Abstract
Objective
To evaluate the relationship of maternal antenatal magnesium sulfate (MgSO4) with neonatal cranial ultrasound abnormalities and cerebral palsy (CP).
Study design
In a randomized trial of MgSO4 or placebo in women at high risk of preterm delivery, up to three cranial ultrasound were obtained in the neonatal period. Images were reviewed by at least two pediatric radiologists masked to treatment and other clinical conditions. Diagnoses were predefined for intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), intracerebral echolucency or echodensity, and ventriculomegaly. CP was diagnosed at two years by standardized neurological examination.
Results
Intraventricular hemorrhage, PVL, intracerebral echolucency or echodensity, and ventriculomegaly were all strongly associated with an increased risk of CP. MgSO4 administration did not affect the risk of cranial ultrasound abnormality observed at 35 weeks post-menstrual age or later. However, for the 82% of infants born at <32 weeks gestation, MgSO4 was associated with a reduction in risk of echolucency or echodensity. The reduction in risk for echolucency explained 21% of the effect of MgSO4 on CP (p=0.04), and for echodensity explained 20% of the effect (p=0.02).
Conclusions
MgSO4 given prior to preterm delivery was associated with decreased risk of developing echodensities and echolucencies at <32 weeks gestation. However, this effect can only partially explain the effect of MgSO4 on CP at two years of age.
Keywords: cranial ultrasound, magnesium sulfate, echodensity, echolucency, intraventricular hemorrhage, periventricular leukomalacia, germinal matrix
Infants born before 32 weeks of gestation account for almost 2% of all births in the United States (1). These infants are at the highest risk for poor neurodevelopmental outcomes, most frequently poor intellectual outcome and cerebral palsy (CP) (2–9).
In prematurely born infants, cerebral lesions detected by neuroimaging are strongly associated with adverse neurodevelopmental outcome (10). Cystic periventricular white matter injury, ventricular dilatation, and germinal matrix hemorrhage can readily be detected by cranial ultrasound, although noncystic white matter injury is not as well visualized. Because cystic manifestations of injuries may take 2–5 weeks to develop, it is important to repeat ultrasound scans over time (11). In infants whose findings are not cystic and resolve in the neonatal period, prognosis is improved over those who develop cystic lesions (12). Not all children who develop CP have identifiable neonatal cranial ultrasound lesions. In one study, 6% of infants with no lesions born at gestational age 28–32 weeks and 8% at gestational age < 28 weeks developed CP (13); in another study, 4% of those with no lesions born at gestational age < 30 weeks had CP (14), and in a third study of infants <1000 grams at birth, CP occurred in 9.4% (15) of those with no lesions. In contrast, 28 to 52% of those who do have cranial ultrasound lesions will develop CP (10, 14).
A meta-analysis of several trials has shown that magnesium sulfate (MgSO4) administered to women at risk of delivering before 32 weeks of gestation reduces CP in their infants (16). As part of a randomized trial to test whether antenatal MgSO4 administered to women about to deliver prematurely reduces the risk of CP in their children, serial neonatal cranial ultrasounds were obtained on the infants (17). Because it is not known whether MgSO4 has an effect on the cerebral lesions detected during the neonatal period or whether the reduction of the risk of CP is through some other pathway, in this secondary outcome study we sought to evaluate whether there is a protective effect of MgSO4 on cranial ultrasound abnormalities that may mediate the decrease seen in CP at age two years.
Methods
The primary trial was conducted by the Maternal Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (ClinicalTrials.gov: NCT00014989). A total of 2241 women between 24 and 32 weeks gestation were randomized to receive either MgSO4 (loading dose 6 gm infused over 20–30 minutes followed by maintenance infusion of 2 gm/hr) or an identical appearing placebo. Eligible women were at high risk of preterm delivery due to ruptured membranes or advanced preterm labor, or fetal indication for delivery (eg, severe growth restriction). Neurodevelopmental outcome was evaluated at 6 months, 12 months, and 24 months, corrected for prematurity. CP was diagnosed at 24 months by examiners masked to study treatment assignment (17). Examiners attended central training sessions and were annually certified through videotaped exams. The study was approved by the institutional review boards of all participating clinical centers, and all enrolled women gave written informed consent. Details of the protocol are available elsewhere (17).
All infants received up to three cranial ultrasound per study protocol. Those born at term had a head ultrasound during the delivery admission or within a week of discharge, and those born at 32 weeks 0 days to 36 weeks 6 days had a head ultrasound at 35 – 40 weeks post-menstrual age. Those born before 32 weeks of gestation could have up to three ultrasounds: the first before 3 days of age, the second at 3 to 4 weeks of age and a third at 35 weeks post-menstrual age or at discharge, whichever was later. At least three coronal and three sagittal views were obtained for each study via the anterior fontanelle. For the purposes of this report the cranial ultrasound that was conducted at 35 weeks or later for each infant is designated as the “term” ultrasound.
Images were reviewed centrally by a team of three pediatric radiologists very experienced in cranial ultrasound interpretation. Each image was reviewed independently by two of the three. When there was disagreement, the third radiologist adjudicated the reading and a consensus diagnosis was determined (among the three radiologists). Prior to each reading session, a set of 10 reference cases with various abnormalities were reviewed to reinforce the agreed-upon scoring criteria. The radiologists were masked to any clinical findings including study treatment assignment.
