Abstract
Cholecystokinin (CCK) is released in response to lipid feeding and regulates pancreatic digestive enzymes vital to the absorption of nutrients. Our previous reports demonstrated that cholecystokinin knockout (CCK-KO) mice fed a 10 weeks of HFD had reduced body fat mass, but comparable glucose uptake by white adipose tissues and skeletal muscles. We hypothesized that CCK is involved in energy homeostasis and lipid transport from small intestine to tissues in response to acute treatment with dietary lipids. CCK-KO mice with comparable fat absorption had increased energy expenditure and were resistant to HFD-induced obesity. Using intraduodenal infusion of butter fat and intravenous infusion using Liposyn III, we determined the mechanism of lipid transport from small intestine to deposition in lymph and adipocytes in CCK-KO mice. CCK-KO mice had delayed secretion of Apo B48-chylomicrons, lipid transport to the lymphatic system, and triglyceride (TG)-derived fatty acid uptake by epididymal fat in response to acute treatment of introdudenal lipids. In contrast, CCK-KO mice had comparable TG clearance and lipid uptake by white adipocytes in response to TG in chylomicron-like emulsion. Thus, we concluded that CCK is important for lipid transport and energy expenditure to control body weight in response to dietary lipid feeding.
Keywords: fat absorption, lymph, fatty acid uptake, white adipose tissues
Introduction
Cholecystokinin (CCK) is secreted by intestinal endocrine cells in response to lipid and protein [1–3]. This fat-induced stimulation of CCK is dependent on the formation and secretion of chylomicrons [4]. CCK is involved in stimulating gallbladder contraction, intestinal motility and pancreatic secretion of insulin and pancreatic enzymes [5–11]. In addition, CCK reduces meal size and energy expenditure and delays gastric emptying [4, 12–16]. Peripheral CCK acts on the CCK receptor 1 (CCK-1R) on vagal afferent nerves projecting to the brain and consequently reduces meal size [12, 16–19]. Conversely, the application of either CCK-1R antagonists or vagal deafferentation abolishes the satiating effect of peripheral CCK [20–22]. Duodenal administration of dietary lipids stimulates brown adipose tissue (BAT) thermogenesis via an intestinal CCK-1R [23]. Peripheral CCK has been shown to attenuate whole-body energy expenditure via CCK-1R, but not via CCK receptor 2 (CCK-2R) [14, 15, 24–28]. Central CCK also regulates energy expenditure [13, 26, 28–31]. Thus, fat-induced CCK plays important roles in lipid transport and metabolism, glucose homeostasis and energy homeostasis between food intake and energy expenditure.
Consumption of a high-fat diet (HFD) increases peripheral CCK secretion in both humans and rodents [32–34]. Controversially, HFD does not increase plasma level of CCK in humans [35]. It also reportedly reduces sensitivity to the satiating effect of peripheral CCK in HFD-induced obese animals [36–38]. CCK knockout (CCK-KO) mice have comparable food intake, enhanced slightly energy expenditure and malabsorption of saturated fatty acids after chronic consumption of HFD [39]. Although CCK-KO animals on HFD have enhanced insulin sensitivity, CCK has no effect on glucose uptake by white adipose tissue and skeletal muscle [39]. Thus, lipid metabolism in CCK-KO mice might be an important regulatory mechanism to improve insulin sensitivity. The objectives of the present experiments were to determine the involvement of endogenous CCK in energy homeostasis and lipid transport from small intestine to tissues using the CCK-KO mouse model.
Materials and Methods
Animals
Male CCK-KO mice and their wild-type (WT) littermates were generated onto a C57BL/6J genetic background [39–41] and housed in an AAALAC-accredited facility under conditions of controlled illumination (12:12-h light-dark cycle, lights on from 0600 to 1800 h). All animal protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Body weight, food intake and metabolic rate
Based on our published protocols [11, 39], CCK-KO and WT mice (n = 10 per group) were individually housed at 10 wk of age and received a semi-purified high-fat pelleted diet (HFD, 20% butter fat by weight; Research Diets, Inc, New Brunswick, NJ) or pelleted chow (LFD, 5% fat diet, Harlan Teklad, Madison, WI) for 10 wk. Body weights and food intake were recorded twice a week using a top-loading balance (± 0.01 g, Adenturer SL, Ohaus Corp, Pine Brook, NJ). Cohorts of CCK-KO and WT mice were acclimatized to individual metabolic cages in an Oxymax system (Columbus Instruments, Columbus, OH) for 3 d. Metabolic rate and food intake were recorded at 15-min intervals for 2 d using the manufacturer’s software.
Fat mass and parameters in the plasma and liver
Fat and lean body masses were determined using an EchoMRI whole body composition analyzer (Houston, TX) [39, 41]. After 10 wk on LFD or the HFD, fat pads, liver and plasma were carefully collected and weighed from 5-h fasted mice. Glucose level was determined in tail blood using a Freestyle glucometer (Abbot Diabetes Care, Alameda, CA). Hepatic lipids were extracted using the Folch method [42]. Triacylglycerides (TG) and cholesterol (CHOL) in the plasma and the liver were determined using Randox triglyceride kits (Antrim, UK) and Infinity cholesterol kits (Thermo Electron, Noble Park, Victoria, Australia), respectively. According to the manufacturer’s protocol, diluted plasma was mixed with 200 µl enzyme reagent and then incubated at 37° C for 30 min [11]. The absorbance was read at 500 nm using a microplate reader (Synergy HT; BioTek Instruments, Richmond, VA). Plasma insulin and leptin were determined using commercial enzyme-linked immunosorbent assay kits (Millipore, St. Charles, MO)[11]. Briefly, 10-µl samples were added to each well of a precoated microtiter plate, and the detection antibody was added to the captured molecules. After incubation, absorbance was measured with a microplate reader and the final concentrations were calculated using standards provided with the enzyme-linked immunosorbent assay kits.
