Abstract
The weight-bearing test is one method to assess pain in rodent animal models; however, the acceptance of this convenient method is limited by the low throughput data acquisition and necessity of confining the rodents to a small chamber.
New methods
We developed novel data acquisition hardware and software, data analysis software, and a conditioning protocol for an automated high throughput static weight-bearing assessment of pain. With this device, the rats voluntarily enter the weighing chamber, precluding the necessity to restrain the animals and thereby removing the potential stress-induced confounds as well as operator selection bias during data collection. We name this device the Voluntarily Accessed Static Incapacitance Chamber (VASIC).
Results
Control rats subjected to the VASIC device provided hundreds of weight-bearing data points in a single behavioral assay. Chronic constriction injury (CCI) surgery and paw pad injection of complete Freund's adjuvant (CFA) or carrageenan in rats generated hundreds of weight-bearing data during a 30 minute recording session. Rats subjected to CCI, CFA, or carrageenan demonstrated the expected bias in weight distribution favoring the un-operated leg, and the analgesic effect of i.p. morphine was demonstrated. In comparison with existing methods, brief water restriction encouraged the rats to enter the weighing chamber to access water, and an infrared detector confirmed the rat position with feet properly positioned on the footplates, triggering data collection. This allowed hands-off measurement of weight distribution data reducing operator selection bias.
Conclusion
The VASIC device should enhance the hands-free parallel collection of unbiased weight-bearing data in a high throughput manner, allowing further testing of this behavioral measure as an effective assessment of pain in rodents.
Keywords: Pain, Behavior, Neuropathy, Inflammation, Weight-bearing, Automation, Unbiased data, Rodents, Chronic constriction injury
1. Introduction
Pain affects more Americans than diabetes, heart disease, and cancer combined and is a leading cause of disability and a major contributor to health care costs [1]. A clear medical need exists for the discovery of more effective, better-tolerated, and safer analgesics [2,3]. Thus, extensive research and development efforts in both academia and industry have been directed towards the discovery of novel analgesics. However, despite significant effort, there has been a lack of breakthrough discoveries during the past half century [4,5]. Of the potential drugs that go through the development pipeline, the leading cause of failure is lack of efficacy in humans. This number is estimated to be the cause of about 30% of all failures in the very costly clinical phase of drug development [6]. Tetreault [5] points out that the weak preclinical prediction of clinical efficacy may be partially a result of limitations of pain assessment in animal models.
The dominant paradigm in analgesic drug development has relied heavily on behavioral pharmacology in laboratory animals. Since nonhuman animals cannot self-report, acute behaviors in response to noxious stimuli were used as an index to gauge pain in experimental animal models. Traditional preclinical assessment of pain in animals has relied on measuring the reflexive withdrawal of a limb to physical stimuli, including punctate tactile stimuli, deep pressure, heat or cold [7–9]. However, there has been ongoing discussion and criticism in the field that these traditional assays may be insufficient to fully encompass the range of clinical pain and do not test the nature of spontaneous pain. Traditional assays such as the von Frey assay and plantar test rely on a behavior paradigm which requires an acute stimulation to induce an evoked readout of reflexive responses. These assays are prone to confounding factors due to forcefully stimulating the animal for a reflex response and the potential observer bias due to often unintentional incomplete blinding of the operator [10]. The issue of whether we can rely on these tests to have a predictive validity for a pre-clinical pain study is in question since they: 1) can be evoked without supraspinal processing as in spinalized animals and the reflexes are not necessarily cerebral-mediated pain responses (for tail-flick reflex), 2) are affected by surgical damage to axons of motor neurons, and 3) lack predictive value [11–15]. Noxious stimulus-evoked reflexive responses most likely do not involve cognitive and emotional aspects of clinical pain, although these dimensions of pain are not directly assessed by the traditional behavioral assays. While the field still lacks clearly defined and well-accepted behavioral paradigms most appropriate for assessing pain in animal models, there have been new attempts to incorporate the affective component of pain with various behavioral paradigms (reviewed in Li [16]).
