Summary
Objectives
This survey aims at highlighting the latest trends (2012-2014) on the development, use, and evaluation of Information and Communication Technologies (ICT) based decision support systems (DSSs) in medicine, with a particular focus on patient-centered and personalized care.
Methods
We considered papers published on scientific journals, by querying PubMed and Web of Science™. Included studies focused on the implementation or evaluation of ICT-based tools used in clinical practice. A separate search was performed on computerized physician order entry systems (CPOEs), since they are increasingly embedding patient-tailored decision support.
Results
We found 73 papers on DSSs (53 on specific ICT tools) and 72 papers on CPOEs. Although decision support through the delivery of recommendations is frequent (28/53 papers), our review highlighted also DSSs only based on efficient information presentation (25/53). Patient participation in making decisions is still limited (9/53), and mostly focused on risk communication. The most represented medical area is cancer (12%). Policy makers are beginning to be included among stakeholders (6/73), but integration with hospital information systems is still low. Concerning knowledge representation/management issues, we identified a trend towards building inference engines on top of standard data models. Most of the tools (57%) underwent a formal assessment study, even if half of them aimed at evaluating usability and not effectiveness.
Conclusions
Overall, we have noticed interesting evolutions of medical DSSs to improve communication with the patient, consider the economic and organizational impact, and use standard models for knowledge representation. However, systems focusing on patient-centered care still do not seem to be available at large.
Keywords: Decision support systems, clinical, decision aids, decision making, shared, patient-centered care, patient empowerment
1 Introduction
Decision support systems (DSSs) in medicine refer to a wide range of applications, based on methodologies at the intersection between Artificial Intelligence (AI) and Information and Communication Technologies (ICT) [1]. The first DSSs to be used for medical practice, appeared in the 1970s as applications of AI paradigms to the medical field, and operated as stand-alone systems targeting almost exclusively medical doctors. In the following forty years, the significant progress in ICT allowed revolutionizing the abovementioned scenario, making those applications available first on the web, and then through widely used devices (e.g. smartphones), able to impact an ever increasing number of users.
While technology is the primary perceived aspect that reflects the changes occurred in the DSS area, there are additional issues, equally important, that must be considered to understand the latest years trends. First, advances in -omics research are now calling for an individualization of diagnosis and treatment. Second, being able to access a lot of information on the internet, patients have become aware of treatment choices and quality of healthcare institutions. Thanks to this awareness, those subjects who wish to, may demand to be involved in their own care process. Third, mass media allowed disclosing the problem of medical errors, making citizens more demanding for quality in healthcare, and more vigilant about malpractice. As a consequence, increased attention has been given to patient’s rights, and there has been a rapid cultural change that led physicians to closely deal with legal and ethical issues. On the one hand, this caused the onset of the so-called defensive medicine [2], but on the other, it fostered physicians’ compliance with evidence-based practice guidelines. In this framework, the well-known concept of “informed consent” is not only gaining more relevance, but it is also acquiring a new meaning, i.e. to provide patients with a deep understanding of the motivations underlying a medical decision. Interestingly, while the typical informed consent procedure does not necessarily imply a close interaction with the patient, new ways of communication are emerging, and terms such as Shared Decision Making (SDM) and Patient Decision Aid (DA) are becoming popular in the medical (and medical informatics) literature (Fig. 1).
Fig. 1.
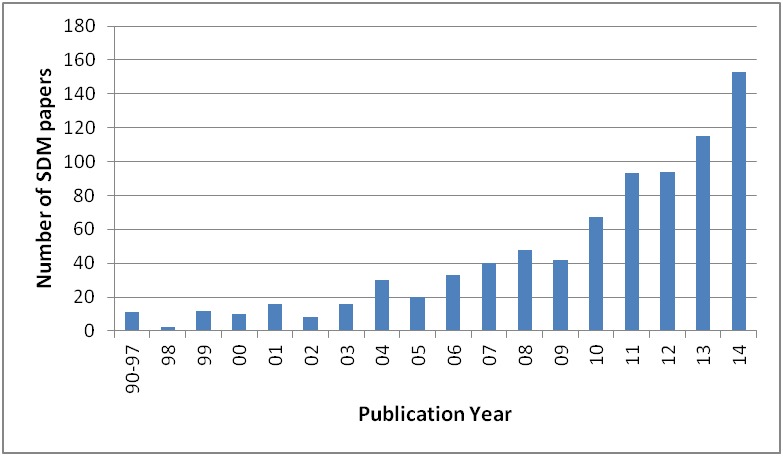
Papers including “shared decision making” (SDM) in their title, published in the last 15 years (source: PubMed).
SDM refers to the practice of making medical decisions while involving the patient (and/or his relatives) in the evaluation of pros and cons of alternative decision options, especially when there is limited scientific evidence about the superiority of an option with respect to the other ones [3]. DAs are artifacts that support SDM by providing information about the alternative decision options, helping patients assess the value they give to benefits versus risks, and ultimately improving communication with caregivers. A recent Cochrane review, based on 115 randomized clinical trials, compared care delivered with and without DAs [4]. While concluding that further research is needed to assess DAs impact on primary endpoints such as clinical outcomes and costs, the review showed that they improve users’ knowledge and awareness of personal values for outcomes, create realistic expectations, raise active participation in decision making with a positive effect on patient-practitioner communication, and reduce decisional conflicts (e.g. feeling uncertain, uninformed, unsupported).
All the mentioned aspects have considerably broadened the focus and extent of DSSs, which can thus no longer be assessed by simply relying on their ability to suggest an appropriate diagnosis or treatment. Their main objectives have been widened to include further dimensions, such as the integration of information coming from multiple sources, and their ability to provide a better understanding of the case at hand and a means for improving communication with patients.
This paper aims at identifying the latest trends in the development, use, and evaluation of ICT-based DSSs in medicine, focusing in particular on the personalization of decision support (decisions tailored to the specific patient’s data) and on patient-centered care (patient’s involvement in decisions about his/her own health). To perform such classification, we have reviewed the literature published between 2012 and 2014.
2 Methods
The literature review presented in this paper relies on two repositories: PubMed and Web of Science™. Although our main search was focused on DSSs, given the progressive inclusion of these tools into CPOEs, an additional search addressing CPOEs has been run. The search has been carried out according to the criteria presented in Table 1.
Table 1.
Criteria used for searching DSS- and CPOE-related papers published between 2012 and 2014.
| General search criteria | Exclusion criteria | Repository-specific conditions | |
|---|---|---|---|
| Search on DSSs | Search on DSSs | ||
|
Publication date: From Jan 1, 2012 to Dec 1, 2014 Publication type: Journal article Language: English |
|
PubMed (MeSH terms):
|
Web of Science™ (title):
research areas: Medical Informatics, Computer Science |
| Search on CPOEs | Search on CPOEs | ||
|
PubMed (title):
|
Web of Science (title):
|
|
Concerning general search criteria for the two repositories, the publication date was constrained to the interval 01/01/2012-01/12/2014, and only papers written in English were considered. To control the quality of the published works, we limited our search to journal papers. Finally, since the starting point of our analysis was to assess the abstract of returned papers, we decided to exclude the papers without abstract.
Regarding DSSs, since our survey was focused on systems facilitating patient-centered care, we refined the search with some repository-specific criteria. In particular, PubMed was queried using the following constraints on MeSH terms: Decision Support System, Clinical plus at least one term among Patient-centered care, Continuity of care, Shared Decision Making, Individualized Medicine or Health Information Exchange. Web of Science™, which does not provide a filter on MeSH terms, was instead searched for the presence of one of the same terms in the paper title. While PubMed is already focused on healthcare-related applications, Web of Science™ includes works regarding several scientific fields. However, it allows constraining the search within broad areas by applying filters. To refine our search and limit it to the fields of interest, we used the areas Medical Informatics and Computer Science.
Regarding CPOEs, considering the maturity of the technology and its diffusion, we decided to limit our search to the acronym and the extended terms in paper titles. This consideration is supported by Fig. 2, which shows that, after the initial outbreak that occurred around the mid 2000s, the publication rate of papers reporting on CPOEs has reached stability.
Fig. 2.
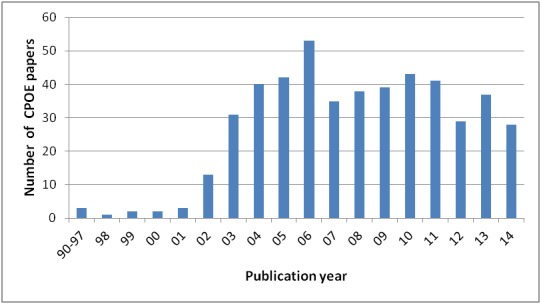
Publication trend of CPOE-related papers in the last 15 years (search by title, source PubMed)
The papers extracted according to the criteria presented in Table 1 were first evaluated on the basis of their abstract. Papers passing the abstract assessment were then reviewed in full text, to identify those that would be included in the analysis.
As regards the search on DSSs, we chose to focus on ICT applications directly delivering recommendations or explicitly targeting information delivery prior to decision making. On this basis, we excluded all tools and methods that were not meant to be used for supporting the decisional process in the medical routine. For example, even if machine learning and other statistical methods may produce predictive models that are potentially useful for decision making, we excluded those papers whose models were not embedded within a tool able to deliver targeted information, suggestions or recommendations for actual patient management. We also excluded papers reporting on alert or reminder systems for patients, such as homecare systems that prompt patients to take their drugs or to take some measurements. The rationale is that, in these cases, the medical decision has already been taken (which drug to prescribe, which parameter to monitor), and the goal of the system is only to enforce and monitor patient compliance with that decision. Therefore, in the perspective of supporting physicians in treatment decision (even in a SDM framework), reminder systems for patients cannot be considered as decision support tools. On the other hand, systems delivering alerts and reminders to physicians (or to both physicians and patients) during the decision process have been considered. We also excluded any tool that was not computer-based, such as DAs delivered through paper-based brochures. To further point out the increasing role covered by DSSs in healthcare settings, other papers were retained in addition to those describing specific tools, including papers that discuss acceptance, compliance, and general methodologies for DSS implementation.
