Abstract
Objective
To credential Stathmin 1 (STMN1) and p16INK4A (p16) as adjunct markers for the diagnosis of serous tubal intraepithelial carcinoma (STIC), and to compare STMN1 and p16 expression in p53-positive and p53-negative STIC and invasive high-grade serous carcinoma (HGSC).
Methods
Immunohistochemistry (IHC) was used to examine STMN1 and p16 expression in fallopian tube specimens (n=31) containing p53-positive and p53-negative STICs, invasive HGSCs, and morphologically normal FTE (fallopian tube epithelium). STMN1 and p16 expression was scored semiquantitatively by four individuals. The semiquantitative scores were dichotomized, and reported as positive or negative. Pooled siRNA was used to knockdown p53 in a panel of cell lines derived from immortalized FTE and HGSC.
Results
STMN1 and p16 were expressed in the majority of p53-positive and p53-negative STICs and concomitant invasive HGSCs, but only scattered positive cells were positive in morphologically normal FTE. Both proteins were expressed consistently across multiple STICs from the same patient and in concomitant invasive HGSC. Knockdown of p53 in immortalized FTE cells and in four HGSC-derived cell lines expressing different missense p53 mutations did not affect STMN1 protein levels.
Conclusions
This study demonstrates that STMN1 and p16 are sensitive and specific adjunct biomarkers that, when used with p53 and Ki-67, improve the diagnostic accuracy of STIC. The addition of STMN1 and p16 helps to compensate for practical limitations of p53 and Ki-67 that complicate the diagnosis in up to one third of STICs.
INTRODUCTION
High-grade serous carcinoma (HGSC) is the most common form of epithelial ovarian cancer and typically presents at an advanced stage when current therapies are rarely curative[1]. A growing body of literature now supports the fallopian tube fimbria as the site of origin for a majority of HGSCs[2–8]. The chief argument for a tubal origin of HGSC is the presence of occult non-invasive carcinomas in the distal end of the fallopian tube (i.e., fimbria), designated serous tubal intraepithelial carcinoma (STIC). Morphologic and genetic evaluation of STICs have shown a high degree of similarity to concomitant ovarian or peritoneal carcinomas[1]. In particular, similar to HGSC, virtually all STICs harbor TP53 mutations, which are identical to TP53 mutations in the affiliated ovarian carcinomas, supporting their clonal relationship [9]. A similar precursor lesion in the ovary containing a TP53 mutation has not been shown. The most common TP53 mutations are missense (61%), while non-sense mutations are present in the remainder of cases [9, 10]. Missense TP53 mutations are correlated with strong diffuse staining of p53 in STICs and HGSCs, while complete absence of p53 immunoreactivity correlates with non-sense mutations, which produce a truncated protein that is not detected by the p53 antibody (“null mutation”)[9].
In addition to invasive HGSCs cases, STICs are also found in 5–10% of fallopian tubes removed prophylactically from women who are at high risk for developing ovarian cancer, including those women with BRCA mutations and/or those with a strong family history of ovarian cancer[11, 12]. Detection of STICs in the high risk population has been greatly enhanced by comprehensive pathologic assessment of fallopian tubes through the use of the SEE-FIM (Sectioning and Extensively Examining the FIMbriated end) protocol, and risk-reducing bilateral salpingo-oophorectomy (RRSO)[7]. An accurate and sensitive diagnosis of STICs in the high-risk population may impact their subsequent clinical management, and can prompt early clinical action including increased surveillance, additional surgical staging or adjuvant chemotherapy[13, 14].
Currently, STICs are diagnosed in many cases using an algorithm that combines morphologic evaluation and immunohistochemistry for p53 and Ki-67[13]. However, the histologic diagnosis can be challenging when the morphologic changes are subtle and lack reproducibility[13, 15]. Accordingly, strong and diffuse p53 immunoreactivity may be the most contributory component of the diagnostic algorithm. However, in the presence of a null mutation, p53 immunoreactivity is completely absent, and this can occur in 20%–50% of STICs [11]. This diagnostic pitfall necessitates the development of additional biomarkers to aid in diagnosis of p53-negative STICs.
Previous studies have shown that overexpression of other oncogenic proteins can also be associated with STIC and HGSC. Karst et al reported that Stathmin 1 (STMN1), a cytoplasmic phosphoprotein that regulates microtubule dynamics, is strongly and diffusely immunoreactive in STICs and a large proportion of HGSCs, but not in non-neoplastic FT epithelium [16]. Similarly, p16INK4A (p16), a cyclin-dependent kinase IV inhibitor, has been shown to be overexpressed in STIC[17] and HGSC [18, 19]. However, neither of these studies specifically addressed the expression of these proteins in p53-negative STICs. The primary objective of this study was to compare STMN1 and p16 expression in p53-positive and p53-negative STICs and HGSCs, and to credential these proteins as adjunct biomarkers for the diagnosis of STIC.
MATERIALS AND METHODS
This study was approved by the Institutional Review Boards at the Cedars-Sinai Medical Center (CSMC), Brigham and Women’s Hospital (BWH), Dana-Farber Cancer Institute (DFCI), and Yale University.