Fat absorption
Using our published protocol [39, 43], animals (n = 7–10 per group) consumed the HFD mixed with sucrose polybehenate (Research Diets, Inc) for 4 d and their fecal pellets were collected for analysis on the final day. Fatty acids in fecal pellets were extracted, methylated and analyzed by a gas chromatography system (Shimadzu GC 2010) equipped with a DB-23 Column (J & W Scientific, Folsom, CA) and Schimadzu Class EZStart 7.4 software [39, 43]. The percentage of fat absorption was determined based on the ratio of total fatty acids to behenic acid in the diet and in the feces.
Lymphatic TG output
Male CCK-KO and WT mice (n= 4–5 per group) fed LFD received a surgery for duodenal cannulation and lymphatic cannulation in the main mesenteric lymph duct based on our published methods [44, 45]. After surgery, the animals were infused via the duodenal catheter with a saline solution containing 5% glucose at a rate of 0.3 ml per h to compensate for fluid and electrolyte loss due to lymphatic drainage. The animals were allowed to recover for 24 h in a warm chamber (approximately 30 °C). Fasting state lymph was collected after a 1-h infusion of saline before a lipid emulsion consisting of 4 moles/h butter fat (The Kroger Corp, Cincinnati, OH) in a 19 mM sodium taurocholate solution was infused. To remove gastric emptying as a potential confounding factor for the transport of lipids to the lymph, the lipid emulsion was infused intraduodenally at a constant rate of 0.3 ml per h for 6 h. Lymphatic levels of TG and CHOL were determined using Randox triglycerides and Infinity cholesterol Kits.
Lipids uptake by adipocytes and lipid clearance assay
Two experiments (either intraduodenal or intravenous infusion of TG) were performed to determine lipid uptake by adipose tissue. For intraduodenal TG infusions, CCK-KO and WT mice (n= 5 per group) maintained on LFD received a duodenal infusion of a lipid emulsion containing 4 µmoles/h butter fat and labeled with [9,10-3H]oleic acid, 1µCi/0.3 ml (Perkin Elmer, Boston, MA) 24 h after recovery from the duodenal cannulation. After the 2-h infusion, inguinal and epididymal adipose tissue was collected on dry-ice. For intravenous infusions, we used Liposyn III (intravenous fat emulsion), which it acts like a chylomicron and can transport TG directly to all tissues including adipose tissues, liver and heart [46]. The 5-h fasted mice (n=6–7 per group) received 100 µl of Lyposyn III (20% fat) mixed with (0.25 µCi, 3H-TG per mouse) over 30 min and tail blood (20 µl) was collected at 2, 4, 6, 10, 20 and 30 min post-injection. Based on previous reports [47, 48], chylomicrons in the plasma are cleared in the rodents within 30 min. After 30 min, adipose tissue was collected on dry-ice. According to our published protocols [45, 48], tissue lipids were extracted using the Folch method [42] and radioactivity in the plasma and tissues was measured by liquid scintillation counting.
Lymph Apolipoproteins Determination
Based on our published protocol [45], lymph samples were loaded into a 4–20% Mini-Protean TGX gel (Bio-Rad Laboratories, Hercules, CA) and gels were run at a constant voltage (60 V) until the protein standards were well separated. Proteins were then transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories) for 2 h at 300 mA. After blocking nonspecific binding sites on the membranes, membranes were then incubated with polyclonal rabbit anti-rat Apo B antibody (1:6,000 dilution) and then incubated either with horseradish peroxidase-conjugated goat anti-rabbit antibodies (Dako, Cytomation). Detection of Apo B48 was achieved by using the enhanced chemiluminescence system (ECL Western Blotting Detection Reagents, Amersham Biosciences, Buckinghamshire, UK), and HyBlot CL films (Denville Scientific Inc) were used for development and visualization of the membranes. Apo B secretion during each subsequent hour of infusion was quantified by subtracting the 0 h (fasting) lymph Apo B content from relative Apo B levels at each hourly time point.
Statistical analysis
