Abstract
Aim:
To assess the phagocytic activity of neutrophils in subjects with chronic obstructive pulmonary disease (COPD).
Background/Need of Study:
There is a paucity of data in relation to phagocytic function in COPD. By this multidisciplinary study, a better understanding about the etiology of lung destruction among COPD patients is being sought.
Materials and Methods:
The study was conducted among 28 subjects with COPD and 25 controls in a private tertiary hospital in Chennai after obtaining Institutional Ethical Clearance. Known cases of COPD as proven by clinical findings and spirometry were included in the study, and subjects with any other source of infection, recent surgery, or chronic granulomatous disease were excluded. The study subjects were divided into three groups based on the severity of COPD as determined by spirometry, and healthy volunteers were taken as Group 4. After obtaining informed consent, validated respiratory health questionnaire was administered. The phagocytic function was assessed by Candida phagocytic test and Nitroblue Tetrazolium (NBT) Reduction Test.
Results:
Significantly impaired phagocytic function as indicated by lower phagocytic, lytic indices and decreased NBT reduction of neutrophils was seen in COPD subjects compared to normal healthy controls (P < .001).
Conclusion:
This study showed that there is phagocytic dysfunction in COPD subjects when compared with normal subjects. This could be due to underlying inflammation in human airway. Understanding the role of neutrophils may lead to improved understanding of the pathogenesis of COPD, which in turn may pave way for implementing modified therapeutic intervention strategies.
KEY WORDS: Chronic obstructive pulmonary disease, neutrophils, phagocytosis
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of mortality worldwide.[1] It has been estimated that COPD will be the third biggest cause of mortality worldwide, by 2030.[2] In India, it affects approximately 5% of adults. COPD is characterized by chronic obstruction of lung airflow due to inflammatory response in the airways and lung to noxious particles and gases.[3] Multifactorial factors such as smoking, intravenous drug use, environmental pollutants, occupational chemicals/dusts, and HIV infection are the known risk factors associated with the development of COPD.[4] Pathophysiologically increased production of free radicals, compromised protease activity and cytokine release predisposes macrophages and neutrophils to reduced phagocytic activity.
Neutrophils are the key regulatory cells in COPD that are increased in numbers with abnormal apoptotic/phagocytic function. Lipopolysaccharide and inflammatory cytokines such as granulocyte macrophage colony stimulating factor can inhibit the apoptosis of neutrophils.[5] Reduction in the apoptosis of neutrophils or disturbed regulation of macrophage uptake of neutrophils, which have undergone apoptosis can lead to chronic inflammation and lung tissue injury in COPD.[6] Thus in this study, the phagocytic activity of neutrophils in COPD and healthy subjects were evaluated to understand the pathogenesis and moreover to explore if it can be used as a biomarker to identify the disease at an earlier stage, which in turn may help in planning the management strategies.
MATERIALS AND METHODS
This cross-sectional study was conducted among 28 COPD subjects who visited the outpatient department of Chest Medicine, in a private tertiary hospital in Chennai and 25 healthy volunteers after getting the approval from the Institutional Ethical Committee. Peripheral blood samples were collected from the study subjects after obtaining the informed consent. Clinically and spirometrically proven cases of COPD with no exacerbations in the last 3 months were included in the study. Subjects with any other source of infection, recent surgery, systemic lupus erythematosus, chronic granulomatous diseases, and immunosuppressant therapy were excluded from the study. Based on GOLD 2010 guidelines, study groups were classified as Group 1: Mild COPD characterized by FEV1/FVC <70%, but FEV1 is still >80% predicted, Group 2: Moderate COPD characterized by 50% <FEV1 <80% predicted, Group 3: Severe COPD characterized by 30% <FEV1 <50% predicted and Group 4: Healthy volunteers as controls.
