Abstract
Initial identification of osteoactivin (OA)/glycoprotein non-melanoma clone B (gpnmb) was demonstrated in an osteopetrotic rat model, where OA expression was increased 3-fold in mutant bones, compared to normal. OA mRNA and protein expression increase during active bone regeneration post-fracture, and primary rat osteoblasts show increased OA expression during differentiation in vitro. To further examine OA/gpnmb as an osteoinductive agent, we characterized the skeletal phenotype of transgenic mouse overexpressing OA/gpnmb under the CMV-promoter (OA-Tg). Western blot analysis showed increased OA/gpnmb in OA-Tg osteoblasts, compared to wild-type (WT). In OA-Tg mouse femurs versus WT littermates, micro-CT analysis showed increased trabecular bone volume and thickness, and cortical bone thickness; histomorphometry showed increased osteoblast numbers, bone formation and mineral apposition rates in OA-Tg mice; and biomechanical testing showed higher peak moment and stiffness. Given that OA/gpnmb is also over-expressed in osteoclasts in OA-Tg mice, we evaluated bone resorption by ELISA and histomorphometry, and observed decreased serum CTX-1 and RANK-L, and decreased osteoclast numbers in OA-Tg, compared to WT mice, indicating decreased bone remodeling in OA-Tg mice. The proliferation rate of OA-Tg osteoblasts in vitro was higher, compared to WT, as was alkaline phosphatase staining and activity, the latter indicating enhanced differentiation of OA-Tg osteoprogenitors. Quantitative RT-PCR analysis showed increased TGF-β1 and TGF-β receptors I and II expression in OA-Tg osteoblasts, compared to WT. Together, these data suggest that OA overexpression has an osteoinductive effect on bone mass in vivo and stimulates osteoprogenitor differentiation ex vivo.
Keywords: Osteoactivin/Gpnmb, Bone, Osteoblast differentiation, Growth factors
Introduction
In normal bone remodeling, osteoclast-mediated bone resorption is coupled with an equivalent amount of osteoblast-mediated bone formation. However, low bone density with both osteopenia and osteoporosis is the result of an imbalance in the bone remodeling process in which the rate of bone resorption exceeds the rate of bone formation. There are two basic approaches to potentially reverse this: the inhibition of bone resorbing cells or the stimulation of bone forming cells, and if each alone is not sufficient, the synergistic effect of the combining both approaches may be useful.
Osteoactivin (OA)/gpnmb has been shown to be a prominent factor in bone and plays a novel role in bone formation. To date, studies have identified OA/gpnmb as an osteoinductive agent that enhances bone formation and stimulates osteoblast differentiation (Abdelmagid et al., 2008; Abdelmagid et al., 2014; Safadi et al., 2001; Selim et al., 2003). High levels of OA/gpnmb expression in bone were initially described in a rat model of osteopetrosis in which its expression was increased during osteoblast differentiation (Safadi et al., 2001). In primary rat osteoblast cultures, OA/gpnmb expression increases over time, with maximal expression during matrix mineralization, the final stage of differentiation (Abdelmagid et al., 2008; Owen et al., 2003; Safadi et al., 2001). In a rat femur fracture repair model, OA/gpnmb mRNA expression was higher in the callus at 3- and 10-days post-fracture, and OA/gpnmb protein immunoexpression was elevated throughout the reparative phase of the hard callus, compared to intact femurs suggesting that the OA/gpnmb plays a role in osteogenesis (Abdelmagid et al., 2010). Furthermore, using a model of local delivery system in which recombinant OA/gpnmb (rOA/rgpnmb) was administered via a single injection into the marrow cavity of rat femur, our laboratory showed that rOA/rgpnmb-injected femurs had islands of newly formed woven bone within the marrow cavity (Singh et al., 2010). Angiogenesis was observed adjacent to those islands, and multiple rows of osteoblasts and/or osteoprogenitors were detected near the periphery of the new bone. In contrast, no evidence of an osteogenic response was observed in the control-injected group (Singh et al., 2010).
Few animal models have been generated to demonstrate the function of OA/gpnmb in various tissues. The importance of OA/gpnmb in osteogenesis was confirmed in mice with a natural mutation in the OA/gpnmb gene in which a premature stop codon resulted in the generation of a truncated OA/gpnmb protein. These mice also exhibited decreased bone mass (Abdelmagid et al., 2014). Transgenic mice that overexpress OA/gpnmb under the control of the tartrate-resistant acid phosphatase (TRAP) exon 1B/C promoter show significant bone loss due to increased bone resorption (Sheng et al., 2012). Histomorphometric bone formation parameters and plasma levels of bone formation biomarkers were not different between transgenic mice and WT littermates, supporting the idea that bone loss in OA-Tg mice is due to an increase in bone resorption and not to a reduction in bone formation (Sheng et al., 2012).
In the current study, given that OA/gpnmb is a vital glycoprotein required for the differentiation and function of both osteoblasts and osteoclasts, osteoactivin transgenic mice were generated (under the control of the CMV promoter) in order to examine the effects of OA/gpnmb overexpression on skeletogenesis. Specifically, our aim was to characterize the skeletal phenotype of this transgenic model. We hypothesized that over-expression of OA/gpnmb positively regulates bone mass in vivo in a transgenic mouse model, and enhances osteoblast differentiation in vitro, using cells from these transgenic mice.
Material and Methods
Animals
The colony of OA-transgenic (OA-Tg) mice was generated by over-expression of OA/gpnmb under control of the CMV promoter. The targeting vector was confirmed for the presence of the OA transgene using MC3T3-E1 osteoblast-like cells; we found that OA/gpnmb is over-expressed over 3-fold, compared to empty-vector control cells (data not shown). This targeting vector was used to generate the OA/gpnmb transgenic (OA-Tg) mice using the University of Pennsylvania Transgenic Core Facility. Six lines were established by breeding the founders with CD-1 wild type mice. Breeding of the hemizygous OA-transgenic mice with CD-1 mice showed Mendelian inheritance of the transgene with an equal sex distribution among the litters. Mice were backcrossed for 8–10 generations using C57BL/6 mice. OA/gpnmb-expression in the transgene was examined and was markedly up-regulated. A second line of OA-transgenic mice was then established and used for further analysis of the skeletal phenotype. Mice from this line survive and breed well. OA/gpnmb expression was increased over 3-fold in transgenic mice, compared to age-matched wild type mice (data not shown). A C57BL/6 mouse strain was used as wild-type (WT) controls. All animals were maintained at Temple University School of Medicine Central Animal Barrier Facility, and used according to the principles in the NIH Guide for the Care and Use of Laboratory Animals, and with the respect to the guidelines established by the Institutional Animal Care and Use Committee.
Micro-Computed Tomography (Micro-CT) Analysis
Femurs from WT and OA-Tg mice that were 4- and 12-weeks of age, and 12 months of age, were scanned (n≥4/group), utilizing a Skyscan 1172 system (Bruker, Billerica, MA), at a pixel size of 7.5 μm, with a source voltage and current of 70 kV and 114 μA, respectively. A 0.5 mm Al Filter was used to minimize the beam hardening from the polychromatic nature of the sealed X-ray source. Following scanning, three-dimensional (3D) microstructural images were reconstructed using SkyscanNRecon software, and structural indices of bone were computed using a marching-cubes algorithm in 3D. Volumes of interest were defined and structural indices calculated using Skyscan CT Analyzer software. The region of interest for trabecular micro-architectural variables started 0.375 mm below the growth plate of the femoral distal epiphysis and then extended 2.5 mm proximally towards the mid-diaphysis to measure trabecular bone volume (BV) per total volume (BV/TV), mean trabecular thickness (Tb.Th), mean trabecular number (Tb.N), and mean trabecular separation (Tb.Sp) indices. The volume of interest for cortical micro-architectural variables started 3 mm below the growth plate of the femoral distal epiphysis and then extended 1.5 mm proximally towards the central diaphysis to measure mid-diaphyseal cortical thickness (Ct.Th), bone perimeter (B.Pm), and percent cortical porosity (Ct.Po) indices. 3D reconstructed images of the sagittal and axial planes of the femoral metaphysis and diaphysis were generated using SkyScan CTvox software.
