Abstract
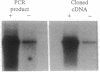
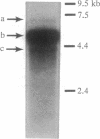
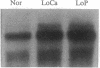
The effect of vitamin D and other variables on the synthesis of the chicken intestinal plasma membrane calcium pump (PMCA) mRNA was assessed. The DNA probe for Northern analysis was obtained by reverse transcription and PCR with intestinal poly(A)+ RNA, using two 20-mer oligonucleotide primers homologous to the 3' coding region of the human teratoma PMCA. An EcoRI restriction fragment of the PCR product was cloned into the pBluescript II KS(-) phagemid vector, and the chimeric plasmid was used to transform Escherichia coli. The amino acid sequence deduced from the nucleotide DNA sequence of the PCR product and the cloned DNA were 96% homologous with the teratoma sequence. Northern blots of intestinal poly(A)+ RNA with 32P-labeled DNA showed the presence of three major species of chicken PMCA mRNAs at about 6.6, 5.4, and 4.5 kb. Northern analysis with the chicken PMCA DNA indicated that repletion of vitamin D-deficient chickens with vitamin D increased PMCA mRNAs in the duodenum, jejunum, ileum, and colon. After injection of 1,25-dihydroxyvitamin D3 intravenously into vitamin D-deficient chickens, duodenal PMCA mRNA tended to increase by 2 hr, reached a maximum at about 16 hr, and returned to baseline levels at 48 hr. Adaptation of chickens to either a calcium- or phosphorus-deficient diet resulted in a 2- to 3-fold increase in duodenal PMCA mRNA. These results indicate that vitamin D and specific variables that affect calcium absorption through the vitamin D-endocrine system increase intestinal PMCA gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armbrecht H. J., Zenser T. V., Bruns M. E., Davis B. B. Effect of age on intestinal calcium absorption and adaptation to dietary calcium. Am J Physiol. 1979 Jun;236(6):E769–E774. doi: 10.1152/ajpendo.1979.236.6.E769. [DOI] [PubMed] [Google Scholar]
- Bar A., Hurwitz S. The interaction between dietary calcium and gonadal hormones in their effect on plasma calcium, bone, 25-hydroxycholecalciferol-1-hydroxylase, and duodenal calcium-binding protein, measured by a radioimmunoassay in chicks. Endocrinology. 1979 May;104(5):1455–1460. doi: 10.1210/endo-104-5-1455. [DOI] [PubMed] [Google Scholar]
- Bar A., Wasserman R. H. Control of calcium absorption and intestinal calcium-binding protein synthesis. Biochem Biophys Res Commun. 1973 Sep 5;54(1):191–196. doi: 10.1016/0006-291x(73)90907-8. [DOI] [PubMed] [Google Scholar]
- Borke J. L., Caride A., Verma A. K., Kelley L. K., Smith C. H., Penniston J. T., Kumar R. Calcium pump epitopes in placental trophoblast basal plasma membranes. Am J Physiol. 1989 Aug;257(2 Pt 1):c341–c346. doi: 10.1152/ajpcell.1989.257.2.C341. [DOI] [PubMed] [Google Scholar]
- Borke J. L., Caride A., Verma A. K., Penniston J. T., Kumar R. Cellular and segmental distribution of Ca2(+)-pump epitopes in rat intestine. Pflugers Arch. 1990 Sep;417(1):120–122. doi: 10.1007/BF00370781. [DOI] [PubMed] [Google Scholar]
- Borke J. L., Caride A., Verma A. K., Penniston J. T., Kumar R. Plasma membrane calcium pump and 28-kDa calcium binding protein in cells of rat kidney distal tubules. Am J Physiol. 1989 Nov;257(5 Pt 2):F842–F849. doi: 10.1152/ajprenal.1989.257.5.F842. [DOI] [PubMed] [Google Scholar]