Case Selection
The cases for this study were obtained from the Departments of Pathology at CSMC, BWH, and Yale University. Tubal sections were cut from paraffin blocks from 31 patients whose original pathology reports indicated the presence of STIC. These H&E slides were reviewed concurrently by two pathologists (MSH, RD) to confirm the presence of STICs and possibly invasive carcinoma in the deeper tissue sections cut for this study. STICs were diagnosed based on established morphologic features, including loss of ciliated cells, loss of cell polarity, epithelial stratification and tufting, nuclear enlargement and pleomorphism, nuclear hyperchromasia, prominent nucleoli, and increased mitotic figures. Lesions fulfilling morphologic criteria were then examined for p53 and Ki-67 reactivity in subsequent serial sections. Lesions with >10% Ki-67-positive nuclei were considered proliferative. p53 expression was evaluated for strong diffuse immunoreactivity (positive for mutation, “p53 positive STIC”) or a complete absence of staining (positive for a null mutation, “p53-negative STIC”) in the area of atypia; scattered cells immunoreactive for p53 was considered a negative result (p53 wild type). For p53-null lesions, the presence of scattered immunoreactive stromal cells and/or non-neoplastic fallopian tube cells were noted to confirm that the antibody and immunostaining technique were adequate (i.e., positive internal control).
The tissue sections in this study were obtained from patients whose age at the time of surgery ranged between 44-75 years (mean=63, median=65). For detailed information about the patients’ age, FIGO stage, and BRCA1/2 status please refer to Supplemental table 1. Of the 31 cases, 20 cases contained p53-positive STICs, and 11 contained p53-negative (null) STICs. Six of the 31 cases contained only one STIC (four p53-positive, two p53-negative), while the remaining 25 cases contained at least two STICs (16 p53-positive, nine p53-negative) of which two per case were used for analysis; therefore, 56 STICs in total were examined in this study. Multiple STICs in one tissue section presented an opportunity to examine whether STMN1 and p16 immunoreactivity is concordant across multiple lesions in the same patient. In the 25 cases where two arbitrary STICs were evaluated, the in situ lesions were marked “A” and “B” by one author (MN) to ensure the same lesions was evaluated by multiple subsequent reviewers. For statistical analyses, only one STIC per case was used (unless comparing group A to group B), and the STICs used from either group A or B were selected randomly using the “RANDBETWEEN” function in Microsoft Excel. Twenty-four of the 31 total cases with STIC also contained an invasive tumor component (17 p53-positive, seven p53-negative). Of note, there were three cases, which contained STICs with no evidence of invasive HGSC in the tubes or in the peritoneum (marked “in situ” in Supplemental table 1). Morphologically normal tubal epithelium consisting of secretory and ciliated cells was represented in every case.
Immunohistochemistry
Immunohistochemical staining was performed using Envision Plus Horseradish Peroxidase system (DAKO, Carpinteria, CA, USA) as previously described [16]. Sections were incubated with primary antibody using the conditions specified in Supplemental Table 2. Secondary antibody was applied for 30 minutes, followed by DAB. Studies were interpreted in conjunction with appropriate positive (Supplemental Table 2) and negative (incubation without a primary antibody) controls. Additionally, scattered cell immunoreactivity by all biomarkers in non-neoplastic epithelium was used an internal positive control.
Analysis of p16 and STMN1 immunostaining
The p16 and STMN1 immunostains were scored independently by four individuals (MN, AMK, MSH, RD), to evaluate the extent of immunoreactivity (percent of positive cells). STICs (single or multiple) and invasive carcinomas were marked accordingly on the H&E slides as described above. The scoring criteria for p16 and STMN1 were adopted from Phillips et al[19] and Karst et al[16], respectively (summarized in Supplemental Table 3). In brief, the distribution of immunoreactivity for both p16 and STMN1 was scored semiquantitatively as follows: 0 (negative or occasional positive cells), 1+ (<10% cells positive), 2+ (10%–75% cells positive), 3+ (76%–100% cells positive). Lesions scored as 0 and 1+ were considered “negative”, and those scored as 2+ and 3+ were considered “positive”. All cases of discordant scoring were reviewed simultaneously and the final score was determined by consensus.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA). Differences in STMN1 or p16 expression between p53-positive and p53-negative STICS were examined using Fisher’s exact test. Difference between STMN1-positive and p16-positive STICs regardless of p53 status was examined using Fisher’s exact test. Difference in immunoreactivity for p16 or STMN1 in morphologically normal epithelium, STIC, and invasive carcinoma was examined using a Chi-square test. Concordance between STMN1 or p16 expression in paired STICs from the same case was calculated as the ratio of concordant cases to total cases, and McNemar’s test was used to compare the paired proportions. For all analyses, p values < 0.05 were considered statistically significant.