Three-Point Bending
Breaking strength of the right femur was measured under 3-point bending using a material testing machine (Bose, Eden Prairie, MN) fitted with a 1,000 N load cell. Wild-type and OA-Tg mice were sacrificed at 12 weeks of age and femurs were collected (n ≥ 4/group). Superficial muscles were dissected off and the femurs were placed in 1X PBS (Cellgro-Mediatech, Manassas, VA). Samples were preserved at −20°C until testing. Prior to testing, the bones were thawed in saline at room temperature to ensure hydration. At the time of testing, femurs were individually positioned on the loading fixture anterior side down and loaded first in the anterior–posterior plane, and then loaded to failure at a rate of 0.05 mm/s, during which displacement and force were collected (100 Hz). Bending moments were calculated from the force (F) data (M=FL/4; N mm), as previously described (Joshi et al., 2011). Displacement data were divided by L2/12 (mm/mm2), where L was the distance between the lower supports. Whole-bone mechanical properties were then determined from the moment versus normalized displacement curves, including peak moment (N mm, ultimate load the specimen sustained) and stiffness (N mm2, the slope of the initial linear portion of the moment–displacement curve).
Histological and Histomorphometric Analyses
After sacrifice, femurs of 12-weeks-old WT and OA-Tg mice were dissected out (n ≥ 4/group), fixed in buffered 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), dehydrated, and embedded undecalcified in methylmethacrylate. To measure dynamic bone formation parameters, calcein (i.p., 10 mg/kg body weight) had been previously injected at 7 and 2 days before euthanasia. Bones were sectioned into 3 μm longitudinal sections, placed onto slides, and dried at 60°C overnight. Unstained plasticized longitudinal sections were used for the measurement of calcein fluorochrome label, using a Nikon microscope, digital camera, and image analysis system (Bioquant Osteo 2012, v12.1, Nashville, TN). Adjacent sections were deplasticized before staining with von Kossa and toluidine blue, or Masson’s Trichrome staining. Static and dynamic histomorphometric analyses were then performed, as described previously (Abdelmagid et al., 2014).
Biochemical Analysis
Sera were collected from 4-, 8-, and 12-week old WT and OA-Tg mice (n≥5/group). ELISA was conducted using the manufacturer’s protocol to determine serum levels of RANKL (receptor activator of nuclear factor kappa-B ligand) and OPG (osteoprotegrin) using Quantikine® ELISA (R&D Systems, Minneapolis, MN), and for levels of CTX (C-terminal telopeptide) using BioTang (Waltham, MA). The optical absorbance of the solution was measured at 450 nm using a micro-plate reader (iMark, Bio-Rad). The concentration of proteins present in the sera was quantified from the slope standard curve estimation and normalized to μg protein per ml of serum assayed.
Primary Osteoblast and Bone Marrow-Derived Mesenchymal Stem Cell Isolation and Culture
For calvarial primary osteoprogenitors, parietal calvaria were dissected out from WT and OA-Tg neonatal pups (approximately 3–6 days of age). Following dissection, calvaria pieces were digested using 0.1% Collagenase (Sigma) in 2.5% trypsin (Invitrogen) at 37°C. Cells released from subsequent digestions were plated in 100 mm dishes (Corning Life Sciences, Lowell, MA) at a density of 5×105 cells/plate in Alpha Minimal Essential Medium (α-MEM; Cellgro-Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS; HyClone), hence called growth medium, and 1% penicillin/streptomycin solution (Invitrogen). The cells were incubated at 37°C with 5% CO2 and culture media were changed every three days until they reached ~80% confluence. For mesenchymal stem cell (MSC) isolation, bone marrow was flushed out of the long bones of 8-week-old WT and OA-Tg mice under aseptic conditions, centrifuged and resuspended into growth media and plated in 100 mm dishes in an incubator at 37°C with 5% CO2. The next day, non-adherent cells were washed off the plates with 1X PBS. The adherent cells were maintained and the culture media were changed every three days. In order to differentiate osteoblasts from osteoprogenitors, cells were cultured in the growth medium containing 50 μg/ml ascorbic acid (Sigma Aldrich) and 10 mM β-glycerophosphate (Sigma Aldrich), hence called osteogenic medium. In addition to osteogenic media, stromal cell cultures were also supplemented with 0.1 μM dexamethasone (Sigma; Australia Register Number: 16375; Melbourne, Victoria, Australia). Osteoblast cultures were maintained by changing the osteogenic medium every third day and stopped at specific time points according to the desired assay.
Cell Proliferation
To determine cell numbers, a CyQUANT® NF Cell Proliferation Assay Kit (Molecular Probes) was used according to the manufacturer’s protocol. Briefly, WT and OA-Tg primary osteoblasts were plated at a density of 4 × 103 cells/well in a 96-well plate (Falcon), with a minimum of six replicates per condition per independent experiment, in growth media and incubated at 37°C in the presence of 5% CO2. On day 3, the culture media was aspirated from all wells to be tested and replaced with DNA binding dye solution. Cells were incubated at 37°C for 1 hour and samples were measured using a Wallac 1420 fluorometer. Cell numbers were calculated based on a standard curve generated for primary osteoblasts.
Alkaline Phosphatase Staining and Activity
For alkaline phosphatase (ALP) staining, WT and OA-Tg osteoprogenitors or MSCs were plated at a density of 5 × 104 cells/well in a 24-well plate (Corning) with osteogenic media (n ≥ 4 replicates/condition/experiment). On day 14, osteoblast cultures were stained for ALP using the Leukocyte Alkaline Phosphatase Kit (Sigma) according to the manufacturer’s protocol. Briefly, wells were washed with 1X Hank’s Balanced Salt Solution (HBSS) (Cellgro-Mediatech), fixed, rinsed twice with ddH2O, and incubated for 15 minutes at RT in a staining solution (sodium nitrate, FRV-Alkaline, and Naphthol AS-B Alkaline solutions). Following incubation, wells were rinsed with ddH2O and allowed to air dry. To examine the staining pattern and to capture images, a Nikon Eclipse TE300 inverted microscope interfaced with a Nikon Digital Sight DS Camera and NIS Elements F software was used. For ALP activity, osteoblast cultures at day 14 were evaluated using a LabAssay™ALP kit (Wako, Richmond, VA). Briefly, wells were washed with 1X HBSS and lysed using assay buffer with 0.2% Triton X-100, and the ALP assay was carried out according to the manufacturer’s protocol. Prior to beginning the assay, the following reagents were prepared: working assay solution and a dilution series of the standard buffer solution. Briefly, samples were incubated with the working solution (p-Nitrophenylphosphate Disodium 6.7 mmol/L) for 15 minutes at 37°C. After incubation, Stop Solution was added and the absorbance was measured at 405 nm using a microplate reader (Bio-Rad). The absorbance readings were used to generate the standard curve. ALP activity was calculated and normalized to the total μg protein/sample. Protein concentrations were quantified by BCA protein assay kit (Pierce).
RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated from cell cultures of WT and OA-Tg osteoblasts at days 7, 14, and 21 using TRIzol reagent (Invitrogen). RNA was purified using the RNeasy Mini Kit (Qiagen) and treated with RNase-Free DNase (Qiagen). RNA concentrations were measured using DU®640 spectrophotometry (Coulter, Jersey, NJ). RNA integrity of all samples was confirmed using 1% formaldehyde-agarose gels. For cDNA synthesis, 1 μg of cDNA was transcribed from total RNA using SuperScript® First-Strand Synthesis kit (Invitrogen). Gene expression profile for alkaline phosphatase (ALP), runt-related transcription factor 2 (Runx-2), collagen type I alpha 1 (Col1a1), transforming growth factor beta 1 (TGF-β1), transforming growth factor beta receptors I and II (TGF-β RI, TGF-β RII) was determined by quantitative real time PCR (qPCR) using SYBER Green Master Mix (Applied Biosystems, Foster City, CA). qPCR was performed using an ABI Model 7500 Real-Time PCR system (Applied Biosystems) in triplicate. Specific PCR primers were synthesized and optimized for amplification of each cDNA. Primers are listed in Table 2. The cycling parameters were as follows: an initial step of 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 sec, and 60°C for 1 min. Gene expression analysis was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), using delta delta CT. At least three independent experiments were performed for each gene expression.
Table 2.
qPCR primers
| Gene Symbol | Primer Sequence (5′-3′) Forward, Reverse | Product size: bp | Accession number |
|---|---|---|---|
| GAPDH | ATCTTGGGCTACACTGAGGA CAGGAAATGAGCTTGACAAAGT |
122 | NM_008084 |
| ALP | TCCTGACCAAAAACCTCAAAGG TGCTTCATGCAGAGCCTGC |
101 | NM_007431 |
| Runx-2 | CCGTGGCCTTCAAGGTTGT TTCATAACAGCGGAGGCATTT |
118 | NM_001146038 |
| Col1a1 | TGGCAAAGACGGACTCAAC GGCAGGAAGCTGAAGTCATAA |
156 | NM_007742 |
| TGF-β1 | GCTAATGGTGGACCGCAACAACG CTTGCTGTACTGTGTGTCCAGGC |
682 | NM_011577 |
| TGFβ RI | AGTGGTCTTGCCCATCTTC GGCAATAGCTGGTTTTCCT |
60 | NM_009370 |
| TGFβ RII | AGATGGCTCGCTGAACACTACCAA AGAATCCTGCTGCCTCTGGTCTTT |
100 | NM_009371 |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; ALP: alkaline phosphatase; Runx-2: runt-related transcription factor 2; Col1a1: collagen type 1 alpha 1; TGF-β1: transforming growth factor beta 1; TGFβ RI: transforming growth factor beta receptor I; TGFβ RII: transforming growth factor beta receptor II.
Osteogenesis Array
Total RNA from WT and OA-Tg osteoblast cultures was isolated, purified, and quantified, as described above. cDNA was synthesized using RT2 First Strand Kit (SABiosciences; Qiagen). Gene expression profiling was performed using RT2 Profiler PCR Array; mouse osteogenesis (SABiosciences; Qiagen) and qRT-PCR was performed, as described (Abdelmagid et al., 2014).
Protein Isolation and Western Blotting
WT and OA-Tg cells were washed with 1X PBS and lysed in 1X RIPA (Millipore). Protein concentrations in the cell lysates were determined using a BCA Protein Assay Reagent Kit (Pierce, IL). Protein Samples (30μg) were mixed with denaturing buffer, heated to 100°C, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Total proteins were transferred to nitrocellulose membrane (Bio-Rad) using semi-dry transfer apparatus (Bio-Rad). The membranes were blocked with 5% milk in Tris-buffered saline (TBS)-0.1% Tween-20 (TBST) for 1 h and then incubated with the following primary antibodies: custom made anti-chicken OA (concentration of 1:250), produced as previously described (Abdelmagid et al., 2007), and anti-rabbit actin (concentration of 1:1000; Sigma), overnight. Blots were washed with 1X TBS-0.1% Tween-20 (TBST) and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies as appropriate (donkey anti-chicken or anti-rabbit IgG, Jackson Immunoresearch), in a blocking buffer. Blots were washed with TBST, incubated with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific), and exposed to X-ray film, which was developed using Konica SRX-101A Medical Film Processor (Taiwan). Quantification of the immunoblots was performed using Image J software (version 1.49n, NIH Image), and results of OA were normalized to β-actin.
Immunocytochemistry
Primary osteoblasts from WT and OA-Tg mice were plated in 2-well chamber slides (Lab-Tek, Naperville, IL) at a density of 3,000 cells per well. The next day, cells were washed with 1X PBS, fixed with 4% PFA, washed with (1X PBS-0.05% Tween). Cells were permeabilized by 1X PBS-0.1% Triton-X100, rinsed twice with wash buffer, blocked with (1X PBS-2.5% BSA), and incubated with anti-chicken OA primary antibody (concentration of 1:250) at 4°C overnight. The next day, chambers were washed with wash buffer and incubated with the appropriate secondary antibody (concentration of 1:500; Jackson Immunoresearch) for 1hr. Cells were then rinsed with wash buffer, mounted with DAPI-Vectashield (Vector Laboratories, CA) and coverslipped. Slides were examined using confocal microscope. Captured images were merged using Image J software (version 1.49n, NIH Image).
Statistical Analysis
The statistical differences between WT and OA transgenic groups for the in vivo and in vitro data were determined using unpaired, two-tailed Student’s t-tests. One-way analysis of variance (ANOVA) with Bonferroni post-hoc tests were used for multiple comparisons and adjusted p-values are reported. All statistical analyses were generated using GraphPad Prism Software version 4 (San Diego, CA). The differences were considered statistically significant if the p-value < 0.05. All data are represented as mean plus standard error of the mean (SEM). All in vitro data are representative of at least three independent experiments.
Results
Generation of osteoactivin/gpnmb transgenic (OA-Tg) mice
Our group previously showed that OA/gpnmb is a principal bone anabolic factor (Abdelmagid et al., 2008; Abdelmagid et al., 2014; Safadi et al., 2001). To confirm the importance of OA in osteogenesis, osteoactivin/gpnmb transgenic mice (OA-Tg) were generated (under the control of CMV promoter). The targeted over-expression vector was confirmed for the presence of OA in MC3T3-E1 osteoblast-like cells (data not shown). Our results demonstrating that OA is over-expressed > 3 fold when compared to control untransfected control cells (data not shown). The targeted over-expression vector was used to generate the OA-Tg transgenic mice. To examine expression of the OA-transgene, multiple tissues were used to examine OA-transgene mRNA using PCR. The OA-transgene expression was confirmed using Northern blot analysis. Transgene expression was markedly significant in all tissues examined (data not shown).
To confirm that OA-Tg mice over-expressed OA/gpnmb in bone, the cellular OA/gpnmb protein levels were measured via Western blot in osteoblast cultures derived either from neonatal calvaria of OA-Tg and WT or from bone marrow of 8-week-old OA-Tg mice and WT littermates. Fig. 1A and B show that the different molecular weights (115, 65, and 47 kDa) of the OA protein were increased in OA-Tg osteoblasts, compared to WT. OA/gpnmb overexpression in OA-Tg versus WT mice was also confirmed via immunofluorescent staining in primary osteoblasts cultured for 24 hours, then fixed and stained with the anti-OA/gpnmb antibody (red signal) (Fig. 1C).