Studies were evaluated for the presence of germinal matrix hemorrhage, periventricular leukomalacia (echodense and/or echolucent), intracerebral echolucency or echodensity, and ventriculomegaly. The Papile and Burnstein scoring system was used for germinal matrix hemorrhage (18). For this study, grade III was diagnosed when the amount of blood was sufficient to fill and distend the ventricles, i.e., a small intraventricular hemorrhage (IVH) within an enlarged ventricle due to post hemorrhagic hydrocephalus and not due to further distension by subsequent hemorrhage was not upgraded to grade III. However, if more bleeding occurred and the additional blood distended the ventricles, the IVH was upgraded to grade III. All parenchymal abnormal lesions were categorized as echogenic or echolucent. These were further categorized as grade IV periventricular hemorrhagic infarcts or periventricular leukomalacia (PVL) when appropriate. Ventriculomegaly was defined as temporal and lateral horn dilatation, with or without cortical thinning.
Analyses were conducted on two cohorts of infants: the whole cohort comprising data from the term ultrasound, and the cohort of those born at less than 32 weeks of gestation using data from all the ultrasounds (up to three). For the babies born at less than 32 weeks of gestation, a finding was considered present if diagnosed at any of the ultrasounds.
Statistical Analyses
For each analysis cohort, the effect of MgSO4 versus placebo on each cranial ultrasound abnormality was analyzed by log binomial regression, taking into account the correlation between twins. The association of each cranial ultrasound abnormality with CP was also analyzed, with similar methods. Lastly, mediation analysis was used to examine the relationship of both MgSO4 and cranial ultrasound abnormalities with CP. In order to compute the mediation effect, first the main effect of MgSO4 on CP was estimated. Second, a separate model with each cranial ultrasound abnormality added was constructed, and the change to the main effect of MgSO4 was computed. The Sobel test was used to assess if the amount of mediation was different from zero (19). To assess the potential effect of missing CP outcomes due to death after neonatal discharge (a competing risk), analyses were repeated with a combined outcome of CP or death. A nominal p-value of <0.05 was chosen to denote significance. No adjustments were made for multiple comparisons.
Results
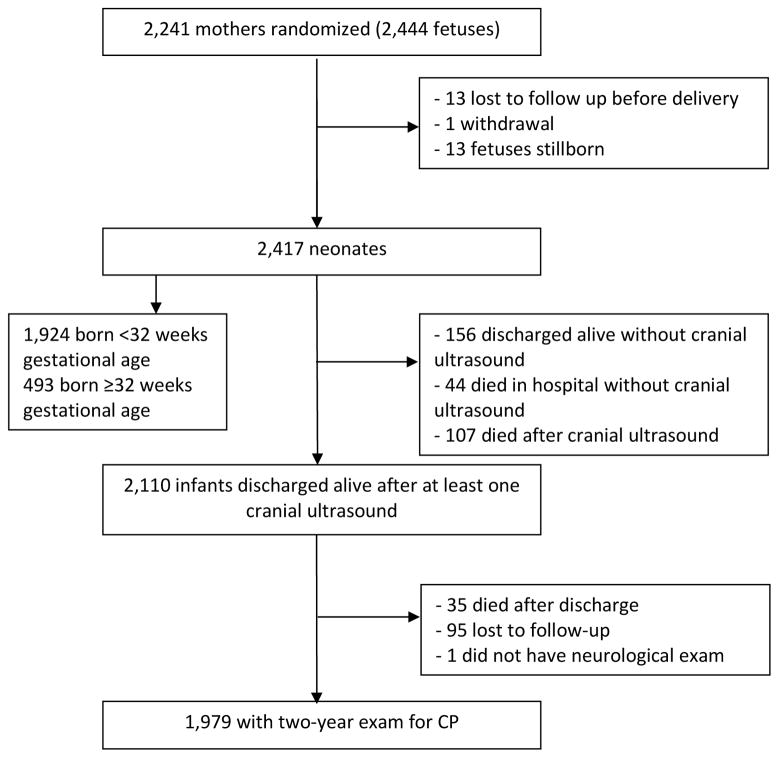
Enrollment lasted from December 1997 to May 2004. Of the 2241 women (2444 fetuses) who were included in the trial, 2110 infants were discharged alive and had at least one cranial ultrasound (Figure; available at www.jpeds.com). An assessment for CP was performed on 1979 of the children (94%) at two years of age. Table I shows the characteristics of the children in the cohort: 953 of the children (48%) were in the MgSO4 group, and 1026 in the placebo group; 1613 (82%) were born before 32 weeks of gestation and 17% were twins. In the 1979 children in this analysis, as in the overall trial, MgSO4 was associated with a reduction in CP in the children at two years of age: 4.2% in the MgSO4 group versus 6.9% in the placebo group (OR 0.59; 95% CI 0.39–0.88). MgSO4 was also associated with a reduced risk of CP (OR 0.63, 95% CI 0.42–0.95) among the 1613 infants born before 32 weeks.
Figure 1.
Selection of study cohort
Table 1.