The Patient Safety Network of the US Agency for Health Research and Quality defines CPOEs as “… any system in which clinicians directly enter medication orders (and, increasingly, tests and procedures) into a computer system, which then transmits the order directly to the pharmacy”, in such a way that “… at a minimum, it ensures standardized, legible, and complete orders and thus has the potential to greatly reduce errors at the ordering and transcribing stages” (http://psnet.ahrq.gov/). According to this definition, these systems should not be considered as “real” DSSs. However, CPOEs are increasingly being coupled with some type of DSSs, to support the ordering process by considering some important safety issues, such as allergies, previous adverse effects, or comorbidities. CPOEs are thus increasingly being linked to Electronic Health Records (EHRs), to consider not only a specific drug information, but also any interaction of that drug with the overall clinical condition of a single patient, acting as a bridge for integrating information from multiple sources. As regards our search on CPOEs, we only retained papers describing specific CPOEs, and we discarded review papers.
3 Results
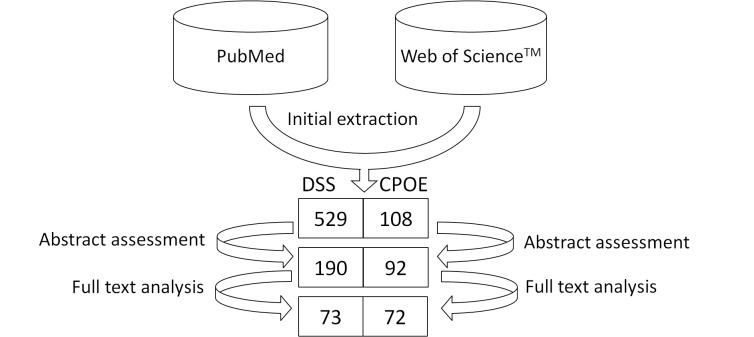
A total of 529 papers were extracted relying on our search criteria concerning DSSs, among which 339 were dropped after reading their abstracts since they did not concern ICT applications. The remaining 190 papers were further analyzed and 73 were kept for final classification according to the aims of the review. One hundred and eight papers were found when searching for “CPOE”, “Computerized Physician Order Entry” or “Computerized Provider Order Entry” in the paper title. After a manual selection of abstracts, 92 papers were retained for the analysis, and 72 papers were finally included in the review after full-text assessment. These results are summarized in Figure 3.
Fig. 3.
Review steps and results for DSSs and CPOEs.
Reading the papers helped us tune the semantics of classification and identify a set of useful attributes to analyze them. In particular, we have identified the following features:
Type of decision support, to describe whether decision support is provided through the delivery of recommendations, or through the filtering and display of information to facilitate the decision making process;
Patient-centered care, to indicate to which extent a patient is actively involved in the use of a specific tool;
Medical area and goal, to describe the addressed pathologies and goals of the intervention;
Design and development, to identify the methods used for decision support and knowledge representation (e.g., ontologies, rules, probabilistic models);
Target users of the system (e.g., patients, physicians, healthcare managers);
Integration with hospital information system (HIS), to identify to what extent a system is integrated within the HIS;
System assessment, to indicate whether and how the tool has been validated and/or evaluated.
One of the features initially considered as a potential classification dimension, i.e. the use of genomic information, was eventually dropped because it was addressed by few papers. This could indicate that this type of evidence, while highly referenced in the medical and bioinformatics literature, has not yet been widely translated into DSSs.
For the sake of clarity, we report two result sections, one for DSSs/DAs, and one for CPOEs.
3.1 Decision Support Systems/Decision Aids
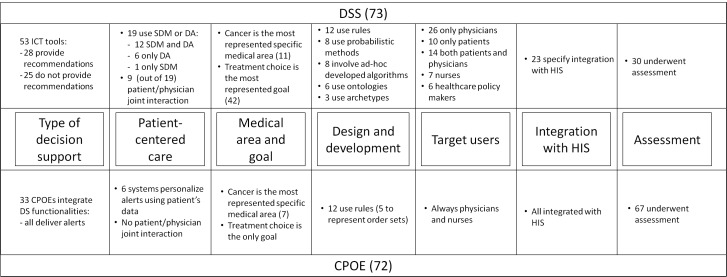
As shown in Figure 3, 73 papers were finally chosen for inclusion in the review. Fifty-three of them describe specific ICT tools, while the remaining 20 discuss facilitators and barriers to DSS acceptance, methods for measuring compliance with DSSs, and general methodologies for DSSs implementation.
The following sections report results according to the 7 dimensions listed above.
3.1.1 Type of Decision Support
Out of the 53 papers describing ICT tools, 28 describe the implementation and/or the evaluation of original DSSs that deliver some type of recommendations [5-32]. The remaining 25 tools do not deliver recommendations, but present the information in a way that facilitates the decision making process [33-57]. Eighteen tools show both functionalities [5-22]. Thirteen papers explicitly deliver decision support using SDM tools [8-11, 33, 35-41, 56].
As a prototypical example of the first category (systems that deliver recommendations), the work by Yu et al. reports on a sharable, cloud-based, collaborative database for a major pancreatic surgery with a high complication rate [12]. Suggestions and recommendations to physicians are produced both by diagnostic rules, implementing the pancreatic cancer staging system, and by therapeutic rules, delivered according to evidence-based guidelines. Moreover, data collected on different patients are used to train a machine learning model.
As a prototypical example of the second category, the paper by Kuru et al. describes a system for the production of computerized medical reports, necessary for the communication among healthcare professionals that participate to the patient’s management [55]. One medical report is proposed in natural language, while another one provides a structured, hierarchical format, using standard terms, in which abnormalities are easily highlighted. Moreover, the report can be produced in multiple languages. Another interesting example is given by Carney, who describes a web-based tool, MyCancerGenome, that provides up-to-date information on both gene mutations affecting different cancers, and mutation-specific treatment options [57]. The web site is freely available to any clinician, patient, or researcher on the internet (http://www.mycancergenome.org/).
3.1.2 Patient-centered Care
Systems that implement SDM and DAs consider the patient as a subject actively participating in the decision making process. However, this does not necessarily mean that patients directly interact with the DSS. Most of these systems are meant to be used by physicians during the SDM process, also accounting for patient choices. As a matter of fact, out of 19 papers dealing with SDM and/or DAs [5-11, 33-41, 52, 56, 57], only 9 describe tools where both doctors and patients jointly interact with the system during the process of preferences elicitation [5, 9-11, 34-38].
In 12 papers, DAs are used as a support for SDM [8-11, 35-41, 56]. For example, the system by Chi et al. suggests how lifestyle should be changed to reduce cardiovascular risk, according to a predictive model based on very large observational studies [9]. Different levels of risk reduction are presented to the patient according to a number of possible behavioral changes. Ozanne et al. describe a web-based tool that women can access together with their doctors to discuss how to reduce breast cancer risk [41]. Different options, all evidence-based, are suggested, spanning from behavioral changes to drug treatment and surgical interventions. Users are provided with an interface where personal and family history may be entered to refine risk calculation. Another example of a web-based DA specifically addressing patient self-assessment is given by Wu et al. [56]. The tool is intended to be used by patients at home to better understand the pros and cons of two different types of surgical interventions for colorectal cancer. This is a paradigmatic example of a situation where SDM is necessary, since no evidence exists to prove the superiority of an intervention over the other, neither in terms of survival nor of quality of life.
Personalization is tackled at several levels in the papers retrieved by our search. For example, some systems allow the physician to retrieve patient-tailored material to be handed to the patient [6, 39, 56]. In other works, a number of critical patient parameters, including for example delays in follow-up visits, are monitored over time [14, 16, 33], and/or questionnaires are delivered for evaluating the patient quality of life to assess the treatment outcome and, possibly, re-consider the treatment decision previously taken [33]. Finally, Welch and Kawamoto report on genetically guided personalized medicine [58].
3.1.3 Medical Area and Goal
Twelve papers report on general purpose systems. Among specific medical areas, cancer is the most common topic [11, 12, 18, 33, 41, 56, 57, 59-62], followed by diabetes [10, 32, 36, 51, 63] and cardiovascular diseases [5, 8, 9, 30]. Radiology [44, 55, 60], HIV [16, 31, 52], chronic diseases [14, 64, 65], and mental health [29, 38, 47] are tackled in three papers each. Other medical areas are represented with less than 3 papers. Regarding the clinical setting, the majority of systems are supposed to be used in hospitals, while only 4 papers address systems implemented in primary care [66-69].
Among the papers that clearly define the medical goal of decision support, 66% address treatment selection, 19% are related to diagnostic support, 6% address both treatment and diagnosis, and 9% are related to prevention.
3.1.4 Design and Development
This dimension refers to the knowledge representation formalism, knowledge extraction algorithm, or inference engine type on which the DSS is based. Concerning the knowledge representation, 12 papers describe systems based on production rules [5, 6, 12, 16, 17, 21, 23, 26, 28, 30, 46, 55]; 8 describe systems based on probabilistic methods, such as Bayesian networks, decision trees, and Markov models [8, 10, 15, 19, 24, 35, 49, 70]; 8 involve ad-hoc developed algorithms [6, 7, 11, 20, 27, 31, 53, 54]; ontologies are used in 6 systems [14, 18, 22, 28, 30, 71]; openEHR archetypes are used in 3 papers [18, 71, 72]. Other approaches, such as case-based reasoning, machine learning and mathematical models of biological systems are mentioned in less than 3 papers each.