Evaluation of pulmonary functions
A detailed respiratory health questionnaire was administered to each subject to assess their smoking/alcoholic status and respiratory health. In addition, pulmonary function test was done by using a portable data logging spirometer. The test was performed in a sitting position and a mouthpiece was placed in mouth making sure that the lips were sealed around the mouthpiece and that the tongue did not occlude it. The subject was then asked to inhale completely and rapidly and exhale maximally until no more air can be expelled. The subjects were verbally encouraged to continue to exhale the air at the end of the maneuver to obtain optimal effort. The same was repeated for a minimum of three maneuvers and not more than eight maneuvers were performed for acceptability and repeatability following American Thoracic Society guidelines (Miller, et al., 2005). Forced vital capacity (FVC), forced expiratory volume in one second (FEV1), peak expiratory flow rate (PEFR), and mid-expiratory volume (FEF25%–75%) values were recorded. The values of the best of the three reproducible curves were taken. A complete flow–volume loop was obtained from the spirometer. Most of the sources of variation in pulmonary function assessment such as motivation, effort, and body position were controlled.
Spirometric testing including bronchodilation after inhalation of 200 µg of salbutamol, was done in order to grade COPD. COPD cases were defined as follows: reversibility test result of <12% improvement in FEV1 compared with prebronchodilator FEV1; or postbronchodilator improvement of FEV1 <200 mL, and FEV1/FVC <70% and no history of atopy or pattern of disease suggestive of asthma.
Total leukocyte count was done by manual method. A sample of blood was diluted with a diluting fluid, which destroys the red cells and stains the nuclei of the leukocytes. The cells were then counted in a counting chamber and their number in undiluted blood reported as leukocytes/mm3 (Bagby, et al). To determine the differential count, a drop of blood was thinly spread over a glass slide, air dried, and stained with a Romanowsky stain (Leishman stain). One hundred cells were counted and classified.
Assessment of phagocytic function
Candida phagocytosis test
This test relies on the uptake of heat killed Candida albicans by neutrophils over a brief period of time. The intracellular Candida that stains intensely can be identified within the neutrophils and counted. Heat-killed Candida suspended in phosphate-buffered saline was adjusted to ×108 cells/mL and stored at –20°C. A 0.1mL of Hank's balanced salt solution, 0.1mL of pooled serum, and 0.1mL of heat-killed Candida were added to 0.2mL of buffy coat and centrifuged for 5 min at 1000 rpm. The supernatant was removed. Then the sediment was smeared, fixed with methanol, stained with Leishman's stain and viewed under microscope (100×). One hundred neutrophils/slide were counted. Number of Candida-engulfed neutrophils were counted as positive cells.
Phagocytic index = Number of positive cells/100 cells.
Lytic index = Total number of Candida/100. This is also called as Avidity index.
Nitroblue tetrazolium reduction test
In this assay, 0.5 mL of heparinized blood was taken on a clean glass slide, incubated at 37°C for 30 min. It was gently washed with cold saline (should not be dried), tapped gently, and excess saline was removed. Nitroblue tetrazolium (NBT) medium was added, a coverslip was placed, and incubated for 30 min at 37°C. Then it was washed with cold saline, air dried, fixed with methanol for 3 min, and washed with distilled water. Then the slide was air dried and stained with safranin (0.77%) for 7 min and washed with distilled water. The percentage of formazon-positive cells was counted using light microscopy under 100× magnification (VISION 2000, LABOMED, Chennai). NBT freely enters into the cells and intracellular reduction of the dye by phagosomes converts it to an insoluble blue crystalline form (formazon crystals). Intracellular reduction is essential for microbicidal activity and it depends on activation of hexose monophosphate shunt.
Statistical analysis
The data were analyzed using SPSS software. Results of the numerical data are expressed as mean and standard deviation. Statistical significance was determined by using unpaired t test and one way ANOVA. P < 0.05 was taken as the level of significance. Categorical data are expressed as proportions and Chi-square test was used to find the association between categorical variables.
RESULTS
Based on pulmonary function tests, among 28 COPD cases, 6 belong to mild COPD category (21%), 10 were moderate COPD (36%), and 12 were severe COPD cases(43%).
The mean age of the COPD group was 62.39 ± 11.31 years and that of the controls 63.04 ± 11.2 years. Of the 28 COPD cases, 25 were smokers (89%) and 7 were alcoholics (25%). On the other hand, in healthy volunteers (n = 25), 17 were smokers (68%) and 2 were alcoholics (8%). Pack years of smoking for COPD cases was 14.66 ± 15.5 and for controls was 0.79 ± 0.68. COPD subjects showed decreased weight (mean 57.1kg) as compared to healthy volunteers (mean 66.1kg, P = 0.005).