Patient Characteristics
| MgSO4 (n=953) | Placebo (n=1026) | |
|---|---|---|
|
| ||
| Number of cranial ultrasounds, n (%) | ||
| One | 257 (27.0%) | 263 (25.6%) |
| More than one | 696 (73.0%) | 763 (74.4%) |
| Cranial ultrasound at 35 weeks or greater, n (%) | 854 (89.6%) | 922 (89.9%) |
|
| ||
| Gestational age at birth, n (%) | ||
| < 32 weeks | 777 (81.5%) | 836 (81.5%) |
| 32 weeks or greater | 176 (18.5%) | 190 (18.5%) |
|
| ||
| Birth weight, g, mean (SD) | 1417 (537) | 1428 (556) |
|
| ||
| Twins, n (%) | 151 (15.8%) | 188 (18.3%) |
|
| ||
| Male, n (%) | 508 (53.3%) | 519 (50.6%) |
| Female, n (%) | 445 (46.7%) | 507 (49.4%) |
|
| ||
| Maternal self-reported race, n (%) | ||
| African American | 431 (45.2%) | 442 (43.1%) |
| Caucasian | 343 (36.0%) | 376 (36.7%) |
| Hispanic | 157 (16.5%) | 185 (18.0%) |
| Other | 22 (2.3%) | 23 (2.2%) |
|
| ||
| Maternal education, yr, median (interquartile range) | 12 (11 – 13) | 12 (11 – 13) |
|
| ||
| Antenatal steroids, n (%) | 924 (97.0%) | 998 (97.3%) |
An initial discrepancy between the two radiologists assigned to read each ultrasound was found in 14% of the films. This included both disagreement on the presence or absence of any ultrasound finding, and a difference in degree (eg, a discrepancy between the reviewers of IVH grade III versus IV). Ninety percent (n=1776) had the term ultrasound. The remaining 10% did not differ by rate of MgSO4 assignment, but were born at a later gestational age and had a shorter hospital stay than those who did have the term ultrasound.
There was no effect of MgSO4 on any of the cranial abnormalities detected on the term ultrasound (Table II). Echolucency, PVL, ventriculomegaly, and IVH observed in the term ultrasound were all strongly associated with CP (all p < 0.001), with odds ratios varying from 3.1 (any IVH) to 70.9 (PVL) (Table III). However, there was no mediation effect of term-ultrasound cranial ultrasound abnormalities on CP (all p>0.05).
Table 2.
Effect of Magnesium Sulfate on Abnormalities Detected by Neonatal Cranial Ultrasound
| All children: Term ultrasounds | Children born before 32 weeks: most severe findings in serial ultrasounds | |||||
|---|---|---|---|---|---|---|
| MgSO4 N=854 n (%) | Placebo N=922 n (%) | Adjusted OR (95% CI)* | MgSO4 N=777 n (%) | Placebo N=836 n (%) | Adjusted OR (95% CI)* | |
| Echodensity** | n/a | n/a | n/a | 10 (1.3) | 28 (3.3) | 0.38 (0.19, 0.79) |
| Echolucency | 23 (2.7) | 40 (4.3) | 0.60 (0.36, 1.02) | 26 (3.3) | 46 (5.5) | 0.59 (0.36, 0.97) |
| PVL | 10 (1.2) | 16 (1.7) | 0.67 (0.30, 1.49) | 14 (1.8) | 19 (2.3) | 0.79 (0.39, 1.59) |
| Ventriculomegaly | 26 (3.0) | 32 (3.5) | 0.81 (0.47, 1.40) | 39 (5.0) | 45 (5.4) | 0.87 (0.55, 1.37) |
| IVH (all) | 92 (10.8) | 113 (12.3) | 0.86 (0.64, 1.16) | 160 (20.6) | 181 (21.7) | 0.93 (0.73, 1.18) |
| IVH grades III/IV | 6 (0.7) | 12 (1.3) | 0.54 (0.20, 1.45) | 15 (1.9) | 27 (3.2) | 0.57 (0.30, 1.09) |
| Any of the above | 123 (14.4) | 156 (16.9) | 0.82 (0.63, 1.06) | 186 (23.9) | 217 (26.0) | 0.89 (0.71, 1.12) |
MgSO4 = magnesium sulfate; PVL = periventricular leukomalacia; IVH = intraventricular hemorrhage
Adjusted for correlations between twins
Since only three children had an echodensity detectable at the term ultrasound, these data are omitted.
Table 3.
Effect of Findings from the Term Ultrasound on Cerebral Palsy, and Mediation of the Association of MgSO4 with Cerebral Palsy
| MgSO4 | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect of Ultrasound Abnormality on the risk of CP (OR and 95% CI)* | CP (n=35) | No CP (n=819) | CP (n=65) | No CP (n=857) | Effect of MgSO4 on the risk of CP after adjusting for CUS finding (OR and 95% CI)** | % of the total effect explained by the CUS finding | p-value for effect of CUS finding† | |
| Echolucency | 17.0 (9.7, 29.5) | 7 | 16 | 20 | 20 | 0.61 (0.39, 0.94) | 16% | 0.06 |
| PVL | 70.9 (27.3, 184) | 7 | 3 | 13 | 3 | 0.56 (0.36, 0.88) | 3% | 0.33 |
| Ventriculomegaly | 9.9 (5.6, 17.6) | 4 | 22 | 15 | 17 | 0.56 (0.36, 0.87) | 2% | 0.45 |
| IVH (all) | 3.1 (1.9, 5.0) | 8 | 84 | 19 | 94 | 0.57 (0.37, 0.87) | 4% | 0.33 |
| IVH grades III/IV | 18.0 (6.8, 47.4) | 0 | 6 | 9 | 3 | 0.58 (0.38, 0.89) | 6% | 0.23 |
| Any of the above | 6.0 (4.0, 9.1) | 16 | 107 | 33 | 123 | 0.59 (0.38, 0.90) | 13% | 0.14 |
MgSO4 = magnesium sulfate; CP = cerebral palsy; PVL = periventricular leukomalacia; IVH = intraventricular hemorrhage; CUS = cranial ultrasound
Odds ratio (95% CI) adjusted for correlations between twins.