Figure 1. Over-expression of OA/gpnmb in osteoblasts from transgenic mice.
(A) Representative Western blot of OA/gpnmb protein level in primary osteoblasts from OA/gpnmb transgenic (OA-Tg) mice and wild-type (WT) littermates. (B) Densitometric analysis of the relative density of a three OA protein bands (115, 65 and 47 kDa). OA/gpnmb expression levels were normalized to β-actin. (C) Immunofluorescent staining of OA/gpnmb (red) and nuclei (DAPI stained blue) in WT and OA-Tg primary osteoblasts using anti-OA/gpnmb antibody. Images were obtained using a confocal microscope with a 60 × objective. Experiments were repeated three times with similar results. * p < 0.05, ** p < 0.01, *** p < 0.001., compared to WT mice.
Over-expression of osteoactivin enhances bone mass in vivo
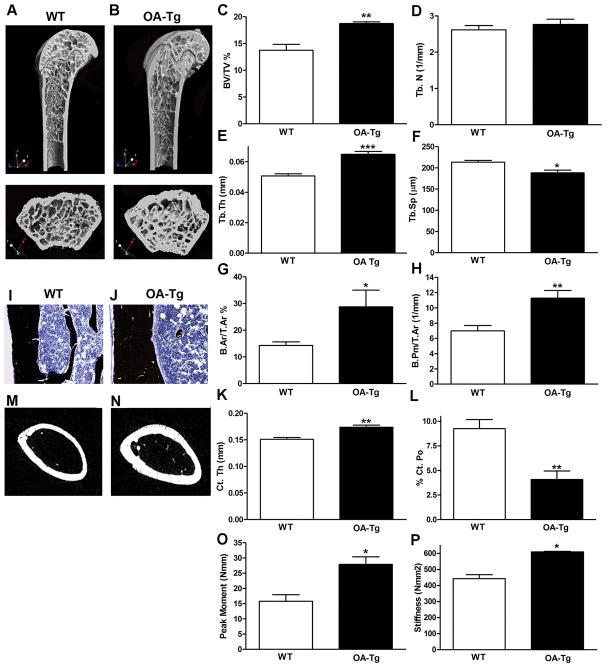
Given that OA/gpnmb expression is increased in bones in a rat model of osteopetrosis (Safadi et al., 2001), we sought to evaluate the effect of systemic OA over-expression in bones in vivo. Femurs harvested from WT and OA-Tg mice that were 4- and 12-weeks of age as well as 12-months of age, of both genders, were scanned using micro-CT, and the distal metaphyseal region was analyzed for trabecular bone parameters. All data are listed in Table 1, while data from 12-week old males is shown in Figure 2. Cross-sectional and longitudinal sections from distal metaphyseal regions of OA-Tg femurs showed increased bone, compared to WT mice (Fig. 2A and B). Specifically, we observed increased trabecular bone volume (BV/TV) and trabecular thickness (Tb. Th) in OA-Tg femurs, compared to WT femurs (Fig. 2C and E). In contrast, there was a reciprocal reduction in trabecular separation (Tb. Sp) in OA-Tg mice, compared to WT littermates (Fig. 2F). The increase in trabecular bone in OA-Tg mice was also demonstrated using histomorphometry, which showed increased trabecular bone area as a percentage of total bone area (B.Ar/T.Ar %), and increased trabecular bone perimeter (B.Pm/T.Ar) (Fig. 2G and H).
Table 1.
Micro-CT analysis of long bone microstructure in wild type (WT) and osteoactivin transgenic (OA-Tg) femora
| μ-CT Parameters Genotype |
4-week | 12-week | 12-month | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | ||
| BV/TV (%) | WT | 5.22±0.26 | 4.11±0.27 | 13.76±1.08 | 6.23±0.64 | 4.14±0.31 | 3.78±0.25 |
|
| |||||||
| OA-Tg | 8.57±0.68*** | 9.02±0.83*** | 18.70±0.36** | 6.30±0.53 | 5.90±0.40* | 4.22±0.30** | |
|
| |||||||
| Tb.Th (mm) | WT | 0.04±0.00 | 0.04±0.00 | 0.05±0.00 | 0.04±0.001 | 0.04±0.002 | 0.05±0.005 |
|
| |||||||
| OA-Tg | 0.04±0.00 | 0.04±0.00 | 0.06±0.00*** | 0.05±0.001 | 0.06±0.002* | 0.06±0.003 | |
|
| |||||||
| Tb.N (1/mm) | WT | 1.31±0.05 | 1.07±0.08 | 2.62±0.12 | 1.10±0.14 | 0.82±0.05 | 0.13±0.03 |
|
| |||||||
| OA-Tg | 2.07±0.24** | 2.25±0.17*** | 3.00±0.17 | 1.54±0.07* | 0.96±0.07 | 0.70±0.05*** | |
|
| |||||||
| Tb. Sp (mm) | WT | 0.50±0.01 | 0.53±0.01 | 0.21±0.00 | 0.25±0.01 | 0.30±0.005 | 0.62±0.04 |
|
| |||||||
| OA-Tg | 0.36±0.03*** | 0.28±0.03*** | 0.18±0.00* | 0.26±0.006 | 0.32±0.01* | 0.36±0.03** | |
|
| |||||||
| Co. Pm (mm) | WT | 3.45±0.09 | 3.40±0.05 | 4.52±0.15 | 4.32±0.08 | 4.51±0.17 | 4.35±0.10 |
|
| |||||||
| OA-Tg | 4.17±0.05*** | 9.65±1.04*** | 5.40±0.0.17* | 4.33±0.14 | 5.76±0.16** | 5.20±0.17** | |
|
| |||||||
| Ct. Th (mm) | WT | 0.08±0.00 | 0.08±0.00 | 0.15±0.00 | 0.15±0.00 | 0.14±0.00 | 0.16±0.00 |
|
| |||||||
| OA-Tg | 0.09±0.00* | 0.10±0.01 | 0.17±0.00** | 0.15±0.00 | 0.17±0.00* | 0.18±0.00* | |
|
| |||||||
| Po. N (1/mm) | WT | 1.81±0.16 | 1.75±0.11 | 9.25±0.91 | 2.85±0.32 | 1.57±0.24 | 1.46±0.15 |
|
| |||||||
| OA-Tg | 3.02±0.53 | 2.94±0.49* | 3.21±0.09** | 1.18±0.04** | 1.41±0.17 | 1.41±0.17 | |
Values are shown as mean ± SEM;
P < 0.001;
P < 0.01;
P < 0.05
Figure 2. OA Over-expression increases trabecular and cortical bone mass in vivo.
Femurs harvested from 12 week-old WT and OA-Tg mice (n = 6/group) were scanned using a Skyscan 1172 system. For trabecular and cortical micro-CT analyses, the metaphysis of distal femur and mid-diaphysis regions were analyzed, respectively. (A, B) 3D micro-CT-reconstructed images, in the sagittal and transaxial planes, of metaphyseal regions of WT and OA-Tg distal femurs. (C–F) Micro-CT analysis results of trabecular metaphysis: BV/TV, Tb.N, Tb.Th and Tb.Sp. (G, H) Micro-CT analysis results of cortical diaphyseal areas and perimeter: B.Ar/T.Ar and B.Pm/T.Ar. (I, J) Von Kossa stained sections showing mineralized bone in WT and OA-Tg femurs, images taken using a 20X objective. (K, L) Micro-CT analysis results of diaphyseal Ct.Th and Ct.Po. (M, N) Micro-CT reconstructed images, in the axial plane, of mid-diaphyseal region of WT and OA-Tg femurs. (O, P) Biomechanical testing results of failure moment and stiffness of 12 week-old WT and OA-Tg femurs (n = 3/group). * p < 0.05, ** p < 0.01, *** p < 0.001, compared to WT mice.
