Abstract
Purpose.
To identify risk factors associated with primary angle closure (AC) using anterior segment optical coherence tomography (ASOCT) measurements of the iris and to determine if these risk factors differ according to geographic origin.
Methods.
Anterior segment OCT images were collected on 267 persons (eyes) whose family origin was determined by a standardized method. In the 257 eyes with pupil diameter increase in the dark of 0.5 mm or more, findings were compared between bright light conditions and those in a dark room. In 130 eyes, comparison was made after pharmacological pupil dilation. After marking the position of the scleral spur, an automated program quantified many angle and iris parameters, with use of a manual method for a minority that the software could not analyze.
Results.
Iris area in bright light was larger with increasing age (univariate regression, P = 0.0005), largest in European and African-derived, and smallest in Korean and Chinese eyes (multivariable regression, P = 0.0001), and was significantly larger in AC groups compared with normal and open angle glaucoma groups (univariate regression, P < 0.0001). The absolute iris area loss per mm pupil dilation was significantly less in Chinese persons than African-derived persons (multivariable regression, P < 0.05 adjusted Tukey). Furthermore, in persons with past acute AC attack, the baseline iris area was not different from others, but their iris area lost per millimeter dilation was significantly less than in persons without past acute AC attack (multivariable regression, P = 0.04). The odds of AC disease significantly increased in eyes with smaller percent iris area lost and percent iris area lost per millimeter pupil increase, but when adjusted for geographic origin, this was significant only in persons of Chinese origin (interaction regression model). Apparent gain of iris volume on pupil dilation, due to an artifact in calculation from iris area loss, may indicate a detrimental shift in iris tissue toward the angle.
Conclusions.
Chinese persons in this cohort had relatively low baseline iris area, but less loss of iris area on pupil dilation than other groups, a feature also associated with greater prevalence of past acute AC attack. Disproportionate peripheral redistribution of iris area on dilation may contribute to AC.
Keywords: glaucoma, iris volume, origin, angle closure, anterior segment optical coherence tomography
In a clinic-based study of iris physiology in patients of different geographic origins, Chinese subjects were more likely to have angle closure disease if their iris failed to decrease in area on pupil dilation.
Introduction
Primary angle closure glaucoma (PACG) affects up to 16 million people and proportionally causes more blindness than open angle glaucoma (OAG).1 Anatomical measurements identified as PACG risk factors include shallow anterior chamber depth, short axial length, smaller anterior chamber width, and thicker, more anteriorly placed lens,2–5 yet most people with these static anatomical characteristics do not develop the various forms of angle closure (AC).6 Studies have shown AC affects more Asian- and Indian-derived patients than African-, Japanese-, and European-derived ones.7–16 However, various regional populations are not dramatically different in mean values of the biometric risk factors for AC.17 Likewise, the greater prevalence of AC disease among women is not yet fully explained by anatomic measurements alone. Using anterior segment optical coherence tomography (ASOCT) imaging, Wang and coworkers18 found that those with narrow angles, as judged by gonioscopy, had thicker irides at set distances from the scleral spur19 and larger iris cross-sectional areas than those with open angles, although this was analyzed independently of pupil size and only in dark conditions. Most of their subjects were Chinese, who had thicker/larger irides than a comparison group of Malay and Indian persons.
A more complete understanding of AC may lie in the dynamic behavior of ocular structures. The choroid has a tendency toward dynamic expansion that is greater in AC eyes than normal or OAG eyes.20–23 This is particularly evident in AC eyes that have undergone acute attacks and their fellow eyes and is greater in AC than OAG eyes when provoked by the water-drinking test.24 Anterior segment OCT and ultrasonic biomicroscopy (UBM)25–27 provide qualitative and quantitative information about the anterior chamber of the eye and compare favorably with gonioscopy.28 It is well known that the angle narrows in dark compared with bright illumination. Furthermore, using ASOCT, the iris cross-sectional area decreases with pupil dilation, and it has been reported that there is less iris area loss in those with AC, especially those who undergo acute attacks.29–31 We sought to investigate whether the dynamic change in iris or angle structures with pupil dilation might be different among persons with different geographical origins, particularly comparing those groups who differ in AC prevalence.
We measured baseline findings and changes in anterior segment parameters by ASOCT at different states of pupil dilation (light, dark, and pharmacologically dilated) to look for differences that might be associated with regional origin or with AC status.
Methods
Patients
The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board and conformed to the tenets of the Declaration of Helsinki. A total of 267 patients were recruited during scheduled appointments at the Wilmer Institute Glaucoma Center of Excellence or Comprehensive Eye Service; 202 were new study evaluations and 65 were included from a prior ASOCT study image database.29 Informed verbal consent was obtained. Patients were included if they were 18 years or older, had vision of 20/200 or greater in the enrolled eye, and were diagnosed or suspected of having primary glaucoma according to classifications published by Foster et al.32 Because the primary study question involved AC disease, we oversampled those with AC compared with OAG subjects in our clinic. Many AC eyes had undergone laser iridotomy and were included. We have previously published that the presence of iridotomy does not significantly alter dynamic iris behavior in AC eyes.30 Eyes were excluded if they had secondary glaucoma, conditions affecting iris dilation (posterior synechiae, iris atrophy, pharmacological mydriasis or miosis), a history of intraocular surgery except laser trabeculoplasty, were of unknown or of multiple-regional origins, and if the patient could not sit in an examination chair for ASOCT imaging. In eyes with past acute attack of AC, we preferentially selected the fellow eye. The eye with the greater vertical cup-to-disc ratio from each patient, as determined by a glaucoma specialist, was enrolled, as we wished to investigate associations with degree of damage and initial evaluations showed that there would be a need to oversample for greater than mild damage. If discs were similar, the eye with the worse mean deviation (MD) and/or pattern standard deviation (PSD) on visual field testing (Humphrey Field Analyzer 750i, 24-2, Swedish interactive threshold algorithm; Carl Zeiss Meditec, Inc., Dublin, CA) was selected.
The regional origin of patients was determined by asking patients to provide the regional origin of both of their parents, their grandparents, or more distant progenitors. To be eligible, both parents had to share the same regional origin. We initially grouped subjects into nine categories: European, African, Chinese, Japanese, Indian (Asia), Korean, Asian Other, Hispanic, and Middle Eastern. Due to lower frequency of visits in our clinic of some groups, a category “Other” was made by grouping together the Japanese, Asian Other, Hispanic, and Middle Eastern persons. Thus, we had six final origin groups.
Examinations
Patient demographics, ocular history, and ocular measurement data were recorded, including age, central corneal thickness (PachPen; Accutome, Inc., Malvern, PA), phoropter refractive error, vertical cup/disc ratio (the larger value of glaucoma specialist estimate or the value from SDOCT (Cirrus; Carl Zeiss Meditec, Inc.), and standard automated perimetry (indexes: MD, PSD, and glaucoma hemifield test [GHT]).
Intraocular pressure (Icare tonometer; Tiolat, Helsinki, Finland) was recorded as the mean of three consecutive measurements at the time of imaging. Imaging by the IOLMaster (Carl Zeiss Meditec, Inc.) provided measurements of axial length and anterior chamber depth (ACD). Blood pressure was recorded at imaging time as the mean of three consecutive measurements (Datascope Accutorr Plus, Paramus, NJ). Iris color was noted by the examiner as being blue (containing no brown elements other than freckles), intermediate (<50% brown iris, >50% blue or green), or brown (uniform light or dark brown).
All 267 participants underwent imaging using the Visante ASOCT (Model 1000; Carl Zeiss Meditec, Inc.) in the enrolled eye under standardized illumination conditions as detailed below. Anterior segment OCT images were analyzed with the Anterior Segment Analysis Program (Anterior Segment Analysis Program; National University Health System, Singapore),33 after manual identification of the scleral spur (SS). In a minority of images, manual identification of some anatomic features was carried out due to the inability of ASAP to identify structures automatically. Anterior Segment Analysis Program measures a variety of anterior chamber parameters, including pupil diameter, iris area, iris volume, trabecular iris space area, anterior chamber area and volume, and lens vault.
