Abstract
Background:
Acute exacerbations of chronic obstructive pulmonary disorder (AECOPD) are known to be associated with increased morbidity and mortality and have a significant socioeconomic impact. The factors that determine frequent hospital readmissions for AECOPD are poorly understood. The present study was done to ascertain failures rates following AECOPD and to evaluate factors associated with frequent readmissions.
Materials and Methods:
We conducted a prospective study among 186 patients with COPD with one or more admissions for acute exacerbations in a tertiary care hospital. Frequency of previous re-admissions for AECOPD in the past year, and clinical characteristics, including spirometry were ascertained in the stable state both before discharge and at 6-month post-discharge. Failure rates following treatment were ascertained during the follow-up period. All the patients were followed up for a period of 2 years after discharge to evaluate re-admissions for the AECOPD.
Results:
Of 186 COPD patients admitted for AECOPD, 54% had one or more readmission, and another 45% had two or more readmissions over a period of 2 years. There was a high prevalence of current or ex-heavy smokers, associated co-morbidity, underweight patients, low vaccination prevalence and use of domiciliary oxygen therapy among COPD patients. A total of 12% mortality was observed in the present study. Immediate failure rates after first exacerbation was observed to be 34.8%. Multivariate analysis showed that duration >20 years (OR = 0.37; 95% CI: 0.10-0.86), use of Tiotropium (OR = 2.29; 95% CI: 1.12-4.69) and use of co-amoxiclav during first admission (OR = 2.41; 95% CI: 1.21-4.79) were significantly associated with higher immediate failure rates. The multivariate analysis for repeated admissions revealed that disease duration >10 years (OR = 0.50; 95% CI: 0.27-0.93), low usage of inhaled ICS + LABA (OR = 2.21; 95% CI: 1.08-4.54), and MRC dyspnea grade >3 (OR = 2.51; 95% CI: 1.08-5.82) were independently associated with frequent re-admissions for AECOPD.
Conclusions:
The outcomes of patients admitted for an acute exacerbation of COPD were poor. The major factors influencing frequency of repeated COPD exacerbations were disease duration, low usage of inhaled ICS + LABA, and MRC dyspnea grade >3.
KEY WORDS: Acute exacerbation, chronic obstructive pulmonary disease, failure rates, hospitalization, repeated exacerbation
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) and acute exacerbations of COPD (AECOPD) have major impacts both on the population and on health care costs. The burden of COPD and the impact of exacerbation on the health economy vary between countries and within regions of the same country, likely due to inconsistent reporting and diagnosis as well as to the differences in the risk factors for COPD. COPD morbidity and mortality, however, have increased over the past 40 years and this trend will continue. COPD, with its social, health care utilization, and economic consequences is currently the fourth leading cause of death in the United States and it is projected to be the third leading cause of death by 2020, with only cardiovascular diseases and cancer projected to be ahead.[1]
Although COPD and AECOPD are important economically, they are significant at the level of the patients. From a long-term prospective, repeated exacerbations of COPD are associated with more pejorative course of the disease, characterized by an accelerated decline in forced expiratory volume in 1 second (FEV1), decreased quality of life, persistent tachypnea, development of co-morbid disease and premature mortality.[2] AECOPD requiring inpatient interventions are particularly important in that they incur a large cost, and each one has the potential for a fatal outcome.[3] A better understanding of long-term outcomes of COPD patients following hospitalization, may help patients and their physicians to better manage the disease and in making healthcare decisions. The factors that determine acute exacerbations and hospitalization in COPD patients are poorly understood. Factors that have been studied as predictors of mortality and other outcomes include FEV1, blood gases, co-morbidity, chronic mucus hypersecretion (CMH), muscle weakness, poor nutritional status, low BMI, socioeconomic status and support, number of previous physician visits or hospital admissions, influenza vaccination, pneumococcal vaccination, pulmonary rehabilitation, inhaled corticosteroids and long-term oxygen therapy.[4] However, these studies have produced inconsistent results with respect to the influence of FEV1, BMI and smoking.[5] Most investigators have studied a limited number of specific risk factors in a single study, some of which did not fully take into account potential confounding among variables. Other potential risk or protective factors such as psychological well-being, patient adherence to care and social support have not been reported or rarely so.
Hence the present prospective study was done to evaluate the outcome variables that are responsible for the AECOPD. The aims of this study were to ascertain in a cross-sectional sample survey in hospitalized COPD patients the rates of hospital re-admissions for acute exacerbations, the frequency of modifiable and non-modifiable risk factors; and to evaluate the risk factors associated with recurrent hospital admissions for AECOPD.
