Abstract
Background:
Diffuse parenchymal lung diseases (DPLD) are a group of disorders characterized by chest radiological findings of bilateral diffuse shadowing. Lung biopsy is generally required to make an etiological diagnosis of DPLD's. Transbronchial lung biopsy (TBLB) is a minimally invasive method to achieve a lung sample which has been found to be a useful diagnostic tool in patients with DPLD. As per American Thoracic Society guidelines for management of idiopathic interstitial pneumonias, TBLB is not required in patients who have findings consistent with idiopathic pulmonary fibrosis (IPF) on HRCT scan thorax. Some Indian researchers have evaluated, on a small number of subjects, the role of TBLB in patients with DPLD, but they had not excluded patients with ‘IPF pattern’. This study was planned to assess TBLB in patients with DPLD after excluding patients with ‘IPF pattern’.
Materials and Methods:
A prospective non-randomized study on 49 patients with DPLD without a characteristic ‘IPF pattern’ were subjected to TBLB.
Results:
The overall diagnostic yield of TBLB was 85.7%. Non-specific interstitial pneumonitis, tuberculosis and sarcoidosis were the most common histology patterns found (22.4, 18.4 and 16.3%, respectively). Procedure-related mortality was nil. Iatrogenic pneumothorax occurred in five patients (10.2%). Minor complications included hemorrhage and transient hypoxia.
Conclusion:
TBLB is a safe and effective tool in the diagnosis of DPLD.
KEY WORDS: Diffuse parenchymal lung disease, idiopathic pulmonary fibrosis, transbronchial lung biopsy
INTRODUCTION
DPLD is a wide spectrum of more than 200 disorders, which have a similar clinical presentation with diffuse shadowing on chest skiagram.[1] HRCT scan is one of the most useful investigations in the diagnostic tool kit of DPLD. In fact, some patterns on HRCT scan have high sensitivity and specificity for diagnosing some conditions with DPLD, one of them being the ‘IPF pattern’ in which, as per recent ATS-ERS consensus statement,[2] histopathologic confirmation is not required. However, rest of the DPLDs require histopathology for their diagnosis. Open lung biopsy, considered as the ‘gold standard’ for DPLD, is associated with significant morbidity and mortality, so a safer method, the transbronchial lung biopsy, is gradually gaining wide acceptance.[3] Few authors from India have published their experience of TBLB in patients with DPLD, but they have not excluded patients with ‘IPF pattern’ so a significant proportion of patients in their studies were found to be having IPF.[4,5,6] This study was aimed at assessing TBLB in patients with DPLD who did not have ‘IPF pattern’ on HRCT thorax.
MATERIALS AND METHODS
This is a prospective, non-randomized study conducted at our center to assess the role of TBLB in patients with DPLD without IPF pattern on HRCT thorax. During the study period of 3 years, 49 patients who had the following inclusion and exclusion criteria were enrolled.
Inclusion criteria
All consecutive patients of DPLD were enrolled. DPLD pattern included diffuse nodular and/or infiltrative pattern and alveolar and/or interstitial involvement on HRCT scan thorax.
Exclusion criteria
’IPF pattern’ on HRCT scan thorax which comprised of bilateral, predominantly basal, subpleural reticular pattern, with subpleural cysts (honeycombing) and/or traction bronchiectasis. Patients, who had contraindications for undergoing bronchoscopy, were also excluded.
Procedure details
Patients were enrolled in the study after written informed consent. Detailed history including occupation, drug intake, smoking and relevant medical history were recorded. Bronchoscopy was performed under local anesthesia (nebulization with 4% xylocaine + 2% xylocaine instillation through working channel on vocal cords and central airways) and conscious sedation (midazolam and fentanyl as per protocol). Flexible bronchoscope (Olympus BF 1T 150) was used. TBLB was done under C-arm fluoroscopy guidance with biopsy forceps (Olympus FB-19C-1). The selection of lobe/segment for biopsy was based on the disease pattern on HRCT scan of thorax. Upper lobe biopsy was generally avoided because of proximity to pleura and theoretically higher chances of pneumothorax. Middle lobe and lower lobe were sampled in most of the cases. On an average 6-8 biopsies were taken in each patient. Only one lung was sampled to avoid a possibility of bilateral pneumothoraces. Skiagram chest was done one hour after the procedure to rule out pneumothorax. Patients were hospitalized for 24 hrs to monitor for complications.
The demographic profile and histology reports of selected patients were recorded.