- Borke J. L., Minami J., Verma A. K., Penniston J. T., Kumar R. Co-localization of erythrocyte Ca++-Mg++ ATPase and vitamin D-dependent 28-kDa-calcium binding protein. Kidney Int. 1988 Aug;34(2):262–267. doi: 10.1038/ki.1988.174. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Dudeja P. K., Eby B., Lau K. Correction by 1-25-dihydroxycholecalciferol of the abnormal fluidity and lipid composition of enterocyte brush border membranes in vitamin D-deprived rats. J Biol Chem. 1986 Dec 15;261(35):16404–16409. [PubMed] [Google Scholar]
- Chandler J. S., Calnek D., Quaroni A. Identification and characterization of rat intestinal keratins. Molecular cloning of cDNAs encoding cytokeratins 8, 19, and a new 49-kDa type I cytokeratin (cytokeratin 21) expressed by differentiated intestinal epithelial cells. J Biol Chem. 1991 Jun 25;266(18):11932–11938. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Corradino R. A. Embryonic chick intestine in organ culture: interaction of adenylate cyclase system and vitamin D3-mediated calcium absorptive mechanism. Endocrinology. 1974 Jun;94(6):1607–1614. doi: 10.1210/endo-94-6-1607. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J. 1988 Mar 1;2(3):224–236. [PubMed] [Google Scholar]
- Dupret J. M., Brun P., Perret C., Lomri N., Thomasset M., Cuisinier-Gleizes P. Transcriptional and post-transcriptional regulation of vitamin D-dependent calcium-binding protein gene expression in the rat duodenum by 1,25-dihydroxycholecalciferol. J Biol Chem. 1987 Dec 5;262(34):16553–16557. [PubMed] [Google Scholar]
- Favus M. J., Tembe V., Ambrosic K. A., Nellans H. N. Effects of 1,25(OH)2D3 on enterocyte basolateral membrane Ca transport in rats. Am J Physiol. 1989 Mar;256(3 Pt 1):G613–G617. doi: 10.1152/ajpgi.1989.256.3.G613. [DOI] [PubMed] [Google Scholar]
- Fox J., Pickard D. W., Care A. D., Murray T. M. Effect of low phosphorus diets on intestinal calcium absorption and the concentration of calcium-binding protein in intact and parathyroidectomized pigs. J Endocrinol. 1978 Sep;78(3):379–387. doi: 10.1677/joe.0.0780379. [DOI] [PubMed] [Google Scholar]
- Friedlander E. J., Henry H. L., Norman A. W. Studies on the mode of action of calciferol. Effects of dietary calcium and phosphorus on the relationship between the 25-hydroxyvitamin D3-1alpha-hydroxylase and production of chick intestinal calcium binding protein. J Biol Chem. 1977 Dec 10;252(23):8677–8683. [PubMed] [Google Scholar]
- Ghijsen W. E., Van Os C. H. 1 alpha, 25-Dihydroxy-vitamin D-3 regulates ATP-dependent calcium transport in basolateral plasma membranes of rat enterocytes. Biochim Biophys Acta. 1982 Jul 14;689(1):170–172. doi: 10.1016/0005-2736(82)90202-4. [DOI] [PubMed] [Google Scholar]
- Goff J. P., Reinhardt T. A., Engstrom G. W., Horst R. L. Effect of dietary calcium or phosphorus restriction and 1,25-dihydroxyvitamin D administration on rat intestinal 24-hydroxylase. Endocrinology. 1992 Jul;131(1):101–104. doi: 10.1210/endo.131.1.1611988. [DOI] [PubMed] [Google Scholar]
- Greeb J., Shull G. E. Molecular cloning of a third isoform of the calmodulin-sensitive plasma membrane Ca2+-transporting ATPase that is expressed predominantly in brain and skeletal muscle. J Biol Chem. 1989 Nov 5;264(31):18569–18576. [PubMed] [Google Scholar]
