Abstract
Rats received NMDA lesions of the bed nucleus of the stria terminalis (BNST) and then ten aversive conditioning trials in which exposure to a context was paired with footshock. For half the animals, shock was presented one minute after the onset of each context exposure; for the other half, shock was presented after 10 minutes. With the one-min context duration, aversive conditioning (measured by freezing) was unaffected by BNST lesion. In contrast, at the 10-min duration, lesioned animals froze substantially less than sham controls. When one-minute-conditioned animals were left in the context for 10 minutes, freezing that was evident (though declining) throughout the test was not affected by the BNST lesion. When freezing over 10 mins was similarly examined in the 10-min-conditioned animals, BNST lesions caused a deficit that was consistently evident over time. The results indicate that the BNST is involved in aversive conditioning to long-duration, but not merely contextual, conditional stimuli. Results may be less consistent with the view that BNST becomes activated after prolonged fear than the view that it is involved when a cue’s onset has a remote temporal relation to shock.
Keywords: Anxiety, Aversive Conditioning, BNST, Conditional Stimulus Duration, Extended Amygdala, Lesion, Stress
The bed nucleus of the stria termialis (BNST) has emerged as an important brain structure associated with stress and emotional responding. Hence, BNST activity has been implicated in anxiety-like behavior (Waddell, Bouton, & Falls, 2008; Waddell, Morris, & Bouton, 2006; Walker & Davis, 2008; Walker, Miles, & Davis, 2009; Walker, Toufexis, & Davis, 2003), depression-like behavior (Hammack, Richey, Watkins, & Maier, 2004; Stout, Mortas, Owens, Nemeroff, & Moreau, 2000), addiction processes (e.g. (Erb, Shaham, & Stewart, 2001)), the response to stressors (see (Crestani et al., 2013) for review), stress-induced anorexia (Roman et al., 2012), and the affective component of pain (Tran, Schulkin, & Greenwood-Van Meerveld, 2014). A role for BNST activity in emotional behavior has also been observed in nonhuman primates (e.g. (Kalin, Shelton, Fox, Oakes, & Davidson, 2005)) and humans (Somerville, Whalen, & Kelley, 2010; Straube, Mentzel, & Miltner, 2007).
In an effort to delineate the behavioral systems dependent on BNST activity, Davis and colleagues originally argued that the BNST mediates a sluggish behavioral response system to diffuse stimuli that influences behavior long after the stimulus has terminated, which they likened to human states of anxiety as distinct from fear (Walker et al., 2003). In support, electrical BNST stimulation produces many of the endocrine, cardiovascular and respiratory responses that are elicited by anxiogenic stimuli (Casada & Dafny, 1991; Dunn, 1987), the BNST is activated following anxiogenic treatments (Choi et al., 2006; Singewald, Salchner, & Sharp, 2003), and BNST lesions reduce anxiety-like, but not fear-like behavioral responding (Lee & Davis, 1997; Waddell et al., 2006). Waddell et al. (2006) further clarified that the BNST mediates behavioral responding to cues that predict a temporally-distant threat; BNST lesions blocked fear-like responding to a 10-min clicking noise that was paired with shock, but not a 1-min clicking noise paired with shock. The idea was that, from a behavioral systems perspective on learning (e.g., (Domjan, 1994; Fanselow, 1994; Timberlake, 2001)), conditioned responses that develop during Pavlovian conditioning depend on the timescale over which conditioned stimuli predict the biologically-significant stimulus. Conditioned responses to stimuli that predict an aversive event in the immediate future may be more akin to a fear-like behavioral state, whereas those to stimuli that predict an aversive event more remotely in time may be more like anxiety (Bouton, 2005; Bouton, Mineka, & Barlow, 2001). The idea that there are two distinct (though somewhat overlapping) aversive emotional states is consistent with analysis of the symptoms of human participants, which can be “characterized by a subjective sense of extreme fear or impending doom… [vs.] apprehension and worry” (e.g., Bouton et al., 2001, p. 8).
