Abstract
Presence of miliary shadows in chest imaging in the appropriate clinical setting is often taken as a marker of miliary tuberculosis. If sputum is negative for acid -fast bacillus, empirical anti-tubercular therapy is given without securing a histological or microbiological diagnosis. We report a young female with human immunodeficiency virus infection who had miliary infiltrates on chest radiography. She was started on empirical anti-tubercular therapy. But an alternate diagnosis was achieved later with invasive sampling and ATT was stopped. This case illustrates the need for physicians to remain alert to diseases which mimic tuberculosis in presentation.
KEY WORDS: Histoplasmosis, human immunodeficiency virus infection, miliary shadows, miliary tuberculosis
INTRODUCTION
India is a tuberculosis–endemic country. Presence of miliary shadows in chest imaging is often attributed to tuberculosis without further etiological investigation in our country. Patients are started on empirical anti-tubercular therapy (ATT). Alternate diagnoses are considered only if patient does not respond to ATT. While this approach will prove correct in majority of cases, failure occurs in a significant fraction. Our case illustrates a less commonly encountered infection causing miliary mottling on chest radiograph.
CASE REPORT
A 23-year-old housewife, native of Bengal, presented to our hospital in north-west India with fever with chills and evening rise of temperature of three months, cough and breathlessness of three weeks and one episode hematemesis two days back followed by melaena. She had been recently detected to be human immunodeficiency virus (HIV) positive and her husband was also seropositive. Patient was tachypnoeic and tachycardic but, maintaining oxygen saturation on room air. She had pallor and palpable liver 2 cm under right costal margin. Her initial investigations showed anemia (4.5 g/dL), thrombocytopenia (105 × 103/µL), low white cell count (4200/µL), raised AST (120 U/L), ALP (412 U/L) and LDH (944 U/L) and low serum albumin (2.5 g/dL). Her CD4+ T-cell count was 23 cells/µL. There were miliary shadows in both lung fields on her chest X-ray which was confirmed on computed tomography (CT) scan of chest [Figure 1a and b]. Contrast CT of abdomen showed hepatomegaly and multiple enlarged retroperitoneal lymph nodes some of which were necrotic [Figure 2a and b]. A working diagnosis of disseminated tuberculosis was made and patient was started on anti-tubercular therapy (ATT).
Figure 1.
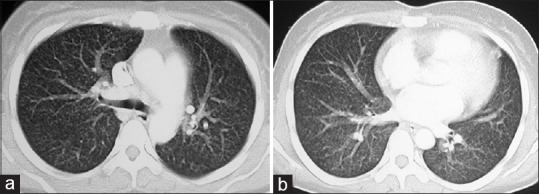
Contrast-enhanced CT axial images in lung window settings through upper (a) And lower lobes (b) Reveal randomly distributed miliary nodules in the both lungs
Figure 2.
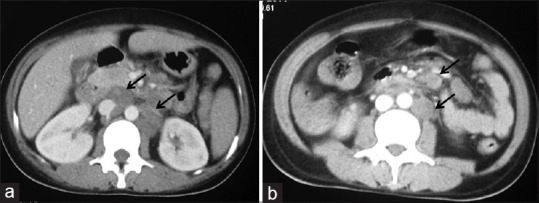
Contrast-enhanced CT axial images at the level of renal hilum (a) And infra-renal levels. (b) Reveal discrete necrotic enlarged lymph nodes in aorto-caval, para-aortic locations and in the mesentery
Cytopenias were suspected to be due to bone marrow involvement by TB. However, bone marrow trephine biopsy showed presence of histiocytes laden with histoplasma [Figures 3 and 4a and b] Patient was started on intravenous amphotericin deoxycholate at 1 mg/kg/day and ATT was stopped after 6 days. Patient became afebrile on fourth day of starting amphotericin and cytopenias started improving. During hospital stay, patient had one episode of hemetemesis and an episode of hematochezia. Tc99m-RBC scan was done which showed evidence of active gastrointestinal bleeding involving distal jejunum/proximal ileum. Patient was managed conservatively and PRBC transfusions were given. Amphotericin infusion was continued and patient improved. A bronchoalveolar lavage and guided fine needle aspiration from retroperitoneal lymph nodes were planned to exclude co-infection with tuberculosis. But, patient and relatives were not willing for further evaluation and she was discharged on oral itraconazole after starting highly active anti-retroviral therapy (HAART). On follow-up at 6 months patient had improved. Her chest X-ray was normal and her cytopenias had resolved.
Figure 3.