3.1.5 Target Users
Target users of DSSs are mostly physicians but may also involve other healthcare professionals, patients, and home caregivers. These users, although in different ways, exploit decision support to select and adopt optimal diagnostic or treatment options. Healthcare policy makers and administrative people have also emerged as novel DSSs users, as they may benefit from DSSs to decide the best population-related organizational strategies or investments. Considering the reviewed papers, 26 are targeted at physicians only [8, 15, 17, 19, 21, 23-26, 28, 30-32, 42, 44, 45, 47, 48, 51, 55, 59, 67-69, 73, 74], 10 at patients only [7, 9, 11, 34, 38, 39, 43, 60, 63, 64], and 14 at both physicians and patients [5, 6, 10, 33, 35-37, 40, 41, 50, 57, 61, 66, 70]. Seven are targeted at nurses or other healthcare professionals [14, 16, 20, 27, 29, 46, 75], and six at healthcare policy makers, directors of healthcare institutions or administrative personnel [13, 22, 49, 53, 54, 72]. Since the latter are not typical DSS users, it is interesting to briefly introduce the goals of systems targeting these users. Chiarini Tremblay et al., borrowing from the finance literature, propose an Information Volatility Measure to complement business intelligence tools when considering aggregated data, or when observing trends in data [53]. The authors argue that decisions are often taken by considering only point estimates, and the measure they propose is intended to provide more insight into data variability and outliers. Peirson et al.’s report on the registry of knowledge translation methods and tools developed under the Government of Canada’s commitment, aimed at facilitating and improving evidence-informed decision making throughout public health systems [54]. Ip et al. show that a provider-led radiology medical management program equipped with accountability tools produces a significant reduction in inappropriate, high-cost, imaging ordering in radiology departments [13]. Five papers present DSSs that address also researchers as users [12, 18, 56, 58, 76]. For example, Yu et al. describe a classification tree that is continuously trained to allow researchers to investigate the risk factors associated with post-operative complications [12].
3.1.6 Integration with Hospital Information System
Twenty-three DSSs show some kind of integration with HIS [5, 8, 12-17, 21, 23, 28-31, 33, 40, 47, 48, 51, 52, 57, 60, 72]. For example, the experiment by Anani et al. proves that it is possible to share the same guideline across different institutions exploiting the openEHR Guideline Definition Language, reference terminologies, and the Data Archetype Definition Language [72]. Another system, described by Yu et al., allows sharing patient data through secure connections among five medical centers in Taiwan and two cancer centers in Mongolia [12]. Dixon et al. illustrate a successful experiment in which a subset of data referring to primary care patients was securely sent to an engine adopting the production rule formalism hosted in the cloud [23]. The same authors describe in a second paper a system for assessing patient adherence with prescribed medications, integrating the hospital medical record with a personal health record and pharmacy claims [51]. Another system maps the data from an electronic patient record to an ontology about the treatment of chronic illnesses [14]. Finally, Schnall et al. present a system for allowing case managers of HIV patients to make decisions. The system leverages on a continuity of care record enhanced with infobuttons that link clinical information to certified information resources [52].
3.1.7 Assessment
Thirty out of the 53 tools considered in this survey underwent some kind of formal assessment. Among them, 13 underwent an evaluation through different methodologies such as focus groups, questionnaires, and interviews with stakeholders [5, 6, 11, 14, 23, 27, 33, 40, 41, 43, 52-54]. The remaining systems underwent more comprehensive evaluation studies, using either retrospective [13, 18, 24, 38, 42, 46, 55] or prospective data (on real or simulated patients) [15-17, 25, 26, 29, 31, 34, 47, 75].
The goals of the evaluation are different among the 30 papers. Fifteen address usability, acceptability, and perceived usefulness of the presented tools [5, 6, 11, 14, 17, 23, 27, 33, 40, 41, 43, 52-55]. These are mostly measured in terms of scores derived from questionnaires or surveys. Only one paper reports results in terms of size effect of the patient exposure to the system on some factors, such as knowledge about the disease, and attitude towards screening [11]. Fifteen papers present an evaluation of the effectiveness of the system, either in terms of correctness of the decision [16, 18, 24, 25, 26, 42, 46, 75], clinical outcome [31], care flow [15, 29, 34], compliance [38, 47] or costs [13]. As far as correctness of the decision is concerned, in six cases the system output is compared with expert opinion, using a number of patients ranging from 20 to 99 (fake subjects in two cases), and results are given in terms of a variety of indicators, such as recall, accuracy, sensitivity and specificity [18, 24, 25, 26, 42, 46].
Twelve studies (five retrospective and seven prospective) specify the number of patients involved in the evaluation. The average number of patients considered by the retrospective studies is 595 (range: 19-1240) [18, 24, 38, 42, 55]. The prospective studies result in an average of 1,023 patients (range 230-2480) [15-17, 31, 34, 47, 75].
Seven papers state the duration of the study, resulting in an overall average of about 15 months [15-17, 29, 31, 34, 47].
As regards the results of the assessment studies, only one reports negative results [40], while two report no significant effects on the outcomes [15, 38]. All the other studies report positive results. However, to properly interpret this finding, we must consider that 2/3 of the assessments are based on retrospective studies, which could have caused a bias towards positive results.
3.2 Computerized Physician Order Entry
We have used the dimensions identified for DSSs for assessing CPOE papers. Given the nature of this kind of systems, some of the dimensions were more meaningful than others to consider. For example, the target users are always physicians and nurses, while patients are not considered as direct users in any of the reviewed papers. While patient-centered care is not mentioned in any of the considered papers, personalization is addressed by those CPOEs embedding some kind of decision support. In addition, the medical goal of the decision support is always related to treatment. As a consequence of the maturity of the technology, a small number of the selected articles describe newly implemented systems [77-81]. All these papers describe systems integrated with the HIS. In the following, we report on the type of decision support for those systems that are coupled with a DSS, on the medical area, on the methodology for knowledge representation, and on the assessment dimension.
3.2.1 Type of Decision Support
Traditional CPOEs are intended to support drug prescription. The most common functionalities are related to the smart visualization of the summary of product characteristics such as contraindications and drug-drug interactions. Other drug-related aspects are now also addressed. For example, Hsu et al. report on the pill splitting issue, which can arise for drugs originally intended not to be split [82]. The system, which fires an alert when a drug is ordered with the wrong dosage, produced a behavioral change on physicians, significantly decreasing inappropriate splitting.
In addition to drug prescription, modern CPOEs are also intended to improve ordering of other medical procedures, such as imaging, transfusions and diagnostic tests. For example, Thrall describes a system aimed at reducing unnecessary costs and the radiation burden related to the overuse of imaging [83]. McCrory et al. show that the use of a CPOE in a pediatric intensive care unit (ICU) both improves compliance with protocols for red blood cell exchange transfusions and hemoglobin level control [84]. Howell et al. describe an alert system to reduce the number of inappropriate follow-up Pap-tests, according to published screening guidelines [85]. In this case, results were not completely satisfying, as the compliance with guidelines was improved only for some age groups.
In our search, 33 papers deal with adding decision support functionalities to CPOEs [78-82, 85-112]. This has been found as a way to tailor these systems to individual patient’s data. For example, McWilliams et al. and Yazer et al. report on a tool intended to improve compliance with clinical practice guidelines for plasma transfusions, taking into account the coagulation levels of the recipients [78, 101]. Netherton et al. describe a CPOE enriched with orders related to renal colic that takes into account the patient’s blood pressure to compute analgesic dosing options [94]. In the same way, the already mentioned paper by Howell et al. is tailored on the age of the individual patient [85]. Wang et al. present a CPOE enhanced with a calculator for antibiotic dosages that takes into account the patient renal function [91]. Long and Chang describe the impact of coupling the health smart card, issued for all 23 million citizens in Taiwan, to a CPOE implemented at a 700-bed teaching medical center in Taipei [106]. The resulting system shows the promising potential to decrease harmful medication prescribed to pregnant patients. Pulley et al. present a decision support tool based on genotyping results that, embedded into a CPOE, recommends alternative drugs for patients with decreased clopidogrel effectiveness [5].
Even if there is evidence that adding decision support to CPOEs can improve patient outcomes [96], the well-known problem of alert fatigue is still debated [73]. For example, Jung et al. identify a number of context factors useful to prioritize and filter alerts [98]. Interestingly, the top-ranked features are all related to specific patient characteristics (e.g. severity of symptoms and risk factors).
3.2.2 Medical Area
Thirty five papers report on general purpose systems [77, 80, 81, 86, 88-90, 96-99, 105, 107-109, 113-132]. Twelve papers specifically deal with children care [84, 89, 95, 99, 100, 107, 110, 113, 115, 116, 133, 134]. Among specific medical areas, cancer is the most common topic [92, 104, 111, 135-138], followed by cardiology [78, 87, 139, 140]. Other medical areas, including treatments with a specific drug, are represented with less than 3 papers.
Regarding the clinical setting, most of the systems are meant to be used in hospitals. In particular, 7 papers address systems implemented in the ICU [84, 87, 100, 133, 134, 141, 142]. In some cases, CPOEs embedding DSSs are also addressing outpatients. For example, Sampedro et al. describe a system to notify general practitioners of the potential risk of viral reactivation when prescribing biological therapies, thereby facilitating the request for a serological profile [103]. Aita et al. illustrate a system for decreasing prescription errors in outpatients undergoing chemotherapy [104], whereas Al-Rowibah et al. present a system for decreasing adverse drug effects in a very large hospital outpatient service [105].
With respect to DSSs/DAs, specific topics such as pediatrics and intensive care are more represented with CPOEs. As a matter of fact, the choice of treatment dose and delivery are particularly critical in these areas.
3.2.3 Design and Development
Only 12 papers specify some kind of knowledge representation methodology [79, 85, 100, 103, 107-112, 136, 139]. All of these exploit rules, and 5 of them specifically use rule-based techniques to represent the so-called order sets [107, 108, 110, 111, 112]. Besides single drug prescriptions, order-sets allow representing more complex procedures, such as sets of drugs to be administered in sequence, guidelines-based drug protocols, or conditioning regimens to be performed before specific interventions.
3.2.4 Assessment
Most of the papers selected in this search are about the evaluation of already existing systems. Of these, 8 consider a cost/effectiveness perspective [86, 89, 100, 113, 114, 143-145] and 9 consider the impact of CPOEs on the clinical workflow with a particular emphasis on nurses’ tasks [87, 108, 115, 119, 128, 131, 134, 138, 146]. Some papers also report on system re-designing or re-engineering after realizing pitfalls in the former systems [107, 120, 133, 135]. Slightly surprising, no papers report on mobile implementation of CPOE.
Results on CPOEs evaluation demonstrate that there is still no definite answer for CPOE effectiveness. First of all, not all the papers (61, i.e. 85%) conclude with a clear judgment about the impact of the described system. Out of them, 40 (66%) report a positive conclusion (examples are [81, 84, 87, 89, 108, 110, 123, 136, 140, 111, 121, 123, 136, 138, 139, 147]). Despite this encouraging number of positive evaluations, other papers conclude that using the system lead to irrelevant or even harmful results [102, 112, 119, 122, 134, 148], or to CPOE-induced errors [91, 115, 124, 125, 137, 141]. Uncertainty about CPOE effectiveness is also reported in two recent reviews [149, 150].