Walker and Davis (2008, 2009) subsequently also argued that the critical feature of a BNST-dependent behavioral paradigm was temporal, suggesting that the BNST mediates responding to threats if that response sustained for a long duration. These models are consistent with the characterization of anxiety states as apprehension, tension and worry to threats that may occur in the more distant future (Bouton, 2005; Bouton et al., 2001).
In contrast, Sullivan et al. (2004) found that BNST lesions reduced endocrine and behavioral responding to contextual cues, but not discrete cues, previously paired with shock (Sullivan et al., 2004). Since this report, several others have investigated the role of the BNST in mediating fear conditioning to context (e.g. (Resstel et al., 2008; Sink, Davis, & Walker, 2013; Zimmerman & Maren, 2011)), and this potential role for BNST activity in the behavioral response to threat has garnered more attention in the literature than the temporal distinction described above. However, in studies in which the BNST has been implicated in fear conditioning to contextual, but not discrete, cues, the duration of the cues represents a confounding factor. That is, context exposure is typically for 8 min or more, whereas whereas discrete cues are typically presented for 60 s or less. If the BNST mediates responding to long-duration cues rather than context, BNST lesions should still attenuate responding in these studies only if animals are exposed to the context for a long period of time.
In order to clarify whether BNST activity mediates responding to either contextual stimuli or instead the duration in which stimuli are presented, BNST- or sham-lesioned rats were aversively conditioned to contexts presented either for 1-min or 10-min during training. BNST lesions attenuated context conditioning only in the 10-min group, supporting the argument that the BNST mediates a behavioral responding to cues that predict a temporally-distant threat, independent of whether the cue is contextual or discrete.
Method
Subjects
The subjects were 32 naive female Wistar rats purchased from Charles River Laboratories (St. Constance, Quebec). They were between 75 and 90 days old at the start of the experiment and were individually housed in a room maintained on a 16:8h light/dark cycle. Animals were allowed access to food and water ad libitum. All procedures were approved by the Institutional Animal care and Use Committee of the University of Vermont.
Surgery
Upon arrival, subjects were allowed a minimum of 7 days to acclimate before undergoing surgery. After having the incision area shaved, subjects were anesthetized with isofluane vapor (1.5–3.5%) and secured in a stereotaxic apparatus (David Kopf Instruments, Tujuanga, CA) with blunt earbars. The skull surface was exposed via a midline head incision, and was leveled prior to determining stereotactic coordinates. Coordinates aiming at the BNST were based on the Paxinos and Watson rat brain atlas (Paxinos & Watson, 2007), and were as follows; AP, −0.5 mm from bregma; ML, ±1.7 mm from bregma; DV, −7.0 mm from scull surface. While we targeted anterolateral BNST regions with these coordinates, it is important to note that multiple BNST subregions were lesioned in the present study. Bilateral infusions were performed by lowering a stainless steel 30-gauge cannula that was connected to a Hamilton 10uL microsyringe (Model 701, Hamilton Company, Reno, NV), via PE10 standard wall tubing, which was mounted on an infusion pump (Model 310, Stoelting, Wood Dale, IL). Excitotoxic lesions of the BNST were produced by infusions of 5.0 μg N-methyl-D-aspartate (NMDA) (Sigma-Aldrich Co., St. Louis, MO) in 0.5 μL saline. Animals in the control group were given sham lesions, which consisted of an infusion of saline, equal in volume. Bilateral infusions of 0.2 μL of either NMDA or saline were given over a 4-minute period. Once infused, the cannula remained in place for 5 minutes before being slowly removed. The midline head incision was closed with 5–6 surgical sutures. Finally, animals received a 1 mL subcutaneous injection lactated ringers immediately after surgery, and two injections of the pain reliever meloxicam (Metacam, Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO) at a dose of 1.5 mg/kg, one immediately after surgery and the other the next day. All subjects were allowed to recover for a minimum of 7 days prior to behavioral training, during which they were weighed and handled daily.