- Guillemant J., Guillemant S. Early rise in cyclic GMP after 1,25-dihydroxycholecalciferol administration in the chick intestinal mucosa. Biochem Biophys Res Commun. 1980 Apr 14;93(3):906–911. doi: 10.1016/0006-291x(80)91161-4. [DOI] [PubMed] [Google Scholar]
- Lomri A., Baron R. 1 alpha,25-dihydroxyvitamin D3 regulates the transcription of carbonic anhydrase II mRNA in avian myelomonocytes. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4688–4692. doi: 10.1073/pnas.89.10.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Fullmer C. S., Wasserman R. H., Komm B. S., Haussler M. R. Dietary restriction of calcium, phosphorus, and vitamin D elicits differential regulation of the mRNAs for avian intestinal calbindin-D28k and the 1,25-dihydroxyvitamin D3 receptor. J Bone Miner Res. 1992 Apr;7(4):441–448. doi: 10.1002/jbmr.5650070412. [DOI] [PubMed] [Google Scholar]
- Morrissey R. L., Wasserman R. H. Calcium absorption and calcium-binding protein in chicks on differing calcium and phosphorus intakes. Am J Physiol. 1971 May;220(5):1509–1515. doi: 10.1152/ajplegacy.1971.220.5.1509. [DOI] [PubMed] [Google Scholar]
- Mykkanen H. M., Wasserman R. H. Reactivity of sulfhydryl groups in the brush-border membranes of chick duodena is increased by 1,25-dihydroxycholecalciferol. Biochim Biophys Acta. 1990 Mar 26;1033(3):282–286. doi: 10.1016/0304-4165(90)90134-i. [DOI] [PubMed] [Google Scholar]
- Mykkänen H. M., Wasserman R. H. Effect of vitamin D on the intestinal absorption of 203Pb and 47Ca in chicks. J Nutr. 1982 Mar;112(3):520–527. doi: 10.1093/jn/112.3.520. [DOI] [PubMed] [Google Scholar]
- Norman A. W. Hormonal actions of vitamin D. Curr Top Cell Regul. 1984;24:35–49. doi: 10.1016/b978-0-12-152824-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Ozono K., Sone T., Pike J. W. The genomic mechanism of action of 1,25-dihydroxyvitamin D3. J Bone Miner Res. 1991 Oct;6(10):1021–1027. doi: 10.1002/jbmr.5650061002. [DOI] [PubMed] [Google Scholar]
- Pansu D., Bellaton C., Bronner F. Effect of Ca intake on saturable and nonsaturable components of duodenal Ca transport. Am J Physiol. 1981 Jan;240(1):G32–G37. doi: 10.1152/ajpgi.1981.240.1.G32. [DOI] [PubMed] [Google Scholar]
- Rader J. I., Baylink D. J., Hughes M. R., Safilian E. F., Haussler M. R. Calcium and phosphorus deficiency in rats: effects on PTH and 1,25-dihydroxyvitamin D3. Am J Physiol. 1979 Feb;236(2):E118–E122. doi: 10.1152/ajpendo.1979.236.2.E118. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Matsumoto T., Fontaine O., Goodman D. B. Role of changes in membrane lipid structure in the action of 1,25-dihydroxyvitamin D3. Fed Proc. 1982 Jan;41(1):72–77. [PubMed] [Google Scholar]
- Ribovich M. L., DeLuca H. F. Effect of dietary calcium and phosphorus on intestinal calcium absorption and vitamin D metabolism. Arch Biochem Biophys. 1978 May;188(1):145–156. doi: 10.1016/0003-9861(78)90367-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler E. E. Recent advances in the molecular characterization of plasma membrane Ca2+ pumps. J Membr Biol. 1991 Feb;120(1):1–15. doi: 10.1007/BF01868586. [DOI] [PubMed] [Google Scholar]
- Swaminathan R., Sommerville B. A., Care A. D. The effect of dietary calcium on the activity of 25-hydroxycholecalciferol-1-hydroxylase and Ca absorption in vitamin D-replete chicks. Br J Nutr. 1977 Jul;38(1):47–54. doi: 10.1079/bjn19770060. [DOI] [PubMed] [Google Scholar]