Cell culture, Gene Silencing and Western Blot
FT282-CCNE1, an immortalized human tubal epithelial line stably expressing human telomerase reverse transcriptase (hTERT), mutant p53R175H, and cyclin E1 (CCNE1) was derived and grown as previously described[20]. COV318, Kuramochi, OVKATE, EFO27, and JHOS2 are human ovarian cancer lines, which were recently identified as good models for high-grade serous ovarian cancer based on their genomic profiles[21]. These cell lines were grown in DMEM/F12 supplemented with 10% FBS. For siRNA-mediated silencing of TP53, the cells were plated and 24 hours later transfected with pooled siRNAs targeting TP53, or with non-targeting control pool using Lipofectamine RNAiMAX (Life Technologies, Carlsbad, CA, USA). The siRNAs (SMARTpool ON-TARGET Plus TP53 siRNA, and Control pool siRNA) were purchased from Dharmacon (Lafayette, CO, USA). Three days after transfection with siRNA, the cells were lysed using NETN 150 lysis buffer, prepared as previously described[16]. Proteins were separated by SDS-PAGE on 4–12% Bis-Tris gels and transferred onto nitrocellulose membranes (Invitrogen, Carlsbad, CA, USA). The membranes were blocked with 5% BSA in PBS-Tween20 for one hour and immunoblotted overnight at 4°C with primary antibodies as described in Supplemental Table 2. The blots were then incubated with the appropriate HRP-conjugated secondary antibodies diluted in 5% non-fat dry milk, for one hour at room temperature. Pierce ECL2 chemiluminescent substrate (Thermo, Rockford, IL, USA) was used to visualize the bound antibody. To compare STMN1 protein levels in the presence or absence of endogenous mutant p53, p53-null cell ovarian cell lines SKOV3 and OVCAR5, and mutant-p53-expressing OVCAR3 were grown and assayed by immunoblot as described above. To prepare cytologic cell blocks, primary HGSC cells (DF23, DF30, DF59, and DF94), derived from malignant ascites, were fixed in 10% buffered formalin and embedded in HistoGel (Richard-Allan Scientific). The HistoGel-embedded cells were then processed, sectioned, and stained as described above for regular tissue sections.
RESULTS
Overall biomarker expression in STICs and invasive carcinomas
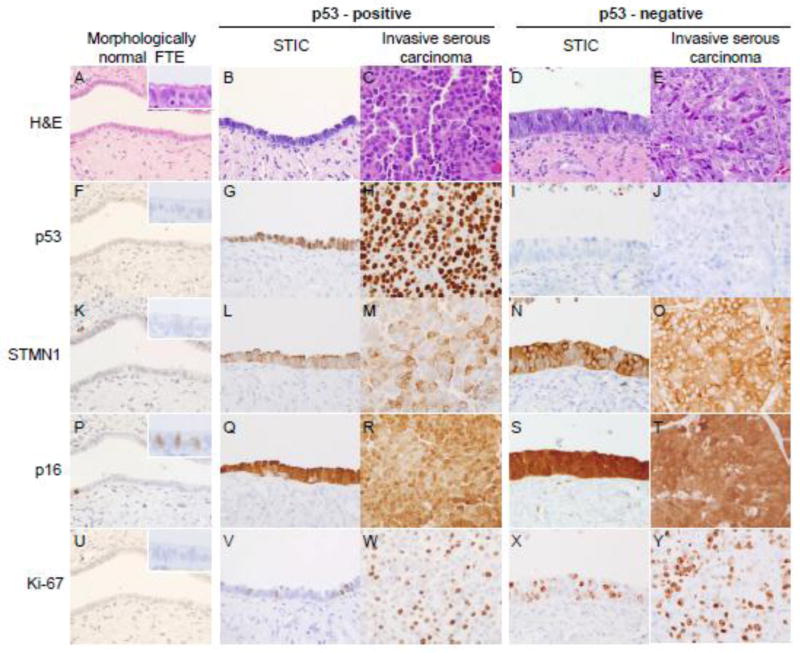
The primary objective of this study was to compare STMN1 and p16 expression in non-neoplastic FTE, p53-positive and p53-negative STICs, and associated invasive HGSCs. As expected, morphologically normal FT epithelium showed heterogeneous nuclear immunoreactivity for p53 in scattered cells, and the Ki-67 proliferation index was below 5% (Figure 1A, F, U). In contrast, 20 of 31 (65%) STICs, and 17 of 24 (71%) invasive carcinomas overexpressed p53, while Ki-67 was elevated in all cases, but was generally higher in invasive carcinomas when compared to STICs. p53 expression, when present in an invasive carcinoma, was also positive in the corresponding STIC(s); similarly, all p53-negative invasive carcinomas were associated with p53-negative STICs (Fig 1G–J). In contrast, STMN1 and p16 were immunoreactive in 84% (26/31) and 77% (24/31) of STICs, respectively. Of these cases, 3 lesions were STMN1-positive/p16-negative and 4 additional lesions were STMN1-negative and p16-positive. Invasive carcinomas were positive for both, STMN1 and p16 in all but three cases (21/24, 88%) (Fig 1L–O, and Q–T). These findings demonstrate that STMN1 and p16 have higher sensitivity for STICs and invasive carcinomas when compared to p53, but there is no significant difference when compared to each other (Table 1).
Figure 1.
STMN1 and p16 expression in (A) morphologically benign fallopian tube epithelium (FTE), (B and C) p53-positive STIC and invasive carcinoma, (D and E) p53 negative STIC and invasive carcinoma. (F) Occasional weak nuclear p53 staining in normal FTE, (G, H) strong diffuse nuclear p53 staining in p53-positive STIC and the concomitant invasive carcinoma, (I, J) absent p53 staining in p53-negative STIC and invasive carcinoma (scattered positive stromal cells serve as internal positive control). (K–O) STMN1, and (P–T) p16 staining is absent in normal FTE but strongly induced in both, p53-positive, and p53-negative STICs and invasive carcinomas. (P - inset) p16-positive staining in a subset of ciliated cells present in morphologically benign FTE. (U–Y) focal nuclear Ki-67 staining identifies proliferating cells.