Apparatus
Two sets of four conditioning chambers housed in separate rooms of the laboratory were used. The chambers have been described elsewhere (e.g., (Bouton & Schepers, 2015), with operant manipulanda removed). Each was housed in its own sound attenuation chamber, and all were of the same design (Med Associates model: ENV-008-VP). They measured 30.5 cm × 24.1 × 21.0 cm (l × w × h). The two sets of chambers differed in ways that allowed them to serve as different contexts, but they were not used in that capacity here (and were counterbalanced). In both sets, the sidewalls and ceiling were made of clear acrylic plastic, while the front and rear walls were made of brushed aluminum. Each chamber was illuminated by a 7.5-W incandescent bulb mounted to the ceiling of the sound attenuation chamber, approximately 35 cm from the grid floor at the front of the chamber. Observation of each subject was provided by a video camera mounted to the wall of each experimental room, approximately 1.2 m from the sound attenuation chambers. Clear acrylic plastic windows in the front of each chamber permitted a view of each rat.
In both sets of boxes, a 0.5-s, 1-mA footshock delivered to the grid floor was provided by Med Associate (Georgia, VT) Model ENV-414 shockers.
Procedure
As noted above, aversive conditioning began after the animals had been handled for at least 7 days following surgery. Over 5 consecutive days, rats from each lesion condition (BNST vs. sham) then received trials in which exposure to the context was paired with footshock. On each of 10 trials, the rat was placed in a conditioning box and footshock was presented either 1 or 10 min later. Rats were removed from the conditioning boxes 30 s after footshock. There were two trials each day, with the trials separated by a least 1 hr. Thus, the experimental design was a 2 × 2 factorial. One factor was lesion (BNST vs. Sham) and the other factor was Context Duration (1 min vs. 10 min). There was a final performance test on Day 6 in which rats that had been conditioned with 1 min of context exposure where placed in the conditioning chambers for a10-minute period.
Freezing was scored by two independent observers from videotape. One observer was unaware of the rats’ lesion condition. (It was not possible to “blind” the observer to trial duration.) Each rat’s behavior was sampled every 4 s and scored as either freezing, defined as body immobility with minimal vibrissae movement, or not freezing. The dependent measure was the percentage of observations scored as freezing. Each observer’s freezing scores were averaged for each rat over the 10 trials of conditioning; the correlation between the two observers’ means was r (27) = .92. Only the freezing scores of the “blind” observer are reported here.
Lesion Verification
After the last day of behavioral testing, rats were anesthetized with pentobarbital (Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MI), perfused transcardially with 4% paraformaldehyde, and brain sections were prepared and processed for Neu-N immunohistochemical analysis as previously described (Poulos, Ponnusamy, Dong, & Fanselow, 2010).
Results
Lesions
The largest and smallest lesions are shown in Figure 1. BNST lesions were centered around the region where the anterior commissure crosses the midline of the brain, and typically encompassed the full medial-lateral extent of the BNST at this level. While lesions varied in the degree of spread along the anterio-posterior plane, for all rats the BNST region 0.36 to 0.48 posterior to bregma exhibited significant neuronal loss.
Figure 1.
