by addition of free Cu(I) in solution (Fig. 2A and Fig. S2). Copper transfer was complete at a 2:1 stoichiometry. Human Cu(I)-Sco1, instead, was unable to effectively transfer Cu(I) ions to Tt COX II: cross peaks corresponding to a species different from the apo or the di-Cu(I) form were detected (Fig. S4), suggesting that the process is arrested in a nonproductive form. Similarly, no metal transfer to form a native CuA site was observed when Cu(I)-TtSco1 was added to apo-COX II*2SH (Fig. S5). These results reveal that the engineered loops in COX II* play a role in specific protein–protein recognition by human Sco1 and in Cu(I) transfer. This selectivity validates the use of this chimera as a model for the human protein, and provides evidence on the role of the loops in COX II in this process.
Fig. 2.
Human Sco1 specifically recognizes the loops of human COX II to assemble its CuA site. (A) 15N, 1H HSQC spectrum of Apo-COX II*2SH (green) and upon addition of two equivalents of Cu(I)-Sco1 (red), showing complete copper transfer. (B) Summary of the copper transfer experiments (spectra shown in Supporting Information). The green arrow indicates the stoichiometric transfer of two Cu(I) ions from Sco1 to COX II*, and the red lines indicate unsuccessful Cu(I) transfer assays.
Fig. S4.
Cu(I) transfer from Cu(I)-Sco1 to Apo-TtCOX II2SH followed by NMR. 1H-15N HSQC spectra of TtCOX II2SH (green) and upon addition of two equivalents of Cu(I)-Sco1 (red). Arrows indicate cross peaks that do not correspond neither to apo nor to the metallated form of the protein.
Fig. S5.
Cu(I) transfer from Cu(I)-TtSco1 to Apo-COX II*2SH followed by NMR. 1H-15N HSQC spectra of COX II*2SH (green) and upon addition of two equivalents of Cu(I)-TtSco1 (pink), showing that TtSco1 is not able to transfer Cu(I) ions to the oxidase. The position of the expected cross peaks corresponding to the holo form protein absent in the final spectrum are indicated in blue.
Addition of human Cu(I)-Sco2 to apo-COX II*2SH, instead, led to formation of only 30% of the di-Cu(I) adduct *. This reaction did not proceed further even upon addition of excess Cu(I)-Sco2. Furthermore, resonances corresponding to a species similar to that observed for the nonproductive transfer between human Cu(I)-Sco1 and the bacterial COX II domain (Fig. S6) appeared. The formation of this adduct suggests that this pathway is not efficient enough for copper transfer. We conclude that (i) human Sco1 is able to produce a fully metallated COX II, (ii) metallation by human Sco2 is not efficient, and (iii) Cu(I) insertion by Sco1 is facilitated by protein–protein recognition involving the engineered loops (Fig. 2B).
Fig. S6.
Cu(I) transfer from Cu(I)-Sco2 to Apo-COX II*2SH followed by NMR. 1H-15N HSQC spectra of COX II*2SH (green) and upon addition of two equivalents of Cu(I)-Sco2 (orange). The position of the expected cross peaks corresponding to the holo form protein shifted in the final spectrum are indicated in blue.
Copper-Bound Human Sco2 Is a Thiol Oxidoreductase of the Cys Residues in COX II.