Odds ratio (95% CI) adjusted for correlations between twins. Note that without including the effect of the CUS finding, the odds ratio (95% CI) for CP of MgSO4 is 0.56 (0.37, 0.86)
Sobel test
In the group of infants born before 32 weeks and receiving serial ultrasounds, there was a reduced risk of echodensity (OR 0.38, 95% CI 0.19–0.79) and echolucency (OR 0.59, 95% CI 0.36–0.97) detected on any ultrasound for those receiving MgSO4 (n=777) compared with placebo (n=836; Table II). All reported cranial abnormalities were strongly associated with CP (all p < 0.001), with odds ratios varying from 3.3 (any IVH) to 34.9 (PVL) (Table IV). Twenty-nine percent (24/84) with ventriculomegaly on any scan developed CP, 43% (18/42) with grade III/IV IVH, and 67% (22/33) with PVL. The decrease in echodensity and echolucency associated with MgSO4 partially explains the reduction in CP due to MgSO4. When the presence of echodensity was added to the regression model examining the effect of MgSO4 on CP, the OR of CP increased from 0.63 to 0.69. Reduction of echodensity explains 20% of the effect of MgSO4 on reducing CP (p=0.02). Very similar mediation effects are seen for echolucencies. The effect of MgSO4 on reducing echolucencies explains 21% of the effect of MgSO4 on CP (p=0.04). There were no mediation effects seen with PVL (3%), ventriculomegaly (1%) or IVH (3%). However, 11% (p=0.10) of the effect of MgSO4 on CP is explained by the reduction of IVH grade III or IV.
Table 4.
Effect of Findings from the Most Severe Findings of Three Cranial Ultrasounds of Children Born at <32 weeks on Cerebral Palsy, and Mediation of the Association of MgSO4 with Cerebral Palsy
| MgSO4 | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect of Ultrasound Abnormality on the risk of CP (OR and 95% CI)* | CP (n=40) | No CP (n=737) | CP (n=66) | No CP (n=770) | Effect of MgSO4 on the risk of CP after adjusting for CUS finding (OR and 95% CI)** | % of the total effect explained by the CUS finding | p-value for effect of CUS finding† | |
| Echodensity | 9.6 (4.9, 19.1) | 1 | 9 | 13 | 15 | 0.69 (0.45, 1.05) | 20% | 0.02 |
| Echolucency | 12.6 (7.4, 21.5) | 7 | 19 | 22 | 24 | 0.69 (0.45, 1.05) | 21% | 0.04 |
| PVL | 34.9 (16.6, 73.4) | 8 | 6 | 14 | 5 | 0.63 (0.40, 0.97) | 3% | 0.51 |
| Ventriculomegaly | 6.8 (4.0, 11.5) | 6 | 33 | 18 | 27 | 0.62 (0.41, 0.95) | 1% | 0.54 |
| IVH (all) | 3.3 (2.2, 5.0) | 15 | 145 | 32 | 149 | 0.63 (0.42, 0.96) | 3% | 0.54 |
| IVH grades III/IV | 12.4 (6.4, 24.0) | 2 | 13 | 16 | 11 | 0.66 (0.43, 1.00) | 11% | 0.10 |
| Any of the above | 5.2 (3.4, 7.8) | 21 | 165 | 43 | 174 | 0.64 (0.42, 0.97) | 8% | 0.32 |
MgSO4 = magnesium sulfate; CP = cerebral palsy; PVL = periventricular leukomalacia; IVH = intraventricular hemorrhage; CUS = cranial ultrasound
Odds ratio (95% CI) adjusted for correlations between twins.
Odds ratio (95% CI) adjusted for correlations between twins. Note that without including the effect of the CUS finding, the odds ratio (95% CI) for CP of MgSO4 is 0.63 (0.42, 0.95)
Sobel test
In order to measure any potential effect of missing CP diagnoses for those who died after initial neonatal hospital discharge but before the child’s neurodevelopmental exam (MgSO4: 6 deaths following and 12 not following cranial ultrasound abnormality; Placebo: 3 deaths following and 14 not following cranial ultrasound abnormality), all analyses with CP were repeated with a combined outcome of CP or death. None of the findings were substantially changed from those with CP alone (Tables V and VI; available at www.jpeds.com).
Table 5.
Effect of Findings from the Term Ultrasound on Cerebral Palsy/Death, and Mediation of the Association of MgSO4 with Cerebral Palsy/Death
| MgSO4 | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect of Ultrasound Abnormality on the risk of CP/death (OR and 95% CI)* | CP/death (n=47) | No CP (n=819) | CP/death (n=82) | No CP (n=857) | Effect of MgSO4 on the risk of CP/death after adjusting for CUS finding** | % of the total effect explained by the CUS finding | p-value for effect of CUS finding† | |
| Echodensity | 13.3 (7.8, 22.7) | 8 | 16 | 21 | 20 | 0.64 (0.43, 0.94) | 15% | 0.07 |
| Echolucency | 55.1 (21.5, 141) | 8 | 3 | 13 | 3 | 0.59 (0.40, 0.88) | 1% | 0.45 |
| PVL | 8.7 (5.0, 15.0) | 6 | 22 | 16 | 17 | 0.59 (0.41, 0.87) | 1% | 0.58 |
| IVH (all) | 2.5 (1.6, 4.0) | 9 | 84 | 21 | 94 | 0.61 (0.42, 0.88) | 4% | 0.32 |
| IVH grades III/IV | 13.8 (5.3, 35.8) | 0 | 6 | 9 | 3 | 0.61 (0.42, 0.90) | 6% | 0.24 |
| Any of the above | 4.7 (3.2, 6.8) | 19 | 107 | 36 | 123 | 0.62 (0.43, 0.90) | 11% | 0.16 |
MgSO4 = magnesium sulfate; CP = cerebral palsy; PVL = periventricular leukomalacia; IVH = intraventricular hemorrhage
Odds ratio (95% CI) adjusted for correlations between twins
Odds ratio (95% CI) adjusted for correlations between twins. Note that without including the effect of the CUS finding, this odds ratio (95% CI) for CP/death of MgSO4 is 0.60 (0.41, 0.87)
Sobel test
Table 6.