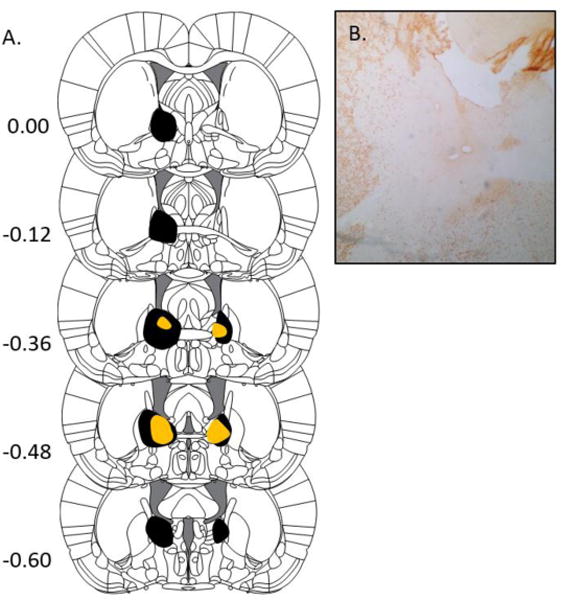
BNST Lesions. A. Extent of the largest (black) and smallest (orange) BNST lesions accepted for analysis, ranging from +0.00 to −0.60 mm from bregma. From The Rat Brain in Stereotaxic Coordinates (6th ed.), Pages 33–38, by G. Paxinos & C. Watson, 2007, New York, NY: Academic Press. Copyright 2005 by Elsevier Academic Press. Adapted (or reprinted) with permission.B. Example of a BNST lesion in a NeuN stained brain slice.
Freezing during conditioning trials
The groups’ freezing on each conditioning trial is summarized in Panels A and B of Figure 2. It is apparent that the BNST lesion reduced freezing in the 10-min context condition (Panel A), but not in the 1-min condition (Panel B). This impression was confirmed by a Lesion × Context Duration × Trial ANOVA. The ANOVA revealed a main effect of trial, F (9, 207) = 50.16, p < .001, no effect of Lesion (but perhaps a trend), F (1,23) = 3.041, p = .095, no main effect of Context Duration F (1,23) < 1, and a near significant Lesion × Context Duration interaction F (1,23) = 4.024, p = .057. Neither Lesion × Trial nor the Duration × Trial interactions approached significance, Fs (9, 207) ≤ 1.60, although the Lesion × Duration × Trial interaction was significant, F (9, 207) = 1.92, p = .05. To assess the effects of the lesion at both context durations, we conducted separate Lesion × Trial ANOVAs with the 1-min and 10-min groups. In the 10-min groups, there was a Trial effect, F (9, 117) = 35.25, p < .001, a Lesion effect, F (1,13) = 11.620, p = .005, and a Lesion × Trial interaction, F (9, 117) = 3.31, p = .001. In contrast, in the 1-min groups, the Trial effect was reliable, F (9, 90) = 18.49, p < .001, but neither the lesion effect, F (1,10) < 1, nor the Lesion × Trial interaction, F (9, 90) < 1, approached reliability. The 1-min groups did not differ on any conditioning trial when the trials were analyzed individually, ps > .10. The results thus suggest that freezing to a contextual CS is not affected by a BNST lesion unless it is longer than 1 min in duration.
Figure 2.
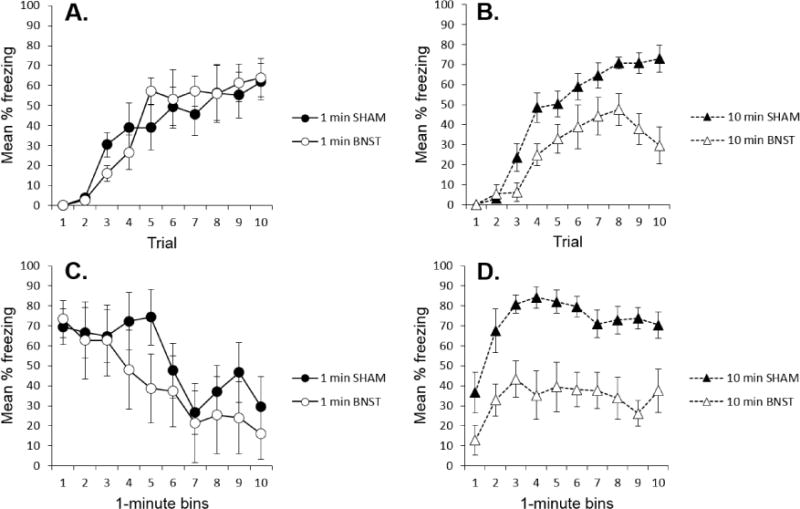
Behavioral results of the experiment. A. Mean percentage freezing (+ SEM) on each conditioning trial for the sham and BNST-lesioned groups given 1-min context exposures. B. Mean percentage freezing (+ SEM) on each conditioning trial for the sham and BNST-lesioned groups given 10-min context exposures. C. Mean percentage freezing (+ SEM) over time during the 10-min test of the groups previously conditioned with 1-min context exposures. D. Mean percentage freezing (+ SEM) of the groups conditioned with 10-min context exposures over each min of the last two conditioning trials (averaged).