All values are expressed as mean ± SE. Parametric statistical analyses, one-way and two-way ANOVA, followed by the Bonferroni posttest for multiple comparisons, were analyzed by GraphPad™ Prism (version 6.0, San Diego, CA). Differences were considered significant relative to the WT mice at the same time point if P values were < 0.05.
Results
CCK-KO and WT mice maintained on LFD or HFD
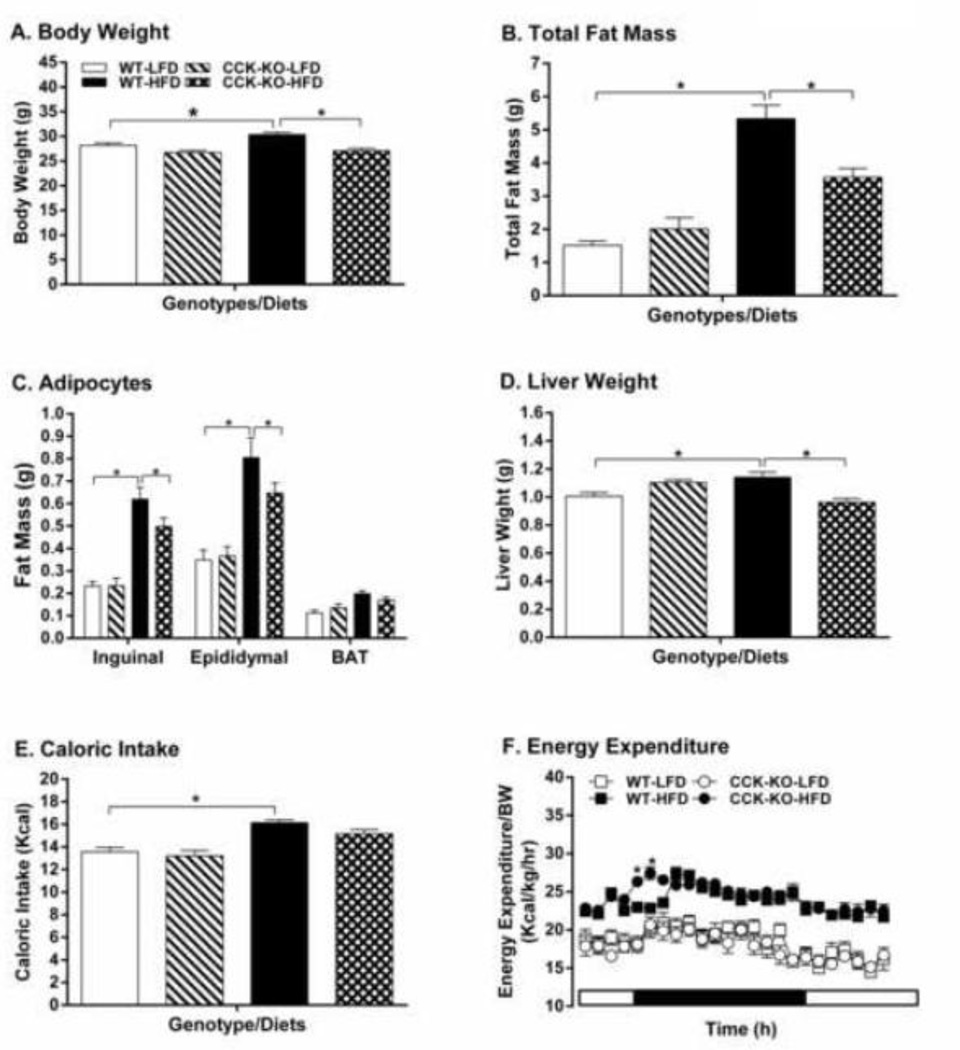
CCK-KO mice maintained on LFD had comparable daily caloric intake, body weight, energy expenditure, fat pad weight and basal plasma levels of lipids, glucose, insulin and leptin relative to their WT control animals (Figure 1A–F, Table 1). After 10 wk of HFD, WT mice had a significant increase in caloric intake, body weight, energy expenditure, fat mass, liver weight, and basal levels of plasma glucose, lipids, insulin and leptin (Figure 1A–F and Table 1, P < 0.05). In contrast, CCK-KO mice had less body weight and fat-pad weight increase (Fig 1A–B). A two-away ANOVA followed by post-hoc tests revealed that there was significant difference in body weight between strains [F(1,35)=6.22, P=0.175] and diets [F(1,35)=22.52, P< 0.0001], but no notable relationship between strains and diets [F(1,35)=3.35, P= 0.075]. The findings indicate that HFD increases body weight of WT mice, but the body weight of CCK-KO mice were not significantly altered by the HFD. There was also a significant effect in the amount of fat tissues between strains [F(2,92)=66.94, P< 0.0001] and diets [F(3,92)=29.93, P< 0.0001], as well as significant interaction between strains and diets [F(6,92)=4.385, P= 0.0006]. These observations suggest that HFD enhances the amount of epididymal and inquinal fats in both WT and CCK-KO mice, but the increase noted in overall fat mass is attenuated in CCK-KO mice.
Figure 1.
Body weight, tissue weight and caloric intake of CCK-KO and WT mice. Body weight (A), Total fat mass (B), fat weight for various fat pads (C), liver weight (D), caloric intake (E), and energy expenditure (F) of CCK-KO and WT mice (n = 8 per group). Animals were fed a low-fat chow diet (LFD) or HFD for 10 wk. Data are expressed as mean ± SEM and values with an asterisk represent significant differences between groups (P < 0.05).
Table 1.
Plasma and hepatic parameters
| WT (LFD) |
CCK-KO (LFD) |
WT (HFD) |
CCK-KO (HFD) |
|
|---|---|---|---|---|
| Plasma Glucose (mg/dl) | 140.67 ± 3.05 | 143.64 ± 5.58 | 168.50 ± 7.56 * | 144.00 ± 5.47 # |
| Plasma Triglyceride (mg/dl) | 50.58 ± 3.94 | 41.22 ± 3.06 | 64.29 ± 5.30* | 48.28 ± 3.82# |
| Plasma Cholesterol (mg/dl) | 83.86 ± 6.93 | 74.11 ± 5.40 | 164.80 ± 3.99* | 144.49 ± 9.49* |
| Plasma Insulin (ng/ml) | 0.69 ± 0.06 | 0.53 ± 0.05 | 1.10 ± 0.08* | 0.77 ± 0.05# |
| Plasma Leptin (ng/ml) | 0.90 ± 0.11 | 0.66 ± 0.17 | 6.40 ± 1.02* | 3.41 ± 0.41 *# |
| Hepatic Triglyceride (mg/g tissue) | 12.02 ± 2.77 | 12.25 ± 3.12 | 57.75 ± 12.29* | 33.71 ± 7.96# |
| Hepatic Cholesterol (mg/g tissue) | 2.48 ± 0.37 | 2.94 ± 0.19 | 3.74 ± 0.48 | 3.39 ± 0.24 |
Plasma (n=7–8 per group) and liver (n= 6 per group) in CCK-KO and WT mice were measured after either consuming low-fat chow diet or a high-fat diet for 10 wk starting at 10 wk of age. Values represent mean ± SEM and an asterisk (*) indicates a significant difference (P < 0.05) relative to chow-treated WT mice in a one-way ANOVA with Bonferroni’s posttest and hashtag (#) represents a significant difference compared to HFD-fed WT mice.
The CCK-KO mice on HFD exhibited significantly reduced plasma levels of glucose, TG, insulin and leptin despite having comparable daily caloric intake of HFD (Figure 1E and Table 1). In addition, there was increased energy expenditure at some time points during HFD feeding (Figure 1F). A two-away ANOVA demonstrated that there was a significant difference in energy expenditure between strains [F(1,336)=10.10, P=0.0016] and time [F(23,336)=8.62, P< 0.0001], as well as significant interaction between strains and time [F(23,336)=1.91, P= 0.0080]. In addition, the respiratory quotient (RQ) in HFD-fed CCK-KO mice (0.85 ± 0.01) was significantly higher than that in WT mice on HFD (0.77 ± 0.01) during dark cycle, indicating that they utilize more carbohydrate as energy substrate rather than fat early in the dark cycle.