Effect of Findings from the Most Severe Findings of Three Cranial Ultrasounds of Children Born at <32 weeks on Cerebral Palsy/Death, and Mediation of the Association of MgSO4 with Cerebral Palsy/Death
| MgSO4 | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect of Ultrasound Abnormality on the risk of CP/death (OR and 95% CI)* | CP/death (n=57) | No CP (n=737) | CP/death (n=82) | No CP (n=770) | Effect of MgSO4 on the risk of CP/death after adjusting for CUS finding** | % of the total effect explained by the CUS finding | p-value for effect of CUS finding† | |
| Echodensity | 7.0 (3.6, 13.9) | 1 | 9 | 13 | 15 | 0.78 (0.54, 1.11) | 22% | 0.02 |
| Echolucency | 9.7 (5.8, 16.1) | 8 | 19 | 23 | 24 | 0.78 (0.54, 1.13) | 26% | 0.045 |
| PVL | 26.7 (12.8, 55.6) | 9 | 6 | 14 | 5 | 0.72 (0.50, 1.05) | 3% | 0.63 |
| Ventriculomegaly | 6.0 (3.7, 9.7) | 9 | 33 | 19 | 27 | 0.72 (0.50, 1.03) | 1% | 0.73 |
| IVH (all) | 2.5 (1.8, 3.7) | 19 | 145 | 34 | 149 | 0.73 (0.51, 1.04) | 3% | 0.62 |
| IVH grades III/IV | 10.3 (5.5, 19.2) | 3 | 13 | 17 | 11 | 0.76 (0.53, 1.09) | 15% | 0.11 |
| Any of the above | 3.8 (2.6, 5.4) | 27 | 165 | 46 | 174 | 0.73 (0.51, 1.05) | 8% | 0.40 |
MgSO4 = magnesium sulfate; CP = cerebral palsy; PVL = periventricular leukomalacia; IVH = intraventricular hemorrhage
Odds ratio (95% CI) adjusted for correlations between twins
Odds ratio (95% CI) adjusted for correlations between twins. Note that without including the effect of the CUS finding, this odds ratio (95% CI) for CP/death of MgSO4 is 0.72 (0.51, 1.03)
Sobel test
Discussion
Our data reaffirm the previously documented strong association of neonatal cranial ultrasound findings (ventricular dilatation, PVL, and IVH) with CP (20–23). Prenatal MgSO4 in prospective and retrospective studies has also been associated with a decreased risk of IVH and white matter disease in infants born preterm (24–27). Other studies did not find a decrease in IVH with MgSO4 (28,29). In the French PREMAG study, which had a higher rate of maternal-fetal infection and only enrolled 62% of the planned sample size, there were non-significant decreases seen in white matter disease in the group treated with MgSO4 compared with placebo (30).
Randomized controlled trials and observational studies have found a decreased risk of CP when MgSO4 is administered to women with threatened preterm delivery (16,22,23). Previously, we determined in our primary trial that MgSO4 administration was associated with a reduced rate of CP among survivors to age 2 years (17). In this study we explored whether the decreased risk of CP following MgSO4 administered before delivery was mediated by the relationship of treatment to abnormalities seen on early cranial ultrasound examinations. We did find a decrease in clinically important cranial ultrasound lesions in those who had been treated with MgSO4 compared with placebo. Although the effect on echolucencies and echodensities was statistically significant for our early preterm group, the point estimates of the odds ratios for echolucency were very similar in our early preterm group (OR 0.59, p=0.04) and for the term ultrasound in the entire population (OR 0.60, p=0.06). Furthermore, we found that this reduction of cranial ultrasound abnormalities partially explained the reduction of CP from MgSO4.
There are a number of possible mechanisms by which MgSO4 could mediate its protective effect, including blocking the N-methyl-D-aspartate receptors that allow glutamate release and influx of calcium (16), increasing platelet aggregation and adhesion, enhancing immune response and modulating inflammatory reactions, and stabilizing membranes with reduction in free radicals (31). MgSO4 can reduce the risk of cell death by decreasing proinflammatory cytokines or free radicals produced during hypoxic-ischemic reperfusion (32). The compound may also have a beneficial effect by lowering neonatal cerebral blood flow (33), especially in IVH, when fluctuations of cerebral blood flow and fragility of the germinal matrix cerebrovasculature may have a role in causing damage (34).