MATERIALS AND METHODS
Study design and patient selection
This study was a prospective study in a tertiary care hospital over a period of 48 months from January 2008 to December 2011. During the initial 24 months, the patients were recruited in the study and for the next 24 months all these patients were followed up systematically for stability of the disease or any adverse impact. Patients who had acute exacerbation of COPD during the initial entry period of 24 months were included in the study. Diagnosis of COPD was made according to the criteria set by GOLD.[6] Severity of COPD condition was stratified based on post-bronchodilator FEV1 percent predicted using GOLD guidelines (Stage I - FEV1 >80%; Stage II - FEV1 50%-80%; Stage III -FEV1 30%-50%; and Stage IV -FEV1 <30% or FEV1 <50% along with signs of right ventricular failure). The inclusion criteria were: (i) patients with a principal diagnosis of AECOPD; (ii) age over 50 years; (iii) current smokers or ex-smokers with a history of smoking equivalent to at least 20 pack-years; and (iv) surviving patients with stable COPD status on discharge. Both COPD patients with first-episode exacerbation and repeat admissions were included. The exclusion criteria were patients with active pulmonary tuberculosis, pulmonary fibrosis, pneumothorax, pulmonary embolism, lung cancer, acute heart failure, acute myocardial infarction, stroke sequelae, severe renal failure, moderate to severe Parkinson's disease, dementia, hearing impairment, or those who were mentally unable to answer a questionnaire. An acute exacerbation was defined using Anthonisen criteria: Increased cough and sputum volume, increased sputum purulence and increased dyspnea. In addition patient may have had fever, malaise, fatigue and chest congestion. Severity of AECOPD was defined as Type I when patients had all three symptoms; Type II when patients had any two symptoms and Type III when patients had any one symptom. Each patient was treated in a standard fashion with 40-80mg of parental methylprednisolone, nebulized bronchodilators (salbutamol and ipratropium bromide), theophyllines and supplemental oxygen. Antibiotics were used when there were signs of bacterial infection. In addition, some patients with respiratory failure were admitted to the ICU and started on mechanical ventilated, if required. All the patients were followed up for a period of 24 months after discharge for any adverse outcome. If any fatality occurred during this period, the cause of death was ascertained as far as possible.
Clinical data
Demographic and clinical data were collected for all patients including: age, sex, socioeconomic and marital status, smoking load (pack-years), history of previous pneumonia, use of home oxygen, co-morbid medical conditions, detailed hemogram and biochemical, spirometric and arterial blood gas analysis at admission. In addition, admission to the ICU, length of hospital stay, BMI, duration of COPD, time elapsed since first hospitalization, use of daily medications including inhaled steroids, long-acting beta-2 agonist (LABA) and tiotropium was also noted for each patient. This information and time elapsed since first hospitalization, were obtained from reviews of personnel and official medical documents and by questioning the patients. Co-morbid conditions were given scores according to Charlson to predict mortality. Severe diseases were assigned higher scores, and milder diseases lower scores (e.g. CCF=1, malignancy=2, AIDS=6). These data were evaluated to determine the factors that may influence overall survival time. Each patient was assessed at intervals of 3 months for 24 months. In the event of death, cause of death was ascertained as far as possible. Low BMI was used to categorize patients in two nutritional status groups: underweight (<20 kg/m2) and normal or overweight (≥20 kg/m2). The number of exacerbations that required hospitalization over 24 months was recorded for each patient. The following data was recorded for each patient: Clinical signs and symptoms, chest X-ray, sputum for gram staining and culture, arterial blood gas analysis, use of antibiotics and steroids. Standard spirometric measurements at admission were performed using Koko spirometer. If spirometric measurements could not be performed just after admission, it was performed as soon as possible within 24 hrs. Severity of COPD was done according to GOLD guidelines. The treatment outcome response for each exacerbation was recorded as follows: Treatment success if they had no return visit in 4 weeks for respiratory problem, treatment failure if they had a return visit in 4 weeks with persistent respiratory symptoms that required change of antibiotics or hospitalization with change of an antibiotic. All the patients were given standard form of therapy during hospitalization, which included: Oxygen supplementation, nebulized bronchodilators consisting of salbutamol and ipratropium bromide, parental steroids, parental broad spectrum antibiotics, mucolytics and diuretics, if indicated. At the time of discharge all the patients were given standard treatment as per GOLD guidelines depending upon the severity of the disease. This included inhaled ICS + LABA (stage III and IV), inhaled tiotropium, oral theophyllines, oral antibiotics, oral steroids (7-10 days) and mucolytics. Long-term oxygen (LTOT) was advised depending upon the standard criteria's for hypoxemias.
Outcome variables
Patient outcomes reported were: (i) Mechanical ventilation requirements; (ii) ICU and hospital lengths of stay; (iii) hospital mortality, home discharge; and (iv) readmission to hospital.
Data analysis
All data was analyzed using the statistical package, SAS (version 6.12; SAS Inc., NC, USA). The distributions of normal variables were compared using the Chi-squared test. Results were expressed as mean ± SD for continuous variables or number and percentage for discrete variables. A P value < 0.05 was considered statistically significant. C 2 tests of contingency tables were used to compare proportions for discrete variables, and t-tests were used to compare the means of normally distributed continuous variables between the two groups. Univariate analyses of risk factors that were associated with frequent re-admissions were performed as for a case-control study, using the Chi-square statistic and estimates of the odds ratio (OR) with their 95% confidence intervals (CI). Multivariate analysis to evaluate the independent risk factors for frequent re-admissions for AECOPD was performed using logistic regression for dichotomous-dependent variables.