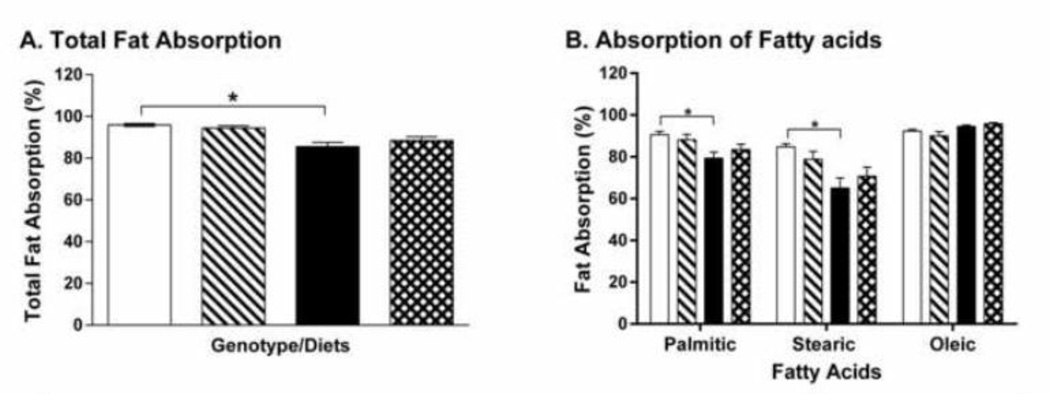
CCK-KO mice fed LFD had comparable total fat absorption (94.4± 1.1 %) relative to their WT counterparts (95.9 ± 0.6 %) (Figure 2A). Similarly, both HFD-fed WT and CCK-KO mice had reduced total fat absorption (85.5 ± 2.2 % and 88.5 ± 1.7 %, respectively), and more specifically, decreased saturated fatabsorption (Figure 2A–B, P < 0.05). Thus, there were no differences in fat absorption between the two genotypes after HFD. These data suggest that the chronic feeding of a HFD increases caloric intake and saturated fat malabsorption in CCK-KO mice as well as in WT mice. Despite these findings, HFD-fed CCK-KO mice ultimately had a significantly reduced increase of total fat mass and body weight compared with the WT counterparts.
Figure 2.
Fat absorption of CCK-KO and WT mice. Total fat absorption (A) and fatty acid profiles (B) in fecal pellets were determined using a GC system. Fecal pellets were collected on the 4th day after animals (n=7–9 per group) had been consuming a 20% butter fat diet mixture with 5% Olestra during the 9th week on LFD or HFD. Data are expressed as mean ± SEM and values with an asterisk represent significant differences between groups (P < 0.05).
Lymphatic output of lipids in WT and CCK-KO mice
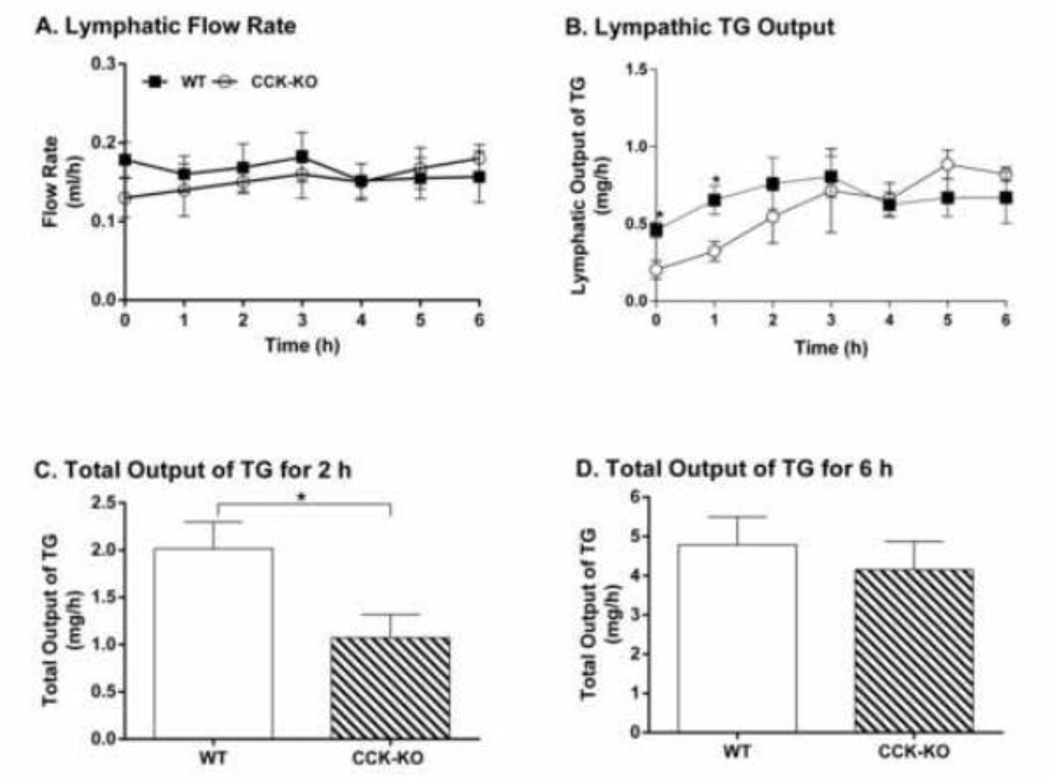
To determine whether the impaired lipid transport from the small intestine to adipocytes contributed to the smaller increase of fat mass in CCK-KO mice fed the HFD, we used WT and CCK-KO mice fed LFD that had comparable body weight and fat mass in these experiments. To mimic acute lipid transport from the small intestine to lymph, all mice received a duodenal infusion of fat emulsion with 20% butter fat (4 µmole/h) at 0.3 ml/h. When compared to WT mice, CCK-KO mice had a similar lymphatic flow rate throughout a 6-h infusion after a 1-h fast (Figure 3A). In WT mice, the transport rate of lymphatic TG reached a steady state after 2-h lipid infusion. CCK-KO mice had slower initial lymphatic TG output than WT mice during the 1-h fast and the first-h infusion (Figure 3B, P < 0.05), but comparable rates of TG appearance in the lymph between the 2nd and 6th hours of the infusion (Figure 3B). Corresponding with the initial attenuation of lymphatic TG output, CCK-KO mice had significantly lower total TG to the lymph during the first 2-h of infusion (Figure 3C). Despite this, there was comparable total TG transport to the lymph between the two groups over 6-h infusion (Figure 3D).