The light condition included both room fluorescent lighting on and a bright flashlight directed at the fellow (nonimaged) eye during imaging. During the dark condition, room lights were off, no stray light was allowed in either eye. In 130 eyes, images were also taken 20 minutes after pharmacological dilation with 1% tropicamide (Bausch & Lomb, Inc., Tampa, FL) and 2.5% phenylephrine hydrochloride (Alcon Laboratories, Inc., Fort Worth, TX) in dark illumination. The scan protocols were Anterior Segment Quad for Visante version 2.0 and Enhanced Anterior Segment Single for Visante 3.0, when it became available. Only images in the 0- to 180-degree (nasal-temporal) meridian were analyzed. Three ASOCT images were obtained per pupil condition before any procedure involving contact with the corneal surface other than eye drops. The refractive error was entered into the viewing system to reduce accommodation, and the fixation target and patient position were set to align the temporal and nasal iris as close to the horizontal axis as possible. Scans were centered on the pupil and proper eye alignment was indicated by the presence of an interference flare right along the visual axis. One image per pupil condition was selected based on clarity, lack of tilt, lack of obscuring structures, such as lids or eyelashes, and presence of an interference flare.
Image conversion From Visante to ImageJ and ASAP
Raw binary images were imported from the ASOCT instrument to ASAP using 16-bit signed format with bilinear interpolation at a size of 256 pixels horizontal × 1024 pixels vertical. The size of ASOCT scans was 16 × 8 mm. Images were rescaled to reflect these dimensions by multiplying the horizontal axis by a factor of 8. Anterior Segment Analysis Program used a segmentation program that traced the iris, cornea, and lens, converted the image to TIF format, and processed it to be 600 pixels wide × 300 pixels high, rotated it 90 degrees to the right, and flipped it horizontally. The resulting images had a size of 2048 × 1024 pixels. Horizontal and vertical linear scales in ASAP were calculated using the formula 16 mm/600 pix = 0.0267 mm/pix and 8 mm/300 pixels = 0.0267 mm/pix, respectively. For manual evaluations using ImageJ software (National Institutes of Health, Bethesda, MD),34 the horizontal and vertical scales were 16 mm/2048 pixels = 0.0078 mm/pix and 8 mm/1024 pixels = 0.0078 mm/pix, respectively, as confirmed by a previous study.35
Description of Image Analysis
For images processed by ASAP, the program made determinations of parameter values. In 27 images (10.1%), the iris area was not correctly identified by ASAP as judged by visual inspection of the image and the marked outline; hence, the iris area and pupil diameter was marked manually in these images. To do this, the iris limits were marked according to ASAP protocol33 as follows: (1) peripherally, by a line drawn from the SS through the iris at an angle perpendicular to the corneal endothelial surface; (2) posteriorly, just posterior to a bright linear zone that represents the iris pigment epithelium; (3) anteriorly and medially, along the iris–anterior chamber interface. For both manual and automated analysis, the pupil diameter (PD) was measured between the two nearest edges of the nasal and temporal iris borders. Iris volume was calculated by using the centroid of cross-sectional iris area. The centroid is the estimated center of mass as calculated from a two-dimensional image by the ImageJ program, as described by Aptel and Denis.30 Briefly, the centroid-to-centroid distance for the iris was divided by 2 to obtain the centroid-to-centroid radius. Iris volume (V) was calculated using the formula: V = π (R*Anasal + R*Atemporal), where R is the centroid-to-centroid radius and A is iris area, nasally and temporally.
Biostatistical Methods
All baseline values were defined as those under bright lighting conditions. The primary outcome variables were baseline iris area and comparisons between baseline and dark conditions for loss in iris area, percent loss in iris area, loss in iris area per millimeter change in pupil diameter, and percent loss in iris area per millimeter change in pupil diameter. Also estimated were iris volume by a published method and made the same calculations to evaluate loss in iris volume as for iris area. The hypotheses were that these outcomes might be different by origin, type of glaucoma, or by one or more of other variables. Covariates that were considered relevant to evaluate in this study included age, sex, origin, eye (right/left), diagnosis as detailed below, past laser iridotomy, eye color, presenting spherical equivalent refractive error (for phakic eyes), IOP, central corneal thickness, cup-to-disc ratio, blood pressure, Swedish Interactive Thresholding Algorithm Standard 24-2 visual field result (MD, PSD, GHT), ACD, axial length, pupil condition at time of imaging (light, dark, pharmacologically dilated), and pupil diameter. The ASOCT through ASAP provided for each image the nasal and temporal parameters called trabecular iris space area 500 μm and 750 μm from the SS (trabecular iris space area [TISA] 500 and TISA 750, respectively), iris thickness measured 500 μm and 750 μm from SS, angle opening distance at 500 μm or 750 μm (angle opening distance [AOD] 500 and AOD 750, respectively), angle recess area at 500 μm or 750 μm (angle recess area [ARA] 500 and ARA 750, respectively), anterior chamber area (ACA), anterior chamber volume (ACV), and iris concavity ratio. These ASAP parameters were not separately evaluated in images requiring manual ImageJ evaluation due to failed ASAP analysis.
Baseline data were available for 267 eyes. For change from light to dark, data were available for 257 eyes with pupil diameter expansion of 0.5 mm or more; and for change from light to pharmacological pupil dilation, data were available for 130 eyes, all of which had pupil diameter expansion of 0.5 mm or more.
Descriptive statistics (mean and SD) were tabulated for each iris parameter by selected categorical variables of interest, such as origin and diagnosis. Diagnosis was analyzed as six groups or two groups. The six groupings were normal, OAG, OAG suspects (OAGS), primary angle closure (PAC), PAC suspect (PACS), and 6) angle closure glaucoma (ACG). The analysis by two groups consisted of (1) normal, OAG, and OAGS; and (2) PAC, PACS, and ACG. Descriptive statistics were also tabulated for selected variables by origin (number/percent for categorical variables and mean/SD for continuous variables). Origin groups were compared using the χ2 test for categorical variables and analysis of variance or the Kruskall-Wallis test for continuous variables. Univariate linear regression models were used to identify variables associated with each iris parameter. Variables with univariate significance of 0.20 or less were then used to develop a multivariable linear model using the stepwise selection process; the criterion used for a variable to enter a multivariable model and stay in the model was significance of 0.10 or less. Logistic regression models were used to look at the effect of iris parameters on the risk of AC adjusted for origin and the interaction between iris parameters and origin on the risk of AC. For variables with more than two categories and significance of 0.05 or less in a univariate or multivariable model, categories were compared using Tukey-Kramer and Bonferroni adjustments for multiple comparisons in linear and logistic models, respectively. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Demographic Data
The study group consisted of 267 eyes of 267 persons, divided into six regional groups: European, African, Chinese, Korean, Indian, and Other (Table 1). There were significant differences among the origin groupings for mean age. The Indian group was significantly younger than the European or African group (P < 0.05, Tukey). Differences in subgroup diagnoses were borderline significant. Because the study groups were recruited from a clinic, the proportions of various diagnoses are not representative of population-based samples. Sex, baseline pupil size, and change in pupil diameter from light to dark did not differ significantly among groups.
Table 1.
Demographic Data
|
Characteristic |
Origin |
P
Value |
|||||
|
European,
n
= 74 |
African,
n
= 50 |
Chinese,
n
= 48 |
Indian,
n
= 45 |
Other,
n
= 34 |
Korean,
n
= 16 |
||
| Age, y, mean (SD) | 62.7 (9.7) | 62.9 (12.2) | 60.1 (16.3) | 54.0 (13.5) | 60.1 (13.3) | 61.8 (13.6) | 0.01* |
| Sex, n (%) | |||||||
| Male | 30 (41) | 17 (34) | 18 (38) | 21 (47) | 18 (53) | 7 (44) | 0.57† |
| Female | 44 (59) | 33 (66) | 30 (62) | 24 (53) | 16 (47) | 9 (56) | |
| Diagnosis, 6 groups, n (%) | |||||||
| Normal | 9 (12) | 2 (4) | 8 (17) | 9 (20) | 9 (26) | 2 (12) | — |
| OAGS | 19 (26) | 16 (32) | 11 (23) | 13 (29) | 4 (12) | 4 (25) | |
| OAG | 13 (18) | 12 (24) | 15 (31) | 14 (31) | 10 (29) | 7 (44) | |
| PACS | 9 (12) | 2 (4) | 5 (10) | 3 (7) | 2 (6) | 1 (6) | |
| PAC | 14 (19) | 7 (14) | 3 (6) | 3 (7) | 6 (18) | 1 (6) | |
| PACG | 10 (14) | 11 (22) | 6 (12) | 3 (7) | 3 (9) | 1 (6) | |
| Diagnosis, 2 groups, n (%) | |||||||
| Normal/OAGS/OAG | 41 (55) | 30 (60) | 34 (71) | 36 (80) | 23 (68) | 13 (81) | 0.06† |
| PACS/PAC/PACG | 33 (45) | 20 (40) | 14 (29) | 9 (20) | 11 (32) | 3 (19) | |
| Pupil diameter, mm | |||||||
| Baseline (light), median | 2.28 | 2.43 | 2.42 | 2.43 | 2.48 | 2.68 | 0.24‡ |
| Change (light − dark), mean (SD) | 1.79 (0.71) | 1.62 (0.71) | 1.93 (0.70) | 1.89 (0.79) | 1.79 (0.75) | 184 (0.83) | 0.42* |
χ2 P value for diagnosis (six groups) is not presented due to small number of subjects for some categories.