A static weight-bearing device using a dual-channel weighing apparatus was developed and historically used as a clinical tool in orthopedics as an indicator of pain [17], as well as to monitor changes in postsurgical recovery and gait [18]. The use of the weight-bearing test to assess arthritic pain in rodents was first introduced by Schöt et al. [19] and subsequently utilized to assess pain in various experimental models including neuropathic, inflammatory, and cancer pain [20–22]. The advantage of the static weight-bearing test is that it is allegedly an objective measure of pain that does not involve artificial external stimuli and is applicable to a large spectrum of animal pain models in hind limb. Also, unlike evoked pain response paradigms, the weight-bearing distribution has been often included in the repertoire of behaviors that assess spontaneous pain as well as guarding behavior of the site of pain or injury [14]. However, the conventional static weight-bearing test requires restraining the animals in a small cage which confines them to an unnatural posture during the period of measurement. Two issues arise from conventional behavior assays such as the incapacitance meter. Such restriction of movement against the animals' desire during the test may obscure the data by either invoking acute stress-induced analgesia [23, 24] or chronic stress-induced hyperalgesia [25,26]. Also, repeated acute restraint of rodents has been shown to induce modulation of conventional pain readouts [27–29]. As an alternate solution, a dynamic weight-bearing (DWB) device that reduces the potential restraintstress was introduced and validated [5,30] for its compatibility with conventional pain models. Although DWB is a more sensitive free-moving behavior system requiring less handling, a major disadvantage of the DWB device is that the analysis requires labor intensive manual integration of the multiple video and sensor data [30].
We introduce a novel automated, free-moving, and high-throughput weight-bearing device as a convenient method for assessing pain in rodents. Our device combines the basic concept of the static weight-bearing test with a novel chamber modification to the measurement apparatus to allow a simple behavioral task and an appropriation of a thirst satiation reward-driven voluntary behavior of the animals to measure its own weight without an operator. As such, the present paradigm incorporates an affective component to the pain assessment since the rats must assess the reward of satiating thirst vs. the discomfort of pain. Therefore, our device circumvents the limitation of conventional behavioral assays requiring a restraint or extensive handling of animals while allowing the experimenter to obtain hundreds of consistent and independently replicable unbiased weight distribution data. The implemented hardware, software, and behavioral paradigm allow rapid accumulation of unbiased weight-bearing measures with minimal user intervention suited for a large-scale automated pain assessment in rodents required for analgesic development.
2. Materials and methods
2.1. Voluntarily Accessed Static Incapacitance Chamber (VASIC)
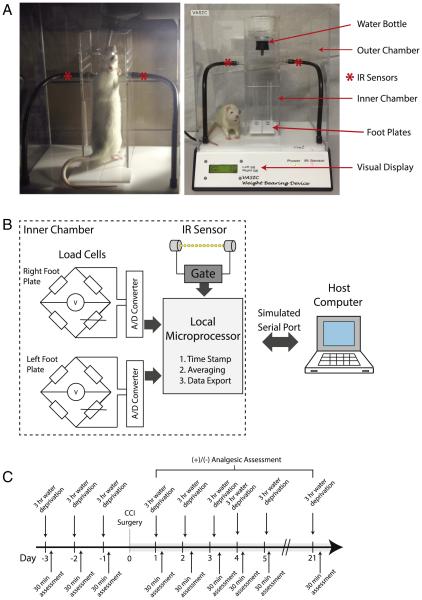
The analog weight information obtained from the load cells updated at a 100 millisecond interval was converted to digital data by an onboard analog-to-digital converter. The local microprocessor averaged the weight data over a user-defined time interval, and the averaged weight data was transmitted to the host computer via a Windows simulated serial port on a USB port. The detection of correct animal position within the smaller inside chamber was determined by the infrared (IR) beam breaker detector positioned below the water source that can only be reached when the animal positions itself on the weighing platforms. Once captured by the host computer, the weight data for right and left sides, along with a time stamp, were collated as a text file and saved. An analysis software developed in Excel (Microsoft, Seattle, WA) read the text data, applied user-selected data filters, and wrote out the processed data onto an output column of the spreadsheet. Figures were made, in Excel, from the output text files. Fig. 1 shows photographs of the animal voluntarily positioned in the behavioral chamber to access water as well as a block diagram of the relevant components of the electronic circuit. The current VASIC model allows detection sensitivity of 0.2 g up to 800 g for each foot pad. The inner chamber and software calibration is designed and optimized to accommodate a rat size ranging from a lower limit of approximately 70 g to an upper limit of 500 g by body mass. A schematic of the device along with the physical dimensions and key hardware components is listed in Supplemental Fig. 1 and Supplemental Table 1. The VASIC device and the data analysis software will be commercially available in the near future. Meanwhile, further details of the hardware design and software can be obtained from the corresponding author.
Fig. 1.