RESULTS
Patient baseline characteristics
A total of 186 patients with AECOPD over a period of 24 months were included in the study. Seventy-one patients were dropped from the final analysis due to various reasons - defaulted immediately after inclusion, left the residence and developed other co-morbidity [Figure 1]. Thus a total of 115 patients were finally assessed. The mean age was 66.9 ± 7.8 years. A total of 256 exacerbations were observed over 24 months during the follow-up period. Ninety-seven patients (84.4%) were either current smokers or ex-smokers. Majority of patients (83.5%) had stage II or stage III COPD disease. The incidence of co-morbidity varied significantly: diabetes (15.6%), IHD (51.3%), hypertension (53.9%), renal disease (18.3%), liver disease (10.4%) and history of alcoholism (41.7%). Nearly 30% had more than two co-morbid conditions [Table 1]. Nearly 60% of the patients in the present study were taking inhaled tiotropium, and another 17.5% were on the maintenance inhaled steroids along with beta2 agonists. The duration of the COPD condition had a long duration of illness with 20% having the condition for more than 20 years. Majority of the COPD patients had wasted muscle tissue giving rise to low BMI and the mean serum albumin levels were <3.00g/dl.
Figure 1.
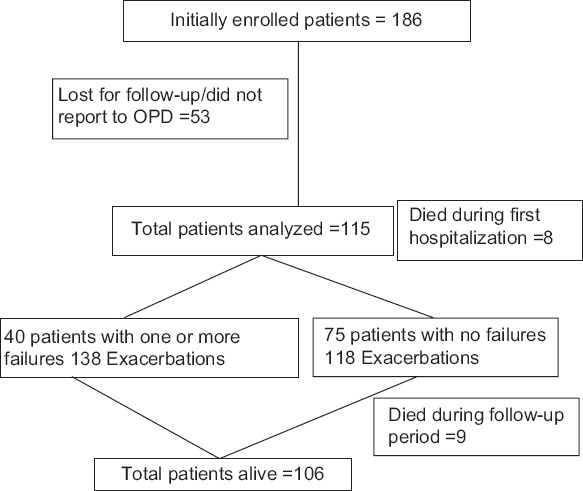
Distribution of all patients with exacerbations and failures
Table 1.
Baseline characteristics of patients hospitalized for chronic obstructive pulmonary disease exacerbations

Outcome variables
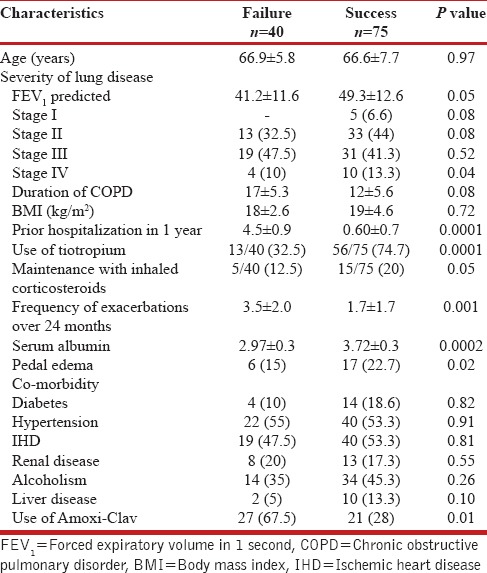
The clinical characteristics of COPD patients with failure and successful exacerbation attack are summarized in Table 2. Many of the subjects were current smokers or ex-smokers with a history of smoking equivalent to at least 20 pack-years. Majority of the patients reported good compliance with the medications. From the Table 2, it was observed that, use of inhaled tiotropium, use of maintenance inhaled steroids along with beta-2 agonists, prior hospitalization during the previous one year, pedal edema and low serum albumin levels were associated with higher failure rates following an exacerbation of COPD. A total of eight patients died following exacerbation. They were given a trial of invasive mechanical ventilation but it was unsuccessful. Comparison of the two groups revealed that age, smoking status, cardiac co-morbidities, and admission blood gas parameters were associated with mortality. A total of 40 patients had failure rates following first exacerbation and they had to be readmitted within one month for the repeat exacerbation. Sixty-five percent of patients who survived the first hospitalization required re-admissions later on for exacerbations. During follow-up period, the mean number of readmissions was 3.4 per subject (range 2-8). The median time to the next exacerbation requiring hospitalization was 165 days (range 1-278). Another nine patients died during the follow-up period during the repeat exacerbations. They were failure cases during the first admission.
Table 2.
Comparison of patient characteristics between failure and success groups
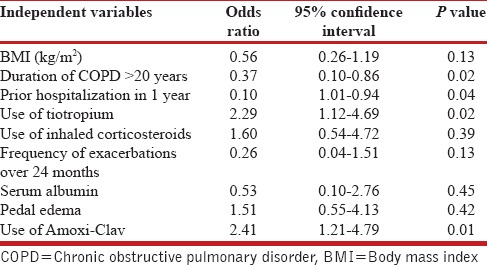
From Table 3, it can be observed that there were 40 failure cases with AECOPD. These patients had to be admitted within 1 month after discharge. Many of these cases were also admitted later on during the follow-up period of 2 years. On multivariate analysis, it was observed that prior use of tiotropium during the stable state of COPD was the independent factor for less number of failure cases (OR 2.29, CI 1.12-4.69, P < 0.02). Another important factor which had a confounding effect on the failure rates is the use of antibiotics during the first episode. It was observed that use of amoxicillin-clavulanic acid combination was significantly associated with higher chances of relapse as compared to the use of third generation cephalosporins or moxifloxacin (OR 2.41, CI 1.21-4.79, P < 0.01).