There are several limitations of our study. Although the most recent American Academy of Neurology practice guideline on neonatal neuroimaging recommends cranial ultrasound serially in preterm neonates <30 weeks of gestation (35), MRI and advanced MRI techniques provide a more detailed assessment, particularly at term and later (36). Our study did not have MRI imaging and thus lesions such as focal noncystic white matter abnormalities may have been missed. However, by performing serial cranial ultrasound in the neonatal period, including term gestational age, most severe abnormal white matter injury can be detected by the development of cystic leukomalacia; moderate and mild injury is less reliably detected. Echodensities in the periventricular white matter, which may be ipsilateral to a hemorrhage (ie, Grade IV IVH) or may occur independently of IVH, often bilaterally (ie, PVL), may gradually change to echolucencies and end up as visible cystic lesions (37). Furthermore, although it would have been informative to perform an analysis of those diagnosed with moderate to severe CP (without mild CP), the relatively low rates in our cohort precluded this more limited analysis.
Although the proportions of study infants without any cranial ultrasound (6.9%) or without a two-year exam for CP (4.6%) were relatively small, it is possible that these missing data could have an impact on our results. Not surprisingly, those without the term scan were more likely to have been born at a later gestational age and to have a shorter postnatal hospital stay. These neonates in general would be less likely to have demonstrated cranial ultrasound abnormalities or have CP. Interestingly, those who did have a cranial ultrasound but subsequently lost to follow-up before the two-year exam (n=95) were more likely to have PVL identified on the term scan (but not any other cranial ultrasound abnormality). However, there was no imbalance by treatment group, either in general between those lost to follow-up and not, or specifically in those with PVL.
This study provides an in-depth analysis of the cranial ultrasound findings not previously described from a large clinical trial of neonates. In summary, about one quarter of the infants examined with cranial ultrasound had one or more of the following sonographic abnormalities: parenchymal echodensities, parenchymal echolucencies, ventriculomegaly, IVH, or any combination. We confirmed that abnormalities identified by cranial ultrasound were strongly associated with CP. We saw no effect of MgSO4 on cranial ultrasound findings done at a late post-conceptual age (equivalent to >35 weeks) for the entire cohort, but for those born <32 weeks gestation, MgSO4 was associated with a lowered risk of specific cranial ultrasound abnormalities, thus providing a possible mechanism by which MgSO4 exerts its protective effect on CP. There was a reduced risk of echodensity or echolucency in those given MgSO4 compared with placebo, and we observed a mediation of the effect on CP attributable to those same abnormalities. This new information from our study adds to the prognostic information available to neonatologists when counseling families, particularly with the increased use of antenatal MgSO4 for CP prevention.
Acknowledgments
Supported by the National Institute of Neurological Disorders and Stroke (NINDS) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; HD27869, HD34208, HD34116, HD40544, HD27915, HD34136, HD21414, HD27917, HD27860, HD40560, HD40545, HD40485, HD40500, HD27905, HD27861, HD34122, HD40512, HD53907, HD34210, HD21410, HD36801, HD19897, and M01-RR-000080). Comments and views of the authors do not necessarily represent the views of the NICHD or NINDS.
The authors thank Allison Todd, MSN, RN (University of Alabama at Birmingham), for protocol development and coordination between clinical research centers; Elizabeth Thom, PhD (George Washington University Biostatistics Center), for protocol development, data management and statistical analysis; and Karin B. Nelson, MD (National Institute of Neurological Disorders and Stroke) and Catherine Y. Spong, MD (Eunice Kennedy Shriver National Institute of Child Health and Human Development) for protocol development and oversight.
Abbreviations
- CP
cerebral palsy
- IVH
intraventricular hemorrhage
- MgSO4
magnesium sulfate
- PVL
periventricular leukomalacia
Appendix
The following investigators, in addition to those listed as authors, are members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network:
University of Alabama at Birmingham, Birmingham, AL: John C. Hauth, MD; Allison Todd, RN, MSN, Tawanda Hill, RN, MSN, Stacy Harris, RN, BSN, Kathleen G. Nelson, MD, Fred J. Biasini, PhD; University of Utah, Salt Lake City, UT: Kristine Anderson, RN, BSN, Marla K. Jensen, BSN, Lori A. Williams, RN (Intermountain Healthcare), Lisa H. Fullmer, BSN, Anna M. Guzman, RN; Case Western Reserve University-MetroHealth Medical Center, Cleveland, OH: Marc Collin, MD, George VanBuren, MD, Cynthia Milluzzi, RN, BSN, BFA, Monica Fundzak, APN, MSN, Cathy Santori, RN; The Ohio State University, Columbus, OH: Francee Johnson, BSN, Mark B. Landon, MD, Cheryl Latimer, BSN, MS, Valerie Curry, BSN, Sandra Meadows, BS, MPH; Thomas Jefferson University, Philadelphia, PA: Anthony Sciscione, DO, Michelle DiVito, RN, MSN, Mary Talucci, RN, MSN, Shobhana A. Desai, MD, David A. Paul, MD; University of Tennessee, Memphis, TN: Baha M. Sibai, MD, Risa D. Ramsey, PhD, RN, BSN, William C. Mabie, MD, Lu Kao, RN, Melanie Cassie, RN; Wayne State University, Detroit, MI: Gwendolyn S. Norman, RN, BSN, MPH, Debra Driscoll, RN, BSN, Barbara Steffy, RN, Mitchell P. Dombrowski, MD; Wake Forest University Health Sciences, Winston-Salem, NC: Paul J. Meis, MD, Melissa Swain, RN, Kurt Klinepeter, MD, T. Michael O’Shea, MD, Linda Steele, RN; University of North Carolina at Chapel Hill, Chapel Hill, NC: Kenneth J. Moise, Jr, MD, Seth Brody, MD, Janice Bernhardt, RN, MS, Karen Dorman, RN, MS; University of Texas Health Science Center at Houston-Children’s Memorial Hermann Hospital, Houston, TX: Larry C. Gilstrap, III, MD, Mary-Clare Day, RN, BSN, Erin Flinn-Gildersleeve, RN, BSN, Felecia Ortiz, RN, BSN; Marcia Kerr, RN; Columbia University, New York, NY: Victoria Pemberton, RN, MS, Lisa Paley, CCRC, Charles Paley, MD, Sabine Bousleiman, MSN, MSPH, Vilmarie Carmona; Brown University, Providence, RI: JoAnn Tillinghast, MSN, Donna Allard, RNC, Betty Vohr, MD, Lucy Noel, AD, Kathleen McCarten, MD; University of Cincinnati, Cincinnati, OH: Menachem Miodovnik, MD, Nancy Elder, MSN, Wendy Girdler, RN, Teresa L. Gratton, PA; University of Chicago, Chicago, IL: Atef Moawad, MD, Marshall Lindheimer, MD, Phyllis L. Jones, RN, BSN, MPH; University of Miami, Miami, FL: Faith Doyle, ARNP, MSN, Carmen Alfonso, RN, May Scott, RN, Ramona Washington, BSN; Northwestern University, Chicago, IL: Gail Mallett, RN, BSN, Mercedes Ramos-Brinson, BS, Paula Simon, RN, BSN; University of Texas Medical Branch, Galveston, TX: Tony Wen, MD, Linda A. Goodrum, MD, George R. Saade, MD, Gayle L. Olson, MD, Hassan M. Harirah, MD, Elizabeth Martin, RN; University of Texas at San Antonio, San Antonio, TX: Elly M-J. Xenakis, MD, Deborah L. Conway, MD; Michael Berkus, MD; University of Pittsburgh, Pittsburgh, PA: Theresa M. Kamon, BSN (deceased), Margaret Cotroneo, RN, Cheryl A. Milford, EdS; University of Texas Southwestern Medical Center, Dallas, TX: M. Lynne Sherman, RN, Jody S. Dax, RN, BSN, Lisa Fay-Randall, RN, Carla F. Melton, RN, Ester Flores; The George Washington University Biostatistics Center, Washington, DC: Elizabeth Thom, PhD, Barbara Jones-Binns, MPH, Maureen Cooney, MPH, Molly L. Fischer, CRNP, MPH, Sarah McLaughlin, MPH, Kimberly Brunette, MPH, Elizabeth Fricks, MBA; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD: Catherine Y. Spong, MD, Susan Tolivaisa, BS, Donald McNellis, MD, Charlotte Catz, MD, Kimberly Howell, MA; National Institute of Neurological Disorders and Stroke, Bethesda, MD: Karin B. Nelson, MD (consultant); University of Pittsburgh, Pittsburgh, PA: James M. Roberts, MD (MFMU Network Steering Committee Chair).
Footnotes
The authors declare no conflicts of interest.
Trial Registration ClinicalTrials.gov: NCT00014989
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1–68. [PubMed] [Google Scholar]
- 2.Schlapbach LJ, Adams M, Proietti E, Aebischer M, Grunt S, Borradori-Tolsa C, et al. Outcome at two years of age in a Swiss national cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatr. 2012;12:198. doi: 10.1186/1471-2431-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonsdottir GM, Georgsdottir I, Haraldsson A, Hardardottir H, Thorkelsson T, Dagbjartsson A. Survival and neurodevelopmental outcome of ELBW children at 5 years of age: comparison of two cohorts born 10 years apart. Acta Paediatr. 2012;101:714–718. doi: 10.1111/j.1651-2227.2012.02645.x. [DOI] [PubMed] [Google Scholar]
- 4.Latal B. Prediction of neurodevelopmental outcome after preterm birth. Pediatr Neurol. 2009;40:413–419. doi: 10.1016/j.pediatrneurol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Marlow N, Wolke D, Bracewell MA, Samara M EPICure Study Group. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 6.Gibson AT. Outcome following preterm birth. Best Pract Res Clin Obstet Gynaecol. 2007;21:869–882. doi: 10.1016/j.bpobgyn.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Beaino G, Khoshnood B, Kaminski M, Marret S, Pierrat V, Vieux R, et al. Predictors of the risk of cognitive deficiency in very preterm infants: the EPIPAGE prospective cohort. Acta Paediatr. 2011;100:370–378. doi: 10.1111/j.1651-2227.2010.02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platt MJ, Cans C, Johnson A, Surman G, Topp M, Torrioli MG, et al. Trends in cerebral palsy among infants of very low birth weight (<1500 g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet. 2007;369:43–50. doi: 10.1016/S0140-6736(07)60030-0. [DOI] [PubMed] [Google Scholar]
- 9.Mikkola K, Ritari N, Tommiska V, Salokorpi T, Lehtonen L, Tammela O, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996–1997. Pediatrics. 2005;116:1391–1400. doi: 10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- 10.Kuban KC, Allred EN, O’Shea TM, Paneth N, Pagano M, Dammann O, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24:63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vries LS, Van Haastert IL, Rademaker KJ, Koopman C, Groenendaal F. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr. 2004;144:815–820. doi: 10.1016/j.jpeds.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Pisani F, Leali L, Moretti S, Turco E, Volante E, Bevilacqua G. Transient periventricular echodensities in preterms and neurodevelopmental outcome. J Child Neurol. 2006;21:230–235. doi: 10.2310/7010.2006.00059. [DOI] [PubMed] [Google Scholar]