Voluntarily Accessed Static Incapacitance Chamber (VASIC). A. Photographs of the VASIC device showing the outer and inner plexiglass chambers, water bottle, IR sensors, and footplates. Photo on the left shows a typical self-positioning of an animal on the footplates inside the inner cage. As the animal accesses the water mounted on top of the inner chamber, the IR beam is broken and recording is initiated. The load cells are positioned underneath the footplates which feed the real-time weight information every 100 ms. B. A block diagram of the major components of the device. The load cells placed under the footplates transduce the weight into analog signals that are converted by the analog-to-digital (A/D) converter and subsequently processed by a local microprocessor for time stamp, signal averaging, and data export via a Windows simulated serial port to the host computer. The IR sensor gates the transfer of data. C. A timeline of a typical experiment indicating the pre-surgery water restriction conditioning for 3 days and a daily assessment of the weight distribution with VASIC.
2.2. Chronic constriction injury rat model
All studies were approved (Protocol M02476) by the local institutional animal care use committee, and all animals were treated in accordance with published NIH standards. Male Sprague Dawley rats weighing 150–200 g, purchased from Harlan Lab (Madison, WI), were used for this study. General anesthesia was induced by delivery of 3–4% isoflurane in oxygen at 3 L/m. Once the animal was unconscious, 1.5% isoflurane in oxygen was introduced at a rate of 2 L/m via a nose cone to maintain anesthesia during the surgery. Upon clipping the hair on the dorsal aspect of the left hind leg, the surgical area was cleaned with 70% ethanol and povidone–iodine scrub solution. An incision of 1.5–2 cm was made dorsal to the pelvis where the biceps femoris and left gluteus superficialis are separated. A small incision was made between the two muscle bellies to expose the sciatic nerve and isolated by blunt dissection with forceps. Using forceps, four loose ligations were made using 4-0 chromic gut suture such that the distance between each knot was less than 1.0 mm apart. The biceps femoris and gluteus superficialis muscle layer and the skin were closed up using a simple interrupted pattern with 5-0 nylon sutures. Animals were assessed daily post-operatively to detect any signs of excessive pain. None of the animals showed behaviors indicative of excessive pain, such as significant appetite change, aggression, or autotomy. In rats receiving morphine sulfate (2 mg/kg), the drug was administered intraperitoneally (i.p.) 30 m before the behavioral assessment.
2.3. Complete Freund's adjuvant or carrageenan injection
Inflammatory pain was induced by injecting 150 μL of CFA 50% m/v 1:1 emulsion of 75 μg Mycobacterium tuberculosis dry cells (Sigma, St. Louis, MO, USA) in 75 μL phosphate buffered saline inter-dermally or 100 μL of 1% w/v λ-carrageenan (Sigma, St. Louis, MO, USA) in normal saline solution in the left hind paw pad using a 25-gauge needle.
2.4. Behavioral paradigm and conditioning
Rats were housed in the institutional vivarium with ad libitum access to food and water. A standard water restriction protocol involved subjecting the rats to a 3 h water restriction in the housing cage prior to a single behavioral assessment session of 30 m in VASIC, where animals were given access to either water or 6% sucrose water. After 3–5 days of acclimatization, the rats were brought to the laboratory and taken off of water for the indicated times. Immediately after the water restriction session ended, the rats were placed in the VASIC device and the behavioral assessment started. The measurement was taken for 30 min (1800 s), and the rats were returned to their housing with, once again, ad libitum access to food and water. The brief water restriction had no apparent ill effect on the health of the rats. Once the rats were placed in the VASIC device, the data acquisition was completely automated with no further user involvement, precluding the risk of operator bias and the need for blinding the behavioral observer. The same behavioral assessment was repeated for a minimum of 3 days to maximally condition water access prior to subjecting the rats to surgery. This protocol was determined as a standard condition that provided sufficient weight distribution data.
2.5. Statistical test
All weight-distribution data are presented as mean ± S.E.M. The statistically significant difference in the mean was evaluated by a one-way or two-way ANOVA, as appropriate, followed by a post-hoc comparison of the means with Sidk–Bonferroni correction for multiple t-tests (Prism 6, GraphPad Software, Inc., La Jolla, CA) and α < 0.05 was defined as a significant difference.