Table 1.
STMN1 and p16 immunostaining in p53-positive and p53-negative STIC and invasive serous carcinoma
| Morphologic feature | p53 status | n | STMN1 | p16 | ||
|---|---|---|---|---|---|---|
| positive | p* | positive | p* | |||
| a STIC (n=31) | positive | 20 | 17 (85%) | 1.000 | 17 (85%) | 0.210 |
| negative | 11 | 9 (82%) | 7 (63%) | |||
|
| ||||||
| b Invasive carcinoma (n=24) | positive | 17 | 16 (94%) | 1.000 | 16 (94%) | 0.507 |
| negative | 7 | 7 (100%) | 6 (86%) | |||
|
| ||||||
| c Morphologically benign FTE (n=31) | positive | 20 | 0 (0%) | 1.000 | 0 (0%) | 1.000 |
| negative | 11 | 0 (0%) | 0 (0%) | |||
a vs. b; (p = 0.158); Fisher’s exact test
a, b vs. c; (p < 0.001), Chi-square
p* (p53 pos vs. p53 neg); Fisher’s exact test
STMN1 and p16 are overexpressed in p53-positive and p53-negative STICs
Next, STMN1 and p16 expression was compared in p53-positive and p53-negative STICs. Results of the scoring are summarized in Table 1 (p*). As expected based on previous reports, morphologically normal epithelium in all cases, regardless of p53-status, secretory cells stained negative for STMN1 and p16, although in several cases p16 was overexpressed in ciliated cells within morphologically normal epithelium (Fig 1P, inset). Immunostaining for STMN1 was positive in 17/20 (85%) p53-positive STICs and in 9/11 (82%) p53-negative STICs; however, this was not significantly different (Table 1). Similar to STMN1, immunostaining for p16 was positive in 17/20 (85%) p53-positive STICs and in 7/11 (63%) p53-negative STICs. As with STMN1, there was no significant difference between p53-positive and p53-negative STICs that were positive for p16 (Table 1). Finally, STMN1 and p16 were each overexpressed in 16/17 (94%) p53-positive invasive carcinomas, while in p53-negative invasive carcinomas STMN1 was overexpressed in 7/7 (100%), and p16 in 6/7 (86%) cases.
Both biomarkers are overexpressed in STICs and corresponding invasive carcinomas
There was no significant difference in STMN1 or p16 overexpression between STIC and corresponding invasive carcinoma. The Chi square statistic showed a strong association between STMN1 or p16 overexpression in STIC or invasive carcinoma versus morphologically normal epithelium (p<0.001, Chi-square, Table 1).
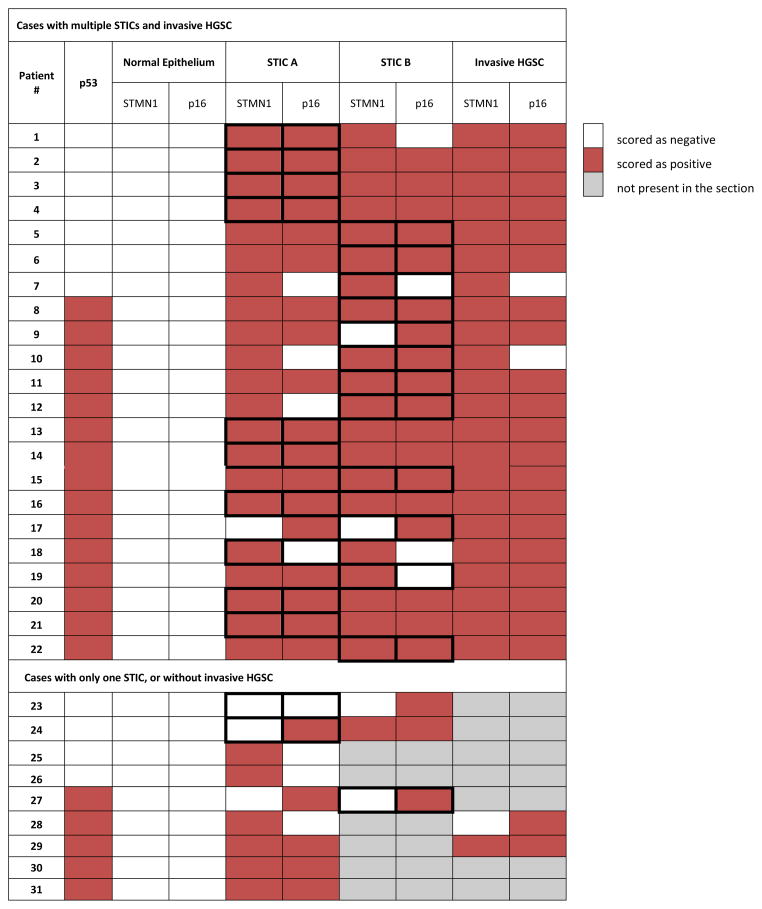
Both markers are concordantly overexpressed in multiple STICs within the same case
The overall STMN1 or p16 concordance rate between two STICs (N=25, group A versus group B) from the same case was 92% and 80%, respectively. Additionally, in the 22 cases with multiple STICs and concomitant invasive carcinoma, STMN1 and p16 overexpression was concordant in all three lesions (both STICS and the invasive HGSC) in 20 (91%) and 17 (77%) cases respectively (Table 3). There was only one case where one STIC was negative for both STMN1 and p16 (Table 3; Patient 23), while the second STIC was p16-positive. Most importantly, of the 25 patients examined with ≥2 STICS, there were no cases where both markers were absent in both STICs. Of all 56 STICs (6 single STICs plus 25 paired STICs), thirty-seven (66%) were positive for both STMN1 and p16. Simultaneous overexpression of STMN1 and p16 in morphologically normal epithelium was not observed.