Table 3.
Final multivariate risk factors for immediate failure of acute exacerbation of COPD
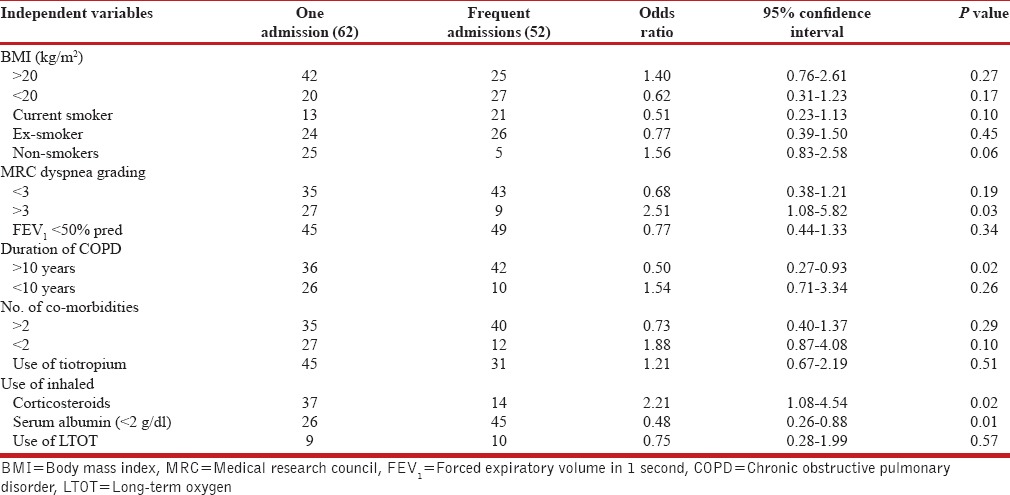
The clinical characteristics of COPD patients with frequent and non-frequent re-admissions are summarized in Table 4. Majority of the subjects were current smokers or ex-smokers with a history of smoking equivalent to at least 20 pack-years. Nearly 40% (47) subjects were underweight (BMI < 20 kg/m2), 74% (85) had at least one co-morbid disease, and 39% (44) had two or more co-morbid diseases. The results of multivariate analysis showed that smoking, co-morbidities or BMI were associated with increased hospital readmissions. On the other hand, patients with MRC dyspnea grade of less than 3 were associated with less chances of exacerbations than those with severe grade of dyspnea. Patients with long duration of COPD(>10 years) were approximately two times more likely to have frequent readmissions than those with short duration (OR = 0.50, CI: 0.27-0.93, P < 0.02). Severity of lung function did not correlated with the repeated exacerbation. Serum albumin levels (<2g/dl) and use of inhaled corticosteroids along with beta-2 agonist combinations were also observed to be independent risk factors for the multiple admissions in patients with acute exacerbation of COPD (OR=0.48, CI: 0.26-0.88, P < 0.01; OR=2.21, CI: 1.08-4.54, P < 0.02, respectively).
Table 4.
Final multivariate risk factors for multiple readmissions to the hospital for acute exacerbation of COPD
DISCUSSION
Exacerbations of COPD are known to be associated with increased morbidity and mortality and to have a significant socioeconomic impact. Patients with frequent exacerbations often experience impaired quality of life and a faster decline in lung function over time. Severe exacerbations that lead to hospitalization constitute a risk for mortality both during the hospitalization and during the subsequent years. In the present study we have tried to analyze various factors leading repeated exacerbations following AECOPD.
The present study described the outcomes of 115 patients who experienced at least one AECOPD resulting in admission in the hospital over a 24-month period during. All the patients were monitored for the next 24 months. Re-admission rates for COPD patients were high. During the study period, 54% of subjects were re-admitted once while others were re-admitted multiple times; and the mean duration from the index admission to the first readmission was only 4 ± 4 months. After one exacerbation the failure rates in the present study was observed to be 34.8%. Failure was considered if the patient was readmitted within 30 days after the discharge. A total of 45% had more than one exacerbation during the follow-up period. A previously described cohort of 345 COPD patients[7] admitted to hospital for COPD exacerbations and followed for re-admission in Spain found a similar rate. The authors reported a first re-admission rate of 63% over a mean follow-up period of 1.1 years and a median time to first readmission of 186 days. Bahodori et al.[8] have also reported high rate of admissions in AECOPD after the discharge for the hospital. The high frequency of re-admission and the short time to re-admission found in our study and that of other studies, highlight the social and economic burden that COPD exerts on society.