To examine the effect of OA/gpnmb over-expression on cortical bone, histological sections stained with von Kossa were examined, and showed an increase in cortical bone thickness in 12 week OA-Tg mice, compared to WT (Fig. 2I and J). This was confirmed using micro-CT in which the central diaphyses were scanned and analyzed for cortical bone parameters (See Table 1 for all data and Figure 2 for 12-week old male mice data). We observed an increase in cortical thickness (Ct.Th) and a significant decrease in total % cortical bone porosity in OA-Tg femurs, compared to WT (Fig. 2K and L). The 2D cross-section images of the mid diaphyseal region of OA-Tg femurs showed thicker cortical bone, compared to WT (Fig. 2M and N). Biomechanical testing showed a significant increase in the three-point bending strength of femurs from OA-Tg mice, compared to WT mice (Fig. 2O and P).
Supplemental Figure 1 shows that OA-Tg and WT mice were similar in weight across weeks (4–52 weeks of age), eliminating weight differences as a contributor to bone mass changes. Taken together, these data suggest that OA overexpression has significant effects on osteogenesis at both trabecular and cortical regions of the femur (Table 1).
Over-expression of osteoactivin/gpnmb enhances bone formation in vivo
To determine if the higher bone mass phenotype in OA-Tg mice was due to an increase in bone formation, plastic embedded bone sections underwent Masson’s Trichrome staining and histomorphometric analysis and showed an increase in osteoblast numbers per bone perimeter (N.Ob/B.Pm) and percent osteoblast surface per bone surface (% Ob.S/B.S) (Fig. 3A and B). Examination of calcein double-labeling in unstained plastic embedded bone sections showed increased width between labeled surfaces in OA-Tg mice, compared to WT mice (Fig. 3C). Dynamic histomorphometry of the calcein labeling in distal femoral trabeculae showed increased bone formation and mineral apposition rates (BFR and MAR) in femurs of OA-Tg mice, compared to WT (Fig. 3D and E), indicative of increased bone mineralization in OA-Tg mice.
Figure 3. Bone formation is enhanced in OA-Tg mice.
(A, B) Histomorphometric results of osteoblast number lining bone surfaces: N.Ob/B.Pm and % Ob.S/B.S (n ≥ 4/group). (C) Calcein labeling showing periosteal mineralized bone width. (D, E) Dynamic histomorphometric results for BFR and MAR. * p < 0.05, compared to WT mice.
Defective bone remodeling in OA-Tg mice
To determine if the higher bone mass phenotype in OA-Tg mice was due to a decrease of bone resorption, histomorphometric analysis for numbers of osteoclasts lining the trabecular bone surface was performed. We observed a significant decrease in osteoclast numbers per bone perimeter (N.Oc/B.Pm) and the percent of osteoclast surface per bone surface (% Oc.S/B.S) (Fig. 4A and B). To confirm that the high bone mass phenotype in OA-Tg mice was due to, at least in part, a generalized decrease in bone resorption, serum was analyzed for levels of C-terminal telopeptide (CTX-1), receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegrin (OPG) using ELISA (Fig. 4C–F). Serum levels of CTX-1, a biomarker of osteoclast activity, were reduced in OA-Tg mice, compared to WT (Fig. 4C), as were serum levels of RANKL, a key factor for osteoclast differentiation and activation, (Fig. 4D). We also evaluated serum levels of OPG, another essential molecule that is synthesized by osteoblasts and plays a regulatory role in the inhibition of osteoclast differentiation, and found that the ratio of RANKL/OPG was significantly reduced in sera of OA-Tg mice, compared to WT (Fig. 4E). However, OPG levels were relatively higher in serum samples from OA-Tg mice, compared to WT littermates, likely due to the increased number of osteoblasts, the source of OPG (Fig. 4F).
Figure 4. Defective osteoclast-mediated bone resorption in OA-Tg mice.
(A, B) Histomorphometry results for osteoclast numbers lining bone surfaces: N.Oc/B.Pm and % Oc.S/B.S. (C–F) Serum analysis of bone resorption markers using ELISA: CTX-1, RANKL, RANKL/OPG ratio, and OPG in 8 week WT and OA-Tg mice (n ≥ 5/group). ** p < 0.01, *** p < 0.001, compared to WT mice.
Together, overexpression of OA/gpnmb lead to increased osteoblast numbers and function (Fig. 3), yet decreased osteoclastic activity (Fig. 4).
Over-expression of osteoactivin stimulates osteoprogenitor proliferation and differentiation in vitro
Previous studies have demonstrated the temporal expression of OA/gpnmb in osteoblast development in vitro (Abdelmagid et al., 2008; Abdelmagid et al., 2014; Safadi et al., 2001). To determine if OA/gpnmb over-expression affects osteoblast number in vitro, WT and OA-Tg primary osteoblast cultures were terminated at day 3 after plating to evaluate their proliferation ability. The number of osteoblasts in 3-day OA-Tg calvarial and bone marrow-derived MSC cultures was significantly higher than in 3-day WT osteoblast cultures (Fig. 5A and B).
Figure 5. Over-expression of OA stimulates osteoprogenitor proliferation and differentiation in vitro.

WT and OA-Tg primary osteoblast cultures were terminated at day three and assayed for proliferation. (A, B) Cell proliferation was measured using CyQUANT NF dye reagent. Cell numbers were based on fluorescence at 520 nm (n≥6/group). WT and OA-Tg primary osteoblast cultures were terminated at day 14. (C) Macro- and microscopic images of ALP staining were evaluated using ALP staining kit and an inverted microscope. (D) ALP activity quantification using ALP substrate. After the incubation period, samples were read on a plate reader at 405 nm. Activity was normalized to the total protein. (E) ALP mRNA gene expression at days 7 and 14. Values were normalized to GAPDH. n 4. (F, G) Runx-2 and Col1a1 mRNA gene expression at day 7. Values were normalized to GAPDH. n≥4. *** p < 0.001, **** p < 0.0001, compared to WT mice.
It has been reported that OA/gpnmb over-expression in osteoprogenitors in vitro stimulates their differentiation and function (Abdelmagid et al., 2008). To investigate the effect of OA/gpnmb over-expression on osteoprogenitor differentiation in our transgenic mouse model, primary osteoblasts from WT and OA-Tg mice were cultured for 7 or 14 days. A subset of cultures was terminated on day 14 and evaluated for the staining and activity of alkaline phosphatase (ALP), a marker of early osteoblast differentiation. We found that both ALP staining (Fig. 5C) and activity (Fig. 5D) were significantly increased in OA-Tg primary osteoblasts, compared to WT. In addition, an increase in ALP mRNA expression was detected in OA-Tg osteoblasts at days 7 and 14, compared to WT osteoblasts (Fig. 5E). In culture terminated on day 7, we further evaluated mRNA levels of runt-related transcription factor 2 (Runx-2), a crucial factor for early osteoblast differentiation, and collagen type 1 alpha 1 (Col1a1), an important protein for osteoblast maturation phase. We found that Runx-2 and Col1a1 mRNA expressions were also increased in 7-day OA-Tg osteoblast cultures, compared to WT (Fig. 5F and G).