Table 3.
Expression patterns of p53, STMN1, and p16 in all scored lesions
|
Bold framing marks a randomly selected STIC (from group A or B)
STMN and p16 overexpression is retained after acute loss of mutant p53
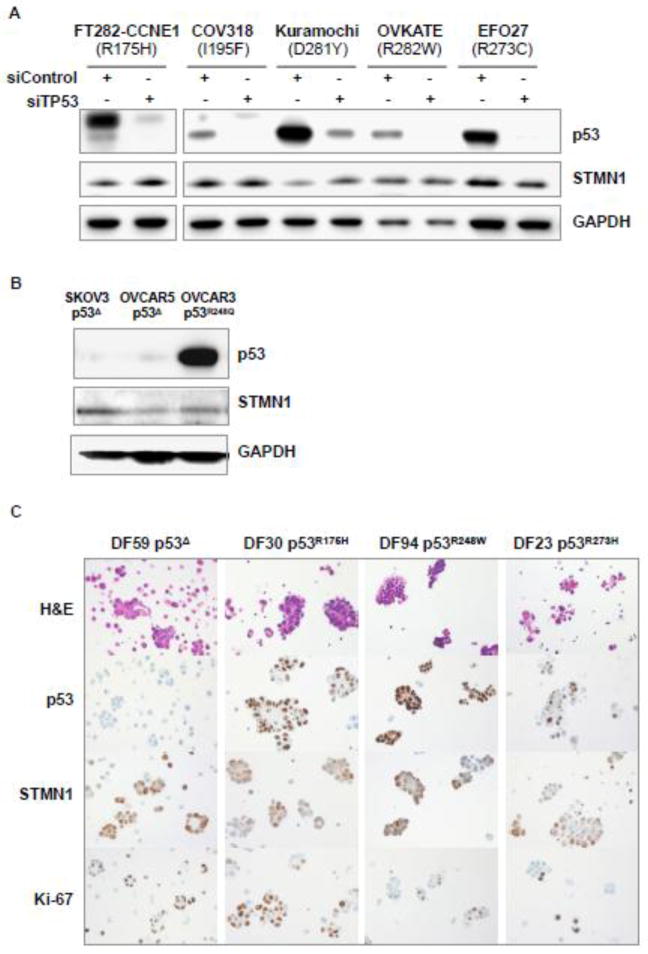
It was previously reported that mutant p53 and STMN1 expression are functionally related. Using two breast adenocarcinoma cell lines, Masciarelli et al. demonstrated that siRNA-mediated knockdown of p53 resulted in a concomitant decrease in STMN1. Masciarelli et al proposed that mutant p53 increases stability of the STMN1 transcript, leading to STMN1 protein overexpression. To address this possibility, STMN1 expression was evaluated in the presence and absence of siRNA-mediated knockdown of p53, using multiple cell lines with known missense mutations in TP53, including an immortalized tubal cell line (FT282-CCNE1), and ovarian cancer cell lines that express four different hotspot p53 mutants (COV318, Kuramochi, OVKATE, and EFCO27). As expected siRNA-mediated knockdown of p53 effectively decreased or abolished p53 protein expression below detection in all examined cell lines (Figure 2A). In contrast, STMN1 protein levels remained unchanged or were modestly elevated with siRNA-mediated knockdown of p53. To further examine the proposed dependence of STMN1 overexpression on mutant p53, we compared STMN1 protein expression in HGSC cell lines and in primary tumor cells expressing common missense (hotspot) p53 mutants and in p53-null cells. Western blot analysis of cell lysates and immunohistochemical analysis of cytology cell blocks showed comparable levels of STMN1 in all cell lines regardless of their p53 status (Figure 2B and C).
Figure 2.
STMN1 is expressed at comparable levels in the presence or absence of common p53 mutant variants. (A) STMN1 does not decrease following TP53 knockdown with siRNAs in immortalized tubal epithelial cells (FT282) or serous ovarian tumor cell lines, expressing common missense p53 mutants. (B) STMN1 expression in serous ovarian tumor cell lines devoid of full-length p53 or expressing a missense p53 mutant. (C) Immunohistochemical analysis of cytologic cell blocks detects comparable levels of STMN1 in p53-null primary HGSC cells and in cells expressing common missense p53 mutants.
DISCUSSION
STIC has emerged as an important precursor lesion in pelvic serous carcinogenesis [15]. Accordingly, an accurate diagnosis of STIC has significant clinical utility in at least two settings: in establishing the putative site of tumor origin in patients with HGSC (which is important for clinical trial eligibility), and for the correct assignment of cancer risk status in high risk women who are undergoing prophylactic RRSO, but are otherwise healthy[15]. Optimal recommendations for women with STIC lesions are still evolving, but accurate diagnosis of the index lesion will be key to developing optimal therapeutic interventions.