The cause of an exacerbation of COPD may be multi-factorial, so that viral infection or levels of air pollution may exacerbate the existing inflammation in the airways, which in turn may predispose to secondary bacterial infections.[9,10] COPD patients have frequent co-morbid conditions, particularly coexistent cardiac disease, hypertension, diabetes, etc. Coexistent cardiac disease has been shown to be a risk factor for increased hospital admission and mortality in patients with COPD exacerbation.[11] Furthermore, ischemic heart disease and/or congestive heart failure were reported to increase the rate of treatment failure, thus contributing to the worsening of the patients’ condition.[12] Patients with moderate to severe COPD patients are more likely to have cardiovascular disease. In the present study, almost 51% had associated cardiac disease. Elevated pulmonary artery pressure can lead to right heart failure and ultimately death. Kessler et al.[13] found that pulmonary artery pressure greater than 18 mmHg doubled the risk of subsequent COPD hospitalizations. In a different population, McGhan et al.[14] demonstrated that a diagnosis of pulmonary hypertension increased the risk of re-hospitalization. In addition to the elevated pulmonary artery pressure, a common finding associated with COPD is diastolic dysfunction. In a retrospective study,[15] it was found that patients with diastolic dysfunction had 1.28 exacerbations compared with 0.67 in the control group. Diabetes mellitus, which is found more frequently in COPD, may also be a risk factor for AECOPD.[16] Elevated blood sugars can increase the growth of pathogens in the airway as well as promote inflammation and predispose to the exacerbations.[17] In large unselected populations of COPD patients, it has been observed that half of those patients who are hospitalized are expected to be readmitted at least once in the ensuing 6 months1-3 with a majority (86%) of re-admissions occurring within the first 3 months after hospital discharge.[18] The high prevalence of known and putative risk factors in the study done by Zhenying et al.[19] also in support of a similar finding in a previous representative sample of COPD patients in the EFRAM study.[20] In particular, there was a high prevalence of current or ex-heavy smokers, malnutrition, depression, and consumption of psychotropic drugs, and low prevalence of caregiver support, pulmonary rehabilitation and vaccination.[21]
Despite evaluating a wide range of both modifiable and non-modifiable potential risk factors for hospital re-admission, only a few clinical factors were found to be independently associated with COPD re-admission in the present study. A number of previous studies.[16] have reported homeO2 as a risk factor associated with an increased risk of admission or frequent re-admissions. It is likely that home O2 use is a marker of the disease severity, and individuals with more advanced stage of disease are at higher risk for re-admission. In the present study very few patients were using long-term oxygen therapy, hence the direct conclusions becomes difficult to arrive in present scenario. Previous lung infections (within 1 year before index admission) and other chronic respiratory disease were also both associated with an increased risk of readmission in the logistic regression analyses.[8] Previous hospitalization is a strong risk factor for repeated exacerbation in COPD patients (P < 0.0001). Garcia-Aymerich et al.[22] found that having more than two hospital admissions in the previous year was an independent risk factor for subsequent admissions for AECOPD. This is further supported by Seemungal et al.,[23] who showed that patients with three or more exacerbations (frequent exacerbators) had an increased risk of hospitalization. The presence of other chronic respiratory disease may increase susceptibility to readmission simply by compounding the impairment in lung function, or may impact overall systemic fitness and, therefore, the global ability to cope with acute illness.[24] Use of initial antibiotics may also play an important role in development of AECOPD within few months after discharge. Both viral and bacterial agents can be involved, and appropriate therapy decreases the risk of subsequent re-hospitalizations. The failure rate in the present study was 35%. It was observed that the use of Amoxicillin-Clavulanic acid as initial therapy had higher chances of failure rates as compared to the use of cephalosporins or Moxifloxacin (P < 0.01). In addition, systemic inflammation, which may be related to chronic infection, increases the risk for moderate to severe COPD exacerbations.[25] In the study done by Dewan et al.,[26] treatment failure was noted in 12.1% of first exacerbations and 14.7% of all exacerbations, with more than half the failures requiring hospitalization. Host factors that were independently associated with treatment failure included FEV1 < 35%, use of home oxygen, frequency of exacerbation, history of previous pneumonia, history of sinusitis and use of maintenance steroids. With increased COPD severity, the likelihood of admission during exacerbations increases.