3. Results
3.1. VASIC device
We present the Voluntarily Accessed Static Incapacitance Chamber (VASIC) as a new device for obtaining an essentially hands-off automated method for acquiring weight-bearing measures in rats. The acquisition device consists of two load cells to measure the weight exerted by the right and left hind feet placed on the footplates, with an additional control such that the data transmission of weight information to the host computer is gated by breaking an IR sensor placed near the top of the inner water chamber (Fig. 1A, B). The IR beam is broken when the rat positions itself to drink out of the water bottle, placing both feet on the weighing platform. A typical experimental protocol is depicted in Fig. 1C. Supplemental Video 1 is a video clip of a rat placed in the VASIC device accessing water.
3.2. The water-seeking behavior after water restriction
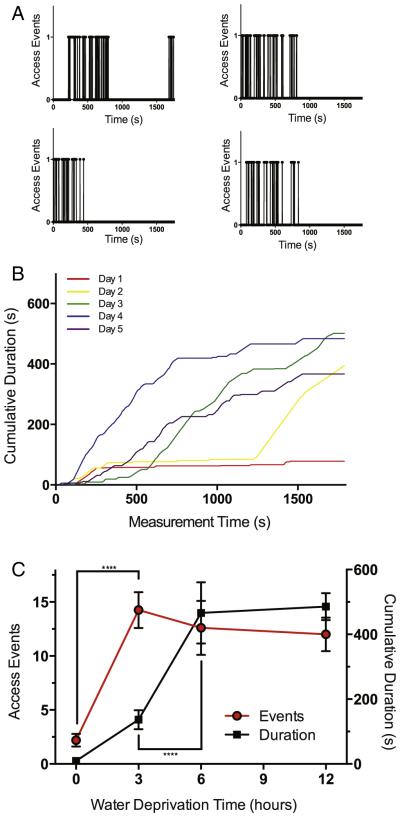
Naive rats placed in the VASIC rarely access the water bottle, but a brief water restriction prior to placing the rats in the chamber greatly increases the access frequency and duration. Fig. 2A shows data of IR beam break-events from water-restricted rats accessing the water bottle. Most rats drank water and positioned themselves on the footplates during the first 1000 s of observation, and the water-seeking behavior was essentially satiated by 2000 s. Repeated water restriction and placement of the rats in the chamber rapidly conditioned the rats (Fig. 2B), and a sufficient number and length of voluntary entries into the inner water chamber and onto the weighing platform were achieved. Water restriction for longer duration up to 12 h did not increase the water-seeking behavior during the observation period (Fig. 2C). Exploration of the water restriction time and repeated conditioning parameters determined a 3 day conditioning with 3 h water restriction as an optimal paradigm for acquisition of sufficient weight-bearing measurements.
Fig. 2.
Brief water restriction and conditioning increase the weighing chamber access frequency and total duration of access. A. Water-access detected by the IR sensor during a 30 m observation period is plotted for 4 different rats. An upward measure at 1 (arbitrary unit) indicates water access and interruption of the IR sensor. Animals were water deprived in the housing cage for 3 h prior to the behavioral measurement. B. A cohort of rats (n = 3) repeatedly conditioned with 6 h water restriction and water access; cumulative duration during a 30 m observation session was plotted. The lines are cumulative access time over 5 days (different colors) of conditioning. C. The graph shows the number of access events (circle, red line) and duration of access (box, black line) for control rats subjected to different durations of water restriction. Both number of events and cumulative duration were significantly different by one-way ANOVA and a post-hoc comparison of the mean was significantly different from the no water restriction control for all deprivation times (****P < 0.0001 n = 5.). No repeated water restriction was imposed in a single 24 h period. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Consecutive data of weight distribution during a typical session
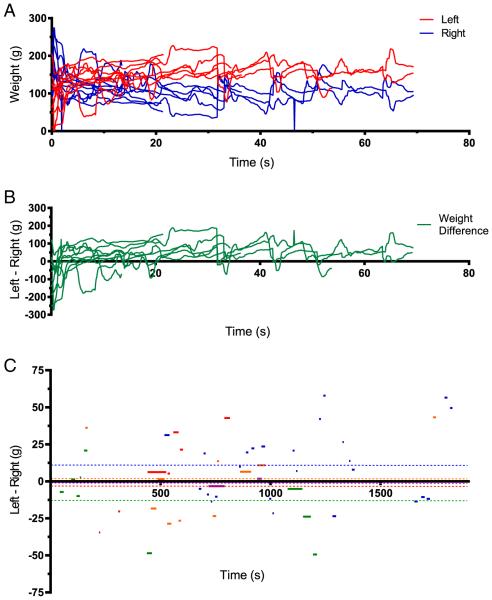
Weight distribution during a typical 30 minute observation session from a single rat is shown in Fig. 3A (actual weight recorded for each hind limb) and B (weight difference reported as Left–Right). The individual lines (an epoch) represent the times when the rat was “in position” as detected by the IR sensor triggering transfer of weight measures to the host computer. Inspection of the data showed weight measurement fluctuations especially at the start and end of an epoch. Observation of the rats confirmed that the rats often shifted weight and turned the body in the process of entering or leaving the water chamber. We developed an Excel routine for a post hoc filtering of weight measurement data, deleting the unstable 2 s of data at the start and end of each epoch. The mean weight data from the stable middle portion of every epoch were calculated, and a grand mean (indicated by the dashed line in Fig. 3C) based on all epochs of data collected during a 30 m session was taken as the final measurement of weight distribution for that session. Different colors represent different rats in this cohort of 5 animals.