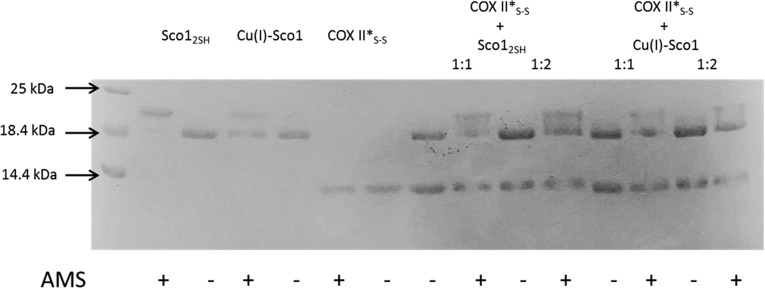
The IMS of mitochondria is an oxidizing environment, with a redox potential of −255 mV (36). The reduction potential of the Cys residues in apo-COX II* is −288 ± 3 mV, identical to that of apo Tt COX II (Fig. S7). Thus, the apo form of a newly synthesized COX II is expected to have the two Cys ligands at least partially oxidized, being therefore unable to bind the Cu(I) ions. Sco proteins have been suggested to act as thiol reductases of the Cys ligands of COX II based on their thioredoxin fold (27, 37), which is indeed supported by in vitro experiments on the bacterial thermophilic Sco protein (32, 37). Based on the reduction potentials of the S-S/2SH redox couple of the human proteins: Cox17 (−198 mV) > Sco1 (−277 mV) > COX II* (−288 mV) > Sco2 (less than −300 mV) (24, 38, 39), Sco2 would be the only protein able to reduce the disulfide bond in COX II*. We tested the redox reactivity of either human Sco1 or human Sco2 in their apo, reduced state with oxidized apo-COX II*S-S. The reactions were followed by SDS/PAGE under nonreducing conditions followed by treatment with 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid, disodium salt (AMS), a reagent that adds 0.5 kDa per each free thiol group. Incubation of oxidized apo-COX II*S-S with apo-Sco12SH did not elicit any reduction, consistent with the redox potentials of these proteins. Instead, treatment with Sco2 led to formation of reduced apo-COX II*2SH, but the reaction was not complete (Fig. 3A).
Fig. S7.
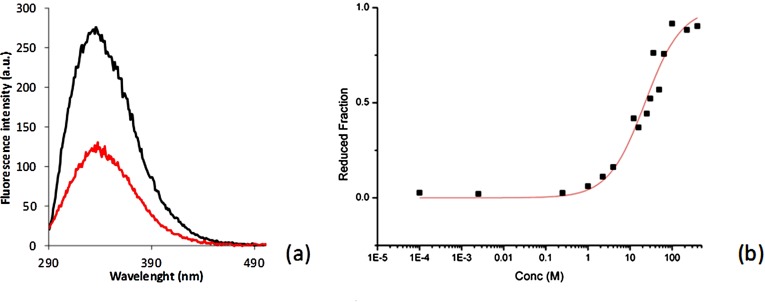
Redox potential of the CX3C motif of Apo-COX II*. (A) Fluorescence emission spectra of oxidized (50 mM phosphate buffer, pH 7.0, 0.01 mM GSSG; black line) and reduced (50 mM phosphate buffer, pH 7.0, 200 mM GSH; red line) Apo-COX II* upon excitation at 280 nm. (B) The redox equilibrium of Apo-COX II* with different [GSH]2/GSSG ratios is shown. Data processing and determination of the equilibrium constant was previously described (3). After nonlinear regression, a value of Keq = 23 ± 2 mM was determined for the Apo-COX II*/glutathione equilibrium, corresponding to a redox potential of −288 ±3 mV for the Apo-COX II* S-S –2SH redox couple.
Fig. 3.
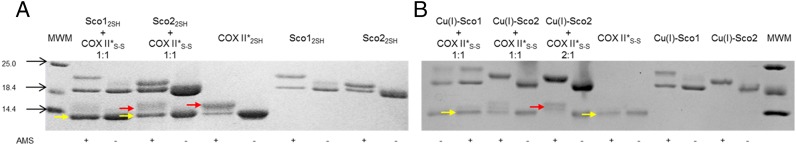
Electron transfer reaction followed by AMS-reacted, nonreducing SDS/PAGE. (A) Sco12SH and Sco22SH mixed with COX II*S-S and (B) Cu(I)-bound forms of the Sco proteins mixed with COX II*S-S. All mixtures were done in a 1:1 ratio, except when indicated. The proteins in their different states are also reported as reference. Signs + and – indicate if the AMS reactive was added to the mixture. Yellow and red arrows indicate where oxidized and reduced COX II* show up, respectively, upon addition of AMS to the mixture.
Biochemical studies have shown that both Sco1 and Sco2 in human mitochondria are present in a mixture of two forms, one of which was reactive with AMS (19). The presence of a Sco1 form with free thiols in the mitochondria is consistent with its redox potential, but Sco2 is expected to be fully oxidized in the IMS. The AMS-reactive form of Sco2 could, however, correspond either to reduced Sco2, or to a copper-bound form, because copper is dissociated in the gel assays. We therefore tested the reactivity of oxidized apo-COX II*S-S with Cu(I)-Sco1 and Cu(I)-Sco2, finding that Cu(I)-Sco2 was able to completely reduce the thiol groups of apo-COX II*S-S (Fig. 3B). This reaction was complete at a 2:1 stoichiometry, indicating that Cu(I)-Sco2 provides one reducing equivalent per molecule. Cu(I) Sco1, instead, did not show any reactivity even at a 2:1 stoichiometric ratio (Fig. S8). Thus, Sco2 is able to act as an efficient disulfide reductase of COX II only in its copper-loaded form.