Patients readmitted to hospital were more likely to have shorter length of stays (LOS). In the present study the patients having disease for more than 20 years had more relapses as compared to shorter duration of the disease. However, previous studies[24] have also indicated that increased disease severity is associated with prolonged LOS. In the present study mean LOS was about 8 ± 4 days. It may be that, shorter LOS was an indication of premature discharge before the clinical and social aspects of the admission were fully resolved and, thus, patients were more likely to return to hospital. It is possible that very short hospital admissions do not allow for appropriate convalescence and discharge planning, while very long LOSs represent individuals with severe disease requiring long-term therapy or those with more precarious social circumstances that make discharge planning more difficult, while very long LOSs represent individuals with severe disease requiring long-term therapy or those with more precarious social circumstances that make discharge planning more difficult.[25]
Smoking is a leading cause of COPD in most developed countries. However, the relation between smoking and AECOPD is less clear. No association was found between current smoking and the risk of COPD admission or re-admission. This finding supports the result of a previous study by Kessler et al.[27] that found no differences between current smokers and the risk of COPD readmission. It has been suggested that severely ill patients with COPD spontaneously quit smoking probably in response to their symptoms and disability, and this may lead to less damage and inflammation to the bronchial mucosa subsequently.[28] Advanced COPD disease is associated with frequent exacerbation, as has been seen in the present study, that stage IV disease had frequent exacerbations as compared to mild to moderate form of disease (P < 0.04). Bahadosi et al.[8] did not observed any relation between FEV1% predicted and the rates of COPD readmissions. Several studies have similarly reported a lack of association between FEV1 %predicted and COPD exacerbations and admissions;[29] however, others[30] have found lower FEV1 or severity of FEV1 impairment to be predictive of a higher risk of COPD admission and readmission. It is possible that the lack of association in the present and other studies was the result of inadequate statistical power rather than a true lack of association. Alternatively, the lack of association between FEV1 and hospital readmission may be due to relatively limited variation in the FEV1 across the study population.[30]
Maintaining muscle mass and activity is another critical aspect in the management of COPD. The mean body mass index (BMI) in the present study is 18 ± 3.5. The relation between BMI and AECOPD is mixed, with some studies[31] showing no effect but other studies showing or suggesting an increased risk.[32] Conversely, pulmonary rehabilitation consistently has shown to improve the patient's quality of life and functional status. In a meta-analysis, Puhan et al.[33] demonstrated that pulmonary rehabilitation after acute exacerbation decreased the risk for hospital admission (OR = 0.13) and decreased mortality (OR = 0.19). Additional studies show that higher activity levels deceases subsequent AECOPD.[34]
On multivariate analysis, it was observed that use of tiotropium is an important protective factor in preventing relapse due to AECOPD (P < 0.02) (OR 2.29, CI 1.12-4.69). After final analysis, it was observed that combination of inhaled corticosteroids and long-acting beta agonist had lower relapse rates due to AECOPD (P < 0.02) (OR 2.21, CI 1.08-4.54). More recent trials have demonstrated the effect of tiotropium on the reduction in the frequency of exacerbations. In one study.[35] involving 1,010 patients, (mean FEV1 of 48%), the frequency of exacerbations was reduced with tiotropium from 2.41 per year in the placebo group to 1.57 in the tiotropium group (P < 0.001). It is of note that in the placebo group up to 61.6% of patients were already receiving ICS and 32.5% were being treated with LABA. The Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) trial,[36] which included 5,993 patients and were followed for 4 years and, compared with controls, tiotropium significantly delayed time-to-first exacerbation (16.7 versus 12.5 months) and time-to-first hospitalization for exacerbations (lower risk of hospitalization: HR 0.86, (95% CI 0.78-0.95); P < 0.002). Tiotropium also reduced the mean number of exacerbations by 14% (rate per patient-year 0.73 versus 0.85; HR 0.86, 95% CI 0.81-0.91; P < 0.001). The addition of a long-acting bronchodilator (salmeterol or formoterol) and an ICS (fluticasone or budesonide) in the same inhaler represents advancement and has been recently studied in COPD. In the TORCH study, a total of 6,112 patients were randomized to receive fluticasone/salmeterol combination (FSC), placebo or one of the two components separately over the course of 3 years.[37] The results demonstrated a reduction in mortality of 2.6% with FSC in absolute terms compared to placebo, representing a relative reduction of 17.5% which was almost statistically significant (P < 0.05). The TORCH study also confirmed the results of previous studies with respect to the reduction in exacerbations and the improvement in the quality of life with combination therapy. Regarding exacerbation, FSC reduced the frequency of moderate or severe exacerbations significantly by 25% as well as hospitalizations by 17%. In patients with stage II COPD, FSC reduced the frequency of exacerbations by 31% (95% CI 19-40) from 0.82 per patient per year to 0.57. In the present study, the patients using ICS + LABA therapy had fewer exacerbations rates, although the number was low.