Fig. 3.
Time course of weight distribution during access epochs. A. The raw weight measures for left (red) and right (blue) are plotted as a function of time where each access epoch is overlapped and plotted from time 0. The access duration varied from some lasting as long as 70 s to some lasting only a few seconds. A change of weight distribution on the left limb is often reflected by a reciprocal change in the right limb. Data is from one rat. B. Weight difference plotted as Left–Right for the data shown in A. C. The mean weight difference (Left–Right) shown for every access during a 30 m observation session. The data was post hoc processed to remove the initial and terminal 2 s of data with a larger variability from every access epoch, and only data within 1 S.D. are plotted. The different colors represent different rats. The dashed colored lines extending for the entire duration are the final mean weight-bearing difference for the individual rats. The overall weight-bearing difference (n = 5, mean = 5.8 ± 14.2 g) for this cohort of control rats was statistically not significantly different from 0 (P > 0.5). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. The aggregate weight distribution data from a typical session
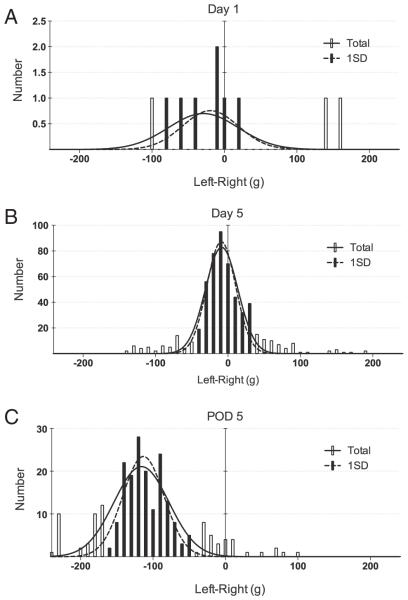
The weight distribution (shown as Left–Right) for a typical session is shown in Fig. 4. An unconditioned rat at day 1 (Fig. 4A) rarely entered the recording chamber, yielding few usable data points (n = 10). However, hundreds of weight distribution data points were obtained by day 5 from a single 30 m recording session, where the data distributed normally with a mean indicating unbiased, near-equal distribution of body weight between left and right (n = 556, mean = − 8.70 ± 0.05 g). The open bars are outliers defined as any data points beyond 1 S.D. Even with 1 S.D. filter applied, excluding outliers, the result showed consistency in the mean value (n = 433, mean = − 9.26 ± 0.04 g) with no difference from the all data value. The outlier data points observed probably resulted from the rats being improperly positioned on the footplates as the animal entered and exited the device. Fitting of a normal distribution curve to all the available data (solid line and open bars), or to data filtered to limit the analysis to data within one S.D. (dashed line and closed bars), both indicated that the baseline weight distribution showed no preference for the control unoperated animal. At day 5 after initiation of the measurements, the same rat was subjected to the chronic constriction injury (CCI) of the left sciatic nerve and the weight distribution recorded. Fig. 4C shows weight distribution on post-operative day (POD) 5 demonstrating a highly significant (P < 0.001) bias in the weight distribution towards the unoperated side, compared to the pre-surgery control (n = 232, mean = − 116.0 ± 2.49 for all data; n = 163, mean = − 113.30 ± 2.23 g after 1 S.D. filter). We have also confirmed, in a limited number of rats, that inflammatory challenge by the injection of complete Freund's adjuvant or carrageenan demonstrated a significant weight distribution bias (*P < 0.05) as recorded by the VASIC device (Supplemental Fig. 2).
Fig. 4.