Fig. S8.
Electron transfer followed by AMS-reacted, nonreducing SDS/PAGE. Sco12SH and Cu(I)-Sco1 mixed with COX II*S-S. Mixtures were done in a 1:1 and 2:1 ratio Sco1:COX II*. The proteins in their different states are also reported as reference. Signs + and – indicate if the AMS reactive was added to the mixture.
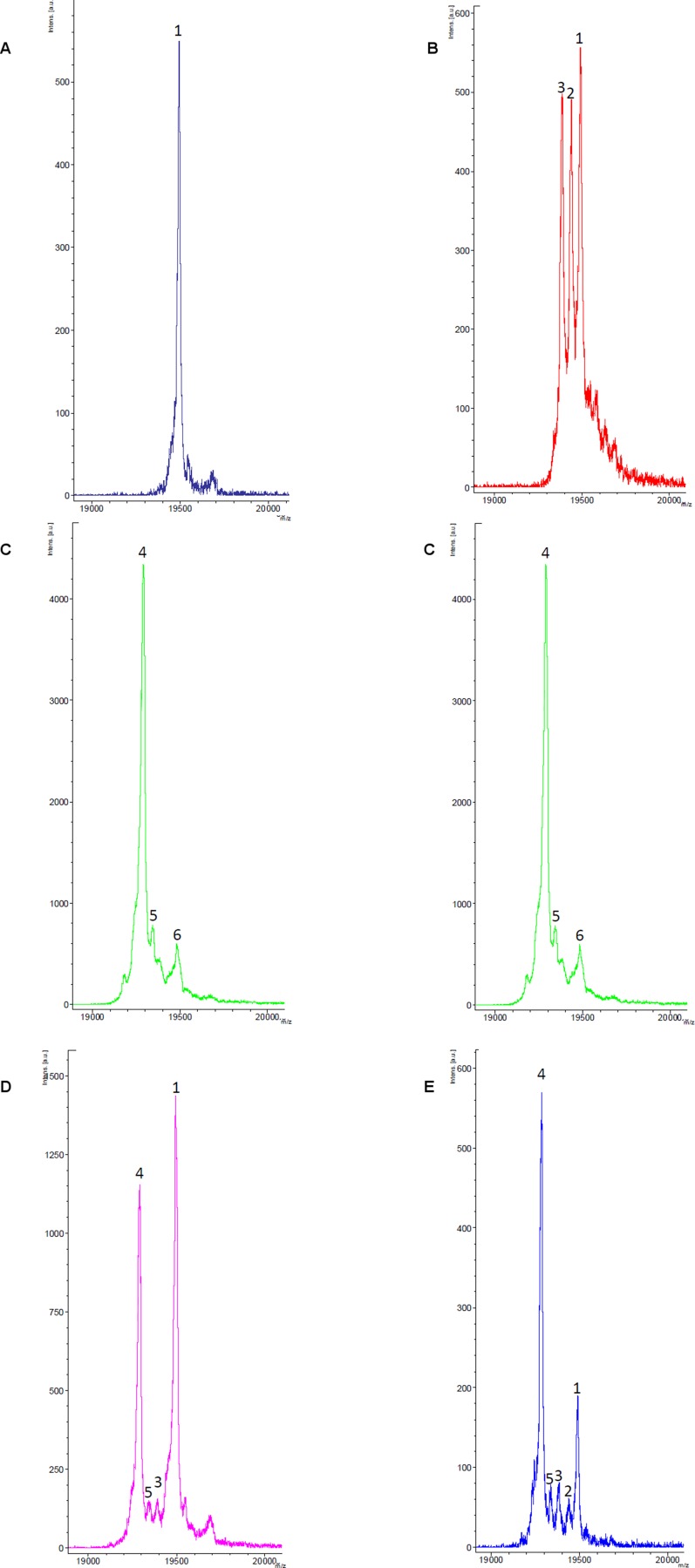
Leary et al. (19) have shown that overexpression of Sco2 in Sco2 cells alters the ratio of oxidized versus reduced Cys of Sco1 in the mitochondria, suggesting a redox interplay between the two Sco proteins. We reacted Sco2S-S with apo-Sco12SH and with Cu(I)-Sco1, and the reaction samples were treated with iodoacetamide (IAM), and studied by MS. No redox reaction was observed in any of these cases (Fig. S9), allowing us to conclude that a direct redox event between the two Sco proteins is not feasible.