RESULTS
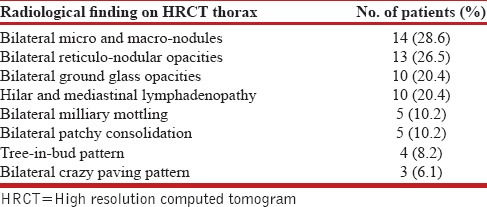
A total of 49 patients fulfilled the inclusion and exclusion criteria. Mean age of the patients was 49.6 (±15.7) yrs. Twenty seven of 49 patients were males and rest 22 were females. Radiological findings of study subjects is given in Table 1. Bilateral nodular and reticulonodular lesions were the most common radiological findings.
Table 1.
Radiological findings of study subjects
Visually, tracheobronchial tree was normal in all the cases except in one patient in whom bronchial tree had nodularity in trachea and major bronchi, which turned out to be due to sarcoidosis. Satisfactory specimens were obtained in all the patients. A diagnosis could be achieved in 42 out of 49 cases (85.7%). The details of histological diagnosis are given in Table 2. Non-specific interstitial pneumonitis, tuberculosis and granulomatous inflammation consistent with sarcoidosis were the most common histologic patterns found. (22.4, 18.4 and 16.3%, respectively) [Table 2]. We also found certain rare but interesting causes of DPLD like lymphangioleiomyomatosis [Figures 1 and 2] and pulmonary alveolar proteinosis [Figures 3 and 4].
Table 2.
Distribution of study population according to histological findings of TBLB
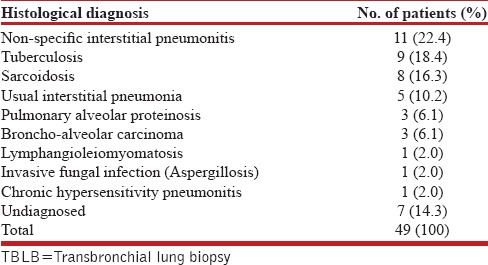
Figure 1.
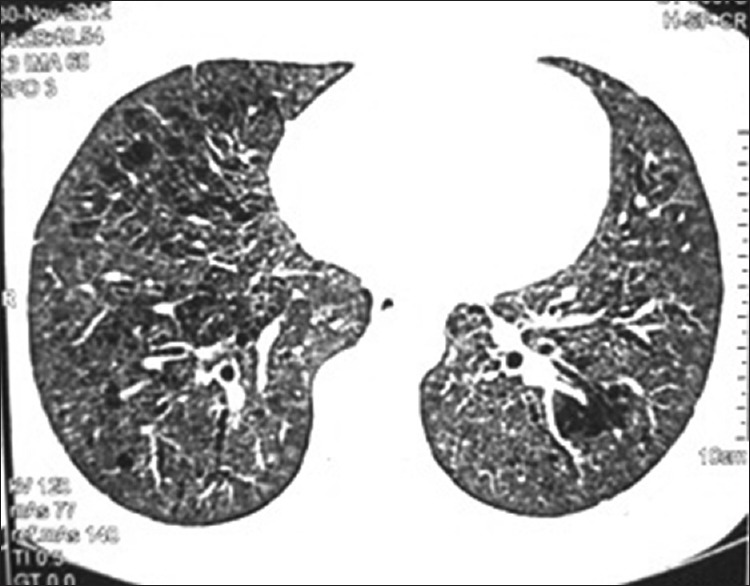
HRCT thorax of patient showing multiple thin-walled cystic areas in both lungs
Figure 2.
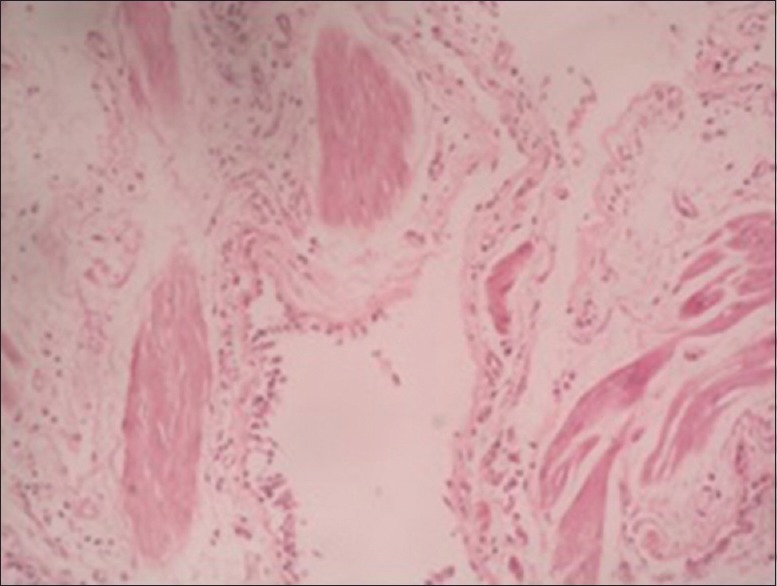
HPE showing cystic chronic interstitial lung disease with smooth muscle bundle with HMB 45 staining
Figure 3.