The weight-bearing data is normally distributed. A. L–R weight distribution data for a naïve rat. B. A significant number of weight data points are generated in the same animal after 5 days of 3 h pre-VASIC water restriction conditioning. All weight data shown are from a single 30 m observation session (n = 556, mean = − 8.70 ± 0.05 g). C. The same rat at post-operative day (POD) 5 after CCI (n = 232, mean = − 116.0 ± 2.49 g). Note the difference in the vertical axis. Pre- and post-CCI means were significantly different (P < 0.001). The open bars are outliers defined as data points beyond 1 S.D. Fitting of a normal distribution to all data points (dashed line) or after exclusion of the outliers (solid line) had no effect on the estimation of the mean. All data is from a single rat conditioned by water restriction for 3 h before each VASIC session.
3.5. The weight distribution shifts after CCI surgery
Fig. 5A and B shows a pre-operative (dashed lines) and post-CCI surgery (solid lines) summary plot of a cohort of rats (n = 5). The figure indicates the number of accesses to the water (Fig. 5A), the cumulative duration of accesses per 30 m recording session (Fig. 5B), and the weight distribution (Fig. 5C). The sham (n = 4) or CCI (n = 5) surgery transiently reduced the cumulative duration, but the access frequency did not change significantly and still provided a sufficient number of valid weight-bearing data even at POD 1. In the CCI rats (solid line), the weight distribution showed the expected bias against the injured leg, with greater weight placed on the non-operated side (Fig. 5C). Sham operated rats where the sciatic nerve was surgically exposed but with no placement of the ligature showed a transient bias in the weight distribution data that completely recovered by POD 5 (dashed line). Finally, in a different cohort of animals (n = 6 for each group), a long-term weight distribution extending for 3 weeks after CCI and the effect of i.p. morphine were assessed (Fig. 5D). Data showed the expected weight distribution bias and statistically significant analgesic effect of morphine over the 3 week observation period post-CCI (Fig. 5D).
Fig. 5.
CCI does not reduce the access frequency, but shows transient reduction of total time accessed per observation session. A and B show the summary number of access and cumulative duration of access per 30 m observation session for a cohort of 5 rats, starting with conditioning for 5 days (dashed line) and after the CCI surgery (solid line). C. Weight distribution data (Left–Right) for CCI (solid square, n = 5) and sham surgery (open square, n = 4) assessed on post-CCI days 1–5. A two-way ANOVA demonstrated significant effects of both group (sham vs. CCI) and time treatments. Post-hoc comparison of the means with Sidk–Bonferroni correction for multiple t-tests showed statistically significant difference between the sham and CCI groups for all time points (**P < 0.01, ***P < 0.001, ****P < 0.0001) with exception of post-operation day 3. D. The weight distribution bias reported as Left–Right over a period of 21 days for CCI animals (n = 6 for each group). The measurements were obtained at the indicated time points after CCI (open circles). The morphine cohort (solid squares) received 2 mg/kg i.p. 30 min prior to the weight distribution assessment. A two-way ANOVA demonstrated significant effects of both group (morphine vs. vehicle) and time treatments, and post-hoc comparison of the means with Sidk–Bonferroni correction for multiple t-tests showed statistically significant difference between the morphine and vehicle groups for all time points (***P < 0.001, ****P < 0.0001).
Although the water restriction period is short (3 h), we acknowledge that there may be a risk of animal stress arising from dehydration during short-term water deprivation. Our preliminary experiment suggested that rewarding the rats with 6% sucrose in the water bottle, but without any water restriction prior to the experiment, might be sufficient for obtaining weight-bearing data (Supplemental Fig. 3) with a trade-off of reduced net duration of access and data quantity. The weight-bearing measure of the water restricted and non-restricted groups did not show a statistically significant difference.