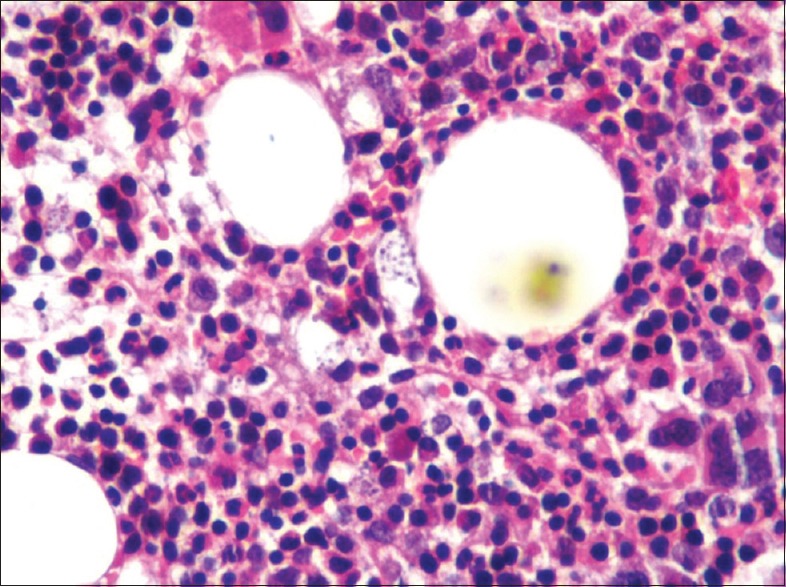
Bone marrow trephine biopsy at high power (×40) showing cellular bone marrow with increase in histiocytes
Figure 4.
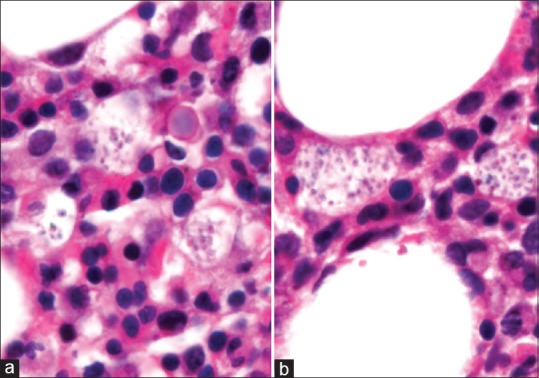
(a and b) Bone marrow trephine biopsy at oil-immersion (×100) showing histiocytes laden with yeast forms of Histoplasma capsulatum
DISCUSSION
Histoplasma capsulatum var. capsulatum, a dimorphic fungus, is an opportunistic pathogen commonly causing infections in the immunocompromised. The microconidia of the fungus present in soil get aerosolized and are infective. The primary site of infection is lungs from where it can disseminate to any other organ.
HIV-positive individuals, due to their poor T-helper cell response are at high risk of developing histoplasmosis. An annual incidence of 4.7% have been observed, the important risk factors being a CD4+ T-cell count below 150/µL, exposure to chicken coops and prior exposure to H. capsulatum as evidenced by sensitivity testing.[1] Diabetes is also an important risk factor for histoplasmosis, considering its high prevalence.[2]
Disseminated histoplasmosis involves multiple organs apart from the lung and may present in acute or chronic form. The acute form usually occurs in immunodeficient and manifests with fever, systemic upset, hepatosplenomegaly, lymphadenopathy and skin and mucus membrane lesions. The patient often has peripheral blood cytopenias and elevated liver enzymes, ferritin and LDH. Tissues if biopsied will show granulomas. On the other hand, the chronic form usually occurs among elderly immunocompetent individuals, runs a course of several months and is less often associated with granulomas in tissues. Pulmonary involvement in disseminated histoplasmosis commonly occurs in the form of miliary nodules. It may be associated with matted, caseating mediastinal lymphadenopathy (mediastinal granuloma), fibrosing mediastinitis and broncholith formation. A hemorrhagic, sterile pericardial effusion may occur as a result of host reaction to the fungus but, tamponade is rare.[3]
Gastrointestinal involvement may occur in upto 80% cases of disseminated histoplasmosis as proven in autopsy series. The fung us localizes to areas rich in submucosal lymphoid tissue. This commonly manifests as ulcers (most common in ileum followed by jejunum), nodules, hemorrhagic spots and hyperplastic lymphoid nodules.[4] There have been case reports of histoplasmosis presenting with upper as well as lower gastrointestinal bleeding.[5,6] Our patient most likely had jejunal/ileal ulcers caused by histoplasma. Hepatic involvement in histoplasmosis has been encountered in about 70% patients on autopsy. In the living, it manifests as hepatomegaly and deranged liver function as seen in our patient.[4]
Histoplasmosis is a diagnosis often overlooked in India. The Gangetic basin is endemic for the fungus and maximum prevalence of histoplasmosis has been noted in Bengal and the Northeast.[7] The clinical presentation resembles tuberculosis and patients may be started on empirical ATT without improvement. In any patient with prolonged fever, hepatosplenomegaly and lymphadenopathy, the possibility of histoplasmosis should be considered and more so if there is no response to empirical ATT.[7] In a study published from South India, the common manifestations were fever, weight loss and oropharyngeal ulcers[2] Constitutional symptoms with primary adrenal insufficiency similar to tubercular involvement of adrenals has been recognized as a presenting manifestation in immunocompetent individuals.[8] In HIV-infected individuals, presence of cough and C-reactive protein above 70 mg/dL are predictors of tuberculosis while, cytopenias (platelet count <150 × 103/µL and neutrophil count <2750/µL), CD4-count <60/µL and raised gamma glutamyl transferase predict histoplasmosis.[9] Coinfection with tuberculosis has been documented in 8-15% patients of HIV infection with histoplasmosis.[10]