The total leukocyte count was found to be 8395.36 ± 1401.41/mm3 in COPD subjects and 8560.80 ± 1509.36/mm3 in controls and it was not statistically significant. However, differential count of polymorphs was 64.25 ± 5.35% in COPD cases and 62.2 ± 5.53% in controls. The lymphocyte count was 28.14 ± 6.09% and 30.33 ± 4.84% in COPD cases and controls, respectively. The eosinophil and monocyte count was 3.58 ± 2.33%, 3.6 ± 1.82%, and 3.91 ± 1.96%, 4.1 ± 3.4% in COPD cases and controls, respectively. Although the difference in total count was not statistically significant, the neutrophils were significantly increased in COPD cases compared with controls.
Number of Candida-engulfed neutrophils was counted and expressed as a percent of positive cells. This is called phagocytic index. The mean percent of phagocytic cells in COPD cases was found to be 0.1 ± 0.06% and 0.22 ± 0.1% in healthy controls. The difference between the phagocytic indices between the cases and controls was found to be highly significant [P < 0.001; Table 1]. Moreover, significant difference in phagocytic activity between the grades of COPD cases in comparison to controls was also found [Table 2].
Table 1.
Various indices among cases and controls
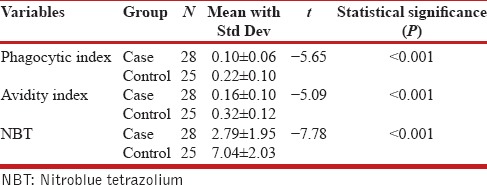
Table 2.
Phagocytic index, Lytic index, and NBT among the different grades of cases and healthy volunteers
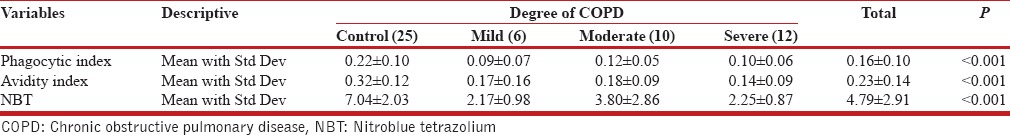
When exposed in NBT, many normal neutrophils were converted into distinctive, degenerate, easily recognizable cells and designated as formazon cells. These positive cells were immediately apparent upon inspection of the slide, because of their blue color. Moreover, there was a rapid increase in cell size, dissolution of the reticulate polymorphic nucleus into homogenous mass, and apparent cell death. The remainder of the granulocytes appeared normal with neither precipitation of formazon nor degeneration. In COPD cases, 2.79 ± 1.95% of the cells were positive for formazon and in healthy controls, 7.04 ± 2.03% cells were positive. This difference in formazon-positive cells between COPD cases and healthy volunteers was highly significant (P < 0.001). In addition, significant difference in formazon positivity (NBT) between the grades of COPD cases in comparison to controls was also observed [Table 2]. The results have shown that there was phagocytic dysfunction, which could be due to underlying inflammation in human airway.[7]
DISCUSSION
COPD is a chronic inflammatory disease that causes cough and sputum production followed by the development of obstructive airways. Chronic inflammatory response in COPD mainly involves neutrophils, macrophages, and CD8+ T-lymphocytes that cause structural changes in airway epithelial cells. Neutrophilic inflammation is increased in many acute and chronic lung diseases, including COPD, acute bronchitis, and adult respiratory distress syndrome. Accumulation of neutrophils at the sites of inflammation is a dynamic process through the recruitment from the bloodstream and clearance from the lungs as a result of phagocytosis of apoptotic cells. This leads to the release of granule proteins, including human neutrophil elastase (HNE) and myeloperoxidase (MPO). MPO and HNE contribute to the inflammation of bronchi and to structural alterations such as peribronchiolar fibrosis and emphysema.[8] Neutrophil recruitment to the airways is controlled by various mediators, most notably CXCL8 (also known as interleukin (IL-8), IL-1β, tumor necrosis factor (TNF)-α, and leukotriene B4. The extensive functional idleness in signaling pathways lead to recruitment of neutrophils in the airways.