A different issue is related to the evaluation of CPOE design and development. Poor consideration of human factors, scarce collaboration between technical and medical partners for knowledge representation, and lack of involvement of final users were identified as the major aspects leading to poorly usable systems. Three papers report on this issue and claim that this could be a major cause of system-induced medication errors [104, 115, 124]. The need for a careful validation phase as the final development task before the system is implemented in the clinical routine is becoming crucial [126].
The economic benefit of CPOEs is debated, too. For example, a study involving four community hospitals in the Massachusetts shows that the return of investment was not significant for traditional CPOEs, probably due to the lack of embedded decision support functionalities [143].
4 Discussion
We have presented a review of the papers reporting on currently implemented DSSs, with a particular emphasis on personalization and patient involvement. Due to the relevance of the topic, the search resulted in a high number of papers showing several heterogeneous features. This posed the problem of identifying a suitable set of dimensions to characterize the current trends and efforts in the research community. Some of the dimensions used to classify papers in our review have been already used in other works. For example, Belle et al. rank systems according to the application areas and knowledge representation methods [151]. Welch and Kawamoto classify papers according to the type of clinical decision support, the application area, and primary users [58]. Due to the sheer size of the search topic, we have introduced additional dimensions, not always mutually exclusive and not all applicable to any system, which, all together, give a picture of the multifaceted state of the art in the field. Figure 4 reports and synthesizes the most relevant results for the considered dimensions.
Fig. 4.
Summary description of DSS (top) and CPOE (bottom) papers according to the identified dimensions
In the following we discuss some general and more specific aspects that have emerged through the full text review of the selected papers.
4.1 General Aspects
It is nowadays established that technological advances can fruitfully be translated into SDM applications when a real need is perceived by both physicians and patients [40]. As a matter of fact, there are still few systems developed to actively and directly involve patients in the decision process. This could be due to the fact that patient involvement requires proper training, willingness and motivation on both sides. This preparatory phase is often lacking, both because doctors and patients are not used to closely interact on those topics, and because the organization does not allow for the required time during regular visits [35]. Among systems dealing with patient-centered care, a considerable number deals with risk communication, indicating a trend towards preventive medicine. Here, the patient’s perception of risk and the consequent behavioral changes are the keys for an effective improvement of the population well-being. Nowadays, given the multi-ethnicity of the society, an additional challenge in SDM is represented by the need of bridging the gap among different cultural dimensions [152, 153].
Our survey highlighted also that the success of CPOEs is highly dependent on the value perceived by physicians. This perception is the basis for their willingness to collaborate to the development of the system and to learn how to appropriately use it. The role of healthcare policy makers in promoting this value is emerging in some countries [54, 66]. Interestingly, the American RAND corporation states that recent developments in health reform, related to the passage of the Affordable Care Act, and ensuing regulations encourage delivery systems to engage in SDM [66]. This means that there will probably be room for further medical informatics research on those themes.
4.2 Methodological Aspects
From our survey, it turns out that providing correct and complete information to users is equally or even more important than supporting them with recommendations. A considerable number of the analyzed tools base decision support only on the presentation of information, and more than a half of those delivering recommendations also consider this aspect. The motivation underlying this trend is twofold. First, it is related to the load of information that physicians have nowadays to consider. Tools able to meaningfully summarize such information are very useful given the limited time dedicated to each medical visit and the variety of data sources to be searched through. Second, as already discussed, since patients are becoming increasingly involved in the medical decision making process, new tools are necessary for tailoring the presentation of information to each of them.
Another interesting observation is related to knowledge representation. The specialized frameworks based on various formalisms developed by individual research teams in the past to represent computerized guidelines are less frequently used in new systems [154]. As a matter of fact, we found only two papers exploiting already published guideline representation languages. One paper uses GLIF [155] to validate the logic of an existing tool, and not for developing the tool itself [46]. Another paper uses PROforma [156] to represent eligibility criteria for colorectal cancer screening. In this paper, the data model for concept representation is based on openEHR archetypes [71]. This highlights the current trend to replace specialized environments by inference engines built on top of standardized frameworks that allow managing ontologies (e.g. Protégé - http://protege.stanford.edu/) and data models (e.g., openEHR archetypes, and the related GDL-Guideline Definition Language - http://www.openehr.org/downloads/ds_and_guidelines). Moreover, many of the methods that in previous reviews were identified as potentially able to provide useful results for decision support (e.g. neural networks and other machine learning techniques) [1], are rarely used in the tools currently exploited in clinical practice. The specific focus of our review could explain the differences in the highlighted trends. As a matter of fact, we included only real-world implementations of DSSs and not the models validated only retrospectively that have not been further exploited in a DS tool.
The small number of methodological papers on CPOEs could be explained by the fact that the majority of commercial and academic CPOE systems are based on proprietary software. Nevertheless, efforts are directed to the use of standard terminologies for dosages, active principles, and laboratory examinations orders [157].
4.3 Technological Aspects
Despite the growing interest for e-health and m-health, we found no mobile DSSs or systems to be used by patients at home in our review. This is not surprising because our search excluded reminder systems, which are the ones that maximally exploit mobile technology. Furthermore, mobile devices are not widely used for the dissemination of clinical DSSs, as pointed out by Charani et al. [158]. This result may indicate that, in settings where computers are available, these are still preferred to mobile devices, being able to provide better interaction and user experience. However, general practitioners who perform home visits could benefit from mobile CPOEs, and the lack of tools in this area may indicate that the interest of professionals for such systems is still poor.
As for the near future, the evolution is likely to lead to a more mature scenario. Manufacturers will provide fully integrated smart devices such as clinical sensors, which will exploit network communication capabilities to enable long-term acquisition and integration of patient data. As it emerges from our review, data acquisition is still mainly performed manually, thus potentially leading to missing data and/or input errors. Moreover, the currently adopted strategies to improve the quality of manually entered data often further increase the burden for the user, negatively impacting their experience with the system [159]. Technological advances will create a huge demand in terms of interoperability and health information exchange. The ultimate challenge will be to achieve a real continuity of care throughout the whole lifespan of patients, which from our results doesn’t seem to be yet mature enough. To face this challenge, several organizational and methodological aspects need to be addressed, such as legal and safety issues, as well as the associated regulatory constraints [160, 161].
4.4 Limitations
Our study has some limitations. First of all, it is not intended to give an insight to the trends of medical DSSs in general, but only of those that focus on patient personalization and/or involvement in the decision process. This choice was based on the growing interest for SDM in the medical community. For this reason, there might have been some works that have not been extracted because patient’s involvement was not explicitly included in the keywords. Examples are the paper by García-Sáez et al. that describes a patient-oriented guidance system for gestational diabetes [162], and the paper by Sacchi et al. that presents a framework for embedding SDM into a guideline-based DSS [163].
Moreover, we limited our search to two scientific literature repositories. Even though these are very comprehensive archives, some potentially interesting works might not have been included.
Furthermore, limiting our search to journal papers prevented the extraction of potentially interesting works existing in conferences proceedings, as for example the paper by Quaglini et al. about achievements in SDM in an ongoing EU-funded project [164].
Even if we considered a number of dimensions able to represent a variety of features of the systems, there might be some aspects that have not been tackled in detail.
Examples are the factors related to the success of the application of the systems and the integration of the considered systems into clinical workflows [1, 165].
Unlike other reviews, we did not limit our search to papers focusing on a specific study design [166, 167]. This allowed defining a broad classification framework, mostly centered on methodological aspects rather than on specific outcomes. On the other hand, the large number of papers extracted and their heterogeneity have not allowed a systematic classification on the basis of the quality of the reported studies, as instead it is proposed by more restricted reviews [168].
The restrictions that we have considered for paper selection, i.e. the actual presentation of a DSS tool, prevented us to analyze a number of papers dealing with information exchange and continuity of care but not describing specific tools that implement decision support in patient care coordination. As a matter of fact, ICT papers dealing with this topic are mostly focused on standards for interoperability among different EHRs and not on medical decision support as meant by our definition. Still, some examples of systems enriching health information exchange platforms with decision support have been found [16].
5 Conclusion
In this work, we have presented a review of papers related to decision support systems used in clinical environments. The aim was to discuss the state of the art as it emerges from the literature of the last three years. Starting from a search focused on the emerging topic of patient-centered care, we had the opportunity of exploring the recent trends also related to other dimensions, such as knowledge representation and systems stakeholders. In addition, the integration of decision support was shown to allow effective personalization of the traditionally physician-oriented CPOEs. Overall, we have noticed interesting evolutions of decision support systems towards a better communication with the patient, attention to the economic and organizational impact, and use of standard models for knowledge representation.