- Takito J., Shinki T., Sasaki T., Suda T. Calcium uptake by brush-border and basolateral membrane vesicles in chick duodenum. Am J Physiol. 1990 Jan;258(1 Pt 1):G16–G23. doi: 10.1152/ajpgi.1990.258.1.G16. [DOI] [PubMed] [Google Scholar]
- Takito J., Shinki T., Tanaka H., Suda T. Mechanism of regulation of calcium-pumping activity in chick intestine. Am J Physiol. 1992 May;262(5 Pt 1):G797–G805. doi: 10.1152/ajpgi.1992.262.5.G797. [DOI] [PubMed] [Google Scholar]
- Theofan G., Norman A. W. Effects of alpha-amanitin and cycloheximide on 1,25-dihydroxyvitamin D3-dependent calbindin-D28K and its mRNA in vitamin D3-replete chick intestine. J Biol Chem. 1986 Jun 5;261(16):7311–7315. [PubMed] [Google Scholar]
- Thomasset M., Cuisinier-Gleizes P., Mathieu H. Differences in duodenal calcium-binding protein (CaBP) in response to a low-calcium or a low-phosphorus intake. Calcif Tissue Res. 1977 May;22 (Suppl):45–50. doi: 10.1007/BF02064039. [DOI] [PubMed] [Google Scholar]
- Timmermans J. A., Kaune R., Bindels R. J., van Os C. H. Quantification of Ca(2+)-ATPases in porcine duodenum. Effects of 1,25(OH)2D3 deficiency. Biochim Biophys Acta. 1991 Jun 18;1065(2):177–184. doi: 10.1016/0005-2736(91)90228-z. [DOI] [PubMed] [Google Scholar]
- Varghese S., Deaven L. L., Huang Y. C., Gill R. K., Iacopino A. M., Christakos S. Transcriptional regulation and chromosomal assignment of the mammalian calbindin-D28k gene. Mol Endocrinol. 1989 Mar;3(3):495–502. doi: 10.1210/mend-3-3-495. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Filoteo A. G., Stanford D. R., Wieben E. D., Penniston J. T., Strehler E. E., Fischer R., Heim R., Vogel G., Mathews S. Complete primary structure of a human plasma membrane Ca2+ pump. J Biol Chem. 1988 Oct 5;263(28):14152–14159. [PubMed] [Google Scholar]
- Walling M. W., Brasitus T. A., Kimberg D. V. Elevation of cyclic AMP levels and adenylate cyclase activity in duodenal mucosa from vitamin D-deficient rats by 1alpha,25-dihydroxycholecalciferol (1alpha,25-(OH)2D3). Endocr Res Commun. 1976;3(1):83–91. doi: 10.3109/07435807609057743. [DOI] [PubMed] [Google Scholar]
- Walters J. R., Weiser M. M. Calcium transport by rat duodenal villus and crypt basolateral membranes. Am J Physiol. 1987 Feb;252(2 Pt 1):G170–G177. doi: 10.1152/ajpgi.1987.252.2.G170. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Smith C. A., Brindak M. E., De Talamoni N., Fullmer C. S., Penniston J. T., Kumar R. Vitamin D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology. 1992 Mar;102(3):886–894. doi: 10.1016/0016-5085(92)90174-w. [DOI] [PubMed] [Google Scholar]
- Zelinski J. M., Sykes D. E., Weiser M. M. The effect of vitamin D on rat intestinal plasma membrane CA-pump mRNA. Biochem Biophys Res Commun. 1991 Sep 16;179(2):749–755. doi: 10.1016/0006-291x(91)91880-l. [DOI] [PubMed] [Google Scholar]
- de Boland A. R., Norman A. Evidence for involvement of protein kinase C and cyclic adenosine 3',5' monophosphate-dependent protein kinase in the 1,25-dihydroxy-vitamin D3-mediated rapid stimulation of intestinal calcium transport, (transcaltachia). Endocrinology. 1990 Jul;127(1):39–45. doi: 10.1210/endo-127-1-39. [DOI] [PubMed] [Google Scholar]