Previous studies have revealed considerable interobserver variability in diagnosing STICs based exclusively on histologic criteria [13, 15]. Carlsson et al[15] demonstrated that the inherent complexities of the salpingeal epithelium, including non-neoplastic histologic features, increase interobserver variability when STICs are diagnosed based on morphology alone. The study emphasized incorporation of confirmatory IHC to the diagnosis of STIC[15]. This prompted the development of a more reproducible, diagnostic algorithm for STIC, which in addition to the presence of high-grade nuclear atypia, also requires coordinate immunohistochemical expression of p53 and Ki-67[13, 15]. However, the use of these biomarkers has several practical limitations. In our experience, many STICs exhibit only a marginally elevated Ki-67 proliferation index (>10% positive nuclei), not uncommonly against a background of scattered Ki-67-positive cells in adjacent morphologically normal epithelium. Moreover, 20%–50% of STICs stain completely negative for p53, due to mutations that result in truncated or complete loss of the p53 protein, neither of which would be detected by the p53 antibody. Consequently, diagnosis of “p53-negative” STICs with marginal Ki-67 index is problematic, which necessitates the development of adjunct immunohistochemical markers.
The findings of this study support that STMN1 and p16, two proteins previously, yet independently, shown to be upregulated in p53-positive early and advanced serous carcinoma[13, 16, 17, 22, 23], are overexpressed in the majority of p53-positive and p53-negative STICs, and in concomitant invasive carcinomas. In this study, 84% of STICs and 96% of invasive carcinomas were positive for STMN1, compared to 100% of positive STICs and invasive carcinomas evaluated in a prior study [16]. In contrast, Sehdev and colleagues reported diffuse p16 immunoreactivity in only 55% (18/33) of STICs[17], which is lower than the 87% observed in this study. However, unlike the Sehdev study, in the current study, STICs were scored p16-positive when both diffuse and focal p16 immunoreactivity was present. This more inclusive categorization explains the higher incidence of p16-positive STICs. Furthermore, the incidence of p16-positive invasive HGSCs (92%) reported here corresponds well with the study by Phillips et al[19], in which exactly 95% of HGSCs contained 10% or more p16-positive cells. In the current study, both STMN1 and p16 were evaluated concurrently, and it is important to note that although not every STICs or invasive carcinoma scored double-positive for STMN1 and p16, no case was negative for both proteins. Therefore, overexpression of at least one of the proteins positively identified 100% of STICs, as well as invasive carcinomas, regardless of their p53 status. These findings demonstrate the potential of the combined use of STMN1 and p16 as sensitive and universal markers for intraepithelial and invasive serous carcinoma. However, a potential pitfall includes overexpression of STMN1 in the absence of p16 and p53 immunoreactivity, as STMN1 immunoreactivity also marks benign epithelial proliferations, termed secretory cell outgrowths (SCOUTs)[24]. However, unlike STICs and invasive carcinomas, SCOUTs do not contain TP53 mutations or evidence of a DNA damage response, and do not appear directly linked to HGSC [24]. To avoid potential overdiagnosis of SCOUTs rather that the precursor STIC, we recommend that lesions have simultaneous overexpression of STMN1 and p16, rather than STMN1 alone, and this be used when either diffuse p53 positivity (or complete loss) is present. Lastly, STMN1 and p16 were found to be overexpressed in a higher proportion of invasive carcinomas when compared to STICs. Although this difference was not statistically significant due to small numbers, it suggests direct correlation between tumor progression and overexpression of STMN1 and p16.
There is convincing evidence that the molecular status of each neoplasm is related to its biologic behavior [25]. Overexpression of STMN1 and p16 observed in the majority of STICs and invasive serous carcinomas, suggest that aberrant expression of these proteins is not specifically tied to presence of either missense, or non-sense/truncating TP53 mutations characteristic for p53-positive, or p53-negative lesions, respectively. However, recent data indicate that some of the most common mutant p53 proteins have, in addition to losing tumor suppressor function, acquired a gain-of-function: these mutants drive tumor progression and metastasis, which are in part a result of their ability to interfere with DNA repair and with the function of other p53 family members [26]. Recently, Masciarelli et al [27] reported that gain-of-function p53 mutants upregulate STMN1 via transcriptional repression of miR223, as demonstrated by siRNA-mediated knockdown of p53, which resulted in concomitant decrease in STMN1 protein levels. Their studies, conducted in breast cancer cell lines, showed that STMN1 overexpression in this context contributed to the resistance to DNA-damaging chemotherapeutic drugs. Considering these reports, we examined whether acute loss of mutant p53 would elicit a concomitant downregulation of STMN1 in cell lines derived from invasive HGSC and an immortalized tubal cell line FT282. Although siRNA-mediated knockdown of p53 effectively decreased or abolished p53 expression, STMN1 protein levels remained unchanged or were modestly elevated. It is possible that this discrepancy between the results obtained in breast cancer lines and our results is a consequence of lineage-specific function of mutant p53. To go beyond the acute effect of siRNA-mediated p53 knock-down, we also compared STMN1 expression in cultured and primary HGSC cells, which were either p53-null or expressed a known hotspot p53 missense mutant. Using both immunoblot and cell block immunochemistry, we showed that regardless of p53 status (positive, or negative), all cell lines exhibited comparable immunoreactivity to STMN1. It remains to be determined whether STMN1 and p16 expression directly contributes to fallopian tube transformation and chemoresistance, however, as shown in this study; both proteins when co-expressed could serve as robust markers for diagnosis of STICs (or biologically similar lesions) and invasive HGSCs.