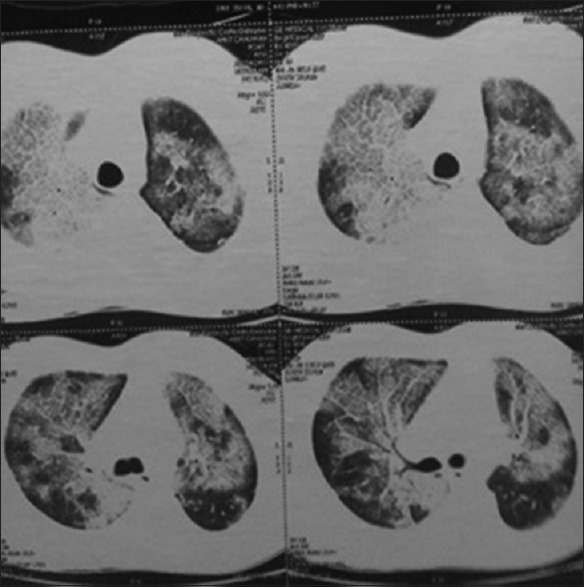
CECT thorax of the patient showing crazy paving
Figure 4.
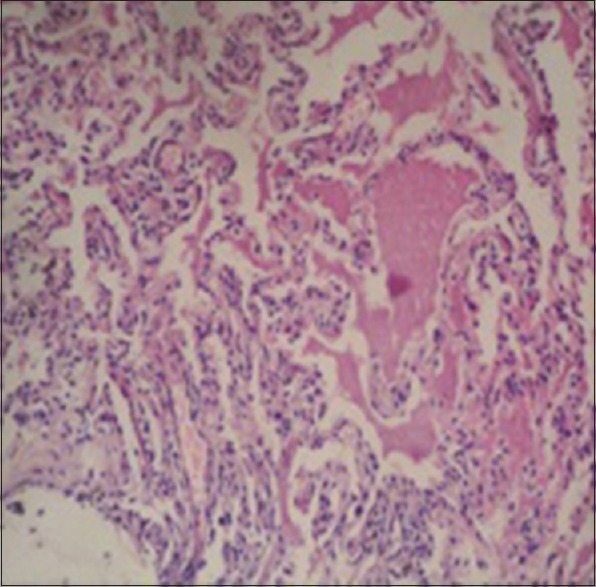
HPE of lung biopsy showing granular pinkish material with normal lung architecture. Material was PAS positive
No procedure-related mortality was observed. No major complications were encountered. Five patients developed pneumothorax, three of whom required chest tube drainage for 2-3 days. Minor bleeding during the procedure was managed by local instillation of adrenaline (1:1000 dilutions), balloon or bronchoscopic wedge tamponade.
DISCUSSION
DPLDs are a group of disorder of lung which usually poses a diagnostic challenge to the pulmonologist as it includes varied number of diseases with similar clinical and radiological features (bilateral diffuse opacification). HRCT scan thorax and lung biopsy are two most important diagnostic tools used for diagnosing patients with DPLD. HRCT scan findings of DPLD can be grossly divided into two patterns: ‘IPF pattern’ and ‘non-IPF pattern’. ‘IPF pattern’ comprises bilateral, predominantly basal and sub-pleural reticular pattern, with sub-pleural cysts (honeycombing) and/or traction bronchiectasis. The positive predictive value of diagnosing IPF with this ‘IPF pattern’ is around 96% when seen by a radiologist confident in HRCT diagnosis.[7,8] Hence, the recent ATS-ERS statement does not recommend lung biopsy in patients with ‘IPF pattern’ on HRCT thorax.
Patients, who do not have ‘IPF pattern’ on HRCT thorax, need a tissue diagnosis. Open lung biopsy, considered to be ‘gold standard’ investigation for this purpose, is associated with significant morbidity and mortality, so another approach, the transbronchial approach (TBLB) is gaining acceptance as the investigation of choice for most of DPLD cases.[3]
At our center, we are performing TBLB in patients of DPLD who do not have ‘IPF pattern’ on HRCT thorax following the recent ATS-ERS statement. In this paper, we are presenting our 3 years experience of TBLB in above-mentioned patients.
