Sir,
Sarcomatoid carcinoma (SC) of the lung comprises conventional non-small cell lung carcinoma (NSCLC) along with a sarcoma-like (pleomorphic/spindle and/or giant cell) component.[1] It is extremely rare, and has worse outcome than conventional NSCLCs.[1] Although not yet standard of care, use of targeted therapy in the form of tyrosine kinase inhibitors (TKIs) is gaining popularity in NSCLCs harboring specific genetic alterations, epidermal growth factor receptor (EGFR) mutations in particular. Various clinicopathological and molecular characteristics are reported to be associated with response to TKIs, including histopathological type of NSCLC. Morphological correlates of EGFR mutation status in various adenocarcinoma subtypes are well characterized.[2] Role of EGFR mutations in SCs is, however, not well documented.
This 68-year-old male, a chronic smoker, presented with history of intermittent fever since 1 month. There was no history of cough, chest pain, or hemoptysis. On examination, no peripheral lymphadenopathy was present. Computed tomography (CT) revealed a large, heterogenous, peripherally located mass in the posterior segment of upper lobe of right lung involving the parietal pleura, with a broad area of contact with the chest wall. Multiple enlarged right paratracheal and pretracheal lymph nodes were identified. Fine-needle aspiration cytology from the mass showed abundant necrosis along with a malignant tumor composed of atypical polygonal and spindle-shaped cells [Figure 1]. Bronchoscopy did not reveal any endobronchial growth. Trucut biopsy was performed which showed predominantly necrosis with few fragments from a NSCLC, immunopositive for TTF-1 and negative for p40, suggestive of adenocarcinoma. The patient underwent radical right upper lobectomy with mediastinal lymph node dissection. Intraoperatively, a friable mass was seen in the right upper lobe, abutting the horizontal fissure and invading the parietal pleura. Gross examination of the surgical specimen showed a 7-cm-sized solid greyish tumor with necrotic areas. Sections examined showed a biphasic tumor [Figure 2] composed of carcinomatous areas, along with areas composed of malignant spindle cells. The carcinomatous component was composed of sheets of polygonal cells with eosinophilic cytoplasm, large vesicular pleomorphic-appearing nuclei and prominent nucleoli. There was no evidence of gland formation or keratinization, making morphological distinction between squamous and adenocarcinoma difficult. The malignant spindle cell component consisted of epithelioid to slender tapered spindle cells arranged in sheets and fascicles. Nuclei were vesicular with prominent nucleoli, similar to the epithelial component. At places, the two components showed intimate admixture. Foci of necrosis, frequent mitotic figures and inflammatory cell infiltrate were evident throughout the tumor. While the epithelial component showed TTF-1 immunopositivity, the sarcomatous areas were positive for cytokeratin and vimentin. Immunohistochemistry was performed using antibodies specific for the E746-A750 del (6B6, dilution: 1:100; Cell Signaling Technology, Inc, MA, USA) and L858R EGFR mutations (43B2, dilution: 1:100; Cell Signaling Technology, Inc), and was negative for both. FISH using locus-specific probe for EGFR paired with a centromeric probe for chromosome 7, CEP7, (Vysis; Abbott Molecular, Des Plaines, IL, USA) did not show amplification [Figure 3]. A final diagnosis of sarcomatoid carcinoma immunopositive for TTF-1 and lacking EGFR mutation was rendered. The patient received four cycles of chemotherapy (paclitaxel and carboplatin). CT done 5 months postoperatively showed a large mass in the previously operated area of upper lobe, with erosion of the adjacent 3rd and 4th ribs, extending up to the tracheoesophageal groove and involving surrounding soft tissue. Due to the extensive nature of the tumor, the patient was deemed unsuitable for surgery. Second-line chemotherapy and radiotherapy were planned. However, the patient did not return to hospital and expired at home 3 months later.
Figure 1.