Candida phagocytic test relies on the uptake of heat-killed C. albicans by neutrophils over a brief period of time. The intracellular candida that stain intensely can be identified within the neutrophils and counted. This gives Lytic index or Avidity index. When neutrophils are exposed to the yellow dye NBT, it is taken up by the cells into phagosomes and intracellular reduction of the dye converts it to an insoluble blue crystalline form (formazon crystals). This gives the value of NBT reduction test. Intracellular reduction is essential for microbicidal activity and it depends on activation of hexose monophosphate shunt.
In support of earlier studies, our study showed reduced phagocytic activity of neutrophils in COPD cases compared with healthy volunteer controls. This has also been evidenced by Harvey et al.'s study[9] in which patients with COPD have immunological dysfunction in the lung mostly due to defect in the phagocytic activity of macrophages.
Based on disease severity and leukocyte priming, activation of peripheral blood neutrophils increases.[10] In contrary to this report, Pletz et al.,[11] observed lack of difference in neutrophil apoptotic activity in peripheral blood in stable COPD cases. In concordance with this, our study did not show any significant difference in phagocytic activity among the different grades of COPD cases.
CONCLUSION
This study is directed to the evaluation of the phagocytic function of neutrophils among COPD patients and the results have revealed that there is actually a phagocytic dysfunction and that it is not related with the severity of the disease. Although the precise mechanisms responsible for phagocytic dysfunction can vary between severities of disease states, these findings suggest that underlying inflammation might have an important effect on the phagocytic function in the human airway.
Neutrophils play a key role in inflammation in COPD, not only in the stable state but also in progression of disease and in bacterial colonization and infection. The process by which this occurs is complex, but understanding the role of the neutrophil has led to improved knowledge of the pathogenesis of COPD. Modulation of neutrophil recruitment, perpetuation, and clearance in the lungs of patients with COPD provides us with potential targets for modifying the natural history of this disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.World Health Report, WHO: Geneva; Global strategy for the diagnosis, management and prevention of COPD (2006) [Google Scholar]
- 2.World Health Organization. Burden of COPD. Chronic Respiratory Diseases. [Last accessed on 2015 Aug 26]. Available from: http://www.who.int/respiratory/copd/burden/en/
- 3.Global Initiative for Chronic Obstructive Lung Disease. Pathology, Pathogenesis and Pathophysiology. Updated GOLD January, 2015. :6–9. [Google Scholar]
- 4.Global Initiative on Chronic Obstructive Lung Disease. GOLD Updated, January 2015. [Last accessed on 2015 Aug 26]. pp. 4–5. Available from: http://wwwgoldcopdorg/uploads/users/files/GOLD_Report_2015_Apr2.pdf .
- 5.John CR, Douglas RG. Cambridge: Cambridge University Press; 2011. Apoptosis. Physiological Pathology; p. 355. [Google Scholar]
- 6.Plataki M, Tzortzaki E, Rytila P, Demosthenes M, Koutsopoulos A, Siafakas NM. Apoptotic mechanisms in the pathogenesis of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:161–71. doi: 10.2147/copd.2006.1.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safwat T, Saeed A, Dina AF, Weaam AM. The Phagocytic activity of peripheral blood macrophages in COPD patients. Egypt J Bronchol. 2008;2(2):244. [Google Scholar]
- 8.Garnez Y, Tirouvanziam R, Chanez P. Neutrophils in chronic inflammatory airway diseases: Can we target them and how? Eur Respir J. 2010;35:467–9. doi: 10.1183/09031936.00186109. [DOI] [PubMed] [Google Scholar]
- 9.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yasmus L, Brown RH, Feller K D, Wise R, Biswal S. Sci Transl Med. 2011;3:78ra32. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koenderman L, Kanters D, Maesen B, Raaijmakers J, Lammers JW, de Kruif J, et al. Monitoring of neutrophil priming in whole blood by antibodies isolated from a synthetic phage antibody library. J Leukoc Biol. 2000;68:58–64. [PubMed] [Google Scholar]
- 11.Pletz MW, Lode H. Apoptosis of circulating neutrophils in COPD patients. Chest. 2005;127:1464–5. doi: 10.1378/chest.127.4.1464. [DOI] [PubMed] [Google Scholar]