4. Discussion
Static and dynamic weight-bearing has been used to assess nociception in rodents subjected to diverse pain models. Significant differences in hind paw weight distributions were observed up to 21 days following peripheral hind paw inflammation with complete Freund's adjuvant (CFA) in a mouse osteoarthritis model, in the CCI, and femoral cancer model, as well as in the hind limb carrageenan model (reviewed in [31]). However, all the studies mentioned only acquire small data samples obtained with a risk for significant operator bias through data selection during the behavior assay, while exposing the animal to the repeated acute stress of being restrained in a rigid cage. The dynamic weight-bearing assessment precludes the necessity for restraint [5]. However, the weights exerted by the forepaws and the hind paws must be correlated and determined through observation of a video recording after the acquisition of data, increasing the operator time commitment. The VASIC device described in the present work circumvented the necessity for restraining the rats and allowed rapid, parallel, and unbiased automatic collection of a large amount of weight distribution measurements. A brief water restriction encouraged each rat to position itself onto the footplates in order to access the water bottle, and an IR sensor detected the rat drinking water, triggering the data acquisition. The data obtained by the VASIC device demonstrated the expected non-biased weight distribution in control or sham operated rats, while a preference for the non-operated side was clearly observed in rats subjected to CCI. The expected analgesic effect of morphine was captured as well. Limited preliminary experiments confirmed the utility of VASIC for inflammatory models. The quantitative difference in the weight-bearing bias observed for CFA and carrageenan models suggests that VASIC may provide a graded pain readout. We currently have six VASIC devices controlled by one laptop computer, and there is no loss of data at an averaging duration where the microprocessor from each device transmits the updated data to the computer every 0.5 s. While we have not applied the device for measurement of weight distribution in mice, proper scaling down and optimization of the inner chamber and footplate size should allow us to use this device for mice to take full advantage of the gene-targeted mice resource.
We acknowledge that the weight-bearing test could reflect tactile allodynia of the hind limb in contact with the floor, rather than reporting on spontaneous pain. However, VASIC does not require direct external stimulation of the hind limb and should better reflect pain experienced by the rats under normal activity. There are no known methods of measuring pain in rodents which do not evoke a possible tactile allodynia due to contact with a solid surface, and pain from tactile touch on the hard surface would occur during normal activity. What the VASIC device offers is not a solution to the debate on whether weight-bearing is a measure of spontaneous pain or not, but an improved nociceptive behavioral measurement method that involves 1) less chance of introducing subjective selection bias to the data, 2) rapid and high throughput acquisition of a large amount of data from multiple animals, 3) compatibility with standard animal pain models that utilize hind limb injury, and 4) nonreflexive operant behavior controlled by the animal. Further experiments are necessary to determine whether VASIC evokes less experimental stress compared to conventional weight-bearing apparatuses, and whether weight-bearing can be used as an accurate reporter of spontaneous pain. Perhaps validation of analgesic efficacy can occur ultimately in humans only [32]. However, we believe that appropriate assessment of pain in animal models is a necessary step in drug development.
Mogil [33] points out that most existing behavioral data sets aimed at assessing pain in rodents are comprised of low-density, very brief evaluations of animals in their resting circadian phase while placed in a novel observation environment. He asks whether longer evaluations of home-cage behaviors would yield better results more reflective of pain states in rodents. The VASIC device is particularly suited for this type of home-cage measuring application, since the rodents could be placed inside the device and weight distribution measurements could be obtained throughout the circadian cycle every time they seek water. We plan to expand the VASIC device to be able to accommodate a home-cage-like monitoring over an extended period of time.
5. Conclusions
In summary, we developed VASIC as a novel device for automated high-throughput static weight-bearing assessment of pain in free-moving rodents. VASIC provided hands-free collection of abundant objective weight distribution data that should be applicable to a large spectrum of different pain models to study surgical or drug induced neuropathic and inflammatory pain.
Supplementary Material
HIGHLIGHTS.
We developed a novel device (VASIC) for automated weight-bearing pain measurement.
The VASIC device is compatible with conventional hind-limb injury pain models.
The device allows hands-free high-through put collection of unbiased behavioral data.
Acknowledgments
The authors thank Tessa Yang for editing the manuscript. The Bamforth Endowment Funds from the Department of Anesthesiology, University of Wisconsin—Madison, and NIH RO1 GM105665 and GM107054 provided financial support for this study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.physbeh.2015.06.035.