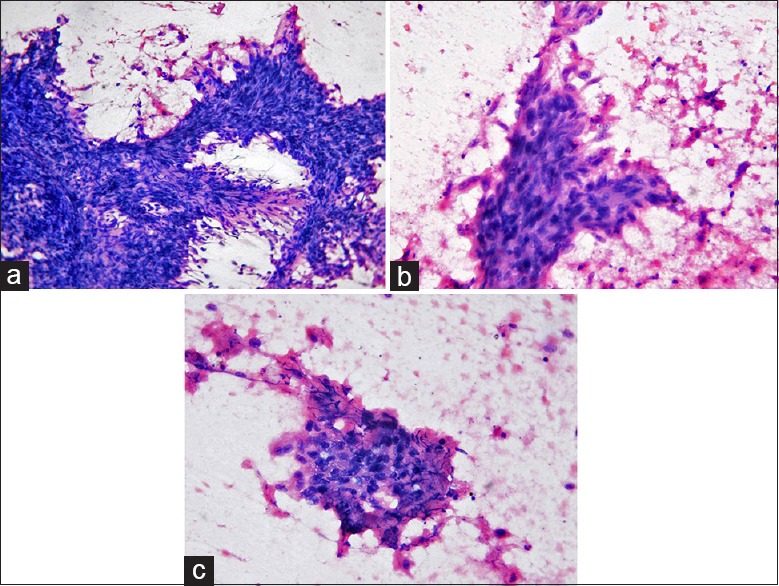
Aspiration cytology of the tumor: Photomicrographs show a malignant tumor composed predominantly of fragments of spindle-shaped cells (a) (HE, ×200) showing nuclear pleomorphism (b) (HE, ×400); few clusters of atypical epithelial cells are seen along with necrosis (c) (HE, ×400)
Figure 2.
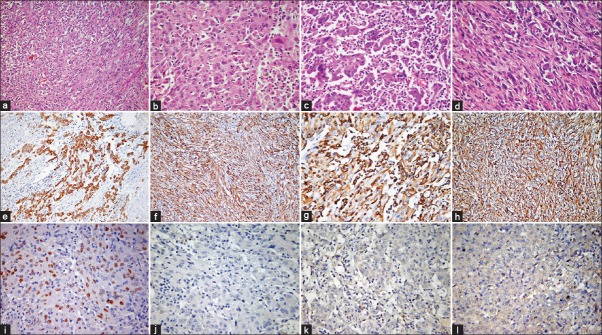
Histopathological features: Photomicrographs showing a biphasic tumor with epithelial and spindle cells (a) (HE, ×200); epithelial component shows nuclear pleomorphism, frequent mitoses (b) (HE, ×400) and inflammatory infiltrate (c) (HE, ×400); sarcomatoid component shows fascicles of malignant spindle cells (d) (HE, ×400); immunohistochemistry for cytokeratin is positive in epithelial (e) and spindle cells (f) vimentin is positive in epithelial (g) and spindle cells (h) (IHC, ×200). TTF1 shows nuclear positivity (i) while p40 (j) EGFR E746-A750del (k) and L858R EGFR (l) are negative (IHC, ×400)
Figure 3.