Fig. S9.
MALDI-TOF-MS spectra of the iodoacetamide (IAM)-derivatized samples of (A) Sco12SH showing the presence a dominant species labeled as 1, corresponding to the Sco1(IAM)2 adduct, indicating that the two reduced Cys at the active site are in the reduced form; (B) Cu(I)-Sco1 showing the presence of three species corresponding to Sco1(IAM)2, Sco1(IAM)1, and Sco1, labeled as 1, 2, and 3, respectively; (C) Sco2S-S with the presence of the presence a dominant species corresponding to the Sco2(IAM) adduct, where Cys115 (far from the active site) is alkylated, and the two Cys at the active site are oxidized, not being alkylated, labeled as 4; minority species 5 and 6 correspond to Sco2(IAM)2 and Sco2(IAM)3; (D) a 1:1 mixture of Sco12SH and Sco2S-S, where the peaks of the reference spectra are preserved, showing that there is no redox reaction; (E) a 1:1 mixture of Cu(I)-Sco12SH and Sco2S-S, where the peaks of the reference spectra are preserved, showing that there is no redox reaction.
Interplay of Sco Proteins in the Maturation of COX II.
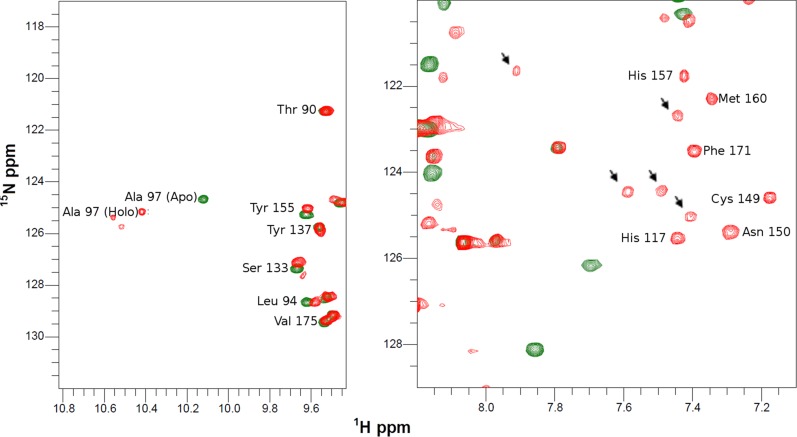
The present results show that (i) only Sco1 is able to deliver the full copper cargo to human COX II with high specificity, and (ii) Cu(I)-Sco2 is able to provide electrons to reduce the Cys ligands at the CuA site. The interplay of both human Sco proteins in the whole redox and copper insertion process was followed by NMR spectroscopy monitoring the HSQC spectra of COX II*. Addition of two equivalents of Cu(I)-Sco1 to 15N apo-COX II*S-S did not elicit any change in its HSQC spectrum, confirming that neither electron nor copper transfer is viable under these conditions. When one equivalent of Cu(I)-Sco2 was added to this mixture, formation of the di-Cu(I)-COX II* form to 50–60% was observed. When Cu(I)-Sco2 was added to oxidized apo-COX II*S-S, spectral changes indicated full reduction to the apo2SH-COX II* form, in agreement with the gel experiments (Fig. S10). There was no evidence of copper transfer in this reaction, confirming the specificity of Sco1 in the metallation event and the selective redox function of Cu(I)-Sco2.
Fig. S10.
Electron transfer from Cu(I)-Sco2 to Apo-COX II*S-S followed by NMR. 1H-15N HSQC spectra of Apo-COX II*S-S (cyan) and upon addition of one equivalent of Cu(I)-Sco2 (blue). The disappearance of several cross peaks compared with the oxidized form of the protein is typical of spectra of COX II*2SH.