The mortality in the present study was about 10%. SolerCataluna[38] showed that AECOPD increases mortality with evidence of dose-response. MacGhan.[14] showed that multiple risk factors for mortality contributed, including metastatic cancer, weight loss, pulmonary hypertension, heart failure, male gender, and previous COPD exacerbation hospitalizations. Fruchter and Yiegla[39] evaluated the predictors for long-term survival in patients with AECOPD, and they observed in-hospital mortality rate of 7.25%. The risk of mortality at 1, 3 and 5 years was 28%, 47% and 54%, respectively. In univariate analysis age, active cancer, current smoking, ischemic heart disease, congestive heart failure, and maintenance use of oral glucocorticosteroids were significantly associated with mortality. In multivariate analysis, only current smoking, ischemic heart disease, PaCO2 on admission, hospital re-admission and FEV1 were independent predictors of mortality. Another study by Wang and Bourbeau[16] showed that the majority (85.5%) of moderate to severe AECOPD patients were discharged home after a median of 10 days of treatment for acute exacerbation of COPD, and the hospital mortality rate was only 9.9%, although the mortality rates at 1 year (24.4%) and 2 years (44.9%) were high. Among the patients who died or required long-term hospital stays, most patients had received ICU treatment (84.2%) and mechanical ventilation (71%). These results suggest that if patients need intensive care or mechanical ventilation during a COPD exacerbation, they will usually have a protracted hospital stay and poor hospital outcomes. Among those discharged home, there was still a 20 and 40% possibility of death in the next 1 and 2 years, respectively.[16]
After the discharge from AECOPD, regular follow-up of these COPD patients was maintained for the next 2 years. It was observed that there was neither improvement in dyspnea level nor improvement in BMI during the follow-up period. COPD exacerbations have a negative effect on quality of life in patients with COPD. The quality of life is impaired not only in patients who are hospitalized for AECOPD, but also in patients who experience frequent exacerbations that are treated in the ambulatory care setting. Hence, while a survival benefit might be expected from a reduction in COPD exacerbations requiring hospitalization, an improvement in quality of life might be an anticipated outcome of a reduction in overall exacerbations, including those that do not require hospitalization. The analysis done in various studies have corroborates the hypothesis that exacerbations impact health-related quality-of-life and demonstrates that interventions that reduce exacerbations are associated with improvements in quality of life.[16] In the study done by Anzueto et al.[40] about 60% of the patients survived for a median of 2 years after hospitalization but their quality of life was quite poor. Many factors, such as air flow limitation, co-morbidity, frequency of COPD exacerbations, and activities of daily living can have impact on health-related quality of life in COPD patients.
Summary
The present study has observed high rate of immediate failure rates following AECOPD, and another 54% of patients had multiple readmissions during follow-up period. Low usage of inhaled steroids (with LABA) and tiotropium, duration of disease and more severe dyspnea grade were significantly associated with a higher likelihood of being readmitted to hospital. These characteristics may be markers of COPD severity or identify individuals with overall more precarious health and/or social circumstances. These results raise the possibility that these variables could be used as identifiers of patients at highest risk for frequent exacerbations and readmissions.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J RespirCrit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Houlguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest 2005. 2005;128:2005–11. doi: 10.1378/chest.128.4.2005. [DOI] [PubMed] [Google Scholar]
- 3.Mnanino DM, Buist AS. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet. 2007;370:765–73. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Anto JM. Estudi del Factors de Riscd’Agudització de la MPOC Investigators. Risk factors of readmission to hospital for a COPD exacerbation: A prospective study. Thorax. 2003;58:100–5. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouw EM, Ten Velde GP, Croonen BH, Kester AD, Schols AH, Wouters EF. Early non-elective re-admission for chronic obstructive pulmonary disease is associated with weight loss. ClinNutr. 2000;19:95–9. doi: 10.1054/clnu.1999.0074. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Roisin R, Anzueto A, Bourbeau J, deGuia TS, Hui DJ, Jenkins C, et al. GOLD. Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: (Updated 2010) 2010:1–117. [Google Scholar]
- 7.Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124:459–67. doi: 10.1378/chest.124.2.459. [DOI] [PubMed] [Google Scholar]
- 8.Bahadori K, FitzGerald JM, Levy RD, Fera T, Swiston J. Risk factors and outcomes associated with chronic obstructive pulmonary disease exacerbations requiring hospitalization. Can Respir J. 2009;16:e43–9. doi: 10.1155/2009/179263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celli BR. Is pulmonary rehabilitation an effective treatment for chronic obstructive pulmonary disease?. Yes. Am J RespirCrit Care Med. 1997;155:781–3. doi: 10.1164/ajrccm.155.3.9117007. [DOI] [PubMed] [Google Scholar]
- 10.Nichols KL, Baken L, Nelson A. Relation between influenza vaccination and outpatient visits, hospitalization, and mortality in elderly persons with chronic lung disease. Ann Intern Med. 1999;130:397–403. doi: 10.7326/0003-4819-130-5-199903020-00003. [DOI] [PubMed] [Google Scholar]
- 11.Vestbo J, Prescott E, Lange P, Schnohr P, Jensen G. Vital prognosis after hospitalization for COPD: A study of a random population sample. Respir Med. 1998;92:772–6. doi: 10.1016/s0954-6111(98)90011-7. [DOI] [PubMed] [Google Scholar]