ANOVA.
χ2 test.
Kruskall-Wallis test.
Baseline Iris Area
For every 10-year increment in age, baseline iris area in the light condition increased by 0.069 mm2 (95% confidence interval [CI]: 0.031–0.107; P = 0.0005, univariate regression).
Univariate regression models (Table 2, n = 267) also were used to analyze baseline iris area according to geographical origin and diagnosis. Baseline iris area varied by origin (P = 0.03); African-derived persons had the largest iris area, whereas Korean and Chinese persons had the smallest iris areas (6%–8% less than African-derived). These differences, however, were not significant in pairwise comparisons with P values adjusted for multiple comparisons (Tukey).
Table 2.
Baseline Iris Area by Origin and Diagnosis
|
Characteristic |
n
(%) |
Iris Area, Mean (SD) |
P
Value |
| Origin | |||
| African | 50 (19) | 3.91 (0.39) | 0.03 |
| European | 74 (28) | 3.83 (0.43) | |
| Other | 34 (13) | 3.78 (0.44) | |
| Indian | 45 (17) | 3.74 (0.40) | |
| Chinese | 48 (18) | 3.69 (0.43) | |
| Korean | 16 (6) | 3.58 (0.44) | |
| Diagnosis, 2 groups | |||
| PACS/PAC/PACG | 90 (34) | 3.93 (0.41) | <0.0001 |
| Normal/OAGS/OAG | 177 (66) | 3.71 (0.42) | |
| Diagnosis, 6 groups | |||
| PAC | 34 (13) | 4.07 (0.42) | <0.0001 |
| PACS | 22 (8) | 3.88 (0.37) | |
| PACG | 34 (13) | 3.82 (0.38) | |
| OAGS | 67 (25) | 3.79 (0.39) | |
| OAG | 71 (27) | 3.66 (0.42) | |
| Normal | 39 (15) | 3.66 (0.46) | |
| Total | 267 |
values from univariate linear regression models.
Iris area was also significantly different when persons were divided into two diagnostic groups. The AC group (PACS, PAC, ACG) had significantly larger iris area compared with the open angle group (normal, OAGS, OAG): 3.93 ± 0.41 vs. 3.71 ± 0.42 mm2 (P < 0.0001). When diagnosis was considered in six categories, there remained a significant relationship between iris area and diagnosis, with larger iris area in PACS, PAC, and ACG than in OAGS, OAG, and normals (P < 0.0001). Eight eyes had a history of acute attacks of AC, but their baseline iris area was not significantly different from that of the remaining 258 eyes: 3.71 ± 0.52 mm2 compared to 3.79 ± 0.42 mm2, respectively (P = 0.63). As would be expected from the above finding of larger area in AC eyes, other features associated with AC disease were associated with larger iris area. These included more hyperopic refractive error, smaller anterior chamber depth, and prior laser iridotomy (P = 0.005, 0.02, and 0.002, respectively, univariate regression).
Iris area did not differ between right and left eyes, nor between men and women, and it was not significantly associated with IOP level, blood pressure parameters, cup-disc ratio, or any visual field index (all P ≥ 0.24). Because we will next consider the iris area loss with pupil size change, it is relevant that the pupil change from light to dark conditions was not significantly related to baseline iris area (P = 0.96). A multivariable model (Table 3, n = 191 eyes) was constructed for baseline iris area, which considered in step-wise fashion the following variables that had univariate associations with iris area of 0.2 or less: age, origin, diagnosis, iris color, anterior chamber depth, and refractive error. We excluded history of iridotomy, as it had near identity with diagnosis. The variables that were selected for the model were origin (six categories; P = 0.0001), diagnosis (two categories; P = 0.03), and iris color (P = 0.0005). Individual comparisons showed that the following groups significantly differed from each other within model parameters (P < 0.05, Tukey): European-derived and African-derived persons each had significantly larger iris area than either Chinese or Korean persons; combined AC group eyes had larger irises than the combined open angle group's eyes, and brown irises were larger than either intermediate or blue irises.
Table 3.
Baseline Iris Area
|
Characteristic |
Regression Parameter (95% CI) |
P Value |
Groups Different, Adjusted P ≤ 0.05 |
| Origin | 0.0001 | ||
| 1 European (r) | 0 | 1, 3 | |
| 2 African | −0.048 (−0.250, 0.154) | 1, 4 | |
| 3 Chinese | −0.388 (−0.603, −0.173) | 2, 3 | |
| 4 Korean | −0.541 (−0.826, −0.257) | 2, 4 | |
| 5 Indian | −0.219 (−0.436, −0.002) | ||
| 6 Other | −0.176 (−0.388, 0.036) | ||
| Iris color | 0.0005 | ||
| 1 Blue | −0.485 (−0.758, −0.213) | 1, 3 | |
| 2 Intermediate | −0.374 (−0.616, −0.133) | 2, 3 | |
| 3 Brown (r) | 0 | ||
| Diagnosis | 0.03 | 1, 2 | |
| 1 Normal/OAGS/OAG (r) | 0 | ||
| 2 PACS/PAC/PACG | 0.144 (0.017, −0.270) |
Multivariable linear regression model, Tukey adjustment for multiple comparisons, n = 191. r, regression coefficient.
Change in Iris Area: Light to Dark Condition
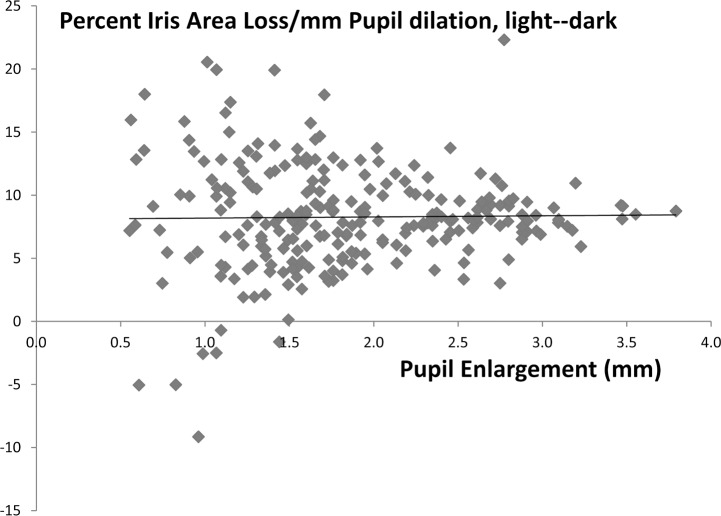
We noted that 10 eyes (3.8%) had essentially no enlargement of the pupil in the dark condition, that is, less than 0.5 mm increase. We excluded these eyes from this portion of the analysis, as the intent was to study the effect of pupil size increase. Even with this exclusion, the variability in percent iris area loss per millimeter pupil change is higher at low pupil dilation values (Fig. 1).
Figure 1.
The relationship between percent iris area loss and the amount of pupil enlargement is shown for each eye in which the pupil increased 0.5 mm or more from light to dark condition. It can be seen that the amount of loss is approximately 8% per millimeter pupil increase, but that the variability of change is much higher in eyes with smaller pupil size increases (linear regression: y = 0.09 + 8.1x; r2 = 0.0002).