Figure 3.
Lymphatic TG transport into lymph. Hourly lymphatic flow rate (A) and lymphatic TG output (B) during continuous intraduodenal infusion of 4 pmoles/h butter fat. Total output of lymphatic TG for 2-h infusion (C) and 6-h infusion. CCK-KO and WT mice (n= 4–5 mice per group) received intraduodenal infusion of a lipid emulsion for 6 h. Data are expressed as mean ± SEM and values with an asterisk represent significant differences between groups (P < 0.05).
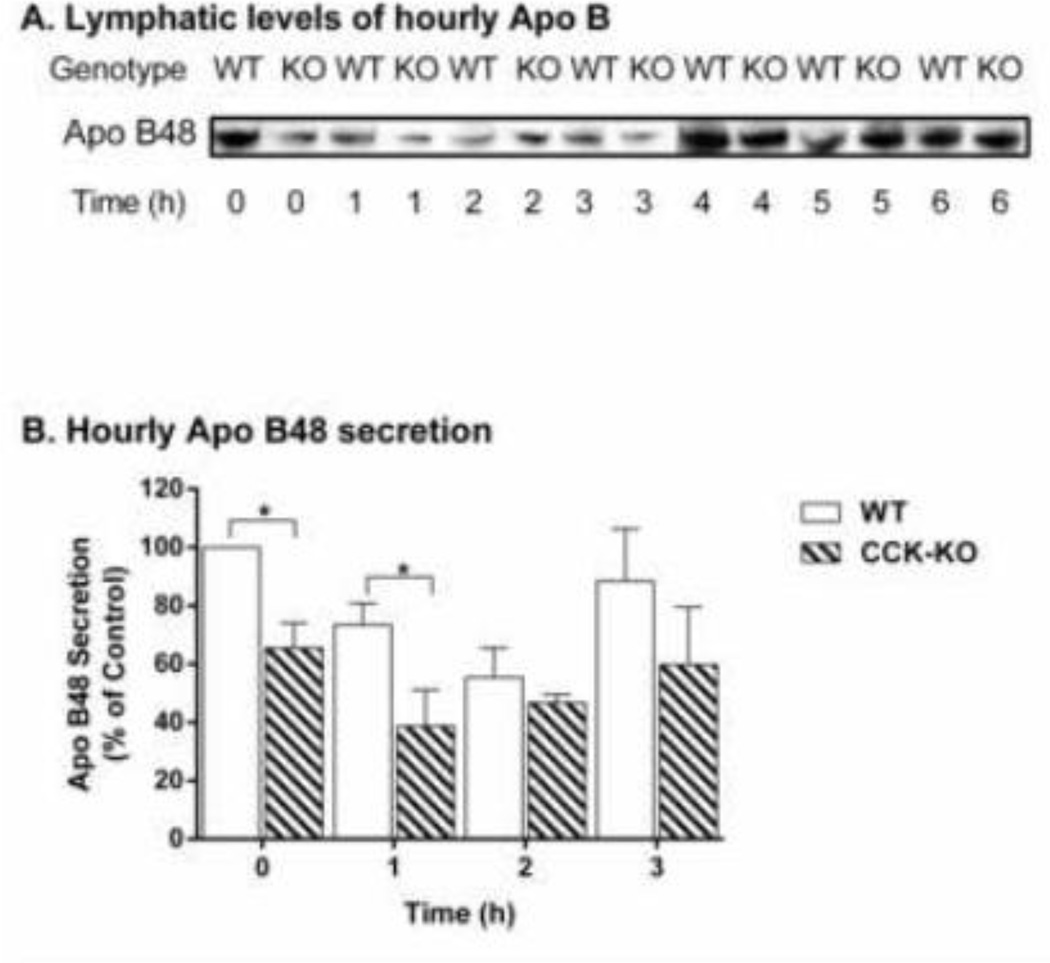
We next examined whether decreased Apo B48 secretion might account for the differences in chylomicron-mediated TG transport observed between WT and CCK-KO mice. The hourly lymphatic Apo B48 mass output of the WT group was higher than that of CCK-KO mice during fasting and at the first-h lipid-infusion time point (Figure 4A–B). These observations suggest that CCK-KO mice have comparable hourly lipid transport from small intestine to lymph, with the exception of a significant difference at fasting and for the first h infusion. In addition, the CCK-KO mice have less TG transport in lymph than WT mice possibly due to the lower number of Apo B48 chylomicron particles.
Figure 4.
Lymphatic apolipoprotein (Apo) B48 secretion in CCK-KO and WT mice. Lymph was collected hourly during the 6 h of continuous intraduodenal infusion of a lipid emulsion for 6 h (n= 4 mice per group); 1-min volumes (based on flow rate) of lymph were analyzed by a 4–20% SDS gel electrophoresis followed by Western blot detection of Apo B48 using a polyclonal antibody in (A). The percentage of hourly Apo B48 secretion divided by base level of Apo B48 (0 h) during the first 3 h of lipid infusion for the CCK-KO and the WT animals (B). Data are expressed as mean ± SEM and values with an asterisk represent significant differences between groups (P < 0.05).