- 12.Prevention and control of influenza: Recommendations of the advisory committee on immunization practices (ACIP). Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–26. [PubMed] [Google Scholar]
- 13.Kesseler R, Faller M, Weitzenblum E, Chaout A, Aykut A, Ducolone A, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J RespirCrit Care Med. 2001;164:219–24. doi: 10.1164/ajrccm.164.2.2006129. [DOI] [PubMed] [Google Scholar]
- 14.McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Lung. 2009;187:128–35. doi: 10.1378/chest.06-3018. [DOI] [PubMed] [Google Scholar]
- 15.Abusaid GH, Barbagelata A, Tuero E, Mahmood A, Sharma G. Diastolic dysfunction and COPD exacerbation. Postgrad Med. 2009;121:76–81. doi: 10.3810/pgm.2009.07.2033. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Bourbeau J. Outcomes and health-related quality of life following hospitalization for an acute exacerbation of COPD. Respirology. 2005;10:334–40. doi: 10.1111/j.1440-1843.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 17.Baker EH, Janaway CH, Philips BJ, Brennan AL, Baines DL, Wood DM, et al. Hyperglecemia is associated with poor outcomes in patients admitted to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2006;61:284–9. doi: 10.1136/thx.2005.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas SL, Daly BJ, Gordon N, Brennan PF. Survival and quality of life: Short-term versus long-term ventilator patients. Crit Care Med. 2002;30:2655–62. doi: 10.1097/00003246-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Cao Z, Ong KC, Eng P, Tan WC, Ng TP. Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11:188–95. doi: 10.1111/j.1440-1843.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Aymerich J, Barreiro E, Farrero E, Marrades RM, Morera J, Antó JM. Patients hospitalized for COPD have a high prevalence of modifiable risk factors for exacerbation (EFRAM study) EurRespir J. 2000;16:1037–42. doi: 10.1034/j.1399-3003.2000.16f03.x. [DOI] [PubMed] [Google Scholar]
- 21.Lacasse Y, Wong E, Guyatt GH, King D, Cook DJ, Goldstein RS. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet. 1996;348:1115–9. doi: 10.1016/S0140-6736(96)04201-8. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Aymerich J, Monsó E, Marrades RM, Escarrabill J, Félez MA, Sunyer J, et al. EFRAMInvestigators. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM study. Am J RespirCrit Care Med. 2001;164:1002–7. doi: 10.1164/ajrccm.164.6.2006012. [DOI] [PubMed] [Google Scholar]
- 23.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J RespirCrit Care Med. 1998;157:1418–22. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 24.Horn SD, Sharkey PD, Buckle JM, Backofen JE, Averill RF, Horn R. The relationship between severity of illness and hospital length of stay and mortality. Med Care. 1991;29:305–17. doi: 10.1097/00005650-199104000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Chenna PR, Mannino DM. Outcomes of severe COPD exacerbations requiring hospitalization. SeminRespirCrit Care Med. 2010;31:286–94. doi: 10.1055/s-0030-1254069. [DOI] [PubMed] [Google Scholar]
- 26.Dewan NA, Rafique S, Kanwar B, Satpathy H, Ryschon K, Tillotson GS, et al. Acute exacerbation of COPD: Factors associated with poor treatment outcome. Chest. 2000;117:662–71. doi: 10.1378/chest.117.3.662. [DOI] [PubMed] [Google Scholar]
- 27.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J RespirCrit Care Med. 1999;159:158–64. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- 28.Anthonisen NR. Smoking, lung function and mortality. Thorax. 2000;55:729–30. doi: 10.1136/thorax.55.9.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decramer M, Gosselink R, Troosters T, Verschueren M, Evers G. Muscle weakness is related to utilization of health care resources in COPD patients. EurRespir J. 1997;10:417–23. doi: 10.1183/09031936.97.10020417. [DOI] [PubMed] [Google Scholar]
- 30.Emerman CL, Effron D, Lukens TW. Spirometric criteria for hospital admission of patients with acute exacerbation of COPD. Chest. 1991;99:595–9. doi: 10.1378/chest.99.3.595. [DOI] [PubMed] [Google Scholar]
- 31.Tsai CL, Camargo CAJr. The role of body mass index in acute exacerbation of chronic obstructive pulmonary disease. Emerg Med J. 2009;26:701–5. doi: 10.1136/emj.2008.068478. [DOI] [PubMed] [Google Scholar]
- 32.Tsimogianni AM, Papiris SA, Stathopoulos GT, Manali ED, Roussos C, Kotanidou A. Predictors of outcome after acute exacerbation of chronic obstructive pulmonary disease. J Gen Intern Med. 2009;24:1043–8. doi: 10.1007/s11606-009-1061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puhan MA, Scharplatz M, Troosters T, Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality--a systematic review. Respir Res. 2005;6:54. doi: 10.1186/1465-9921-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foglio K, Bianchi L, Bruletti G, Battista L, Pagani M, Ambrosino N. Long-term effectiveness of pulmonary rehabilitation in patients with chronic airways obstruction. EurRespir J. 1999;13:125–32. doi: 10.1183/09031936.99.13112599. [DOI] [PubMed] [Google Scholar]
- 35.Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. EurRespir J. 2006;27:547–55. doi: 10.1183/09031936.06.00062705. [DOI] [PubMed] [Google Scholar]
- 36.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFTStudyInvestigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–54. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 37.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCHinvestigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 38.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–31. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fruchter O, Yigla M. Predictors of long-term survival in elderly patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Respirology. 2008;13:851–5. doi: 10.1111/j.1440-1843.2008.01367.x. [DOI] [PubMed] [Google Scholar]
- 40.Anzueto A, Leimer I, Kesten S. Impact of frequency of COPD exacerbations on pulmonary function, health status and clinical outcomes. Int J Chron Obstruct Pulmon Dis. 2009;4:245–51. doi: 10.2147/copd.s4862. [DOI] [PMC free article] [PubMed] [Google Scholar]