Although the majority of STICs analyzed in this study were found in women with concurrent invasive serous carcinoma (N=28), three cases (10%) involved STICs found in isolation, with no evidence of invasive carcinoma (Supplemental Table 1, Patients 23, 30, 31). STMN1 was overexpressed in one, and p16 in two of the three STICs analyzed (Table 3). The proportion of isolated STICs reported here is in line with the reported 5–10% of isolated STICs, which are usually found incidentally or in connection with risk-reducing gynecological surgeries, performed for BRCA1/2-positive women. Indeed, two of the three isolated STICs in this study were BRCA1 positive, while the remaining one was BRCA1 wild-type. It has been shown that HGSCs harboring BRCA1 or BRCA2 mutations are molecularly, biologically, and clinically different from each other and from BRCA wild-type tumors [12, 28, 29]. At the molecular level, BRCA1 or BRCA2 mutations are associated with marked changes in gene expression patterns and with gene copy number alterations [12]. In addition, disruption of the BRCA pathway, due to mutation or promoter methylation, is inversely correlated with amplification of Cyclin E1 or loss of RB1, which are found in approximately 30% of HGSCs [30]. BRCA1/2 pathway dysfunction and Cyclin E1 amplification both promote genomic instability and tumor progression albeit along two mutually exclusive molecular avenues [30]. Unfortunately, the low number of BRCA-positive STICs in this study precludes establishment of significant observations. However, given the distinct molecular biology and potential differences in pathogenesis of BRCA-positive and BRCA wild-type lesions, application of the algorithm developed here to BRCA-positive women with isolated serous intraepithelial neoplasms will require further study to determine the outcome risk in this group.
In summary, we have demonstrated that STMN1 and p16 are overexpressed in both p53-positive and p53-negative STICs and invasive HGSCs, while combined expression of both proteins is rare in morphologically normal tubal epithelium. These results credential STMN1 and p16 as adjunct immunohistochemical markers that could be added to p53 and Ki-67 stains used in the current diagnostic algorithm. The additional redundancy of the IHC arms helps to compensate for practical limitations of p53 and Ki-67 that complicate the diagnosis of approximately one third of STICs. The discriminatory capacity of p16 and STMN1 could be particularly powerful in identifying or diagnosing p53-negative STICs with marginally elevated Ki-67 index and borderline atypia or p53-negative STICs within reactive/proliferative epithelium where the utility of Ki-67 index is limited.
Supplementary Material
Table 2.
STMN1 and p16 expression has high overall concordance rate in multiple STICs from the same patient
| STIC B
|
||||
|---|---|---|---|---|
| STMN1-positive | STMN1-negative | total | ||
| STIC A | STMN1-positive | 20 | 1 | 21 |
| STMN1-negative | 1 | 3 | 4 | |
| total | 21 | 4 | 25 | |
| STIC B
|
||||
|---|---|---|---|---|
| p16-positive | p16-negative | total | ||
| STIC A | p16-positive | 18 | 2 | 20 |
| p16-negative | 3 | 2 | 5 | |
| total | 21 | 4 | 25 | |
Concordance rate: 92% (23/25)
Concordance rate: 80% (20/25)
Highlights.
A significant fraction of STIC lesions can be negative for p53 immunostaining.
STMN1 and p16 are sensitive and specific biomarkers for STIC.
The addition of STMN1 and p16 to Ki-67 and p53 stains improves diagnostic accuracy of STIC.
Acknowledgments
This work was supported by grants from the National Institutes of Health: Pacific Ovarian Cancer Research Consortium NCI P50 CA083636 (BYK, RD), R21 CA156021 (RD), U01 CA152990 (RD); a Miriam and Sheldon G. Adelson Medical Research Foundation Grant (RD), American Cancer Society Early Detection Professorship SIOP-06-258-01-COUN (BYK), and the Honorable Tina Brozman Foundation (RD). We thank Dr. Kai Doberstein for reviewing the manuscript.