Over-expression of OA enhances TGF-β1 expression in osteoblasts
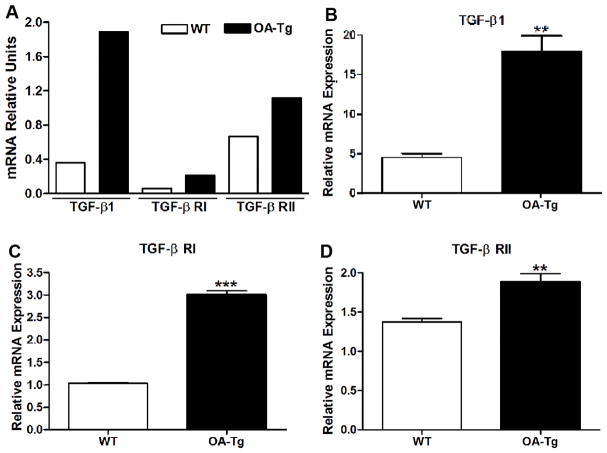
Since OA-Tg osteoblasts displayed enhanced differentiation, we utilized an osteogenesis array on osteoblast cultures terminated on day 7 to screen for differentially expressed genes. We identified that transforming growth factor beta 1 (TGF-β1), and transforming growth factor beta receptors I and II (TGF-β RI and TGF-β RII) were up-regulated in 7-day OA-Tg osteoblast cultures, compared to WT (Fig. 6A). Similarly, gene expression analysis, using qRT-PCR, showed increased TGF-β1 and TGF-β receptors I and II in 7-day cultures of OA-Tg osteoblasts, compared to WT (Fig. 6B–D). Collectively, these data suggested that TGF-β1 and its receptors were enhanced in OA-Tg osteoblasts, which suggests, at least in part, a regulatory effect of OA on the early stages of osteoblast differentiation.
Figure 6. Over-expression of OA enhances TGF-β1 expression in osteoblasts.
(A) Osteogenesis array of TGF-β1, TGF-β receptors I and II (TGF-β R.I and TGF-β R.II) in 7-day osteoblast cultures from WT and OA-Tg mice. (B–D) q-PCR analysis of (B) TGF-β1, (C) TGF-β R.I, and (D) TGF-β R.II in 7-day osteoblast cultures from WT and OA-Tg mice. n≥3. ** p < 0.01, *** p < 0.001, compared to WT mice.
Discussion
Previous studies reported on the anabolic role of osteoactivin/gpnmb in bone formation and matrix mineralization using gain-of-function and loss-of-function approaches (Abdelmagid et al., 2010; Abdelmagid et al., 2008; Safadi et al., 2001). In this study, we sought to examine the function of OA/gpnmb as an osteoinductive agent by overexpressing OA/gpnmb under the CMV-promoter in transgenic mice (OA-Tg). Wild type (WT) mice showed a physiological increase in bone mass at 4 to 12 weeks of age. Yet, overexpression of OA resulted in an exponential increase in trabecular bone mass in OA-Tg mice, compared to WT mice, evident by micro-CT and histomorphometric analyses. Additionally, dynamic bone parameters indicated a substantial increase of bone formation and mineral apposition rates in OA-Tg mice, compared to WT. Further investigations were carried out to identify the mechanism(s) of increased bone volume in the OA-Tg mice. Our data showed that osteoblast numbers were increased, while osteoclast numbers were decreased in OA-Tg mice, compared to WT. Moreover, osteoclast activity markers were significantly lower in OA-Tg than WT mice. Taken together, these results demonstrate an uncoupled bone remodeling process in OA-Tg mice, with net increases in bone formation. These findings were supported by the in vitro data showing a significant increase in osteoprogenitor differentiation, marked by increased ALP staining, activity and mRNA expression in OA-Tg mice, compared to WT. The increased differentiation in OA-Tg osteoblast cultures may be linked to the increased levels of TGF-β1 in OA-Tg osteoblasts, compared to WT.
The continued increase in bone mass of WT mice from 4 to 12 weeks of age can be described as normal bone growth. Physiological increases in trabecular bone volume is known to peak at 3 months of age, while periosteal apposition continues longer in the tibiae and femurs (Glatt et al., 2007). In contrast, overexpression of osteoactivin/gpnmb in the OA-Tg mice resulted in significant increases in bone mass in this same time period, in both distal femoral trabecular and cortical regions, based on micro-CT outcomes. The increased bone mass in OA-Tg mice was evident as increased thickness of trabeculae, increased cortical thickness and perimeter, reduced cortical bone porosity, and increased biomechanical bone strength and stiffness. These findings support those from a previous study (Safadi et al., 2001) in which high levels of OA/gpnmb expression were observed in bones of osteopetrotic mutant rats in association with increased bone mass in those animals, compared to control rats.
On the cellular level, the increased bone mass in the OA-Tg mice was due to increased osteoblast numbers and activity, as indicated by increased bone formation and mineral apposition rates. In addition, the decreased osteoclast numbers and activity likely contributed to the increased bone mass in OA-Tg mice as indicated by histomorphometry, and decreased serum levels of CTX-1 and RANKL/OPG ratio. Thus, both osteoblast and osteoclast numbers and activity were altered by OA/gpnmb overexpression in the OA-Tg mice. Our previous published data showed that intra-medullary injection of recombinant OA/gpnmb (rOA/gpnmb) stimulated the formation of new woven bone within the marrow cavity with multiple tandems of osteoblasts covering the new trabeculae, compared to control-injected mice (Singh et al., 2010).
Another important observation in this study is that the in vitro characterization of osteoprogenitors demonstrated increased proliferation, and earlier and enhanced differentiation into osteoblasts in OA-Tg mice, as marked by ALP expression and activity, for example. These observations confirm our previous published findings that OA/gpnmb over-expression in osteoblasts in vitro stimulated their differentiation (Abdelmagid et al., 2008). We have also shown that OA increases osteoblast markers and decreases myoblast markers in vitro (Sondag et al., 2014). These data combined suggest that OA/gpnmb promotes osteoprogenitor differentiation primarily through up-regulation of osteoblast differentiation markers.
We also observed increased Runx-2 mRNA expression In OA-Tg osteoblast cultures, compared to WT. Our results are in accordance with a prior study reporting that Runx-2 positively regulates early osteoblast differentiation in Runx-2 transgenic mice (Geoffroy et al., 2002). Several gene transfer studies with Runx2 overexpressing cells have supported its capacity to induce osteogenesis in vitro and in vivo (Byers and Garcia, 2004; Zhao et al., 2005; Zheng et al., 2004). Runx2 overexpression in the osteoblast-like cell line UMR-106 leads to the induction of osteoblast ECM protein expression and mineralized nodule formation (Geoffroy et al., 2002). In another study, overexpression of Runx-2 by retroviral infection, induced osteoblast-related gene expression levels, such as mRNA levels of ALP, Col I and BSP in murine fibroblast cell line (NIH3T3) cells and in dental follicle cells (DFC) (Pan et al., 2009). In vitro experiments demonstrate that Runx-2 transfection induce ALP activity in multipotential mesenchymal cells, C3H10T1/2 and C2C12, indicating an important role for Runx-2 in the induction of ALP activity (Harada et al., 1999; Lee et al., 2000). Forced expression of Runx-2 via transfection in nonosteoblastic cells, such as primary fibroblasts, induced the expression of the principal osteoblast specific genes (Ducy et al., 1997).