References
- 1.Peleg M, Tu S. Decision support, knowledge representation and management in medicine. Yearb Med Inform 2006:72-80. [PubMed] [Google Scholar]
- 2.Kachalia A, Mello MM. Defensive medicine--legally necessary but ethically wrong?: Inpatient stress testing for chest pain in low-risk patients. JAMA Intern Med. 2013. June 24;173(12):1056-7. [DOI] [PubMed] [Google Scholar]
- 3.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? Soc Sci Med 1997. March;44(5):681-92. [DOI] [PubMed] [Google Scholar]
- 4.Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014. January 28;1. [DOI] [PubMed] [Google Scholar]
- 5.Pulley JM, Denny JC, Peterson JF, Bernard GR, Vnencak-Jones CL, Ramirez AH, et al. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin Pharmacol Ther 2012. July;92(1):87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kastner M, Straus SE. Application of the Knowledge-to-Action and Medical Research Council frameworks in the development of an osteoporosis clinical decision support tool. J Clin Epidemiol 2012. November;65(11):1163-70. [DOI] [PubMed] [Google Scholar]
- 7.Luo G, Tang C, Thomas SB. Intelligent personal health record: experience and open issues. J Med Syst 2012. August;36(4):2111-28. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh NC, Chang CY, Lee KC, Chen JC, Chan CH. Technological innovations in the development of cardiovascular clinical information systems. J Med Syst 2012. April;36(2):965-78. [DOI] [PubMed] [Google Scholar]
- 9.Chi CL, Nick Street W, Robinson JG, Crawford MA. Individualized patient-centered lifestyle recommendations: an expert system for communicating patient specific cardiovascular risk information and prioritizing lifestyle options. J Biomed Inform 2012. December;45(6):1164-74. [DOI] [PubMed] [Google Scholar]
- 10.Spanakis EG, Chiarugi F, Kouroubali A, Spat S, Beck P, Asanin S, et al. Diabetes Management Using Modern Information and Communication Technologies and New Care Models. Eysenbach G, editor. Interact J Med Res 2012;1(2):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindblom K, Gregory T, Wilson C, Flight IH, Zajac I. The impact of computer self-efficacy, computer anxiety, and perceived usability and acceptability on the efficacy of a decision support tool for colorectal cancer screening. J Am Med Inform Assoc 2012. May-Jun;19(3):407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu HJ, Lai HS, Chen KH, Chou HC, Wu JM, Dorjgochoo S, et al. A sharable cloud-based pancreaticoduodenectomy collaborative database for physicians: emphasis on security and clinical rule supporting. Comput Methods Programs Biomed 2013. August;111(2):488-97. [DOI] [PubMed] [Google Scholar]
- 13.Ip IK, Schneider L, Seltzer S, Smith A, Dudley J, Menard A, et al. Impact of provider-led, technology-enabled radiology management program on imaging. Am J Med 2013. August;126(8):687-92. [DOI] [PubMed] [Google Scholar]
- 14.Riaño D, Real F, López-Vallverdú JA, Campana F, Ercolani S, Mecocci P, et al. An ontology-based personalization of health-care knowledge to support clinical decisions for chronically ill patients. J Biomed Inform 2012. June;45(3):429-46. [DOI] [PubMed] [Google Scholar]
- 15.Dexheimer JW, Abramo TJ, Arnold DH, Johnson K, Shyr Y, Ye F, et al. Implementation and evaluation of an integrated computerized asthma management system in a pediatric emergency department: a randomized clinical trial. Int J Med Inform 2014. November;83(11):805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herwehe J, Wilbright W, Abrams A, Bergson S, Foxhood J, Kaiser M, et al. Implementation of an innovative, integrated electronic medical record (EMR) and public health information exchange for HIV/AIDS. J Am Med Inform Assoc 2012. May-Jun;19(3):448-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldspiel BR, Flegel WA, DiPatrizio G, Sissung T, Adams SD, Penzak SR, et al. Integrating pharmacogenetic information and clinical decision support into the electronic health record. J Am Med Inform Assoc 2014. May-Jun;21(3):522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández-Breis JT, Maldonado JA, Marcos M, Legaz-García Mdel C, Moner D, Torres-Sospedra J, et al. Leveraging electronic healthcare record standards and semantic web technologies for the identification of patient cohorts. J Am Med Inform Assoc 2013. December;20(e2):e288-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klann JG, Anand V, Downs SM. Patient-tailored prioritization for a pediatric care decision support system through machine learning. J Am Med Inform Assoc 2013. December;20(e2):e267-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blank A, Prytherch H, Kaltschmidt J, Krings A, Sukums F, Mensah N, et al. “Quality of prenatal and maternal care: bridging the know-do gap” (QUALMAT study): an electronic clinical decision support system for rural Sub-Saharan Africa. BMC Med Inform Decis Mak 2013. April 10;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duke JD, Morea J, Mamlin B, Martin DK, Simonaitis L, Takesue BY, et al. Regenstrief Institute’s Medical Gopher: a next-generation homegrown electronic medical record system. Int J Med Inform 2014. March;83(3):170-9. [DOI] [PubMed] [Google Scholar]
- 22.Shojanoori R, Juric R. Semantic remote patient monitoring system. Telemed J E Health 2013. February;19(2):129-36. [DOI] [PubMed] [Google Scholar]
- 23.Dixon BE, Simonaitis L, Goldberg HS, Paterno MD, Schaeffer M, Hongsermeier T, et al. A pilot study of distributed knowledge management and clinical decision support in the cloud. Artif Intell Med 2013. September;59(1):45-53. [DOI] [PubMed] [Google Scholar]
- 24.Farmer N. An update and further testing of a knowledge-based diagnostic clinical decision support system for musculoskeletal disorders of the shoulder for use in a primary care setting. J Eval Clin Pract 2014. October;20(5):589-95. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-González A, Torres-Niño J, Mayer MA, Alor-Hernandez G, Wilkinson MD. Analysis of a multilevel diagnosis decision support system and its implications: a case study. Comput Math Methods Med 2012;2012:367345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson KB, Ho YX, Andrew Spooner S, Palmer M, Weinberg ST. Assessing the reliability of an automated dose-rounding algorithm. J Biomed Inform 2013. October;46(5):814-21. [DOI] [PubMed] [Google Scholar]
- 27.Agharezaei Z, Bahaadinbeigy K, Tofighi S, Agharezaei L, Nemati A. Attitude of Iranian physicians and nurses toward a clinical decision support system for pulmonary embolism and deep vein thrombosis. Comput Methods Programs Biomed 2014. July;115(2):95-101. [DOI] [PubMed] [Google Scholar]
- 28.Wang HQ, Li JS, Zhang YF, Suzuki M, Araki K. Creating personalised clinical pathways by semantic interoperability with electronic health records. Artif Intell Med 2013. June;58(2):81-9. [DOI] [PubMed] [Google Scholar]
- 29.Adams P, Nielson H. Evidence based practice: decreasing psychiatric revisits to the emergency department. Issues Ment Health Nurs 2012. August;33(8):536-43. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Romero M, Vázquez-Naya JM, Pereira J, Pereira M, Pazos A, Baños G. The iOSC3 system: using ontologies and SWRL rules for intelligent supervision and care of patients with acute cardiac disorders. Comput Math Methods Med 2013;2013:650671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins GK, Lester W, Johnson KL, Chang Y, Estey G, Surrao D, et al. Successful Outcomes of a Clinical Decision Support System in an HIV Practice: A Randomized Controlled Trial. Ann Intern Med 2012;157(11):757-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu X, Huang Z, Duan H. Supporting adaptive clinical treatment processes through recommendations. Comput Methods Programs Biomed 2012. September;107(3):413-24. [DOI] [PubMed] [Google Scholar]
- 33.Lin HC, Wu HC, Chang CH, Li TC, Liang WM, Wang JY. A real time online assessment system with modelized architecture on clinical infometrics for patient reported outcomes of prostate cancer. Comput Methods Programs Biomed 2012. June;106(3):249-59. [DOI] [PubMed] [Google Scholar]
- 34.Simon D Kriston L, von Wolff A Buchholz A Vietor C Hecke T et al. Effectiveness of a web-based, individually tailored decision aid for depression or acute low back pain: a randomized controlled trial. Patient Educ Couns 2012. June;87(3):360-8. [DOI] [PubMed] [Google Scholar]
- 35.Federer AE, Taylor DC, Mather RC., 3rd Using evidence-based algorithms to improve clinical decision making: the case of a first-time anterior shoulder dislocation. Sports Med Arthrosc 2013. September;21(3):155-65. [DOI] [PubMed] [Google Scholar]
- 36.LeBlanc A, Ruud KL, Branda ME, Tiedje K, Boehmer KR, Pencille LJ, et al. The impact of decision aids to enhance shared decision making for diabetes (the DAD study): protocol of a cluster randomized trial. BMC Health Serv Res 2012. May 28;12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox CE, White DB, Abernethy AP. A universal decision support system. Addressing the decision-making needs of patients, families, and clinicians in the setting of critical illness. Am J Respir Crit Care Med 2014. August 15;190(4):366-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein BD, Kogan JN, Mihalyo MJ, Schuster J, Deegan PE, Sorbero MJ, et al. Use of a computerized medication shared decision making tool in community mental health settings: impact on psychotropic medication adherence. Community Ment Health J 2013. April;49(2):185-92. [DOI] [PubMed] [Google Scholar]
- 39.Ogburn T. Shared decision making and informed consent for hysterectomy. Clin Obstet Gynecol 2014. March;57(1):3-13. [DOI] [PubMed] [Google Scholar]
- 40.Newsome A, Sieber W, Smith M, Lillie D. If you build it, will they come? A qualitative evaluation of the use of video-based decision aids in primary care. Fam Med 2012. January;44(1):26-31. [PubMed] [Google Scholar]
- 41.Ozanne EM, Howe R, Omer Z, Esserman LJ. Development of a personalized decision aid for breast cancer risk reduction and management. BMC Med Inform Decis Mak 2014. January 14;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blau GE, Orcun S, Laínez JM, Reklaitis GV, Suvannasankha A, Fausel C, et al. Validation of a novel approach for dose individualization in pharmacotherapy using gabapentin in a proof of principles study. Pharmacotherapy 2013. July;33(7):727-35. [DOI] [PubMed] [Google Scholar]