For each millimeter of pupil enlargement, the mean iris area loss was 0.30 mm2 or 8% (P < 0.0001, univariate regression). Variables related to greater iris area loss with univariate significance of 0.20 or less included greater pupil enlargement, younger age, smaller cup-disc ratio, and better MD and PSD in visual field data. In a multivariable model for iris area loss, the amount of pupil enlargement and PSD were significant (regression coefficients = 0.29 [0.24–0.34], P < 0.0001, and −0.009 [−0.018–0.000], P = 0.05, respectively). In a multivariable model, factors associated with greater percent iris area loss were larger pupil enlargement (P < 0.0001) and shallower anterior chamber (P = 0.003).
Because important diagnostic groups differed in baseline iris area and because we found that the amount of pupil enlargement significantly affected iris area change, we considered two measures of iris area change using multivariable models to assess iris behavior: percent iris area loss per millimeter pupil enlargement and absolute iris area loss per millimeter pupil enlargement. Shallower anterior chamber was associated with greater percent iris area loss per millimeter dilation (regression coefficient: −1.66 [−2.71 to −0.61], P = 0.002). In the multivariate model for absolute iris area loss per millimeter pupil change, we excluded iridotomy, as it was in near identity with AC. Shallower anterior chamber was also associated with greater absolute iris area loss per millimeter dilation (regression coefficient: −0.076 [−0.118 to −0.034], P = 0.0005). Chinese persons had significantly lower absolute loss/millimeter dilation than did African persons (P < 0.05, adjusted Tukey). Table 4 details the mean (SD) percent and absolute loss per millimeter pupil dilation, light to dark, by origin.
Table 4.
Percent and Absolute Iris Area Loss per Millimeter Pupil Dilation, Light to Dark, by Origin
|
Origin |
n (%) |
Percent Iris Area Loss Per Millimeter Pupil Increase, Mean (SD) |
Absolute Iris Area Loss Per Millimeter Pupil Increase, Mean (SD) |
| Other | 32 (12) | 9.1 (5.5) | 0.36 (0.23) |
| African | 48 (19) | 9.0 (4.4) | 0.36 (0.18) |
| European | 72 (28) | 8.4 (3.5) | 0.32 (0.14) |
| Indian | 44 (17) | 7.7 (3.9) | 0.29 (0.15) |
| Korean | 15 (6) | 7.5 (3.0) | 0.27 (0.11) |
| Chinese | 46 (18) | 7.4 (3.0) | 0.27 (0.11) |
| Total | 257 |
Change in Iris Area: Light to Pharmacologically Dilated Condition
In 130 eyes, data were acquired on the iris area loss between the light condition and a pharmacologically dilated pupil condition. Among these eyes, multivariable models similar to those for the light to dark condition change were constructed. In the model for iris area loss, the only variable selected was pupil enlargement (regression coefficient: 0.366 [0.308–0.424], P < 0.0001), with greater enlargement associated with greater loss. The model for percent iris area loss found significantly less loss with less pupil enlargement, and less loss in those with a history of acute AC attack (−6.60 [−11.86 to −1.34], P = 0.01) and with older age (per 10-year increase: −1.00 [−1.82 to −0.18], P = 0.02). For the model of percent iris loss per millimeter pupil enlargement, only older age related to less percent loss of area (P = 0.004). However, the absolute iris area loss per millimeter pupil enlargement was significantly less in those with a past acute AC attack (regression coefficient: −0.066 [−0.131 to −0.002], P = 0.04).
Interaction Between Odds of AC Disease, Iris Features, and Origin
In the analysis above, we considered what factors determined iris area and its change as the pupil enlarged. Next, we constructed models in which the risk of having AC disease was assessed, taking into account each of the features of the iris as well as the origin of the person. The first model examined baseline iris area and found that larger iris area was significantly associated with AC, adjusted for origin (regression coefficient: 3.29 [1.67–6.48], P = 0.001). This is not surprising, as the model with the dependent and independent variables reversed (baseline iris area and AC disease) found the same relationship. However, there was no interactive effect of geographic origin on the relation of baseline iris area to the odds of AC.
On the other hand, there was interaction between geographic origin and both percent iris area lost and percent iris area lost per millimeter pupil enlargement in light to dark conditions in relation to the odds of AC (f). This adjusted odds ratio estimated the effect of the iris parameter on the odds of AC within an origin group and found significant interaction between percent iris area lost and origin (P = 0.04, Table 5). This interaction model estimated the odds ratio for AC within each ethnic group separately, determining that in Chinese persons, the odds of AC significantly increased for each unit decrease in percent iris area lost. In the other five ethnic groups, the effect of percent iris area lost on the odds of AC was not statistically significant. Using unadjusted P values for pairwise comparisons among ethnicities, the estimated effect found in Chinese was significantly different from that found in Europeans, Africans, and Koreans. However, there was no significant difference using adjusted P values.
Table 5.
Effect of Iris Parameter on Odds of AC, by Origin
|
Iris Parameter |
Origin |
Odds Ratio Estimate (95% CI) per Unit Increase in Parameter |
P Value for Interaction of Parameter and Origin |
Origins Different, Unadjusted P Value |
Origins Different, Adjusted P Value ≤ 0.05 |
| % Iris area lost | 1 European | 0.99 (0.94, 1.04) | 0.04 | 1, 3 | None |
| 2 African | 1.03 (0.96, 1.11) | 2, 3 | |||
| 3 Chinese | 0.85 (0.75, 0.96) | 3, 5 | |||
| 4 Indian | 0.96 (0.88, 1.04) | ||||
| 5 Korean | 1.14 (0.90, 1.45) | ||||
| 6 Other | 0.94 (0.86, 1.03) | ||||
| % Iris area lost per millimeter pupil increase | 1 European | 1.12 (0.99, 1.27) | 0.06 | 1, 3 | 1, 3 |
| 2 African | 1.16 (1.00, 1.35) | 2, 3 | |||
| 3 Chinese | 0.77 (0.60, 0.99) | 3, 4 | |||
| 4 Indian | 1.12 (0.89, 1.41) | 3, 5 | |||
| 5 Korean | 1.72 (0.95, 3.12) | 3, 6 | |||
| 6 Other | 1.05 (0.91, 1.21) |
Logistic interaction models, Bonferroni adjustment, n = 257.
In a similar interaction model that examined the effect on the odds of AC from percent iris area lost/millimeter pupil dilation, the significance of the overall model had a P value of 0.06 (Table 5). For this parameter, Chinese persons who lost less iris area per millimeter pupil enlargement were significantly more likely to have AC, and Chinese persons were significantly different from each of the other groups (unadjusted P < 0.05) and significantly different from Europeans with Bonferroni correction. We estimate that in persons of Chinese origin, the odds of AC increase 30% for each unit decrease in the percent of iris area loss per millimeter pupil enlargement.
Iris Area Change Compared With Iris Volume Change
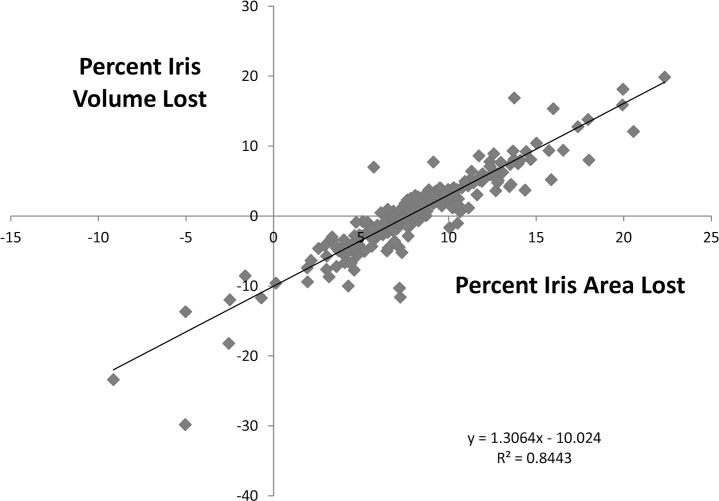
Because it has been reported30,31,36 that iris volume change from the light to the dark condition differs between fellow eyes with acute AC attacks and eyes of non-AC patients, we calculated iris volume from iris area using the formula described in those reports. The eyes with less than 0.5 mm change in pupil were excluded, as above. Figure 2 demonstrates the plot of percent iris area loss versus percent iris volume loss (each presented as loss per angle closure pupil enlargement, light to dark). It shows the high correlation between the values. Although there was a loss of iris area in nearly every eye (251/257, 98%), more than one-third of the eyes (95/257, 37%) were found to have “gained” volume. Likewise, the cited prior reports also found substantial numbers of eyes in which calculated iris volume “increased” on pupil dilation. It seemed illogical that iris volume would increase when iris area decreased; thus, we investigated this paradox further.