Discussion
A series of elegant studies in native and defective human fibroblast cell lines by Leary, Shoubridge, and coworkers (15, 19) have shown that Sco1 and Sco2 play distinct, complementary roles in the maturation process of the CuA site of human COX II. Our experiments allow assessing these roles, therefore providing a mechanism for CuA maturation in eukaryotic COX II. On one hand, only Sco1 is able to efficiently transfer two Cu(I) equivalents, whereas it is unable to reduce the cysteines neither in the apo- nor in the Cu(I)-loaded form. Metal transfer strictly depends on loop recognition between Sco1 and COX II, which is organism dependent (Fig. 2B). This selectivity confirms that these loops are indeed crucial for recognition in the copper transfer event (33). On the other hand, Cu(I)-Sco2 shows impaired metal transfer ability but is able to reduce the cysteines of apo-COX II*S-S at a 2:1 stoichiometry. The essential requirement of two Sco proteins in humans is therefore accounted for by these different, specific functions. Notably, both human Sco proteins are active in their copper-bound forms, accounting for the observation that mutation of any of the copper ligands severely affects the oxidase activity and cellular respiration (40, 41). The most frequent pathological mutations (P174L in Sco1 and E140K in Sco2) are located close to the CX3CXnH motif, and thus expected to alter either the copper-binding or the redox capabilities of these proteins. This has been shown to be the case for mutation P174L in Sco1 (39). The mechanism emerging from this work shows that the same requirements hold for Sco2. Studies aimed to address the specific impact of these mutations on the metal site assembly are required.
The copper-dependent reductase function of Sco2 resembles the disulfide reductase activity of Cox 17 toward Sco1 in its Cu(I)-bound form, which, in the latter case, is coupled with copper transfer (22). Likewise, copper binding to Sco proteins in mitochondria is essential for their function not only as metallochaperones, but also to elicit electron transfer to assist its own delivery to the oxidase. We propose that this aspect has been exploited by evolution to cope with copper toxicity during copper delivery. In the specific case of Sco2, Cu(I) binding not only allows a reaction that does not proceed to completion despite being thermodynamically favored but (most important) also prevents oxidation of its own thiol groups in the IMS. The involvement of thioredoxins in the copper homeostasis network is intimately linked to the onset of metal-binding abilities along the evolution of the thioredoxin fold, still maintaining its redox features. In fact, thioredoxins are not able to bind metal ions due to the presence of a cis-Pro residue, and Sco proteins not only lack this residue but also feature an additional β-hairpin providing a His ligand (42), resulting in a neat gain-of-function.
Our experiments show that Sco2 is not able to directly oxidize Sco1. The fact that this reaction occurs in the mitochondria strongly suggests that there are other cofactors mediating this process. One possible candidate for this role is protein COA6, recently identified as an essential cofactor for oxidase assembly containing a Cys-rich motif, which was proposed to interact with COX II and Sco2 (43, 44).
Analysis of the present in vitro data together with previous evidence (22) support the hypothesis that mitochondrial redox reactions involving copper make extensive use of Cys-rich motifs in their copper-bound form. On one hand, copper acts as a guardian of the thiol groups in the different proteins, preventing oxidation. On the other hand, this allows mitochondrial Sco proteins to regulate copper levels at the IMS, as recently shown (45). Thus, the mechanism herein proposed supports at the molecular level the emerging view that copper and redox homeostasis are entangled and possibly coupled in the mitochondrion.
The chimeric COX II* protein herein designed is of great utility for further in vitro studies. First, it allows pursuing structural studies to assess the effect of the specific defects associated with the different pathogenic SCO alleles, and address the molecular basis of lethal genetic diseases. In addition, the system can be exploited to assess the role of other COX assembly factors to further enrich the understanding of the maturation process of mitochondrial oxidases.
Methods
COX II Clone and Protein Production.
The synthetic gene coding for COX II* was purchased from Genescript, cloned in a pUC57 vector, and subcloned to the isopropyl β-d-1-thiogalactopyranoside–inducible vector pET9a. Human Sco1, human Sco2, and Tt Sco1 were expressed in Escherichia coli BL21-Gold (DE3) cells (Stratagene) to high levels in LB medium. The purification of each protein in its apo form was done as described (24, 26, 32). COX II* and Tt COX II were expressed in E. coli Bl21-Gold (DE3) cells (Stratagene). For uniformly labeled samples, cultures were grown in 15N/13C-enriched media as required. The proteins were purified from cell lysates as described elsewhere.
Redox Potential Determination.