Lipid transport to adipocytes in WT and CCK-KO mice
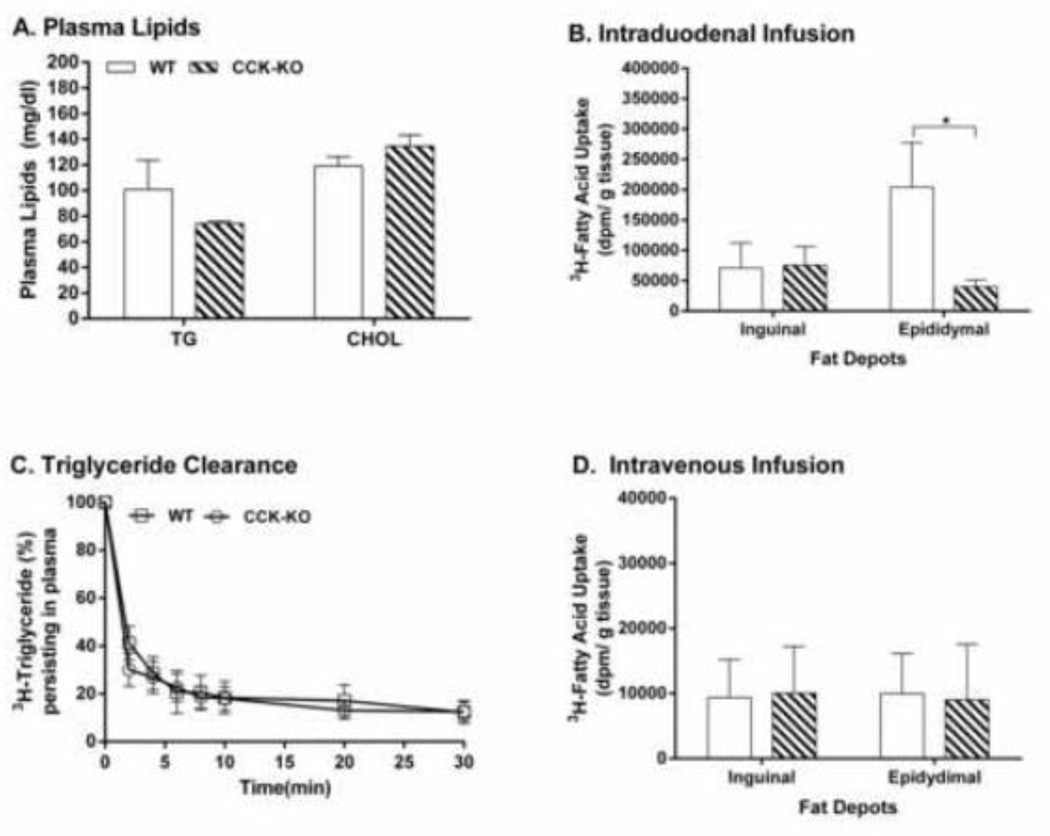
To determine whether CCK-KO mice have reduced fat mass due to impaired intestinal lipid transport to adipocytes, lipid uptake by adipocytes in CCK-KO and WT mice was determined. WT mice produced a steady output of lymphatic TG after 2-h intraduodenal infusion of an emulsion of 20% butter fat and 3H-TG (Figure 3B), indicating that steady-state absorption is achieved and is transported to the circulation [50, 51]. Fatty acid uptake by fat tissues was monitored during the initial two hours of infusion when CCK-KO mice had significantly impaired TG transport relative to WT mice. After 2-h infusion of dietary lipids, CCK-KO mice had comparable levels of plasma TG and CHOL as WT mice (Figure 5A) as well as comparable 3H-TG derived fatty acid uptake by inguinal fat (Figure 5B). In contrast, CCK-KO mice had decreased 3H-fatty acid uptake by epididymal fat pads relative to WT mice (Figure 5B, P < 0.05). Furthermore, to examine whether CCK-KO mice have a reduced clearance of fat emulsion and lipid uptake by adipocytes from the plasma, CCK-KO mice received an intravenous infusion of fat emulsion with radioactive lipids through the jugular vein. Infused 3H-TG in Liposyn III mixture (60–70%) was rapidly cleared within the first 2 min post-injection with WT and CCK-KO mice having comparable rate of plasma TG clearance (Figure 5C). Radioactive TG (88%) in the plasma was removed by tissues at 30-min post-injection. There was no difference in total uptake of TG-derived 3H-fatty acid by epididymal and inguinal fat in CCK-KO mice and WT mice at 30 min post-injection (Figure 5D). This observation suggests that CCK-KO mice have decreased uptake of TG derived fatty acid by epididymal fat, possibly secondary to altered intestinal lipid transport.
Figure 5.
Lipid uptake by adipocytes and lipid clearance in CCK-KO and WT mice. Plasma TG and Cholesterol (A) and 3H-TG-derived fatty acid (B) content in the adipocytes of CCK-KO and WT mice (n = 5 per group) were determined at the end of a 2-h continuously intraduodenal infusion of 4 pmoles/h butter fat and 3H-TG. Plasma clearance of triglyceride (C) in CCK-KO and WT mice were determined at 2–30 min postinjection, and radioactive levels of TG-derived fatty acids (D) in the adipocytes of CCK-KO and WT mice was determined at 30-min postinjection. CCK-KO and WT mice (n= 6–7 per group) intravenously received an intravenous infusion of radioactive fat emulsion (Lyposyn III) and blood was collected 0 to 30 min. Radioactive lipids in plasma and adipocytes were determined by scintillation counting. Data are expressed as mean ± SEM and values with an asterisk represent significant differences between groups (P < 0.05).
Discussion
Fat-induced CCK release plays important roles in the regulation of enzymatic secretion, gastric emptying, insulin action, body temperature, satiation and energy expenditure to influence body weight and preferential fat deposition in specific tissues [4–15, 24]. LFD-fed CCK-KO mice have comparable body weight and fat mass to WT, but HFD-fed CCK-KO mice are shown again to be resistant to HFD-induced obesity which is consistent with previous reports [39, 41]. The present study demonstrates that HFD-fed CCK-KO mice with comparable fat absorption have reduced body weight and fat mass. In the present experiments, we sought to mimic daily consumption of lipids and understand the process of dietary lipids transport from the small intestine to peripheral tissues in CCK-KO mice using intraduodenal and intravenous administration of dietary lipids.