Figure 2.
The measured percent loss of iris area with pupil enlargement is plotted against the calculated percent loss (or gain) in iris volume using a formula depending on iris area and the centroid (center of mass) for the iris. Although nearly all eyes actually lost area as the pupil enlarged, 37% (95/257) paradoxically were judged to have gained iris volume (values below the x-axis). The proposed explanation for this anomaly is shown in Figures 3 and 4.
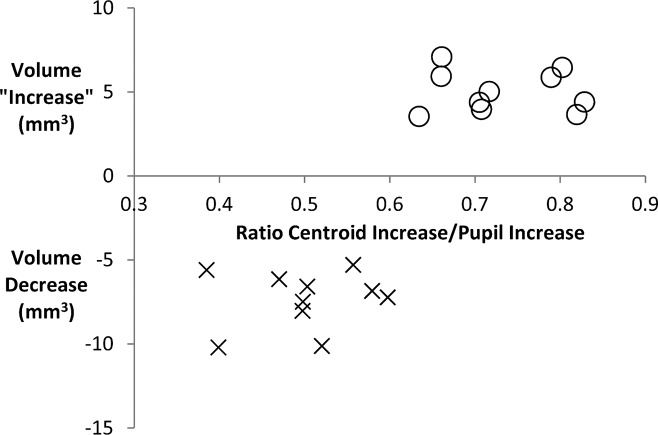
The iris volume formula uses two variables: iris area and the centroid-to-centroid distance. A likely explanation for calculated iris volume increase in eyes with an iris area decrease would be that in these eyes, there was disproportionately larger increase in centroid-to-centroid distance relative to the increase in pupil size. To see if this was the case, we plotted the change in iris volume per millimeter pupil enlargement against the following ratio: (change in centroid-to-centroid distance) divided by (change in pupil size) from light to dark condition. For this comparison, we selected 20 eyes, all of which had a decrease in iris area. There were 10 eyes that had both a loss in iris area and in iris volume, compared with 10 eyes that had loss in iris area, but a calculated increase in iris volume (Fig. 3). In the graph, eyes with iris volume increase are plotted as a positive change (above the x-axis), whereas the more common examples of iris volume change that is negative (volume loss) fall below the x-axis. It is clearly seen that the 10 eyes with volume increase had greater ratio of change in centroid-to-centroid diameter to change in pupil size than did the eyes with calculated volume decrease. Eyes with a greater centroid-to-centroid diameter for that change in pupil size would have a more peripherally redistributed iris area (Fig. 4).
Figure 3.
Graph shows the ratio of the increase in the distance between nasal and temporal iris centroids (centers of mass) to the increase in pupil diameter as eyes go from light to dark (x-axis) versus calculated change in iris volume using a published method (y-axis). Twenty eyes were selected; all eyes had iris area loss, 10 with iris volume loss (X), and 10 with iris volume increase (O). It is clear that the anomalous eyes (those with volume increase) had higher ratios of centroid-to-pupil increase, indicating that the shift in iris area toward the anterior chamber angle was disproportionately greater in these eyes, placing more iris tissue in apposition to the angle and increasing the chance of AC.
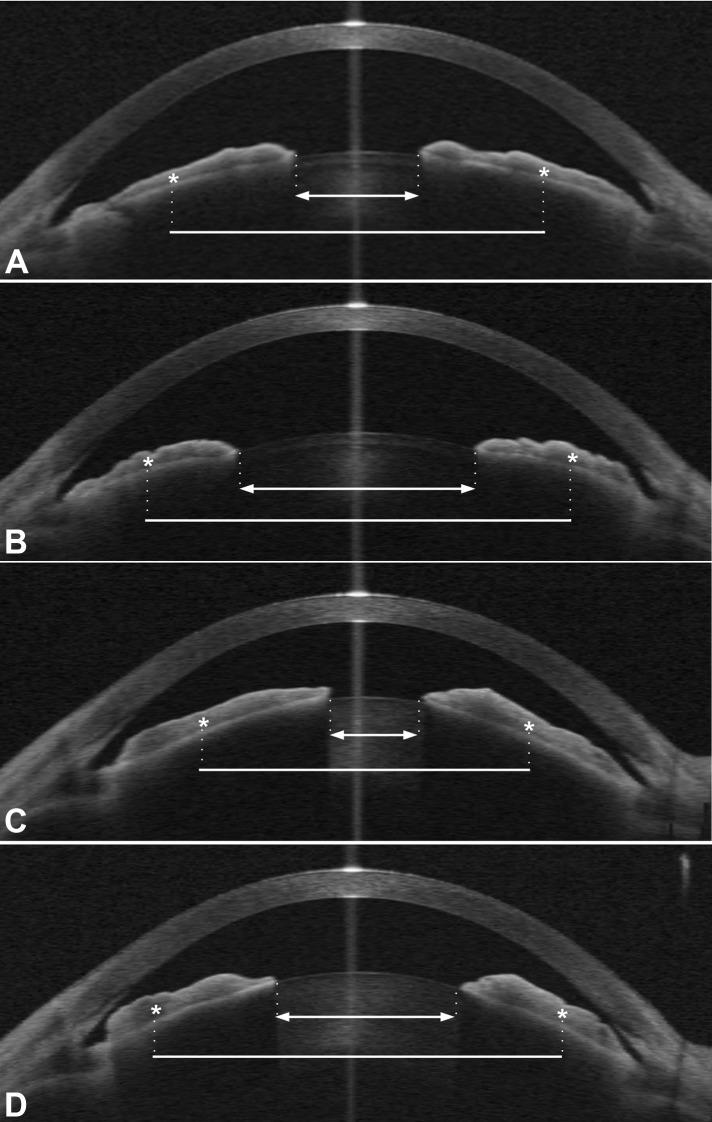
Figure 4.
Examples from two patients (patient 1 in [A, B], patient 2 in [C, D]), comparing the amount of pupil enlargement to the amount of centroid-to-centroid distance enlargement in each eye from bright light to dark condition in ASOCT images. The centroid (center of mass) of each half of the iris is marked by an asterisk. Pupil diameter is shown by a double arrow solid line and centroid-to-centroid distance by a plain solid line. In patient 1, (A) (bright light) the pupil is small and the centroids are relatively closer to the optical axis than the angle, whereas in (B) (dim light) the increase in centroid-to-centroid distance is only half as large as the increase in pupil diameter (ratio 0.5). This is the more typical situation in many eyes, in which iris area decreases and calculated iris volume also decreases with pupil enlargement, represented in Figure 3 by the examples marked by X's below the x-axis. In patient 2, the change from bright light (C) to dim light (D) is marked by a greater increase in centroid-to-centroid distance than its pupil enlargement (ratio = 1.1). This is characteristic of eyes that have an anomalous “increase in iris volume” (above the x-axis in Figure 3, marked by O's). Note that the iris cross-section shows a much larger proportion of the iris has moved to the periphery of the angle in this eye compared with patient 1. Although the increase in iris volume is an artifact of the calculation formula, it is more likely that such eyes would occlude the angle, due to peripheral redistribution of iris tissue. This seems consistent with the findings of two reports that linked iris volume “increase” to acute attacks of AC.
Change in Key Angle Parameters With Pupil Dilation
For the 230 eyes in which the ASAP software provided automated analysis of angle and anterior chamber structures, we compared the following parameters among our origin groups and diagnostic groups: lens vault, anterior chamber depth/area/volume, TISA 750, and iris concavity ratio. In summary, origin did not contribute significantly to the multivariable models for any of these parameters, neither in their baseline values in bright light, nor in the change per millimeter of pupil dilation after pupil dilation in the dark.