The reduction potential of the Cys residues in apo-COX II* was estimated from the changes in emission fluorescence at 334 nm during a titration with glutathione. The redox equilibrium was monitored on 5 μM protein samples previously incubated overnight under N2 atmosphere, with different glutathione (GSH)/glutathione disulfide (GSSG) ratios (0.1 mM GSSG and varying concentrations of GSH, 10–200 mM) in 50 mM phosphate buffer, pH 7. Data collection and analysis were performed following the protocol described for human Sco1 (39).
NMR Spectroscopy.
NMR experiments were carried out on a 600-MHz Bruker Avance II spectrometer equipped with a triple resonance inverse (TXI) probe, a 900-MHz Bruker Avance II spectrometer equipped with a triple resonance inverse (TCI) cryoprobe, and a 950-MHz Bruker Avance III spectrometer equipped with a CP TCI CryoProbe. All experiments were carried out at 298 K using standard techniques.
Backbone resonance assignments for holo-COX II* were obtained by analyzing HSQC, HNCO, HN(CA)CO, HNCA, HN(CO)CA, CBCA(CO)NH, and HNCACB experiments acquired on a uniformly labeled 13C and 15N sample prepared in 100 mM potassium chloride and 100 mM potassium phosphate buffer at pH 6. Because all subsequent titrations were performed at pH 7, the backbone 15N and 1H assignments were transferred from pH 6 to 7 by following the position of the N, H cross peaks in HSQC spectra of a pH titration.
Protein–protein titrations were performed in anaerobic conditions using 0.2–1.5 mM protein samples prepared in 50 mM potassium phosphate buffer at pH 7 and followed by NMR spectroscopy. Samples of proteins with their cysteine thiols in the reduced state were obtained by addition of 10 mM DTT for 30 min followed by removal of the reducing agent through desalting in an anaerobic chamber. Cu(I)-bound forms of Sco proteins were obtained by addition of two equivalents of Cu(I) to the reduced samples followed by removal of excess Cu(I) through desalting in anaerobic chamber.
AMS-reacted gels to probe redox state of cysteines.
The redox state of the cysteine residues in protein mixtures was investigated by nonreducing SDS/PAGE, after reaction of the samples with AMS, a reagent that alkylates free thiols adding a Mr of ∼0. 5 kDa for each reduced cysteine. Reduced and oxidized samples and protein mixtures were (20–40 μM) treated in an anaerobic environment with 1% (wt/vol) SDS and 10 mM AMS for 1 h at 37 °C, and resolved by 17% nonreducing SDS/PAGE.
MALDI-TOF experiments.
The 20 μM samples of reference samples and mixtures of Sco1 and Sco2 in different oxidation states were derivatized by addition of 20 mM IAM, followed by 1-h incubation at room temperature in an anaerobic chamber. To remove excess nonreacted IAM, the protein samples were precipitated using 80% acetone and the protein pellets were resuspended in 20 mM phosphate solution at pH 7. Protein adducts were identified by MALDI-TOF MS in a Bruker Daltonics Ultraflex III TOF/TOF instrument.
Supplementary Material
Acknowledgments
We are thankful for the inspiring ideas on the early stages of this work provided by the late Ivano Bertini. We acknowledge Consejo Nacional de Investigaciones Científicas y Técnicas, Agencia Nacional de Promoción Científica y Tecnológica (PICT 2012-1285 and PPL2), and Worldwide NMR for funding NMR time at Magnetic Resonance Center and at Instituto de Biología Molecular y Celular de Rosario, as well as travel expenses and accommodation for M.N.M. in Florence.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505056112/-/DCSupplemental.