Effects on freezing over time
The Walker-Davis perspective predicts that the BNST becomes increasingly important the longer an animal is frightened, whereas the Waddell et al. (2006; (Bouton, 2005)) perspective predicts that the BNST is primarily important in early parts of a long CS, when the CS might predict a shock that is more remote in time. These predictions were evaluated in two ways. First, the animals conditioned with the 1-min context were given a 10-min test trial. Freezing over minutes on this trial is shown in Panel C of Figure 2. The lesioned and sham groups did not differ at any point as time in the CS progressed. A Lesion × Minute ANOVA revealed a Minute effect, F (9,90) = 6.98, p < .001, suggesting the extinction of freezing over the 10-min test, but neither the lesion effect F (1,10) < 1, nor the Lesion × Minute interaction, F (9,90) < 1, approached reliability. Thus, contrary to the Walker-Davis perspective, being frightened for an extended period of time does not necessarily engage the BNST.
Second, we also examined freezing over time in the 10-min groups. Panel D of Figure 2 shows freezing over time during the final two (asymptotic) conditioning trials. Because only the earlier parts of the trial bore a remote temporal relation with the upcoming shock, Waddell et al. (2006) would predict a larger BNST effect early during the trial rather than later. In contrast, the Walker-Davis perspective again predicts a bigger disruption later rather than earlier. As the panel suggests, however, the lesion had a roughly equivalent effect throughout the 10-min trial. Consistent with this description, a Lesion × Minute ANOVA revealed a reliable effect of Lesion, F (1, 13) = 16.61, p = .001, and Minute, F (9, 117) = 8.19, p < .001, but the Lesion × Minute interaction did not approach reliability, F (1, 117) = 1.00.
Discussion
The present results suggest that the status of a CS as a contextual stimulus does not guarantee a role for BNST activity (cf. (Resstel et al., 2008; Sink et al., 2013; Zimmerman & Maren, 2011)). The sham and lesioned 1-min groups did not differ either during the conditioning trials or during the final test trial. The results thus suggest that a long-duration CS, and not merely the CS’s status as a context, is important for involving the BNST. The importance of time rather than contextual status is also suggested by the earlier results of Waddell et al. (2006), who found that lesions of the BNST disrupted aversive responding to a 10-min noise, but not a 1-min noise. Thus, being a context is neither necessary (Waddell et al., 2006) nor sufficient (present results) to involve the BNST.
It is worth noting that our method of inactivating the BNST was to lesion it prior to aversive conditioning (see also, e.g., Waddell et al., 2006). The deficits observed here could therefore have resulted from the lesion affecting either learning processes or the expression of that learning in performance. Performance processes have been implicated by previous work that demonstrate the effects of temporary inactivation of the BNST during tests that were conducted after conditioning (Resstel et al. 2008).
Both Walker and Davis (e.g., (Waddell et al., 2008; Walker et al., 2009)) and Waddell et al. (2006) have correctly emphasized the importance of a CS’s temporal duration. However, other aspects of the data raise challenges for both views. Contrary to Walker and Davis, the results do not confirm that BNST activation becomes more important the longer the animal is frightened (Waddell et al., 2008; Walker et al., 2009). First, when the context had been conditioned with a 1-min interval preceding shock, a 10-min test exposure continuously elicited freezing, but there was no increase in disruption of freezing associated with the BNST lesion. Second, there was little evidence that the BNST-sham difference became larger in the 10-min groups as a function of time within the long-duration conditioning trials. On the other hand, because conditioning with a 10-min context caused freezing that was equally disrupted by the lesion throughout its duration, the results also did not confirm a decrease in importance of an intact BNST as the predicted shock became less remote and more imminent, (Waddell et al., 2006).