In a multivariable model, the baseline lens vault was greater with increasing age, greater in the combined AC group compared with non-AC, and greater with shallower anterior chamber (all P < 0.0001). With pupil dilation in the dark, the decrease in lens vault was significantly less in the combined AC group and less in intermediate and brown eyes than in blue eyes (P = 0.02, 0.04; multivariable model).
Because anterior chamber depth, area, and volume depend largely on similar factors, it is not surprising that their results were similar. For example, baseline chamber volume in bright light was significantly smaller in the AC diagnosis group, in older persons, and in eyes with more normal PSD visual field index (P < 0.0001, < 0.0001, and = 0.01, respectively; multivariable model). With pupil dilation in the dark, both the anterior chamber volume loss in cubic millimeter and the loss per millimeter pupil dilation were significantly less in the AC group (both P < 0.0001, multivariable models).
The TISA 750 baseline area was smaller in the AC group, but the loss with pupil dilation in the dark was not significantly related to other variables when adjusted for the degree of pupil enlargement. The baseline iris concavity ratio was more than twice as high in the AC combined group than in non-AC at baseline, and was also greater with increasing age and in those eyes with better MD in their visual field (P = 0.0002, < 0.0001, and = 0.01, respectively, multivariable model). However, the change in iris concavity ratio in the dark and its change per millimeter pupil enlargement were not significantly different by diagnosis or other variables when adjusted for pupil enlargement (multivariable models).
Discussion
Baseline iris area under bright light conditions was larger in those with AC disease, in brown irises, and in older persons.37 Increased iris area with age may contribute to the increased AC incidence with age. Larger iris area would also be more likely to lead to closure of the angle in eyes with other predisposing features, such as greater lens vault and shallower anterior chamber, which our data also confirm are related to AC. However, we did not expect that iris area would be significantly smaller among Chinese and Korean persons compared with African-derived and European-derived persons. Given higher prevalence of AC disease among Chinese persons, it seemed more likely that they would have larger iris area. Wang et al.38,39 studied European-derived and Chinese persons who had neither glaucoma nor high refractive error, finding that Europeans had significantly greater anterior chamber depth, anterior chamber width, and corneal arc depth, yet smaller iris thickness and iris area than Chinese persons. They used an earlier version of the ASOCT software that fails to analyze 25% of images, whereas we included all eyes by manual iris area measurement in those failing automated analysis. Another clinic-based study40 used ASOCT to compare Chinese to European-derived persons and found no significant difference in iris area, but the data analysis did not account for pupil diameter.
Our study of dynamic iris behavior identified important associations between iris area, AC disease, and origin. Iris area loss per millimeter pupil dilation was significantly less in Chinese persons. Furthermore, in persons with past acute AC attack, the iris lost significantly less area per millimeter dilation. Similarly, the odds of AC disease significantly increased in eyes in which the percent iris area lost or percent lost per millimeter pupil increase were smaller, but this was true only in Chinese persons. Combining all origin groups and all types of AC (from suspects to glaucoma) showed an inconsistent relationship between iris area loss with pupil dilation and prevalent AC. The present data suggest that the more acute form of AC, acute attack, may be identified by greater retention of iris area as the pupil widens, but that not every AC eye shares this feature. We included in this study persons with PACS, who may have different features from those with definitive evidence of AC disease. Aptel et al.30 and Narayanaswamy and colleagues36 have confirmed that fellow eyes of acute AC attack patients lose less iris area per volume on pupil dilation than do other PAC/PACG subjects. Lee et al.41 found that among Korean persons, iris area decreased with pupil dilation and that the loss of iris area on dilation declined with age; yet, they found no difference between AC and control normal subjects in the slope of iris area loss. However, they excluded acute attack eyes and may have included eyes with iris atrophy from advanced AC disease. The striking differences between Chinese persons and those of other origins in dynamic iris behavior merit further study, especially in larger representative samples.
Although AC is more common in Chinese persons, PACG prevalence in adult Chinese is only 1.4% by meta-analysis of population studies.42 Risk factors for AC may be present in only a small proportion of all persons in an origin group and that feature, although contributing to AC in those with the disease, may still be uncommon overall in an origin group despite its higher prevalence. Thus, larger irises are associated with AC, but iris area was smaller among our Chinese subjects than other groups. Larger iris area may contribute to AC in Chinese persons as in other groups, but we found no interaction between baseline iris area and origin in interaction modeling. If our clinic-based data are representative, larger iris area is not a general feature of Chinese eyes. But, the interaction models found that Chinese eyes are more likely to develop AC when they have failure to lose iris area on dilation, compared with other groups. This reinforces the conclusion that AC, like most complex diseases, has a multifactorial pathogenesis. Thus, Chinese persons who have larger iris area, shallower chamber, and high lens vault are not typical of the Chinese population generally, but are at greater AC risk due to a general Chinese tendency to less iris area loss on dilation. Simple baseline anatomic differences from normal among an entire group, or even differences in dynamic behavior, may be informative only when considered in the interactive format presented.
Previous studies have determined some interorigin differences in anterior segment anatomy by ASOCT. Wang et al.38 reported baseline iris area compared with pupil size in European and Chinese persons. As expected, iris area was smaller in images with larger pupils, and the slope of iris area to pupil diameter for Chinese was flatter, suggesting retention of more iris area on dilation. Leung et al.40 found that Chinese and European persons did not differ in baseline iris area. Chinese had shallower and narrower anterior chambers than Europeans. One meta-analysis of slit lamp measurements suggested that Mongolian and Chinese persons have shallower anterior chambers than Belgians.43 Wang et al.44 reported that the iris insertion in UBM images differed between Chinese and European controls. A community-based, cross-sectional study in Singapore by Nongpiur and colleagues45,46 showed that the ACW was significantly smaller in Chinese persons compared to Indian and Malay persons, in older individuals, and in eyes with narrow angles. Although our data confirm the association between AC disease and smaller anterior chamber dimensions, and the association of smaller chamber volume with increasing age, we did not detect any significant associations between origin and anterior chamber size, iris concavity ratio, TISA 750, or lens vault.
Prior publications calculated iris volume from the iris area measurements provided by ASOCT. We found that this method suggested “increases” in volume in eyes that had actually lost iris cross-sectional area. As shown above, this iris volume “increase” is an artifact of the calculation method. Eyes with increased volume had disproportionately greater centroid-to-centroid width increases compared with pupil enlargement between light and dark conditions. The most likely explanation is that on dilation they redistribute iris area more into the peripheral iris, producing a more peripheral center of iris mass (centroid). Two groups30,31,36 found that acute AC attack subjects were more likely to have iris volume “increase” than controls. Although the increase in volume is, in our opinion, derived from the calculation method and does not indicate an actual volume increase (otherwise area would increase as well), such a peripheral redistribution of iris area would make closure of the angle more likely. Thus, it is a valuable observation and logical risk factor, although it is not indicative of a true “increase in volume.” There may be value in comparing the centroid–centroid increase to the pupil diameter increase as we have done here, to identify disproportionate peripheral redistribution of iris tissue as a risk factor in AC.
Shallower anterior chamber increased the risk of AC,47,48 and Nongpiur et al.49 found that lens vault was another strong predictor of narrow angle. Using hierarchical cluster analysis, Baek et al.50 found that Korean PAC/PACG eyes could be divided into two clusters, one characterized by higher ACD, ACA, iris thickness at 750 microns, ACW, and axial length, and lower lens vault [LV], compared with a second cluster with higher LV and lower ACA. We found that lens vault, iris concavity ratio, ACV, ACA, ACD, and TISA750 were each highly associated with AC compared with controls at baseline (data not shown). We report for the first time that, with pupil enlargement, the lens vault loss was less and loss in anterior chamber dimensions remained smaller in AC eyes. We did not find a significant difference among AC eyes in change in iris concavity or change in TISA 750 with dilation. However, nearly all of our AC eyes had already undergone iridotomy, which would alter changes in these parameters with dilation. Another investigation found that in Chinese persons with PAC/PACG compared with healthy Chinese subjects, the iris of AC eyes stretched less and developed a more convex configuration as the pupil constricted.51 Cheung et al.52 found that nonglaucomatous eyes with narrow angles more often retain a bowed forward configuration on pupil enlargement in the dark. Dynamic change in parameters like lens vault might provide new diagnostic information, and the size of the pupil must be taken into account in such measurements.