Dietary lipids absorbed by enterocytes are delivered into the circulation system via chylomicrons [49]. Intestinal TGs are hydrolyzed by pancreatic lipases to monoacylglycerol and fatty acids and these digested products are taken up by enterocytes where they are converted back into triglycerides [49]. CCK stimulates pancreatic enzyme secretion and induces intestinal transit of lipids, resulting in the modulation of cholesterol absorption [2, 50]. Conversely, CCK-KO mice maintained on HFD have up-regulated pancreatic enzyme activities, possibly due to a compensatory response [39, 40]. HFD-fed CCK-KO mice at 30 weeks of age have previously been shown to have lower absorption of saturated fat than their WT control after 10 weeks of HFD [39]. In contrast, the present study demonstrates that CCK-KO mice at 20 weeks of age have comparable absorption of saturated fats compared with WT mice on the same duration of the HFD. The WT and CCK-KO mice gained a smaller percentage of inguinal and epididymal fat pads per body weight (2.0 and 1.8, 3.0 and 2.4%, respectively) in the present study compared to the animals at 30 weeks of age [39]. Due to the same diet composition and duration of HFD in these two studies, the age difference in these animals appear be a key factor for altered fat absorption in the small intestine. Although CCK-KO mice at 20 weeks of age have comparable fat absorption in the present study and glucose uptake by white adipose tissues and skeletal muscles during euglycemic-hyperinsulinemic clamp [11], the CCK-KO mice are resistant to HFD-induced obesity. These observations suggest that CCK-KO mice might have impaired lipid transport to lipid depots.
We have performed a series of experiments to determine the effect of acute lipid intake on lipid transport and fat mass in CCK-KO mice. CCK-containing neurons and CCK-1R are found in the small intestine [51–54], and CCK promotes release of acetylcholine in postganglionic neurons of the small intestine and causes smooth muscle contraction [50, 55]. CCK-KO and CCK-1R KO mice have been reported to have slower transit time of lipids in the small intestine [56, 57]. The TGs are packaged into chylomicrons which are then released from intestinal cells, drained into the lymphatic system and released from the thoracic duct into the bloodstream [49]. Otsuka Long-Evans Tokushima fatty rats lacking of CCK-1R have been reported to have comparable lymphatic output of TG and intestinal microsomal triglyceride transfer protein activity compared to their control Long-Evans Tokushima Otsuka rats when they are at age of 12–14 wk (before the onset of noninsulin-dependent diabetes mellitus)[58]. The present study demonstrates that CCK-KO mice fed LFD have normal lymphatic flow rate, but have a transiently attenuated rate of TG transport to the lymph during a fat-emulsion infusion which resolves after 2 h and does not impact the total TG transport to lymph during a 2-h or 6-h lipid infusion. This suggests that TG transport into lymph is delayed in CCK-KO mice, potentially due to lack of CCK-induced smooth muscle contraction in the small intestine.
Furthermore, we determined that CCK-KO mice secrete a reduced quantity of TG-rich Apo B48-chylomicrons into the lymph than their control counterparts during the first 3 hours of lipid infusion into the gut. Because only one molecule of Apo B48 is associated with each chylomicron particle [59], the mass of lymphatic Apo B48 secretion can be used as an indirect measure of the production of chylomicron particles by the gut [60] and thus the rate of TG transport to the lymph. Therefore, reduced secretion of Apo B48-chylomicron in CCK-KO mice contributes to the delay of TG transport from the small intestine to lymph.
Mesenteric and omental depots that drain into the portal circulation and then liver are visceral fats [61]. Epididymal fat pad drains into systemic circulation and it should not be considered a visceral depot [62]. Due to smaller mass of visceral depot in LFD-fed rodents [61], fatty acid uptake by epididymal (intra-abdominal) and inguinal (subcutaneous) fat are determined in the present study. CCK-KO mice have reduced TG-derived fatty acid uptake by epididymal fat, but have comparable fatty acid uptake by inguinal fat after an acute infusion of lipids into the duodenum. Upon entering the circulation, the TG in chylomicrons are hydrolyzed to free fatty acids by the enzyme lipoprotein lipase, and half of the fatty acids and all of the monoacylglycerols are directly transported to adipose tissue [63–65]. TG in chylomicrons is completely cleared by tissues in mice after 20-min postinjection of chylomicrons [48]. The majority of TG (88% TG) in the fat emulsion is removed by the tissues after 30 min in WT and CCK-KO mice and the CCK-KO mice have comparable plasma clearance of TG in the present study. Our data show that CCK-KO mice have normal uptake of TG-derived fatty acids by all fat pads after intravenous infusion of TG in fat emulsion.
The liver plays an important role in the uptake of TG-derived fatty acids in chylomicron remnants and in the transport of TG in very low density lipoproteins (VLDL) to other tissues [63, 66–69]. Exogenous infusion of CCK reduces liver weight [70] and decreases hepatic glucose production through CCK-1R on vagal nerves [71]. Controversially, CCK-KO mice have reduced hepatic glucose production after a 10-wk of HFD [11]. Thus, CCK regulates glucose homeostasis and lipid metabolism in response to dietary lipids or glucose. Direct effect of CCK on regulating hepatic uptake of fatty acids, TG transport in VLDL, and hepatic fatty acid synthesis from glucose requires further study. In the present study, the HFD-fed CCK-KO mice have reduced liver weight and hepatic TG content while the CCK-KO mice on LFD have normal liver weight and hepatic lipid content. Therefore, CCK-KO mice on HFD have reduced hepatic lipid transport to tissues and it results in the reduction of fat-pad weights.