The confirmatory evidence of histoplasmosis is obtained by demonstrating the organism in tissues such as bone marrow, lymph nodes and liver by staining with Gomori methanamine silver (GMS) or periodic acid Schiff (PAS). The fungus may be cultured in Sabouraud's agar. The urine and serum antigen testing have high sensitivity and specificity in disseminated histoplasmosis.[3] The recommended treatment of moderate to severe disseminated histoplasmosis is intravenous liposomal amphotericin B at 3 mg/kg/day for 1-2 weeks followed by oral itraconazole 200 mg thrice daily for three days and 200 mg twice daily thereafter. In HIV-positive individuals the drug can be stopped after one year if there is good compliance to HAART, HIV RNA levels are less than 400 copies/mL and CD4+ T-cell count is more than 150/µL.[11]
Miliary mottling on chest radiograph does not always mean tuberculosis. Tuberculosis being common, we have a tendency to label all apparently chronic infectious processes as tuberculosis at first instance. Physicians should keep their minds open to other diagnostic possibilities and appropriate tissue samples should be obtained whenever possible. With a significant proportion of the population being immunocompromised due to diabetes, acquired immune deficiency syndrome (AIDS), bone marrow and solid organ transplant, hematological malignancies and newer immunomodulatory drugs, the diagnosis of histoplasmosis should be entertained in any patient presenting with fever of long duration. Treatment of confirmed cases of histoplasmosis with antifungals is gratifying and is associated with excellent outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shirali A, Kini J, Vupputuri A, Kuruvila M, Prabhu MV. Disseminated histoplasmosis with conjunctival involvement in an immunocompromised patient. Indian J Sex Transm Dis. 2010;31:35–8. doi: 10.4103/2589-0557.68999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subramanian S, Abraham OC, Rupali P, Zachariah A, Mathews MS, Mathai D. Disseminated histoplasmosis. J Assoc Physicians India. 2005;53:185–9. [PubMed] [Google Scholar]
- 3.Kauffman CA. Histoplasmosis: A clinical and laboratory update. Clin Microbiol Rev. 2007;20:115–32. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamps LW, Molina CP, West AB, Haggitt RC, Scott MA. The pathologic spectrum of gastrointestinal and hepatic histoplasmosis. Am J Clin Pathol. 2000;113:64–72. doi: 10.1309/X0Y2-P3GY-TWE8-DM02. [DOI] [PubMed] [Google Scholar]
- 5.Spinner MA, Paulin HN, Wester CW. Duodenal histoplasmosis presenting with upper gastrointestinal bleeding in an AIDS patient. Case Rep Gastrointest Med 2012. 2012:515872. doi: 10.1155/2012/515872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane S, Brasitus T. Histoplasmosis capsulatum as a cause of lower gastrointestinal bleeding in common variable immunodeficiency. Dig Dis Sci. 2000;45:2133–5. doi: 10.1023/a:1026680317424. [DOI] [PubMed] [Google Scholar]
- 7.Gopalakrishnan R, Nambi PS, Ramasubramanian V, Abdul Ghafur K, Parameswaran A. Histoplasmosis in India: Truly uncommon or uncommonly recognised? J Assoc Physicians India. 2012;60:25–8. [PubMed] [Google Scholar]
- 8.Bhansali A, Das S, Dutta P, Walia R, Nahar U, Singh SK, et al. Adrenal histoplasmosis: Unusual presentations. J Assoc Physicians India. 2012;60:54–8. [PubMed] [Google Scholar]
- 9.Adenis A, Nacher M, Hanf M, Basurko C, Dufour J, Huber F, et al. Tuberculosis and histoplasmosis among human immunodeficiency virus-infected patients: A comparative study. Am J Trop Med Hyg. 2014;90:216–23. doi: 10.4269/ajtmh.13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agudelo CA, Restrepo CA, Molina DA, Tobón AM, Kauffman CA, Murillo C, et al. Tuberculosis and histoplasmosis co-infection in AIDS patients. Am J Trop Med Hyg. 2012;87:1094–8. doi: 10.4269/ajtmh.2012.12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, et al. Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–25. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]