We found no significant differences between Indian persons and persons of other origins in angle features as studied here, neither at baseline nor after pupil dilation. A study of healthy Indian persons by ASOCT was reported among a population-based study of Singaporean residents53; however, iris area measures were not presented, nor were direct comparisons with persons of other origins shown. Further study of Indian eyes by ASOCT with attention to change with pupil size is merited, especially comparisons to other groups using standardized protocols.
Baseline iris area and pupil size must be taken into account in any analysis of angle and iris structures. For example, the iris area for Chinese subjects in this study and the non-AC subjects in the Singapore Chinese Eye Study54 are within 5% of each other under dark conditions. This is reassuring given the clinic-based recruitment here versus the population-based ascertainment in the Singapore study. Wang et al.44 found no significant differences between Chinese residing in China compared with those in San Francisco in their ASOCT parameters. Substantial differences in iris and angle structures occur under “standardized” lighting conditions. For example, the pupil change in our sample (using light-dilated or light-dark if the eye was not dilated) differed significantly between the combined normal/OAGS/OAG group and the AC combined group (2.92 ± 1.07 vs. 2.39 ± 1.20 mm, P = 0.0004, t-test). Iris area decreases 8% for every millimeter of pupil enlargement. Literature using UBM to analyze anterior structures has rarely included accounting for pupil size at the moment of imaging. One UBM study found no significant difference overall in iris thickness between AC and non-AC eyes,55 but did not account for pupil diameter. Because degree of pupil enlargement is significantly associated with many iris and angle features, it should be measured and included in statistical comparisons.
There are several limitations of this work. The definition of origin is problematic, regardless of the method used. In past reports, groups have been categorized by terms such as race, ethnicity, or nationality. There are no agreed standards to define race, including genetic analysis. Ethnicity refers more to language and culture. We required that both parents, both grandparents, or all distant direct lineal relatives must have been born in or derived from persons from the same geographic locus as defined. African-derived subjects were denoted by self-report. Hispanic persons were included in the Other category. It could be argued that they are an ethnic, not a geographic group; however, the point was moot, as we had few Hispanic subjects. Heterogeneity within origin groups is potentially a confounding feature, illustrated by considering the prevalence of exfoliation OAG, which is more common among Greek56 and Russian persons57 than French or British persons, yet all would be categorized by origin as European. Furthermore, marriages between persons of different origins are increasingly common and will affect the future predictive power of present conclusions.
Our study used clinic-based ascertainment of subjects, and larger, population-based data will be welcome to corroborate or refute these findings. Such studies should use consistent definitions of origin/ethnicity/race and standard methodology. Although we studied a large number of persons, the numbers per subgroup became smaller when comparisons were stratified by multiple diagnostic groups or other complex variables. Iris color is difficult to characterize objectively. Even in our European-derived subjects, most irises in this study were classified as brown. Finally, cross-sectional analysis can make only limited prediction of the relevance of factors that influence incident disease or that affect disease risk differently among origin groups. It is by longitudinal observations that more specific causal association can be determined. A longitudinal study is presently being conducted that has the potential to contribute in this way.58
In conclusion, we found larger irises in those with AC disease and larger irises in African-derived than in Chinese persons. Chinese eyes exhibit less loss of iris area with pupil dilation than persons of other origins, a feature shown to be interactively related to greater prevalence of AC disease. Failure to lose iris area on dilation is associated with acute AC attack. A disproportionate, peripheral redistribution of iris area with pupil dilation may contribute to AC. Important risk factors for AC include both baseline anatomic features and measures of dynamic change.
Acknowledgments
Supported in part by National Eye Institute–National Institutes of Health Research Grant 01765 (Core Facility Grant, Wilmer Institute), and by unrestricted support from Saranne and Livingston Kosberg and William T. Forrester. Research support in the form of instruments was received from Carl Zeiss Meditec. The authors alone are responsible for the content and writing of the paper.
Disclosure: F.E. Seager, None; J.L. Jefferys, None; H.A. Quigley, Carl Zeiss Meditec (F, C, R)
References
- 1. Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001; 85: 1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 1970; 54: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alsbirk PH. Primary angle-closure glaucoma: oculometry, epidemiology, and genetics in a high risk population. Acta Ophthalmol. 1976; 54: 5–31. [PubMed] [Google Scholar]
- 4. George R, Paul PG, Baskaran M, et al. Ocular biometry in occludable angles and angle closure glaucoma: a population based survey. Br J Ophthalmol. 2003; 87: 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nongpiura ME, Kua JYF, Aung T. Angle closure glaucoma: a mechanistic review. Curr Opin Ophthalmol. 2011; 22: 96–101. [DOI] [PubMed] [Google Scholar]
- 6. Quigley HA. Angle-closure glaucoma—simpler answers to complex mechanisms: LXVI Edward Jackson Memorial Lecture. Am J Ophthalmol. 2009; 148: 657–669. [DOI] [PubMed] [Google Scholar]
- 7. Foster PJ, Oen FTS, Machin D, et al. The prevalence of glaucoma in Chinese residents of Singapore. Arch Ophthalmol. 2000; 118: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 8. Dandona L, Dandona R, Mandal P, et al. Angle-closure glaucoma in an urban population in southern India. The Andhra Pradesh Eye Disease Study. Ophthalmology. 2000; 107: 1710–1716. [DOI] [PubMed] [Google Scholar]
- 9. Bonomi L, Marchini G, Marrafa M, et al. Epidemiology of angle-closure glaucoma. Prevalence, clinical types, and association with peripheral anterior chamber depth in the Egna-Neumarkt Glaucoma Study. Ophthalmology. 2000; 107: 998–1003. [DOI] [PubMed] [Google Scholar]
- 10. Buhrmann RR, Quigley HA, Barron Y, et al. The prevalence of glaucoma in a rural east African population. Invest Ophthalmol Vis Sci. 2000; 41: 40–48. [PubMed] [Google Scholar]
- 11. Liang Y, Friedman DS, Zhou Q, et al. Prevalence and characteristics of primary angle-closure diseases in a rural adult Chinese population: The Handan Eye Study. Invest Ophthalmol Vis Sci. 2011; 52: 8672–8679. [DOI] [PubMed] [Google Scholar]
- 12. Wang YX, Xu L, Yang H, et al. Prevalence of glaucoma in North China: the Beijing Eye Study. Am J Ophthalmol. 2010; 150: 917–924. [DOI] [PubMed] [Google Scholar]
- 13. Foster PJ, Baasanhu J, Alsbirk PH, et al. Glaucoma in Mongolia. A population-based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol. 1996; 114: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 14. Qu W, Li Y, Song W, et al. Prevalence and risk factors for angle-closure disease in a rural Northeast China population: a population-based survey in Bin County, Harbin. Acta Ophthalmol. 2011: 89: 515–520. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto T, Iwase A, Araie M, et al. The Tajimi Study report 2: prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology. 2005; 112: 1661–1669. [DOI] [PubMed] [Google Scholar]
- 16. Vijaya L, George R, Arvind H, et al. Prevalence of primary angle closure disease in an urban south Indian population and comparison with a rural population. The Chennai Glaucoma Study. Ophthalmology. 2008; 115: 655–660. [DOI] [PubMed] [Google Scholar]
- 17. Congdon NG, Qi Y, Quigley HA, et al. Biometry and primary angle-closure glaucoma among Chinese, White, and Black populations. Ophthalmology. 1997; 104: 1489–1495. [DOI] [PubMed] [Google Scholar]