Several studies have implicated a growing number of cytokines involved in the regulation of bone formation and turnover (Eingartner et al., 1999; Sato et al., 1999; Sato et al., 1998; Tavakoli et al., 1999). The actions of the transforming growth factor-βs (TGF-β1, - β2, - β3) have been characterized (Linkhart et al., 1996) and studies show that TGF-βs have an essential role in the regulation of bone formation (Bouletreau et al., 2002). Most publications report that TGF-βs enhance bone formation in vivo (Beck et al., 1993; Beck et al., 1991b; Joyce et al., 1989; Marcelli et al., 1990; Noda and Camilliere, 1989). For example, TGF-β1 augments bone healing in endochondral critical size defect models and significantly enhances the biomechanical properties of bone (Lind et al., 1993; Peterson et al., 1997). Beck and colleagues suggested that in vivo injections of TGF-β stimulate osteoblasts to proliferate and increase in number, which in turn, lead to new bone formation (Beck et al., 1991a). Biological effects of stimulating growth and cell numbers suggest that functional TGF-β cell surface receptors are present on primary osteoblast-enriched cultures derived from adult human trabecular bone (Kells et al., 1992). These reports, combined with our observation of increased TGF-β in primary osteoblasts overexpressing OA, might explain the increased bone formation in OA-Tg mice in vivo. With regard to our other findings, it has been reported that TGF-βs increase the number of osteoprogenitor cells and promote matrix production (Centrella et al., 1987b; Farhadieh et al., 1999; Hock et al., 1990). TGF-β1 reduces the ability of osteoblasts to secrete RANKL, thereby decreasing osteoclast formation (Chen et al., 2012). TGF-β induces Runx2 expression in the C2C12 myogenic cell line (Lee et al., 2003), and stimulates mesenchymal cells to increase ALP production (Long et al., 1990). Lastly, TGF-β stimulates synthesis of type I collagen, thus, it enhances the premature osteoblast cell pool and matrix protein synthesis (Centrella et al., 1986; Centrella et al., 1987a; Robey et al., 1987).
Conclusion
Our findings support a role for OA/gpnmb as an osteoinductive agent, with increased bone mass and strength, as well as increased bone formation rates in OA-Tg mice, compared to WT. Several mechanisms underlying the increased bone volume in OA-Tg mice were identified, including increased numbers, differentiation and activity of osteoblasts as well as decreased numbers and activity of osteoclasts. A number of studies have shown promising results of bone loss reversal through the stimulation of bone-forming osteoblasts. A full understanding of osteoactivin’s effect on bone and its promotion of bone formation will be helpful in developing new therapeutic strategies to selectively enhance bone formation in patients with clinically significant bone loss.
Supplementary Material
The body weight of OA-Tg mice and corresponding WT littermates in grams (g) across 4–52 weeks of age. n ≥ 5.
Acknowledgments
Contract Grant Sponsor: National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)-National Institutes of Health (NIH);
Contract Grant Number: RO1-AR048892-06 to F.F.S.;
Contract Grant Sponsor: Ohio Department of Development to F.F.S.
We would like to thank Roshanak Razmpour for providing high quality histological slides. We also thank Dr. Robin A. Pixley for assisting with micro-CT scanning and analysis. This research work was supported by the NIAMS-NIH (RO1-AR048892-06) and a grant from Ohio Department of Development to Fayez F.Safadi.
Footnotes
The authors declare that there are no conflicts of interest.
References
- Abdelmagid SM, Barbe MF, Arango-Hisijara I, Owen TA, Popoff SN, Safadi FF. Osteoactivin acts as downstream mediator of BMP-2 effects on osteoblast function. Journal of cellular physiology. 2007;210(1):26–37. doi: 10.1002/jcp.20841. [DOI] [PubMed] [Google Scholar]
- Abdelmagid SM, Barbe MF, Hadjiargyrou M, Owen TA, Razmpour R, Rehman S, Popoff SN, Safadi FF. Temporal and spatial expression of osteoactivin during fracture repair. Journal of cellular biochemistry. 2010;111(2):295–309. doi: 10.1002/jcb.22702. [DOI] [PubMed] [Google Scholar]
- Abdelmagid SM, Barbe MF, Rico MC, Salihoglu S, Arango-Hisijara I, Selim AH, Anderson MG, Owen TA, Popoff SN, Safadi FF. Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function. Experimental cell research. 2008;314(13):2334–2351. doi: 10.1016/j.yexcr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Abdelmagid SM, Belcher JY, Moussa FM, Lababidi SL, Sondag GR, Novak KM, Sanyurah AS, Frara NA, Razmpour R, Del Carpio-Cano FE, Safadi FF. Mutation in osteoactivin decreases bone formation in vivo and osteoblast differentiation in vitro. The American journal of pathology. 2014;184(3):697–713. doi: 10.1016/j.ajpath.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LS, Amento EP, Xu Y, Deguzman L, Lee WP, Nguyen T, Gillett NA. TGF-beta 1 induces bone closure of skull defects: temporal dynamics of bone formation in defects exposed to rhTGF-beta 1. J Bone Miner Res. 1993;8(6):753–761. doi: 10.1002/jbmr.5650080614. [DOI] [PubMed] [Google Scholar]
- Beck LS, Ammann AJ, Aufdemorte TB, Deguzman L, Xu Y, Lee WP, McFatridge LA, Chen TL. In vivo induction of bone by recombinant human transforming growth factor beta 1. J Bone Miner Res. 1991a;6(9):961–968. doi: 10.1002/jbmr.5650060910. [DOI] [PubMed] [Google Scholar]
- Beck LS, Deguzman L, Lee WP, Xu Y, McFatridge LA, Gillett NA, Amento EP. Rapid publication. TGF-beta 1 induces bone closure of skull defects. J Bone Miner Res. 1991b;6(11):1257–1265. doi: 10.1002/jbmr.5650061117. [DOI] [PubMed] [Google Scholar]
- Bouletreau PJ, Warren SM, Longaker MT. The molecular biology of distraction osteogenesis. Journal of Cranio-Maxillofacial Surgery. 2002;30:1–11. doi: 10.1054/jcms.2001.0263. [DOI] [PubMed] [Google Scholar]
- Byers BA, Garcia AJ. Exogenous Runx2 expression enhances in vitro osteoblastic differentiation and mineralization in primary bone marrow stromal cells. Tissue engineering. 2004;10(11–12):1623–1632. doi: 10.1089/ten.2004.10.1623. [DOI] [PubMed] [Google Scholar]
- Centrella M, Massague J, Canalis E. Human platelet-derived transforming growth factor-beta stimulates parameters of bone growth in fetal rat calvariae. Endocrinology. 1986;119(5):2306–2312. doi: 10.1210/endo-119-5-2306. [DOI] [PubMed] [Google Scholar]
- Centrella M, McCarthy T, Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cultures from fetal rat parietal bone. J Biol Chem. 1987a;(262):2869–2874. [PubMed] [Google Scholar]
- Centrella M, McCarthy TL, Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987b;262(6):2869–2874. [PubMed] [Google Scholar]
- Chen G, Deng C, Li Y. TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. International Journal of Biological Sciences. 2012;8(2):272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Eingartner C, Coerper S, Fritz J, Gaissmaier C, Koveker G, Weise K. Growth factors in distraction osteogenesis. Immuno-histological pattern of TGF-beta1 and IGF-I in human callus induced by distraction osteogenesis. International orthopaedics. 1999;23(5):253–259. doi: 10.1007/s002640050365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadieh RD, Dickinson R, Yu Y, Gianoutsos MP, Walsh WR. The role of transforming growth factor-beta, insulin-like growth factor I, and basic fibroblast growth factor in distraction osteogenesis ofthe mandible. J Craniofac Surg. 1999;10:80–86. doi: 10.1097/00001665-199901000-00016. [DOI] [PubMed] [Google Scholar]