A brief review of published Indian studies is given in Table 3. One large study from India on TBLB was published by Verma et al.[9] The study was on 121 subjects with DPLD, but in their study, the inclusion criteria were not well defined. All the subjects were not subjected to TBLB and baseline HRCT pattern was not considered during enrolment of subjects. A significant proportion of the subjects were of IPF (48.7%) who would not have required lung biopsy as per current guidelines [Table 3].
Table 3.
Review of Indian studies on DPLD
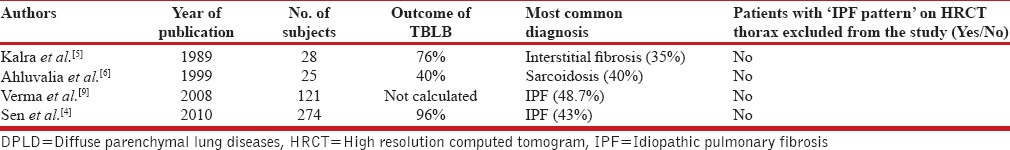
Another retrospective analysis of biopsy proven (both open lung biopsy and TBLB) ILD cases was done by Sen et al.[4] They found a high diagnostic yield of TBLB (96%), which is slightly higher than at our center (85.7%) Another significant finding in their study was a high proportion (43%) of IPF, who would not have required a biopsy, had the HRCT ‘IPF pattern’ been used as a marker of IPF diagnosis [Table 3].
Similar study with lesser number of patients by Kalra et al.[5] on patients with DPLD and TBLB has been published in Indian literature. In this study also, authors did not use the exclusion criteria of ‘IPF pattern’ on HRCT thorax, hence a significant proportion of IPF was found their studies (35%) [Table 3].
In contrast to above-mentioned studies, we have excluded patients with ‘IPF pattern’ on HRCT scan thorax, who as per recent guidelines, do not require a lung biopsy. And because of the same reason, only 6.1% of our study subjects were found to be having usual interstitial pneumonia (UIP) pattern on histology, which is consistent with IPF. This also suggests that despite a ‘non-IPF pattern’ on HRCT thorax, histological evidence of UIP may be found, although we have to keep in mind the lack of expertise of pathologists in examining lung specimen.
CONCLUSIONS
To conclude, this study adds to the previous experiences of other authors that TBLB is a safe investigation with high diagnostic yield and should be performed in patients with DPLD after excluding characteristic ‘IPF pattern’ on HRCT thorax.
ACKNOWLEDGMENT
We acknowledge the support of our bronchoscopy technicians who assisted us in performing bronchoscopy and TBLB.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Walters EH, du Bois R, editors. London: Chapman and Hall; 1995. Immunology and Management of Interstitial Lung Diseases; pp. 19–36. [Google Scholar]
- 2.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 3.Leslie KO, Gruden JF, Parish JM, Scholand MB. Transbronchial biopsy interpretation in the patient with diffuse parenchymal lung disease. Arch Pathol Lab Med. 2007;131:407–23. doi: 10.5858/2007-131-407-TBIITP. [DOI] [PubMed] [Google Scholar]
- 4.Sen T, Udwadia ZF. Retrospective study of interstitial lung disease in a tertiary care centre in India. Indian J Chest Dis Allied Sci. 2010;52:207–11. [PubMed] [Google Scholar]
- 5.Kalra S, D’Souza G, Bhusnurmath B, Jindal SK. Transbronchial lung biopsy in diffuse lung disease–A study of 28 cases. Indian J Chest Dis Allied Sci. 1989;31:265–70. [PubMed] [Google Scholar]
- 6.Ahluwalia G, Sharma SK, Dattagupta S, Pande JN. Role of transbronchial lung biopsy in diffuse pulmonary disease: A review of 25 cases during one year. Indian J Chest Dis Allied Sci. 1999;41:213–7. [PubMed] [Google Scholar]
- 7.Epler GR, McLoud TC, Gaensler EA, Mikus JP, Carrington CB. Normal chest roentgenograms in chronic diffuse infiltrative lung disease. N Engl J Med. 1978;298:934–9. doi: 10.1056/NEJM197804272981703. [DOI] [PubMed] [Google Scholar]
- 8.Martinez FJ. Idiopathic interstitial pneumonias: Usual interstitial pneumonia versus nonspecific interstitial pneumonia. Proc Am Thorac Soc. 2006;3:81–95. doi: 10.1513/pats.200511-123JH. [DOI] [PubMed] [Google Scholar]
- 9.Verma SK, Prasad R, Anant SC, Shukla AD, Surya Kant, Goel MM, et al. A study of diffuse parenchymal lung disease. Pulmon. 2008;10:94–7. [Google Scholar]