- 43.Herrick DB, Nakhasi A, Nelson B, Rice S, Abbott PA, Saber Tehrani AS, et al. Usability characteristics of self-administered computer-assisted interviewing in the emergency department: factors affecting ease of use, efficiency, and entry error. Appl Clin Inform 2013. June 19;4(2):276-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lühr A, Löck S, Roth K, Helmbrecht S, Jakobi A, Petersen JB, et al. Concept for individualized patient allocation: ReCompare--remote comparison of particle and photon treatment plans. Radiat Oncol 2014. February 18;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Was A, Wanderer J. Matching clinicians to operative cases: a novel application of a patient acuity score. Appl Clin Inform 2013. July 10;4(3):445-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi J, Kim H. Enhancement of decision rules to increase generalizability and performance of the rule-based system assessing risk for pressure ulcer. Appl Clin Inform 2013. June 5;4(2):251-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey JE, Wan JY, Mabry LM, Landy SH, Pope RA, Waters TM, et al. Does health information exchange reduce unnecessary neuroimaging and improve quality of headache care in the emergency department? J Gen Intern Med 2013. February;28(2):176-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folks RD, Savir-Baruch B, Garcia EV, Verdes L, Taylor AT. Development of a Relational Database to Capture and Merge Clinical History with the Quantitative Results of Radionuclide Renography. J Nucl Med Technol 2012;40(4):236-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett CC, Hauser K. Artificial intelligence framework for simulating clinical decision-making: a Markov decision process approach. Artif Intell Med 2013. January;57(1):9-19. [DOI] [PubMed] [Google Scholar]
- 50.Galea I, Lederer C, Neuhaus A, Muraro PA, Scalfari A, Koch-Henriksen N, et al. A web-based tool for personalized prediction of long-term disease course in patients with multiple sclerosis. Eur J Neurol 2013. July;20(7):1107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixon BE, Jabour AM, Phillips EO, Marrero DG. An informatics approach to medication adherence assessment and improvement using clinical, billing, and patient-entered data. J Am Med Inform Assoc 2014. May-Jun;21(3):517-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnall R, Cimino JJ, Bakken S. Development of a prototype continuity of care record with context-specific links to meet the information needs of case managers for persons living with HIV. Int J Med Inform 2012. August;81(8):549-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiarini Tremblay M Hevner AR Berndt DJ. Design of an information volatility measure for health care decision making. Decis Support Syst 2012. January;52(2):331–41. [Google Scholar]
- 54.Peirson L, Catallo C, Chera S. The Registry of Knowledge Translation Methods and Tools: a resource to support evidence-informed public health. Int J Public Health Aug 2013; 58(4):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuru K, Girgin S, Arda K, Bozlar U. A novel report generation approach for medical applications: the SISDS methodology and its applications. Int J Med Inform 2013. May;82(5):435-47. [DOI] [PubMed] [Google Scholar]
- 56.Wu R, Boushey R, Potter B, Stacey D. The evaluation of a rectal cancer decision aid and the factors influencing its implementation in clinical practice. BMC Surg 2014. March 21;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carney PH. Information technology and precision medicine. Semin Oncol Nurs 2014. May;30(2):124-9. [DOI] [PubMed] [Google Scholar]
- 58.Welch BM, Kawamoto K. Clinical decision support for genetically guided personalized medicine: a systematic review. J Am Med Inform Assoc 2013. Mar-Apr;20(2):388-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins IM, Breathnach O, Felle P. Electronic clinical decision support systems attitudes and barriers to use in the oncology setting. Ir J Med Sci 2012. December;181(4):521-5. [DOI] [PubMed] [Google Scholar]
- 60.Arnold CW, McNamara M, El-Saden S, Chen S, Taira RK, Bui AA. Imaging informatics for consumer health: towards a radiology patient portal. J Am Med Inform Assoc 2013. Nov-Dec;20(6):1028-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lambin P, van Stiphout RG, Starmans MH, Rios-Velazquez E, Nalbantov G, Aerts HJ, et al. Predicting outcomes in radiation oncology--multifactorial decision support systems. Nat Rev Clin Oncol 2013. January;10(1):27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambin P, Roelofs E, Reymen B, Velazquez ER, Buijsen J, Zegers CM, et al. ‘Rapid Learning health care in oncology’ - an approach towards decision support systems enabling customised radiotherapy’. Radiother Oncol 2013. October;109(1):159-64. [DOI] [PubMed] [Google Scholar]
- 63.Ceriello A, Barkai L, Christiansen JS, Czupryniak L, Gomis R, Harno K, et al. Diabetes as a case study of chronic disease management with a personalized approach: the role of a structured feedback loop. Diabetes Res Clin Pract 2012. October;98(1):5-10. [DOI] [PubMed] [Google Scholar]
- 64.McDermott MS, While AE. Maximizing the healthcare environment: a systematic review exploring the potential of computer technology to promote self-management of chronic illness in healthcare settings. Patient Educ Couns 2013. July;92(1):13-22. [DOI] [PubMed] [Google Scholar]
- 65.Gionfriddo MR, Leppin AL, Brito JP, Leblanc A, Shah ND, Montori VM. Shared decision-making and comparative effectiveness research for patients with chronic conditions: an urgent synergy for better health. J Comp Eff Res 2013. November;2(6):595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedberg MW, Van Busum K, Wexler R, Bowen M, Schneider EC. A demonstration of shared decision making in primary care highlights barriers to adoption and potential remedies. Health Aff (Millwood) 2013. February;32(2):268-75. [DOI] [PubMed] [Google Scholar]
- 67.Meulendijk M, Spruit M, Drenth-van Maanen C, Numans M, Brinkkemper S, Jansen P. General practitioners’ attitudes towards decision-supported prescribing: an analysis of the Dutch primary care sector. Health Informatics J 2013. December;19(4):247-63. [DOI] [PubMed] [Google Scholar]
- 68.Li AC, Kannry JL, Kushniruk A, Chrimes D, McGinn TG, Edonyabo D, et al. Integrating usability testing and think-aloud protocol analysis with “near-live” clinical simulations in evaluating clinical decision support. Int J Med Inform 2012. November;81(11):761-72. [DOI] [PubMed] [Google Scholar]
- 69.King VJ, Davis MM, Gorman PN, Rugge JB, Fagnan LJ. Perceptions of Shared Decision Making and Decision Aids Among Rural Primary Care Clinicians. Med Decis Making 2012;32(4):636-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saverno KR, Rochau U, Stenehjem DD, Morley K, Siebert U, Brixner DI. Application of decision-analytic models in personalized medicine for CML treatment decisions made by payers, providers, and patients. J Manag Care Pharm 2012. Jul-Aug;18(6):457-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marcos M, Maldonado JA, Martínez-Salvador B, Boscá D, Robles M. Interoperability of clinical decision-support systems and electronic health records using archetypes: a case study in clinical trial eligibility. J Biomed Inform 2013. August;46(4):676-89. [DOI] [PubMed] [Google Scholar]
- 72.Anani N, Chen R, Prazeres Moreira T, Koch S. Retrospective checking of compliance with practice guidelines for acute stroke care: a novel experiment using openEHR’s Guideline Definition Language. BMC Med Inform Decis Mak 2014. May 10;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horsky J, Schiff GD, Johnston D, Mercincavage L, Bell D, Middleton B. Interface design principles for usable decision support: a targeted review of best practices for clinical prescribing interventions. J Biomed Inform 2012. December;45(6):1202-16. [DOI] [PubMed] [Google Scholar]
- 74.Rodríguez-González A, Torres-Niño J, Valencia-Garcia R, Mayer MA, Alor-Hernandez G. Using experts feedback in clinical case resolution and arbitration as accuracy diagnosis methodology. Comput Biol Med 2013. September;43(8):975-86. [DOI] [PubMed] [Google Scholar]
- 75.Carroll DL, Dykes PC, Hurley AC. An electronic fall prevention toolkit: effect on documentation quality. Nurs Res 2012. Jul-Aug;61(4):309-13. [DOI] [PubMed] [Google Scholar]
- 76.Kawamoto K, Hongsermeier T, Wright A, Lewis J, Bell DS, Middleton B. Key principles for a national clinical decision support knowledge sharing framework: synthesis of insights from leading subject matter experts. J Am Med Inform Assoc 2013. January 1;20(1):199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenbaum BP, Silkin N, Miller RA. Easily configured real-time CPOE Pick Off Tool supporting focused clinical research and quality improvement. J Am Med Inform Assoc 2014. May-Jun;21(3):564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McWilliams B, Triulzi DJ, Waters JH, Alarcon LH, Reddy V, Yazer MH. Trends in RBC ordering and use after implementing adaptive alerts in the electronic computerized physician order entry system. Am J Clin Pathol 2014. April;141(4):534-41. [DOI] [PubMed] [Google Scholar]
- 79.Levick DL, Stern G, Meyerhoefer CD, Levick A, Pucklavage D. Reducing unnecessary testing in a CPOE system through implementation of a targeted CDS intervention. BMC Med Inform Decis Mak 2013. April 8;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leu MG, Morelli SA, Chung OY, Radford S. Systematic update of computerized physician order entry order sets to improve quality of care: a case study. Pediatrics 2013. March;131 Suppl 1:S60-7. [DOI] [PubMed] [Google Scholar]
- 81.Galanter W, Falck S, Burns M, Laragh M, Lambert BL. Indication-based prescribing prevents wrong-patient medication errors in computerized provider order entry (CPOE). J Am Med Inform Assoc 2013. May 1;20(3):477-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu CC, Chou CY, Chou CL, Ho CC, Chen TJ, Chiang SC, et al. Impact of a Warning CPOE System on the Inappropriate Pill Splitting of Prescribed Medications in Outpatients. Plos One 2014. December 5;9(12):e114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thrall JH. Appropriateness and imaging utilization: “computerized provider order entry and decision support”. Acad Radiol 2014. September;21(9):1083-7. [DOI] [PubMed] [Google Scholar]
- 84.McCrory MC, Strouse JJ, Takemoto CM, Easley RB. Computerized physician order entry improves compliance with a manual exchange transfusion protocol in the pediatric intensive care unit. J Pediatr Hematol Oncol 2014. March;36(2):143-7. [DOI] [PubMed] [Google Scholar]