References
- 1.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci USA. 2009;106(20):8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science. 1999;284(5415):805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 3.O’Halloran TV, Culotta VC. Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem. 2000;275(33):25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 4.Robinson NJ, Winge DR. Copper metallochaperones. Annu Rev Biochem. 2010;79:537–562. doi: 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banci L, et al. The Atx1-Ccc2 complex is a metal-mediated protein-protein interaction. Nat Chem Biol. 2006;2(7):367–368. doi: 10.1038/nchembio797. [DOI] [PubMed] [Google Scholar]
- 6.Banci L, et al. Affinity gradients drive copper to cellular destinations. Nature. 2010;465(7298):645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa S, et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998;280(5370):1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson-Miller S, Babcock GT. Heme/copper terminal oxidases. Chem Rev. 1996;96(7):2889–2908. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 9.Tsukihara T, et al. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996;272(5265):1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- 10.Soto IC, Fontanesi F, Liu J, Barrientos A. Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim Biophys Acta. 2012;1817(6):883–897. doi: 10.1016/j.bbabio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoubridge EA. Cytochrome c oxidase deficiency. Am J Med Genet. 2001;106(1):46–52. doi: 10.1002/ajmg.1378. [DOI] [PubMed] [Google Scholar]
- 12.Barros MH, Johnson A, Tzagoloff A. COX23, a homologue of COX17, is required for cytochrome oxidase assembly. J Biol Chem. 2004;279(30):31943–31947. doi: 10.1074/jbc.M405014200. [DOI] [PubMed] [Google Scholar]
- 13.Oswald C, Krause-Buchholz U, Rödel G. Knockdown of human COX17 affects assembly and supramolecular organization of cytochrome c oxidase. J Mol Biol. 2009;389(3):470–479. doi: 10.1016/j.jmb.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Khalimonchuk O, Rödel G. Biogenesis of cytochrome c oxidase. Mitochondrion. 2005;5(6):363–388. doi: 10.1016/j.mito.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Leary SC, et al. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum Mol Genet. 2004;13(17):1839–1848. doi: 10.1093/hmg/ddh197. [DOI] [PubMed] [Google Scholar]
- 16.Thompson AK, et al. Mutagenic analysis of Cox11 of Rhodobacter sphaeroides: Insights into the assembly of CuB of cytochrome c oxidase. Biochemistry. 2010;49(27):5651–5661. doi: 10.1021/bi1003876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banci L, et al. Solution structure of Cox11, a novel type of beta-immunoglobulin-like fold involved in CuB site formation of cytochrome c oxidase. J Biol Chem. 2004;279(33):34833–34839. doi: 10.1074/jbc.M403655200. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y. Electron transfer: Cupredoxins. In: Que L Jr, Tolman WB, editors. Biocoordination Chemistry, Comprehensive Coordination II: From Biology to Biotechnology. Vol 8. Elsevier; Amsterdam: 2004. pp. 91–122. [Google Scholar]
- 19.Leary SC, Sasarman F, Nishimura T, Shoubridge EA. Human SCO2 is required for the synthesis of CO II and as a thiol-disulphide oxidoreductase for SCO1. Hum Mol Genet. 2009;18(12):2230–2240. doi: 10.1093/hmg/ddp158. [DOI] [PubMed] [Google Scholar]
- 20.Bertini I, Cavallaro G, McGreevy KS. Cellular copper management—a draft user’s guide. Coord Chem Rev. 2010;254(5-6):506–524. [Google Scholar]
- 21.Banci L, et al. A structural-dynamical characterization of human Cox17. J Biol Chem. 2008;283(12):7912–7920. doi: 10.1074/jbc.M708016200. [DOI] [PubMed] [Google Scholar]
- 22.Banci L, et al. Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc Natl Acad Sci USA. 2008;105(19):6803–6808. doi: 10.1073/pnas.0800019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchwald P, Krummeck G, Rödel G. Immunological identification of yeast SCO1 protein as a component of the inner mitochondrial membrane. Mol Gen Genet. 1991;229(3):413–420. doi: 10.1007/BF00267464. [DOI] [PubMed] [Google Scholar]
- 24.Banci L, et al. A structural characterization of human SCO2. Structure. 2007;15(9):1132–1140. doi: 10.1016/j.str.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Abajian C, Rosenzweig AC. Crystal structure of yeast Sco1. J Biol Inorg Chem. 2006;11(4):459–466. doi: 10.1007/s00775-006-0096-7. [DOI] [PubMed] [Google Scholar]