- Geoffroy V, Kneissel M, Fournier B, Boyde A, Matthias P. High bone resorption in adult aging transgenic mice overexpressing cbfa1/runx2 in cells of the osteoblastic lineage. Molecular and cellular biology. 2002;22(17):6222–6233. doi: 10.1128/MCB.22.17.6222-6233.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-Related Changes in Trabecular Architecture Differ in Female and Male C57BL/6J Mice. J Bone Miner Res. 2007;22(8):1197–1207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- Harada H, Tagashira S, Fujiwara M, Ogawa S, Katsumata T, Yamaguchi A, Komori T, Nakatsuka M. Cbfa1 isoforms exert functional differences in osteoblast differentiation. J Biol Chem. 1999;274(11):6972–6978. doi: 10.1074/jbc.274.11.6972. [DOI] [PubMed] [Google Scholar]
- Hock JM, Canalis E, Centrella M. Transforming growth factor-beta stimulates bone matrix apposition and bone cellreplication in cultured fetal rat calvariae. Endocrinology. 1990;(126):421–426. doi: 10.1210/endo-126-1-421. [DOI] [PubMed] [Google Scholar]
- Joshi RN, Safadi FF, Barbe MF, Del Carpio-Cano F, Popoff SN, Yingling VR. Different effects on bone strength and cell differentiation in pre pubertal caloric restriction versus hypothalamic suppression. Bone. 2011;49(4):810–818. doi: 10.1016/j.bone.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce ME, Jinguski S, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-beta initiates cartilage and bone formation in vivo. J Bone Miner Res. 1989;(4):255–259. [Google Scholar]
- Kells AF, Schwartz HS, Bascom CC, Hoover RL. Identification and analysis of transforming growth factor beta receptors on primary osteoblast-enriched cultures derived from adult human bone. Connective tissue research. 1992;27(4):197–209. doi: 10.3109/03008209209006996. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Bae SC. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Molecular and cellular biology. 2000;20(23):8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, Sung JH, Wozney JM, Ryoo HM. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003;278(36):34387–34394. doi: 10.1074/jbc.M211386200. [DOI] [PubMed] [Google Scholar]
- Lind M, Schumacker B, Soballe K, Keller J, Melson F, Bunger C. Transforming growth factor b enhances fracture healing in rabbit tibiae. Acta orthopaedica Scandinavica. 1993;64:553–556. doi: 10.3109/17453679308993691. [DOI] [PubMed] [Google Scholar]
- Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone. 1996;19(1 Suppl):1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- Long MW, Williams JL, Mann KG. Expression of human bone-related proteins in the hematopoietic microenvironment. The Journal of clinical investigation. 1990;86(5):1387–1395. doi: 10.1172/JCI114852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli C, Yates AJ, Mundy GR. In vivo effects of human recombinant transforming growth factor beta on bone turnover in normal mice. J Bone Miner Res. 1990;5(10):1087–1096. doi: 10.1002/jbmr.5650051013. [DOI] [PubMed] [Google Scholar]
- Noda M, Camilliere JJ. In vivo stimulation of bone formation by transforming growth factor-b. Endocrinology. 1989;(124):2991–2994. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- Owen TA, Smock SL, Prakash S, Pinder L, Brees D, Krull D, Castleberry TA, Clancy YC, Marks SC, Jr, Safadi FF, Popoff SN. Identification and characterization of the genes encoding human and mouse osteoactivin. Crit Rev Eukaryot Gene Expr. 2003;13(2–4):205–220. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.130. [DOI] [PubMed] [Google Scholar]
- Pan K, Yan S, Ge S, Li S, Zhao Y, Yang P. Effects of CBFA1 or BMP-2 overexpression in DFCs Effects of core binding factor α1 or bone morphogenic protein-2 overexpression on osteoblast/cementoblast related gene expressions in NIH3T3 mouse cells and dental follicle cells. Cell Prolif. 2009;(42):364–372. doi: 10.1111/j.1365-2184.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DR, Glancy TP, Bacon-Clarke R. A study ofdelivery timing and duration on the transforming growth factor beta 1 induced healing ofcritical size long bone defects. JBR. 1997;12:s304–s305. [Google Scholar]
- Robey PG, Young MF, Flanders KC, Roche NS, Kondaiah P, Reddi AH, Termine JD, Sporn MB, Roberts AB. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987;105(1):457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, Rico MC, Owen TA, Popoff SN. Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. Journal of cellular biochemistry. 2001;84(1):12–26. doi: 10.1002/jcb.1259. [DOI] [PubMed] [Google Scholar]
- Sato M, Ochi T, Nakase T, Hirota S, Kitamura Y, Nomura S, Yasui N. Mechanical tension-stress induces expression of bone morphogenetic protein (BMP)-2 and BMP-4, but not BMP-6, BMP-7, and GDF-5 mRNA, during distraction osteogenesis. J Bone Miner Res. 1999;14(7):1084–1095. doi: 10.1359/jbmr.1999.14.7.1084. [DOI] [PubMed] [Google Scholar]
- Sato M, Yasui N, Nakase T, Kawahata H, Sugimoto M, Hirota S, Kitamura Y, Nomura S, Ochi T. Expression of bone matrix proteins mRNA during distraction osteogenesis. J Bone Miner Res. 1998;13(8):1221–1231. doi: 10.1359/jbmr.1998.13.8.1221. [DOI] [PubMed] [Google Scholar]
- Selim AA, Abdelmagid SM, Kanaan RA, Smock SL, Owen TA, Popoff SN, Safadi FF. Anti-osteoactivin antibody inhibits osteoblast differentiation and function in vitro. Critical reviews in eukaryotic gene expression. 2003;13(2–4):265–275. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.180. [DOI] [PubMed] [Google Scholar]
- Sheng MH, Wergedal JE, Mohan S, Amoui M, Baylink DJ, Lau KH. Targeted overexpression of osteoactivin in cells of osteoclastic lineage promotes osteoclastic resorption and bone loss in mice. PLoS One. 2012;7(4):e35280. doi: 10.1371/journal.pone.0035280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Del Carpio-Cano F, Belcher JY, Crawford K, Frara N, Owen TA, Popoff SN, Safadi FF. Functional roles of osteoactivin in normal and disease processes. Critical reviews in eukaryotic gene expression. 2010;20(4):341–357. doi: 10.1615/critreveukargeneexpr.v20.i4.50. [DOI] [PubMed] [Google Scholar]
- Sondag GR, Salihoglu S, Lababidi SL, Crowder DC, Moussa FM, Abdelmagid SM, Safadi FF. Osteoactivin Induces Transdifferentiation of C2C12 Myoblasts Into Osteoblasts. J Cell Physiol. 2014;229(7):955–966. doi: 10.1002/jcp.24512. [DOI] [PubMed] [Google Scholar]
- Tavakoli K, Shahidi YYS, Bonar F, Walsh WR, Poole MD. expression of growth factors in the mandibular distraction zone. Br J Plast Surg. 1999;52:434–439. doi: 10.1054/bjps.1999.3157. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zhao M, Xiao G, Franceschi RT. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Molecular therapy: the journal of the American Society of Gene Therapy. 2005;12(2):247–253. doi: 10.1016/j.ymthe.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Zheng H, Guo Z, Ma Q, Jia H, Dang G. Cbfa1/osf2 transduced bone marrow stromal cells facilitate bone formation in vitro and in vivo. Calcif Tissue Int. 2004;74(2):194–203. doi: 10.1007/s00223-003-0004-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The body weight of OA-Tg mice and corresponding WT littermates in grams (g) across 4–52 weeks of age. n ≥ 5.