Two possibilities might account for why BNST involvement was not unique to early parts of the long CS. First, it is possible that the rats in the 10-min condition were not actually timing the imminence of the upcoming shock very well. Although the sham rats increased their freezing over the 10-min trial, as if they were timing the shock, the present experiment did not include a control group for which time in the context was not predictive in order to indicate how much the increase was due to timing (it could be due, for example, to sluggish recruitment of the response). Better timing might be required in order for BNST activity to exclusively influence freezing in early parts of the CS. Alternatively, it is possible that the present BNST lesions also compromised brain regions to which the BNST would normally transfer control as shock became more imminent toward the end of the trial. One candidate would the central nucleus of the amygdala (CeA). There are reciprocal connections between the BNST subregions targeted in the present report and the CeA (Bienkowski, Wendel, & Rinaman, 2013; Dong, Petrovich, & Swanson, 2001; Dong, Petrovich, Watts, & Swanson, 2001; Dong & Swanson, 2004).
It is worth observing that the 10-min context appeared to control as much freezing as the 1-min context during conditioning. This contrasts with evidence that longer-duration CSs can appear less effective than shorter ones, even in aversive conditioning (e.g., (Kamin, 1965; Waddell et al., 2006)). However, in contrast to fear conditioning with unimodal CSs such as tones and lights, a complex contextual CS can actually benefit from longer exposure before shock presentation, perhaps because extra time allows the animal to form a more coherent representation of it (e.g., (Fanselow, 1986; Ponnusamy, Poulos, & Fanselow, 2007)). Such a process might offset any tendency for freezing to be weaker with a long-duration context.
The BNST has been divided into several subregions primarily based on afferent and efferent connectivity (Dong, Petrovich, & Swanson, 2001), and BNST neurons display significant heterogeneity in their cytoarchitecture, chemoarchitecture, and physiology (e.g. (Hammack, Mania, & Rainnie, 2007; Larriva-Sahd, 2006)). Haufler et al., 2013 found that fear conditioning led to the increased responsiveness of some BNST neurons, but decreased responsiveness to others, depending on the BNST subregion targeted, and both responses could be observed following exposure to both a short-duration conditioned auditory cue as well as a long-duration conditioned context (Haufler, Nagy, & Pare, 2013). Hence, the role of BNST activity in fear conditioning is complex, but likely does not depend on the CS being a context.
Due to the size of the lesions in the present report, the role of specific BNST subregions in mediating the response to a long-duration CS was not assessed. Nevertheless, the present data suggest that BNST activity is recruited to stimuli that predict a temporally distant threat, and that a stimulus’s duration, rather than its status as a context, is the critical factor that recruits the BNST. These data corroborate studies that have associated BNST activity with anticipatory anxiety-states in humans (e.g. (Somerville et al., 2010; Straube et al., 2007)), and support a large and growing literature implicating the BNST in anxiety-like behavioral states.
Acknowledgments
This research was supported by NIH Grants RO1 DA 033123 to MEB and MH-97988 to SEH. Portions of the work were also supported by the University of Vermont Neuroscience, Behavior and Health Transdisciplinary Research Initiative.