References
- [1].Pain Management Fact Sheet. National Institute of Health; Bethesda, Maryland: http://report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=57. [Google Scholar]
- [2].Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J. Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- [3].Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- [4].Kissin I. The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesth. Analg. 2010;110:780–789. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- [5].Tétreault P, Dansereau MA, Doré-savard L, Beaudet N, Sarret P. Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiol. Behav. 2011;104:495–502. doi: 10.1016/j.physbeh.2011.05.015. [DOI] [PubMed] [Google Scholar]
- [6].Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- [7].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- [8].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- [9].Randall LO, Selitto JJ, Valdes J. Anti-inflammatory effects of xylopropamine. Arch. Int. Pharmacodyn. Ther. 1957;113:233–249. [PubMed] [Google Scholar]
- [10].Woolf CJ. Overcoming obstacles to developing new analgesics. Nat. Med. 2010;16:1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- [11].Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. Pharmacol. Exp. Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- [12].Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112:12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- [13].Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- [14].Blackburn-Munro G. Pain-like behaviours in animals — how human are they? Trends Pharmacol. Sci. 2004;25:299–305. doi: 10.1016/j.tips.2004.04.008. [DOI] [PubMed] [Google Scholar]
- [15].Irwin S, Houde RW, Bennett DR, Hendershot LC, Seevers MH. The effects of morphine, methadone and meperidine on some reflex responses of spinal animals to nociceptive stimulation. J. Pharm. Exp. Therap. 1951;101:132–143. [PubMed] [Google Scholar]
- [16].Li JX. The application of conditioning paradigms in the measurement of pain. Eur. J. Pharmacol. 2013;716:158–168. doi: 10.1016/j.ejphar.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Christiansen CL, Stevens-lapsley JE. Weight-bearing asymmetry in relation to measures of impairment and functional mobility for people with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2010;91:1524–1528. doi: 10.1016/j.apmr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jones ME, Steel JR, Bashford GM, Davidson IR. Static versus dynamic prosthetic weight bearing in elderly trans-tibial amputees. Prosthetics Orthot. Int. 1997;21:100–106. doi: 10.3109/03093649709164537. [DOI] [PubMed] [Google Scholar]
- [19].Schöt E, Berge OG, Angeby-Moller K, Hammarstrom G, Dalsgaard CJ, Brodin E. Weight bearing as an objective measure of arthritic pain in the rat. J. Pharmacol. Toxicol. Methods. 1994;31:79–83. doi: 10.1016/1056-8719(94)90046-9. [DOI] [PubMed] [Google Scholar]
- [20].Medhurst SJ, Walker K, Bowes M, Kidd BL, et al. A rat model of bone cancer pain. Pain. 2002;96:129–140. doi: 10.1016/s0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- [21].Bove SE, Calcaterra SL, Brooker RM, Humber BS, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetetate-induced osteoarthritis. Osteo. Arthritis. Cart. 2003;11:821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- [22].Nakazato-Imasato E, Kurebayashi Y. Pharmacological characteristics of the hind paw weight difference induced by chronic constriction injury of the sciatic nerve in rats. Life Sci. 2009;84:622–626. [PubMed] [Google Scholar]
- [23].Amit Z, Galina ZH. Stress-induced analgesia: adaptive pain suppression. Physiol. Rev. 1986;66:1091–1120. doi: 10.1152/physrev.1986.66.4.1091. [DOI] [PubMed] [Google Scholar]
- [24].Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: activation by stress. Science. 1984;226:1270–1277. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- [25].da Silva-Torres IL, Cucco SN, Bassani M, et al. Long-lasting delayed hyperalgesia after chronic restraint stress in rats—effect of morphine administration. Neurosci. Res. 2003;45:277–283. doi: 10.1016/s0168-0102(02)00232-8. [DOI] [PubMed] [Google Scholar]
- [26].Imbe H, Iwai-liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front. Biosci. 2006;11:2179–2192. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- [27].Gameiro GH, Andrade Ada S, De Castro M, Pereira LF, Tambeli CH, Veiga MC. The effects of restraint stress on nociceptive responses induced by formalin injected in rat's TMJ. Pharmacol. Biochem. Behav. 2005;82:338–344. doi: 10.1016/j.pbb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [28].Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–625. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- [29].Dantas G, Torres IL, Crema LM, Lara DR, Dalmaz C. Repeated restraint stress reduces opioid receptor binding in different rat CNS structures. Neurochem. Res. 2005;30:1–7. doi: 10.1007/s11064-004-9679-2. [DOI] [PubMed] [Google Scholar]
- [30].Robinson I, Sargent B, Hatcher JP. Use of dynamic weight bearing as a novel end-point for the assessment of Freund's complete adjuvant induced hypersensitivity in mice. Neurosci. Lett. 2012;524:107–110. doi: 10.1016/j.neulet.2012.07.017. [DOI] [PubMed] [Google Scholar]
- [31].Tappe-Theodor A, Kuner R. Studying ongoing and spontaneous pain in rodents—challenges and opportunities. Eur. J. Neurosci. 2014;39:1881–1890. doi: 10.1111/ejn.12643. [DOI] [PubMed] [Google Scholar]
- [32].Borsook D, Hargreaves R, Bountra C, Porreca F. Lost but making progress — where will new analgesic drugs come from? Sci. Transl. Med. 2014;6(249):249sr3. doi: 10.1126/scitranslmed.3008320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mogil JS. Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.