- 85.Howell LP, MacDonald S, Jones J, Tancredi DJ, Melnikow J. Can automated alerts within computerized physician order entry improve compliance with laboratory practice guidelines for ordering Pap tests? J Pathol Inform 2014. September 30;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vermeulen KM, van Doormaal JE, Zaal RJ, Mol PG, Lenderink AW, Haaijer-Ruskamp FM, et al. Cost-effectiveness of an electronic medication ordering system (CPOE/CDSS) in hospitalized patients. Int J Med Inform 2014. August;83(8):572-80. [DOI] [PubMed] [Google Scholar]
- 87.Armada ER, Villamañán E, López-de-Sá E, Rosillo S, Rey-Blas JR, Testillano ML, et al. Computerized physician order entry in the cardiac intensive care unit: effects on prescription errors and workflow conditions. J Crit Care 2014. April;29(2):188-93. [DOI] [PubMed] [Google Scholar]
- 88.Mathew G, Kho A, Dexter P, Bloodworth N, Fantz C, Spell N, et al. Concept and development of a discharge alert filter for abnormal laboratory values coupled with computerized provider order entry: a tool for quality improvement and hospital risk management. J Patient Saf 2012. June;8(2):69-75. [DOI] [PubMed] [Google Scholar]
- 89.Shriner AR, Webber EC. Attitudes and perceptions of pediatric residents on transitioning to CPOE. Appl Clin Inform 2014. August 13;5(3):721-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cadwallader J, Asirwa C, Li X, Kesterson J, Tierney WM, Were MC. Using computerized provider order entry to enforce documentation of tests with pending results at hospital discharge. Appl Clin Inform 2012. April 4;3(2):154-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang HY, Lu CL, Wu MP, Huang MH, Huang YB. Effectiveness of an integrated CPOE decision-supporting system with clinical pharmacist monitoring practice in preventing antibiotic dosing errors. Int J Clin Pharmacol Ther 2012. June;50(6):375-82. [DOI] [PubMed] [Google Scholar]
- 92.Devine EB, Lee CJ, Overby CL, Abernethy N, McCune J, Smith JW, et al. Usability evaluation of pharmacogenomics clinical decision support aids and clinical knowledge resources in a computerized provider order entry system: a mixed methods approach. Int J Med Inform 2014. July;83(7):473-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ip IK, Schneider LI, Hanson R, Marchello D, Hultman P, Viera M, et al. Adoption and meaningful use of computerized physician order entry with an integrated clinical decision support system for radiology: ten-year analysis in an urban teaching hospital. J Am Coll Radiol 2012. February;9(2):129-36. [DOI] [PubMed] [Google Scholar]
- 94.Netherton SJ, Lonergan K, Wang D, McRae A, Lang E. Computerized physician order entry and decision support improves ED analgesic ordering for renal colic. Am J Emerg Med 2014. September;32(9):958-61. [DOI] [PubMed] [Google Scholar]
- 95.Mazars N, Milési C, Carbajal R, Mesnage R, Combes C, Rideau Batista Novais A, et al. Implementation of a neonatal pain management module in the computerized physician order entry system. Ann Intensive Care 2012. August 22;2(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Theal J, Protti D. CPOE with evidence-based clinical decision support improves patient outcomes: the journey to date for a Canadian hospital. Healthc Q 2014;17(1):24-9. [DOI] [PubMed] [Google Scholar]
- 97.Cresswell KM, Bates DW, Williams R, Morrison Z, Slee A, Coleman J, et al. Evaluation of medium-term consequences of implementing commercial computerized physician order entry and clinical decision support prescribing systems in two ‘early adopter’ hospitals. J Am Med Inform Assoc 2014. October;21(e2):e194-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jung M, Riedmann D, Hackl WO, Hoerbst A, Jaspers MW, Ferret L, et al. Physicians’ perceptions on the usefulness of contextual information for prioritizing and presenting alerts in Computerized Physician Order Entry systems. BMC Med Inform Decis Mak 2012. October 2;12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Webber EC, Warhurst HM, Smith SS, Cox EG, Crumby AS, Nichols KR. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Inform 2013. November 27;4(4):556-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pageler NM, Franzon D, Longhurst CA, Wood M, Shin AY, Adams ES, et al. Embedding time-limited laboratory orders within computerized provider order entry reduces laboratory utilization. Pediatr Crit Care Med 2013. May;14(4):413-9. [DOI] [PubMed] [Google Scholar]
- 101.Yazer MH, Triulzi DJ, Reddy V, Waters JH. Effectiveness of a real-time clinical decision support system for computerized physician order entry of plasma orders. Transfusion 2013. December;53(12):3120-7. [DOI] [PubMed] [Google Scholar]
- 102.Patterson ME, Marken PA, Simon SD, Hackman JL, Schaefer RS. Associations between the concurrent use of clinical decision support and computerized provider order entry and the rates of appropriate prescribing at discharge. Appl Clin Inform 2012. May 16;3(2):186-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sampedro B, Hernandez-Lapez C, Ferrandiz JR, Illaro A, Fabrega E, Cuadrado A, et al. Computerized physician order entry-based system to prevent HBV reactivation in patients treated with biologic agents: the PRESCRIB project. Hepatology 2014. July;60(1):106-13. [DOI] [PubMed] [Google Scholar]
- 104.Aita M, Belvedere O, De Carlo E, Deroma L, De Pauli F, Gurrieri L, et al. Chemotherapy prescribing errors: an observational study on the role of information technology and computerized physician order entry systems. BMC Health Serv Res 2013. December 17;13:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Al-Rowibah FA, Younis MZ, Parkash J. The impact of computerized physician order entry on medication errors and adverse drug events. J Health Care Finance 2013. Fall;40(1):93-102. [PubMed] [Google Scholar]
- 106.Long AJ, Chang P. The effect of using the health smart card vs. CPOE reminder system on the prescribing practices of non-obstetric physicians during outpatient visits for pregnant women in Taiwan. Int J Med Inform 2012. September;81(9):605-11. [DOI] [PubMed] [Google Scholar]
- 107.Jacobs BR, Hart KW, Rucker DW. Reduction in Clinical Variance Using Targeted Design Changes in Computerized Provider Order Entry (CPOE) Order Sets: Impact on Hospitalized Children with Acute Asthma Exacerbation. Appl Clin Inform 2012. February 8;3(1):52-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davis L, Brunetti L, Lee EK, Yoon N, Cho SH, Suh DC. Effects of computerized physician order entry on medication turnaround time and orders requiring pharmacist intervention. Res Social Adm Pharm 2014. Sep-Oct;10(5):756-67. [DOI] [PubMed] [Google Scholar]
- 109.Woods AD, Mulherin DP, Flynn AJ, Stevenson JG, Zimmerman CR, Chaffee BW. Clinical decision support for atypical orders: detection and warning of atypical medication orders submitted to a computerized provider order entry system. J Am Med Inform Assoc 2014. May-Jun;21(3):569-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Avansino J, Leu MG. Effects of CPOE on provider cognitive workload: a randomized crossover trial. Pediatrics 2012. September;130(3):e547-52. [DOI] [PubMed] [Google Scholar]
- 111.Kadakia KC, Leal AD, Seisler DK, Qin R, Fee-Schroeder KC, Grendahl DC, et al. Anti-emetic prescribing practices using a computerized physician order entry system. Support Care Cancer 2014. January;22(1):217-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brunette DD, Tersteeg J, Brown N, Johnson V, Dunlop S, Karambay J, et al. Implementation of Computerized Physician Order Entry for Critical Patients in an Academic Emergency Department is Not Associated with a Change in Mortality Rate. West J Emerg Med 2013. March;14(2):114-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Teufel RJ, Kazley AS, Basco WT., Jr. Is computerized physician order entry use associated with a decrease in hospital resource utilization in hospitals that care for children? J Med Syst 2012. August;36(4):2411-20. [DOI] [PubMed] [Google Scholar]
- 114.Spaulding TJ, Raghu TS. Impact of CPOE Usage on Medication Management Process Costs and Quality Outcomes. Inquiry 2013. August;50(3):229-47. [DOI] [PubMed] [Google Scholar]
- 115.Househ M, Ahmad A, Alshaikh A, Alsuweed F. Patient safety perspectives: the impact of CPOE on nursing workflow. Stud Health Technol Inform 2013;183:367-71. [PubMed] [Google Scholar]
- 116.Abramson EL, Kaushal R. Computerized provider order entry and patient safety. Pediatr Clin North Am 2012. December;59(6):1247-55. [DOI] [PubMed] [Google Scholar]
- 117.Wright A, Feblowitz JC, Pang JE, Carpenter JD, Krall MA, Middleton B, et al. Use of order sets in inpatient computerized provider order entry systems: a comparative analysis of usage patterns at seven sites. Int J Med Inform 2012. November;81(11):733-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hyman D, Laire M, Redmond D, Kaplan DW. The use of patient pictures and verification screens to reduce computerized provider order entry errors. Pediatrics 2012. July;130(1):e211-9. [DOI] [PubMed] [Google Scholar]
- 119.Pelayo S, Anceaux F, Rogalski J, Elkin P, Beuscart-Zephir MC. A comparison of the impact of CPOE implementation and organizational determinants on doctor-nurse communications and cooperation. Int J Med Inform. 2013. December;82(12):e321-30. [DOI] [PubMed] [Google Scholar]
- 120.Leung AA, Keohane C, Lipsitz S, Zimlichman E, Amato M, Simon SR, et al. Relationship between medication event rates and the Leapfrog computerized physician order entry evaluation tool. J Am Med Inform Assoc 2013. June;20(e1):e85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schneider E, Ruggieri P, Fromwiller L, Underwood R, Gurland B, Yurkschatt C, et al. An electronic safety screening process during inpatient computerized physician order entry improves the efficiency of magnetic resonance imaging exams. Acad Radiol 2013. December;20(12):1592-7. [DOI] [PubMed] [Google Scholar]
- 122.Knight AM, Kravet SJ, Kiyatkin D, Leff B. The effect of computerized provider order entry on medical students’ ability to write orders. Teach Learn Med 2012;24(1):63-70. [DOI] [PubMed] [Google Scholar]
- 123.Muzyk AJ, Rivelli SK, Jiang W, Heinz H, Ray-field A, Gagliardi JP. A computerized physician order entry set designed to improve safety of intravenous haloperidol utilization: a retrospective study in agitated hospitalized patients. Drug Saf 2012. September 1;35(9):725-31. [DOI] [PubMed] [Google Scholar]