- 26.Banci L, et al. A hint for the function of human Sco1 from different structures. Proc Natl Acad Sci USA. 2006;103(23):8595–8600. doi: 10.1073/pnas.0601375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balatri E, Banci L, Bertini I, Cantini F, Ciofi-Baffoni S. Solution structure of Sco1: A thioredoxin-like protein Involved in cytochrome c oxidase assembly. Structure. 2003;11(11):1431–1443. doi: 10.1016/j.str.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Horng YC, et al. Human Sco1 and Sco2 function as copper-binding proteins. J Biol Chem. 2005;280(40):34113–34122. doi: 10.1074/jbc.M506801200. [DOI] [PubMed] [Google Scholar]
- 29.Siluvai GS, Nakano MM, Mayfield M, Nilges MJ, Blackburn NJ. H135A controls the redox activity of the Sco copper center. Kinetic and spectroscopic studies of the His135Ala variant of Bacillus subtilis Sco. Biochemistry. 2009;48(51):12133–12144. doi: 10.1021/bi901480g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slutter CE, et al. Water-soluble, recombinant CuA-domain of the cytochrome ba3 subunit II from Thermus thermophilus. Biochemistry. 1996;35(11):3387–3395. doi: 10.1021/bi9525839. [DOI] [PubMed] [Google Scholar]
- 31.Lappalainen P, Aasa R, Malmström BG, Saraste M. Soluble CuA-binding domain from the Paracoccus cytochrome c oxidase. J Biol Chem. 1993;268(35):26416–26421. [PubMed] [Google Scholar]
- 32.Abriata LA, et al. Mechanism of CuA assembly. Nat Chem Biol. 2008;4(10):599–601. doi: 10.1038/nchembio.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaballa ME, Abriata LA, Donaire A, Vila AJ. Flexibility of the metal-binding region in apo-cupredoxins. Proc Natl Acad Sci USA. 2012;109(24):9254–9259. doi: 10.1073/pnas.1119460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams PA, et al. The CuA domain of Thermus thermophilus ba3-type cytochrome c oxidase at 1.6 Å resolution. Nat Struct Biol. 1999;6(6):509–516. doi: 10.1038/9274. [DOI] [PubMed] [Google Scholar]
- 35.Morgada MN, et al. Control of the electronic ground state on an electron-transfer copper site by second-sphere perturbations. Angew Chem Int Ed Engl. 2014;53(24):6188–6192. doi: 10.1002/anie.201402083. [DOI] [PubMed] [Google Scholar]
- 36.Hu J, Dong L, Outten CE. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J Biol Chem. 2008;283(43):29126–29134. doi: 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banci L, et al. Sco proteins are involved in electron transfer processes. J Biol Inorg Chem. 2011;16(3):391–403. doi: 10.1007/s00775-010-0735-x. [DOI] [PubMed] [Google Scholar]
- 38.Voronova A, et al. Oxidative switches in functioning of mammalian copper chaperone Cox17. Biochem J. 2007;408(1):139–148. doi: 10.1042/BJ20070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banci L, et al. Human Sco1 functional studies and pathological implications of the P174L mutant. Proc Natl Acad Sci USA. 2007;104(1):15–20. doi: 10.1073/pnas.0606189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cobine PA, et al. The P174L mutation in human Sco1 severely compromises Cox17-dependent metallation but does not impair copper binding. J Biol Chem. 2006;281(18):12270–12276. doi: 10.1074/jbc.M600496200. [DOI] [PubMed] [Google Scholar]
- 41.Valnot I, et al. Mutations of the SCO1 gene in mitochondrial cytochrome c oxidase deficiency with neonatal-onset hepatic failure and encephalopathy. Am J Hum Genet. 2000;67(5):1104–1109. doi: 10.1016/s0002-9297(07)62940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su D, Berndt C, Fomenko DE, Holmgren A, Gladyshev VN. A conserved cis-proline precludes metal binding by the active site thiolates in members of the thioredoxin family of proteins. Biochemistry. 2007;46(23):6903–6910. doi: 10.1021/bi700152b. [DOI] [PubMed] [Google Scholar]
- 43.Pacheu-Grau D, et al. Cooperation between COA6 and SCO2 in COX2 maturation during cytochrome c oxidase assembly links two mitochondrial cardiomyopathies. Cell Metab. 2015;21(6):823–833. doi: 10.1016/j.cmet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh A, et al. Copper supplementation restores cytochrome c oxidase assembly defect in a mitochondrial disease model of COA6 deficiency. Hum Mol Genet. 2014;23(13):3596–3606. doi: 10.1093/hmg/ddu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leary SC, et al. The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metab. 2007;5(1):9–20. doi: 10.1016/j.cmet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 47.Soulimane T, et al. Structure and mechanism of the aberrant ba3-cytochrome c oxidase from Thermus thermophilus. EMBO J. 2000;19(8):1766–1776. doi: 10.1093/emboj/19.8.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.