- 18. Wang B, Sakata LM, Friedman DS, et al. Quantitative iris parameters and association with narrow angles. Ophthalmology. 2010; 117: 11–17. [DOI] [PubMed] [Google Scholar]
- 19. Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992; 113: 381–389. [DOI] [PubMed] [Google Scholar]
- 20. Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003; 12: 167–180. [DOI] [PubMed] [Google Scholar]
- 21. Sakai H, Morine-Shinjyo S, Shinzato M, et al. Uveal effusion in primary angle closure glaucoma. Ophthalmology. 2005; 112: 413–419. [DOI] [PubMed] [Google Scholar]
- 22. Kumar RS, Quek D, Lee KY, et al. Confirmation of uveal effusion in eyes with primary angle closure glaucoma: an ultrasound biomicroscopy study. Arch Ophthalmol. 2008; 126: 1647–1651. [DOI] [PubMed] [Google Scholar]
- 23. Yang M, Aung T, Husain R, et al. Choroidal expansion as a mechanism for acute primary angle closure: an investigation into the change of biometric parameters in the first two weeks. Br J Ophthalmol. 2005; 89: 288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arora KS, Jefferys JL, Maul EA, et al. Choroidal thickness change after water drinking is greater in angle closure than in open angle eyes. Invest Ophthalmol Vis Sci. 2012; 53: 6393–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005; 123: 1053–1059. [DOI] [PubMed] [Google Scholar]
- 26. Dada T, Sihota R, Gadia R, et al. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for assessment of the anterior segment. J Cat Refract Surg. 2007; 33: 837–840. [DOI] [PubMed] [Google Scholar]
- 27. Ramos JL, Li Y, Huang D. Clinical and research applications of anterior segment optical coherence tomography – a review. Clin Experiment Ophthalmol. 2009; 37: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakata LM, Lavanya R, Friedman DS, et al. Comparison of gonioscopy and anterior segment ocular coherence tomography in detecting angle closure in different quadrants of the anterior chamber angle. Ophthalmology. 2008; 115: 769–774. [DOI] [PubMed] [Google Scholar]
- 29. Quigley HA, Silver DM, Friedman DS, et al. Iris cross-sectional area decreases with pupil dilation and its dynamic behavior is a risk factor in angle closure. J Glaucoma. 2009; 18: 173–179. [DOI] [PubMed] [Google Scholar]
- 30. Aptel F, Denis P. Optical coherence tomography quantitative analysis of iris volume changes after pharmacologic mydriasis. Ophthalmology. 2010; 117: 3–10. [DOI] [PubMed] [Google Scholar]
- 31. Aptel F, Chiquet C, Beccat S, et al. Biometric evaluation of anterior chamber changes after physiologic pupil dilation using Pentacam and anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 4005–4010. [DOI] [PubMed] [Google Scholar]
- 32. Foster PJ, Buhrmann R, Quigley HA, et al. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002; 86: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng C, Cheung CY, Narayanaswamy A, et al. Pupil dynamics in Chinese subjects with angle closure. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 1353–1359. [DOI] [PubMed] [Google Scholar]
- 34. Rasband WS. ImageJ. Bethesda, MD: US: National Institutes of Health; 1997–2011. Available at: http://imagej.nih.gov/ij/. Accessed January 1, 2014. [Google Scholar]
- 35. Seager FS, Wang J, Arora KS, et al. The effect of scleral spur identification methods on structural measurements by anterior segment optical coherence tomography. J Glaucoma. 2014; 23: e29–e38. [DOI] [PubMed] [Google Scholar]
- 36. Narayanaswamy A, Zheng C, Perera SA, et al. Variations in iris volume with physiologic mydriasis in subtypes of primary angle closure glaucoma. Invest Ophthalmol Vis Sci. 2013; 54: 708–713. [DOI] [PubMed] [Google Scholar]
- 37. Sun JHS, Sung KR, Yun S-C, et al. Factors associated with anterior chamber narrowing with age: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2012; 53: 2607–2610. [DOI] [PubMed] [Google Scholar]
- 38. Wang D, Huang G, He M, et al. Comparison of anterior ocular segment biometry features and related factors among American Caucasians, American Chinese and mainland Chinese. Clin Experiment Ophthalmol. 2012; 40: 542–549. [DOI] [PubMed] [Google Scholar]
- 39. Wang D, He M, Wu L, et al. Differences in iris structural measurements among American Caucasians, American Chinese and mainland Chinese. Clin Experiment Ophthalmol. 2012; 40: 162–169. [DOI] [PubMed] [Google Scholar]
- 40. Leung CK, Palmiero P-M, Weinreb RN, et al. Comparisons of anterior segment biometry tomography between Chinese and Caucasians using anterior segment optical coherence. Br J Ophthalmol. 2010; 94: 1184–1189. [DOI] [PubMed] [Google Scholar]
- 41. Lee Y, Sung KR, Na JH, et al. Dynamic changes in anterior segment (AS) parameters in eyes with primary angle closure (PAC) and PAC glaucoma and open-angle eyes assessed using AS optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 693–697. [DOI] [PubMed] [Google Scholar]
- 42. Cheng JW, Cheng SW, Ma XY, et al. The prevalence of primary glaucoma in mainland China: a systematic review and meta-analysis. J Glaucoma. 2013; 22: 301–306. [DOI] [PubMed] [Google Scholar]
- 43. Foster PJ. The epidemiology of primary angle closure and associated glaucomatous optic neuropathy. Semin Ophthalmol. 2002; 17: 50–58. [DOI] [PubMed] [Google Scholar]
- 44. Wang YE, Li Y, Wang D, et al. Comparison of iris insertion classification among American Caucasian and ethnic Chinese using ultrasound biomicroscopy. Invest Ophthalmol Vis Sci. 2013; 54: 3837–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nongpiur ME, Sakata LM, Friedman DS, et al. Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology. 2010; 117: 1967–1973. [DOI] [PubMed] [Google Scholar]
- 46. Wu RY, Nongpiur ME, He MG, et al. Association of narrow angles with anterior chamber area and volume measured with anterior-segment optical coherence tomography. Arch Ophthalmol. 2011; 129: 569–574. [DOI] [PubMed] [Google Scholar]
- 47. Tornquist R. Shallow anterior chambers in acute glaucoma. Acta Ophthalmol. 1953; 31: 1–74. [PubMed] [Google Scholar]
- 48. Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 1970; 54: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nongpiur ME, He M, Amerasinghe N, et al. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology. 2011; 118: 474–479. [DOI] [PubMed] [Google Scholar]
- 50. Baek S, Sung KR, Sun JH, et al. A hierarchical cluster analysis of primary angle closure classification using anterior segment optical coherence tomography parameters. Invest Ophthalmol Vis Sci. 2013; 54: 848–853. [DOI] [PubMed] [Google Scholar]
- 51. Zheng C, Cheung CY, Aung T, et al. In vivo analysis of vectors involved in pupil constriction in Chinese subjects with angle closure. Invest Ophthalmol Vis Sci. 2012; 53: 6756–6762. [DOI] [PubMed] [Google Scholar]
- 52. Cheung CY, Liu S, Weinreb RN, et al. Dynamic analysis of iris configuration with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2010; 51: 4040–4046. [DOI] [PubMed] [Google Scholar]
- 53. Ang M, Chong W, Tay WT, et al. Anterior segment optical coherence tomography study of the cornea and anterior segment in adult ethnic South Asian Indian eyes. Invest Ophthalmol Vis Sci. 2012; 53: 120–125. [DOI] [PubMed] [Google Scholar]
- 54. Sng CC, Allen JC, Nongpiur ME, et al. Associations of iris structural measurements in a Chinese population: the Singapore Chinese Eye Study. Invest Ophthalmol Vis Sci. 2013; 54: 2829–2835. [DOI] [PubMed] [Google Scholar]
- 55. Jiang Y, He M, Huang W, et al. Qualitative assessment of ultrasound biomicroscopic images using standard photographs: the Liwan Eye Study. Invest Ophthalmol Vis Sci. 2010; 51: 2035–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Anastasopoulos E, Topouzis F, Wilson MR, et al. Characteristics of pseudoexfoliation in the Thessaloniki Eye Study. J Glaucoma. 2011; 20: 160–166. [DOI] [PubMed] [Google Scholar]
- 57. Forsius H. Exfoliation syndrome in various ethnic populations. Acta Ophthalmologica Suppl. 1988; 184: 71–85. [DOI] [PubMed] [Google Scholar]
- 58. Jiang Y, Friedman DS, He M, et al. Design and methodology of a randomized controlled trial of laser iridotomy for the prevention of angle closure in southern China: the Zhongshan Angle Closure Prevention trial. Ophthalmic Epidemiol. 2010; 17: 321–332. [DOI] [PubMed] [Google Scholar]