- 124.Peikari HR, Zakaria MS, Yasin NM, Shah MH, Elhissi A. Role of computerized physician order entry usability in the reduction of prescribing errors. Healthc Inform Res 2013. June;19(2):93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Magid S, Forrer C, Shaha S. Duplicate orders: an unintended consequence of computerized provider/physician order entry (CPOE) implementation: analysis and mitigation strategies. Appl Clin Inform 2012. October 17;3(4):377-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van der Veen W de Gier HJ van der Schaaf T Taxis K, van den Bemt PM. Risk analysis and user satisfaction after implementation of computerized physician order entry in Dutch hospitals. Int J Clin Pharm 2013. April;35(2):195-201. [DOI] [PubMed] [Google Scholar]
- 127.Schreiber R, Peters K, Shaha SH. Computerized provider order entry reduces length of stay in a community hospital. Appl Clin Inform 2014. July 30;5(3):685-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hatfield MD, Cox R, Mhatre SK, Flowers WP, Sansgiry SS. Impact of computerized provider order entry on pharmacist productivity. Hosp Pharm 2014. May;49(5):458-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alsweed F, Alshaikh A, Ahmed A, Yunus F, Househ M. Impact of computerised provider order entry system on nursing workflow, patient safety, and medication errors: perspectives from the front line. Int J Electron Healthc 2014;7(4):287-300. [DOI] [PubMed] [Google Scholar]
- 130.Bedouch P, Tessier A, Baudrant M, Labarere J, Foroni L, Calop J, et al. Computerized physician order entry system combined with on-ward pharmacist: analysis of pharmacists’ interventions. J Eval Clin Pract 2012. August;18(4):911-8. [DOI] [PubMed] [Google Scholar]
- 131.Simon SR, Keohane CA, Amato M, Coffey M, Cadet B, Zimlichman E, et al. Lessons learned from implementation of computerized provider order entry in 5 community hospitals: a qualitative study. BMC Med Inform Decis Mak 2013. June 24;13:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hoonakker PL, Carayon P, Walker JM, Brown RL, Cartmill RS. The effects of Computerized Provider Order Entry implementation on communication in Intensive Care Units. Int J Med Inform 2013. May;82(5):e107-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Castellanos I, Rellensmann G, Scharf J, Barkle T. Computerized Physician Order Entry (CPOE) in pediatric and neonatal intensive care: Recommendations how to meet clinical requirements. Appl Clin Inform 2012. February 15;3(1):64-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chapman AK, Lehmann CU, Donohue PK, Aucott SW. Implementation of computerized provider order entry in a neonatal intensive care unit: Impact on admission workflow. Int J Med Inform 2012. May;81(5):291-5. [DOI] [PubMed] [Google Scholar]
- 135.Jeon J, Taneva S, Kukreti V, Trbovich P, Easty AC, Rossos PG, et al. Toward successful migration to computerized physician order entry for chemotherapy. Curr Oncol 2014. April;21(2):e221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nerich V, Borg C, Villanueva C, Thiery-Vuillemin A, Helias P, Rohrlich PS, et al. Economic impact of prescribing error prevention with computerized physician order entry of injectable antineoplastic drugs. J Oncol Pharm Pract 2013. March;19(1):8-17. [DOI] [PubMed] [Google Scholar]
- 137.Meisenberg BR, Wright RR, Brady-Copertino CJ. Reduction in chemotherapy order errors with computerized physician order entry. J Oncol Pract 2014. January;10(1):e5-9. [DOI] [PubMed] [Google Scholar]
- 138.Hanauer DA, Zheng K, Commiskey EL, Duck MG, Choi SW, Blayney DW. Computerized pre-scriber order entry implementation in a physician assistant-managed hematology and oncology inpatient service: effects on workflow and task switching. J Oncol Pract 2013. July;9(4):e103-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang JM, Park YS, Chung SP, Chung HS, Lee HS, You JS, et al. Implementation of a clinical pathway based on a computerized physician order entry system for ischemic stroke attenuates off-hour and weekend effects in the ED. Am J Emerg Med 2014. August;32(8):884-9. [DOI] [PubMed] [Google Scholar]
- 140.Brokel JM, Ward MM, Wakefield DS, Ludwig A, Schwichtenberg T, Atherton D. Changing patient care orders from paper to computerized provider order entry-based process. Comput Inform Nurs 2012. August;30(8):417-25. [DOI] [PubMed] [Google Scholar]
- 141.Cho I, Park H, Choi YJ, Hwang MH, Bates. Understanding the nature of medication errors in an ICU with a computerized physician order entry system. PLoS One 2014. December 19;9(12):e114243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hundt AS, Adams JA, Schmid JA, Musser LM, Walker JM, Wetterneck TB, et al. Conducting an efficient proactive risk assessment prior to CPOE implementation in an intensive care unit. Int J Med Inform 2013. January;82(1):25-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zimlichman E, Keohane C, Franz C, Everett WL, Seger DL, Yoon C, et al. Return on investment for vendor computerized physician order entry in four community hospitals: the importance of decision support. Jt Comm J Qual Patient Saf 2013. July;39(7):312-8. [DOI] [PubMed] [Google Scholar]
- 144.Forrester SH, Hepp Z, Roth JA, Wirtz HS, Devine EB. Cost-effectiveness of a computerized provider order entry system in improving medication safety ambulatory care. Value Health 2014. June;17(4):340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pettit RS, Zimmerman CR, Alaniz C, Dorsch MP. Cost analysis before and after implementation of a computerized physician order entry order form for enoxaparin. P&T 2012. February;37(2):107-11. [PMC free article] [PubMed] [Google Scholar]
- 146.Slain T, Rickard-Aasen S, Pringle JL, Hegde GG, Shang J, Johnjulio W, et al. Incorporating screening, brief intervention, and referral to treatment into emergency nursing workflow using an existing computerized physician order entry/clinical decision support system. J Emerg Nurs 2014. November;40(6):568-74. [DOI] [PubMed] [Google Scholar]
- 147.Gandhi S, Tyono I, Pasetka M, Trudeau M. Evaluating an oncology systemic therapy computerized physician order entry system using international guidelines. J Oncol Pract 2014. March;10(2):e14-25. [DOI] [PubMed] [Google Scholar]
- 148.Blankenship JF, Rogers L, White J, Carey A, Fosnocht D, Hopkins C, et al. Prospective evaluation of the treatment of pain in the ED using computerized physician order entry. Am J Emerg Med 2012. October;30(8):1613-6. [DOI] [PubMed] [Google Scholar]
- 149.Ranji SR, Rennke S, Wachter RM. Computerised provider order entry combined with clinical decision support systems to improve medication safety: a narrative review. BMJ Qual Saf 2014;23:773-780. [DOI] [PubMed] [Google Scholar]
- 150.Cowan L. Literature review and risk mitigation strategy for unintended consequences of computerized physician order entry. Nurs Econ 2013. Jan-Feb;31(1):27-31. [PubMed] [Google Scholar]
- 151.Belle A, Kon MA, Najarian K. Biomedical informatics for computer-aided decision support systems: a survey. ScientificWorldJournal 2013;2013:769639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miller AC, Ziad-Miller A, Elamin EM. Brain death and Islam: the interface of religion, culture, history, law, and modern medicine. Chest 2014. October;146(4):1092-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Paternotte E, van Dulmen S, van der Lee N, Scherpbier AJ, Scheele F. Factors influencing intercultural doctor-patient communication: a realist review. Patient Educ Couns 2015. April;98(4):420-45. [DOI] [PubMed] [Google Scholar]
- 154.Peleg M. Computer-interpretable clinical guidelines: a methodological review. J Biomed Inform 2013. August;46(4):744-63. [DOI] [PubMed] [Google Scholar]
- 155.Wang D, Peleg M, Tu SW, Boxwala AA, Ogunyemi O, Zeng Q, Greenes, et al. Design and Implementation of the GLIF3 guideline execution engine. J Biomed Inform 2004; 37(3): 305318. [DOI] [PubMed] [Google Scholar]
- 156.Sutton DR, Fox J. The Syntax and Semantics of the PROforma guideline modelling language. J Am Med Inform Assoc 2003. Sep-Oct;10(5):433-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rucker DW, Steele AW, Douglas IS, Couderc CA, Hardel GG. Design and Use of a Joint Order Vocabulary Knowledge Representation Tier in a Multi-tier CPOE Architecture. AMIA Annu Symp Proc 2006:669-73. [PMC free article] [PubMed] [Google Scholar]
- 158.Charani E, Kyratsis Y, Lawson W, Wickens H, Brannigan ET, Moore LS, et al. An analysis of the development and implementation of a smartphone application for the delivery of antimicrobial prescribing policy: lessons learnt. J Antimicrob Chemother 2013. April;68(4):960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lanzola G, Parimbelli E, Micieli G, Cavallini A, Quaglini S. Data quality and completeness in a web stroke registry as the basis for data and process mining. J Healthc Eng 2014;5(2):163-84. [DOI] [PubMed] [Google Scholar]
- 160.Beuscart-Zéphir MC, Borycki E, Carayon P, Jaspers MW, Pelayo S. Evolution of human factors research and studies of health information technologies: the role of patient safety. Yearb Med Inform 2013;8(1):67-77. [PubMed] [Google Scholar]
- 161.Chiarella M, Carney T, Chiarella M, Satchell C, Walton M, Bennett B, et al. Regulating healthcare complaints: a literature review. Int J Health Care Qual Assur 2014;27(6):505-18. [DOI] [PubMed] [Google Scholar]
- 162.García-Sáez G, Rigla M, Martínez-Sarriegui I, Shalom E, Peleg M, Broens T, et al. Patient-oriented Computerized Clinical Guidelines for Mobile Decision Support in Gestational Diabetes. J Diabetes Sci Technol 2014. March 6;8(2):238-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sacchi L, Rubrichi S, Rognoni C, Panzarasa S, Parimbelli E, Mazzanti A, et al. From decision to shared-decision: Introducing patients’ preferences into clinical decision analysis. Artif Intell Med 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 164.Quaglini S, Shahar Y, Peleg M, Miksch S, Napolitano C, Rigla M, et al. Supporting shared decision making within the MobiGuide project. AMIA Annu Symp Proc 2013. November 16;2013:1175-84. [PMC free article] [PubMed] [Google Scholar]
- 165.Roshanov PS, Fernandes N, Wilczynski JM, Hemens BJ, You JJ, Handler SM, et al. Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ 2013. February 14;346. [DOI] [PubMed] [Google Scholar]
- 166.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making 2015. January;35(1):114-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bright TJ, Wong A, Dhurjati R, Bristow E, Bastian L, Coeytaux RR, et al. Effect of clinical decision-support systems: a systematic review. Ann Intern Med 2012. July 3;157(1):29-43. [DOI] [PubMed] [Google Scholar]
- 168.McDermott MS, While AE. Maximizing the healthcare environment: a systematic review exploring the potential of computer technology to promote self-management of chronic illness in healthcare settings. Patient Educ Couns 2013. July;92(1):13-22. [DOI] [PubMed] [Google Scholar]