Footnotes
CONFLICT OF INTEREST
R.D. serves on the scientific advisory board of Siamab Therapeutics. The other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24 (Suppl 10):x16–21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- 2.Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clinical medicine & research. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. The Journal of pathology. 2001;195:451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 4.Gross AL, Kurman RJ, Vang R, Shih Ie M, Visvanathan K. Precursor lesions of high-grade serous ovarian carcinoma: morphological and molecular characteristics. Journal of oncology. 2010;2010:126295. doi: 10.1155/2010/126295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saleemuddin A, Folkins AK, Garrett L, Garber J, Muto MG, Crum CP, et al. Risk factors for a serous cancer precursor (“p53 signature”) in women with inherited BRCA mutations. Gynecologic oncology. 2008;111:226–32. doi: 10.1016/j.ygyno.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carcangiu ML, Radice P, Manoukian S, Spatti G, Gobbo M, Pensotti V, et al. Atypical epithelial proliferation in fallopian tubes in prophylactic salpingo-oophorectomy specimens from BRCA1 and BRCA2 germline mutation carriers. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2004;23:35–40. doi: 10.1097/01.pgp.0000101082.35393.84. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. The American journal of surgical pathology. 2006;30:230–6. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 8.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:3985–90. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn E, Kurman RJ, Vang R, Sehdev AS, Han G, Soslow R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma--evidence supporting the clonal relationship of the two lesions. The Journal of pathology. 2012;226:421–6. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. The Journal of pathology. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 11.Cass I, Walts AE, Barbuto D, Lester J, Karlan B. A cautious view of putative precursors of serous carcinomas in the fallopian tubes of BRCA mutation carriers. Gynecologic oncology. 2014;134:492–7. doi: 10.1016/j.ygyno.2014.07.084. [DOI] [PubMed] [Google Scholar]
- 12.Powell CB, Swisher EM, Cass I, McLennan J, Norquist B, Garcia RL, et al. Long term follow up of BRCA1 and BRCA2 mutation carriers with unsuspected neoplasia identified at risk reducing salpingo-oophorectomy. Gynecologic oncology. 2013;129:364–71. doi: 10.1016/j.ygyno.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visvanathan K, Vang R, Shaw P, Gross A, Soslow R, Parkash V, et al. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. The American journal of surgical pathology. 2011;35:1766–75. doi: 10.1097/PAS.0b013e31822f58bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell CB. Clinical management of patients at inherited risk for gynecologic cancer. Current opinion in obstetrics & gynecology. 2015;27:14–22. doi: 10.1097/GCO.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 15.Carlson JW, Jarboe EA, Kindelberger D, Nucci MR, Hirsch MS, Crum CP. Serous tubal intraepithelial carcinoma: diagnostic reproducibility and its implications. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2010;29:310–4. doi: 10.1097/PGP.0b013e3181c713a8. [DOI] [PubMed] [Google Scholar]
- 16.Karst AM, Levanon K, Duraisamy S, Liu JF, Hirsch MS, Hecht JL, et al. Stathmin 1, a marker of PI3K pathway activation and regulator of microtubule dynamics, is expressed in early pelvic serous carcinomas. Gynecologic oncology. 2011;123:5–12. doi: 10.1016/j.ygyno.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sehdev AS, Kurman RJ, Kuhn E, Shih Ie M. Serous tubal intraepithelial carcinoma upregulates markers associated with high-grade serous carcinomas including Rsf-1 (HBXAP), cyclin E and fatty acid synthase. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23:844–55. doi: 10.1038/modpathol.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Neill CJ, McBride HA, Connolly LE, Deavers MT, Malpica A, McCluggage WG. High-grade ovarian serous carcinoma exhibits significantly higher p16 expression than low-grade serous carcinoma and serous borderline tumour. Histopathology. 2007;50:773–9. doi: 10.1111/j.1365-2559.2007.02682.x. [DOI] [PubMed] [Google Scholar]
- 19.Phillips V, Kelly P, McCluggage WG. Increased p16 expression in high-grade serous and undifferentiated carcinoma compared with other morphologic types of ovarian carcinoma. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2009;28:179–86. doi: 10.1097/PGP.0b013e318182c2d2. [DOI] [PubMed] [Google Scholar]
- 20.Karst AM, Jones PM, Vena N, Ligon AH, Liu JF, Hirsch MS, et al. Cyclin E1 deregulation occurs early in secretory cell transformation to promote formation of fallopian tube-derived high-grade serous ovarian cancers. Cancer research. 2014;74:1141–52. doi: 10.1158/0008-5472.CAN-13-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nature communications. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belletti B, Baldassarre G. Stathmin: a protein with many tasks. New biomarker and potential target in cancer. Expert opinion on therapeutic targets. 2011;15:1249–66. doi: 10.1517/14728222.2011.620951. [DOI] [PubMed] [Google Scholar]
- 23.Witkiewicz AK, Knudsen KE, Dicker AP, Knudsen ES. The meaning of p16(ink4a) expression in tumors: functional significance, clinical associations and future developments. Cell cycle. 2011;10:2497–503. doi: 10.4161/cc.10.15.16776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning G, Bijron JG, Yamamoto Y, Wang X, Howitt BE, Herfs M, et al. The PAX2-null immunophenotype defines multiple lineages with common expression signatures in benign and neoplastic oviductal epithelium. The Journal of pathology. 2014;234:478–87. doi: 10.1002/path.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerdes B, Ramaswamy A, Ziegler A, Lang SA, Kersting M, Baumann R, et al. p16INK4a is a prognostic marker in resected ductal pancreatic cancer: an analysis of p16INK4a, p53, MDM2, an Rb. Annals of surgery. 2002;235:51–9. doi: 10.1097/00000658-200201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harbor perspectives in biology. 2010;2:a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masciarelli S, Fontemaggi G, Di Agostino S, Donzelli S, Carcarino E, Strano S, et al. Gain-of-function mutant p53 downregulates miR-223 contributing to chemoresistance of cultured tumor cells. Oncogene. 2014;33:1601–8. doi: 10.1038/onc.2013.106. [DOI] [PubMed] [Google Scholar]
- 28.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. American journal of human genetics. 2003;72:1117–30. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etemadmoghadam D, Weir BA, Au-Yeung G, Alsop K, Mitchell G, George J, et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19489–94. doi: 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.