References
- Bienkowski MS, Wendel ES, Rinaman L. Organization of multisynaptic circuits within and between the medial and the central extended amygdala. J Comp Neurol. 2013;521(15):3406–3431. doi: 10.1002/cne.23356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Behavior Systems and the contextual control of anxiety, fear, and panic. In: Barratt LF, Niedenthal P, Winkielman P, editors. Emotion: Conscious and unconscious. New York: Guilford Press; 2005. pp. 205–227. [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108(1):4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Schepers ST. Renewal after the punishment of free operant behavior. J Exp Psychol Anim Learn Cogn. 2015;41(1):81–90. doi: 10.1037/xan0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27(2):207–212. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav. 2006;89(3):301–310. doi: 10.1016/j.physbeh.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol. 2013;11(2):141–159. doi: 10.2174/1570159X11311020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domjan M. Formulation of a behavior system for sexual conditioning. Psychon Bull Rev. 1994;1(4):421–428. doi: 10.3758/BF03210946. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38(1–2):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436(4):430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468(2):277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res. 1987;407(2):327–331. doi: 10.1016/0006-8993(87)91111-5. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress-induced relapse to drug seeking in the rat: role of the bed nucleus of the stria terminalis and amygdala. Stress. 2001;4(4):289–303. doi: 10.3109/10253890109014753. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Associative vs. topographical accounts of the immediate shock freezing deficit in rats: Implications for the response selection rules governing species specific defense reactions. Learning and Motivation. 1986;17:16–39. [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1(4):429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98(2):638–656. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118(2):443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem. 2013;20(11):633–641. doi: 10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58(10):796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin LJ. Temporal and intensity characteristics of the conditioned stimulus. In: Prokasy WR, editor. Classical conditioning. New York: Appleton-Century-Crofts; 1965. [Google Scholar]
- Larriva-Sahd J. Histological and cytological study of the bed nuclei of the stria terminalis in adult rat. II. Oval nucleus: extrinsic inputs, cell types, neuropil, and neuronal modules. J Comp Neurol. 2006;497(5):772–807. doi: 10.1002/cne.21011. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17(16):6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th. New York: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Ponnusamy R, Poulos AM, Fanselow MS. Amygdala-dependent and amygdala-independent pathways for contextual fear conditioning. Neuroscience. 2007;147(4):919–927. doi: 10.1016/j.neuroscience.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Ponnusamy R, Dong HW, Fanselow MS. Compensation in the neural circuitry of fear conditioning awakens learning circuits in the bed nuclei of the stria terminalis. Proc Natl Acad Sci U S A. 2010;107(33):14881–14886. doi: 10.1073/pnas.1005754107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Alves FH, Reis DG, Crestani CC, Correa FM, Guimaraes FS. Anxiolytic-like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience. 2008;154(3):869–876. doi: 10.1016/j.neuroscience.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Roman CW, Lezak KR, Kocho-Schellenberg M, Garret MA, Braas K, May V, Hammack SE. Excitotoxic lesions of the bed nucleus of the stria terminalis (BNST) attenuate the effects of repeated stress on weight gain: evidence for the recruitment of BNST activity by repeated, but not acute, stress. Behav Brain Res. 2012;227(1):300–304. doi: 10.1016/j.bbr.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53(4):275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Sink KS, Davis M, Walker DL. CGRP antagonist infused into the bed nucleus of the stria terminalis impairs the acquisition and expression of context but not discretely cued fear. Learn Mem. 2013;20(12):730–739. doi: 10.1101/lm.032482.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68(5):416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. Eur J Pharmacol. 2000;401(1):39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37(4):1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128(1):7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Timberlake W. Integrating niche-related and general process approaches in the study of learning. Behav Processes. 2001;54(1–3):79–94. doi: 10.1016/s0376-6357(01)00151-6. [DOI] [PubMed] [Google Scholar]
- Tran L, Schulkin J, Greenwood-Van Meerveld B. Importance of CRF receptor-mediated mechanisms of the bed nucleus of the stria terminalis in the processing of anxiety and pain. Neuropsychopharmacology. 2014;39(11):2633–2645. doi: 10.1038/npp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Bouton ME, Falls WA. Central CRF receptor antagonist a-helical CRF9–41 blocks reinstatement of extinguished fear: the role of the bed nucleus of the stria terminalis. Behav Neurosci. 2008;122(5):1061–1069. doi: 10.1037/a0013136. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120(2):324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213(1–2):29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463(1–3):199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiol Learn Mem. 2011;95(2):199–205. doi: 10.1016/j.nlm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