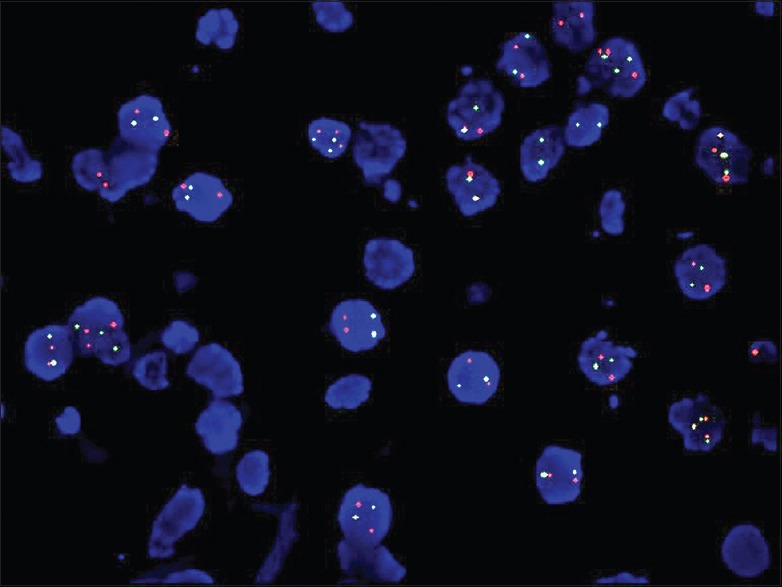
Fluorescence in situ hybridization with EGFR locus specific (red) and CEP7 (green) probes did not show amplification (×1000)
EGFR is a proto-oncogene; abnormal activation of its tyrosine kinase activity by mutation, amplification or protein overexpression leads to increased cell survival, proliferation and invasion.[3] It is well known that the therapeutic effect of TKIs correlates well with mutations of the EGFR gene. Point mutation of Exon 21 (L858R) and deletion mutation of exon 19, including amino-acid residues 747 to 750 (Del 746-750), are the two most frequent, accounting for approximately 90% of all EGFR mutations.[3] SC is a rare NSCLC, optimal therapy for which has not been standardized.[4] The conventional treatment protocol for SC is surgical resection followed by platinum-based double chemotherapy. However, recurrence is common, and survival of these patients remains poor as compared to other NSCLCs. Therefore, there is an urgent need to identify molecular alterations in these tumors that may serve as potential targets for personalized therapy, and the availability of TKIs necessitates the investigation of this group of NSCLCs for presence of EGFR mutations. Italiano et al. were the first to analyze EGFR status of 22 lung SCs.[4] They did not identify EGFR mutations in exons 18, 19, 20 and 21 or amplification in any of their cases, and concluded that the rare occurrence of EGFR mutation in SCs suggests that these patients are not likely to benefit from anti-EGFR therapies. Subsequently, Chang et al. assessed 42 SCs,[5] while Jiang et al. examined 33 SCs for EGFR mutations.[6] They found mutations in 23% and 21% of SCs, respectively, indicating that SCs are genetically heterogenous, and a subset may harbour EGFR mutations, rendering them susceptible to TKI therapy. Recently, EGFR mutation-specific antibodies have been described, providing an alternative method for detection of these alterations. These antibodies specific for exon 21 L858R and exon 19 del 746-750 mutations have been reported to have sensitivity ranging from 24% to 100% and specificity ranging from 77% to 100%. Immunohistochemistry is decidedly useful when scant material is available to extract DNA for molecular testing, and it may serve as a valuable screening tool to evaluate SCs for EGFR mutations.
Thus, although EGFR mutation has been described in lung SC, it is not consistently present. Hence, EGFR testing should be performed in all cases, particularly in those with adenocarcinomatous differentiation, to identify candidates that may benefit from TKI therapy. Immunohistochemistry is a reliable screening method for assessing EGFR mutation status, as it is inexpensive, does not require fresh tissue, can be performed on small tissue samples, and is easy for pathologists to perform as well as interpret.
REFERENCES
- 1.Corrin B, Chang YL, Rossi G, Koss MN, Geisinger K, Wick MR, et al. Sarcomatoid carcinoma. In: Travis WD, Brambilla E, Muller-Hermelink H, Harris CC, editors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC; 2004. pp. 53–8. [Google Scholar]
- 2.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: Role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S24–31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Italiano A, Cortot AB, Ilie M, Martel-Planche G, Fabas T, Pop D, et al. EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: Implications for anti-EGFR treatment of a rare lung malignancy. Int J Cancer. 2009;125:2479–82. doi: 10.1002/ijc.24610. [DOI] [PubMed] [Google Scholar]
- 5.Chang YL, Wu CT, Shih JY, Lee YC. EGFR and p53 status of pulmonary pleomorphic carcinoma: Implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann Surg Oncol. 2011;18:2952–60. doi: 10.1245/s10434-011-1621-7. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X, Liu Y, Chen C, Zhan Z, Yan Q, Guo Y, et al. The value of biomarkers in patients with sarcomatoid carcinoma of the lung: Molecular analysis of 33 cases. Clin Lung Cancer. 2012;13:288–96. doi: 10.1016/j.cllc.2011.11.004. [DOI] [PubMed] [Google Scholar]