- 13.de Vries LS, van Haastert IC, Benders MJ, Groenendaal F. Myth: cerebral palsy cannot be predicted by neonatal brain imaging. Semin Fetal Neonatal Med. 2011;16:279–287. doi: 10.1016/j.siny.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Himpens E, Oostra A, Franki I, Van Maele G, Vanhaesebrouck P, Van den Broeck C. Predictability of cerebral palsy and its characteristics through neonatal cranial ultrasound in a high-risk NICU population. Eur J Pediatr. 2010;169:1213–1219. doi: 10.1007/s00431-010-1207-6. [DOI] [PubMed] [Google Scholar]
- 15.Laptook AR, O’Shea TM, Shankaran S, Bhaskar B NICHD Neonatal Network. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115:673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 16.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009:CD004661. doi: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 19.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev. 1993;17:144–158. [Google Scholar]
- 20.Pinto-Martin JA, Riolo S, Cnaan A, Holzman C, Susser MW, Paneth N. Cranial ultrasound prediction of disabling and nondisabling cerebral palsy at age two in a low birth weight population. Pediatrics. 1995;95:249–254. [PubMed] [Google Scholar]
- 21.O’Shea TM, Kuban KC, Allred EN, Paneth N, Pagano M, Dammann O, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122:e662–e669. doi: 10.1542/peds.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F134–140. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marret S, Marchand-Martin L, Picaud JC, Hascoët JM, Arnaud C, Rozé JC, et al. Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS One. 2013;8:e62683. doi: 10.1371/journal.pone.0062683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuban KC, Leviton A, Pagano M, Fenton T, Strassfeld R, Wolff M. Maternal toxemia is associated with reduced incidence of germinal matrix hemorrhage in premature babies. J Child Neurol. 1992;7:70–76. doi: 10.1177/088307389200700113. [DOI] [PubMed] [Google Scholar]
- 25.FineSmith RB, Roche K, Yellin PB, Walsh KK, Shen C, Zeglis M, et al. Effect of magnesium sulfate on the development of cystic periventricular leukomalacia in preterm infants. Am J Perinatol. 1997;14:303–307. doi: 10.1055/s-2007-994149. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar S, Bhagat I, Dechert R, Schumacher RE, Donn SM. Severe intraventricular hemorrhage in preterm infants: comparison of risk factors and short-term neonatal morbidities between grade 3 and grade 4 intraventricular hemorrhage. Am J Perinatol. 2009;26:419–424. doi: 10.1055/s-0029-1214237. [DOI] [PubMed] [Google Scholar]
- 27.Kimberlin DF, Hauth JC, Goldenberg RL, Bottoms SF, Iams JD, Mercer B, et al. The effect of maternal magnesium sulfate treatment on neonatal morbidity in < or = 1000-gram infants. Am J Perinatol. 1998;15:635–641. doi: 10.1055/s-2007-994082. [DOI] [PubMed] [Google Scholar]
- 28.Paneth N, Jetton J, Pinto-Martin J, Susser M. Magnesium sulfate in labor and risk of neonatal brain lesions and cerebral palsy in low birth weight infants. Pediatrics. 1997;99:E1. doi: 10.1542/peds.99.5.e1. [DOI] [PubMed] [Google Scholar]
- 29.Canterino JC, Verma UL, Visintainer PF, Figueroa R, Klein SA, Tejani NA. Maternal magnesium sulfate and the development of neonatal periventricular leucomalacia and intraventricular hemorrhage. Obstet Gynecol. 1999;93:396–402. doi: 10.1016/s0029-7844(98)00455-4. [DOI] [PubMed] [Google Scholar]
- 30.Marret S, Marpeau L, Follet-Bouhamed C, Cambonie G, Astruc D, Delaporte B, et al. Effect of magnesium sulphate on mortality and neurologic morbidity of the very-preterm newborn (of less than 33 weeks) with two-year neurological outcome: results of the prospective PREMAG trial. Gynecol Obster Fertil. 2008;36:278–288. doi: 10.1016/j.gyobfe.2008.01.012. French. [DOI] [PubMed] [Google Scholar]
- 31.Hirtz DG, Nelson K. Magnesium sulfate and cerebral palsy in premature infants. Curr Opin Pediatr. 1998;10:131–137. doi: 10.1097/00008480-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Shokry M, Elsedfy GO, Bassiouny MM, Anmin M, Abozid H. Effects of antenatal magnesium sulfate therapy on cerebral and systemic hemodynamics in preterm newborns. Acta Obstet Gynecol Scand. 2010;89:801–806. doi: 10.3109/00016341003739542. [DOI] [PubMed] [Google Scholar]
- 33.Marret S, Doyle LW, Crowther CA, Middleton P. Antenatal magnesium sulphate neuroprotection in the preterm infant. Semin Fetal Neonatal Med. 2007;12:311–317. doi: 10.1016/j.siny.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol. 2014;41:47–67. doi: 10.1016/j.clp.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice Parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–1738. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 36.Ferriero DM, Miller SP. Imaging selective vulnerability in the developing nervous system. J Anat. 2010;217:429–435. doi: 10.1111/j.1469-7580.2010.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vries LS, Groenendaal F. Neuroimaging in the preterm infant. Ment Retard Dev Disabil Res Rev. 2002;8:273–280. doi: 10.1002/mrdd.10050. [DOI] [PubMed] [Google Scholar]