Fatty acids are used as energy source in peripheral tissues including BAT and muscles [72–74]. Duodenal lipids have been reported to enhance BAT thermogenesis via a CCK-1R in the small intestine [23]. In response to dietary lipids, gut peptides such as CCK, peptide YY, glucose-dependent insulinotropic peptide and glucagon-like peptide-1 are released from small intestine [3, 44, 75, 76]. The involvement of these gut peptides in the activation of BAT thermogenesis via intestinal CCK-1R requires further experiments. Peripheral CCK decreases whole-body energy expenditure and body temperature [14, 15, 24–28]. The action of peripheral CCK on CCK-1R to attenuate metabolic rate is mediated by promoting decreased heat production and increased heat loss [16, 27]. Lower doses of brain CCK reduces body temperature via 5- hydroxytryptamine (5-HT) receptor [13, 29, 30]. In contrast, higher doses of central CCK induce hyperthermia and increased metabolic rate via CCK-2R [26, 28, 31]. While fed a LFD, CCK-KO and CCK1R–KO mice have normal energy expenditure and body temperature in the present and previous studies [41, 77, 78]. In contrast, CCK2R–KO mice on LFD have more energy expenditure and have higher body temperature than WT mice [77, 79]. In addition, CCK2R–KO mice also had greater energy intake than CCK-1R KO mice. The CCK-2R but not the CCK-1R has been implicated in the regulation of plasma leptin [80, 81]. We therefore speculate that the changes in fat mass and leptin secretion in CCK- KO mice are likely mediated through CCK2R. [77]. Consistent with our previous findings [39], the CCK-KO mice with comparable caloric intake expend more carbohydrate as energy substrate and have slightly elevated energy expenditure during high-fat feeding in the present study. In general, fat feeding attenuates RQ in rodents, indicating that fatty acids are used as a metabolic fuel [39, 82]. In response to high-fat feeding, CCK-KO mice utilize more carbohydrate to generate higher heat production possibly due to reduced TG transport from small intestine to the circulation as found in our previous and present experiments [39]. Direct effect of CCK on the preference of fat versus carbohydrate as metabolic fuels to alter energy homeostasis requires further investigation. Thus, increased energy expenditure in CCK-KO mice also influences the attenuation of HFD-induced obesity.
When fed a LFD, CCK-KO and CCK1R–KO mice have comparable body weight, food intake, total body fat and plasma levels of lipids, insulin and leptin as WT controls in published reports [41, 77, 83, 84] and the present study. CCK2R–KO mice on LFD have increased body weight, but reduced plasma leptin and insulin and fat tissues including visceral fat, retroperitoneal fat and BAT due to increased energy expenditure [77, 79, 81]. Consumption of a HFD increases body weight, fat mass and plasma lipids, glucose, and several hormones related to fat mass in WT rodents [39, 85–94]. Consistent with the previous reports [95, 96], HFD increases white adipose tissues including inguinal and epididymal fat mass in WT mice in the present study. In contrast, CCK-KO mice fed HFD have reduced white adipose tissues in the present study which we have previously demonstrated [39]. In addition, these HFD-fed CCK-KO mice have a comparable levels of plasma leptin, insulin and lipids relative to LFD-fed controls. CCK-KO mice have attenuated HFD-induced increases in the inguinal and epididymal fats, but only have a reduced uptake of fatty acids by epididymal fat in LFD-fed CCK-KO mice in response to introduodenal lipids. We therefore speculate that the rates of fatty acid uptake between epididymal and inquinal fat tissues might be different in response to dietary lipids. In response to duodenal or intravenous infusion of dietary lipids, longer duration of monitoring or variations of the infusion rate to determine the cause of attenuated uptake of fatty acids by inguinal fat also require further study. Alternatively, HFD impaired fatty acid uptake by inguinal fat in CCK-KO mice while CCK-KO mice on LFD have normal uptake of fatty acids. When fed a HFD, CCK1R–KO mice have increased meal size and reduced meal frequency, which results in comparable level of total food intake, weight gain and fat mass as WT mice [84, 97]. The CCK-2R but not the CCK-1R has been implicated in the regulation of fat mass [77, 81, 84]. The findings suggest that CCK-2R likely plays an important role in the changes in fat mass and energy expenditure in CCK-KO mice. Thus, increased energy expenditure and impaired TG transport from small intestine to lymph might contribute to the overall lower fat mass in CCK-KO mice in response to dietary lipids.
Conclusion
Cholecystokinin is well known to be important in several peripheral metabolic actions related to the digestion and absorption of lipids as well as in satiation and energy expenditure. In the present experiments, CCK-KO mice with comparable energy intake and fat absorption have a lower body weight and reduced fat mass relative to WT mice after 10 weeks on a HFD. In addition, these CCK-KO mice on HFD have slightly elevated energy expenditure. CCK-KO mice on LFD have a transiently delayed TG transport into the lymph and have reduced fatty acid uptake by epididymal fat pads at 2-h post-duodenal infusion, but overall CCK-KO mice have comparable total lipid transport. Based on these findings, we conclude that CCK is involved in a short-term lipid transport from small intestine to adipose tissue and also in the regulation of energy homeostasis during high-fat feeding.
Highlights.
Energy homeostasis in cholecystokinin knockout (CCK-KO) mice was determined.
Fat absorption in CCK-KO mice was normal in response to a high-fat feeding.
CCK-KO mice had increased energy expenditure during high-fat feeding.
CCK-KO mice had impaired lipid transport from small intestine to tissues.
CCK-KO mice were resistant to high fat diet-induced obesity.
Acknowledgements
This work was mainly performed at the University of Cincinnati and supported by National Institutes of Health Grants DK83550 and DK97436. We thank Drs.SC Woods, P Tso and RJ Jandacek for suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma Molecular forms, responses to feeding, and relationship to gallbladder contraction. J. Clin. Invest. 1985;75:1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh JH. Gastrointesinal hormones. In: Johnson LR, Christensen J, Jackson MJ, Jacobson ED, Walsh JH, editors. Physiology of the gastrointesinal tract. 2 ed. New York: Raven Press; 1986. pp. 181–253. [Google Scholar]
- 3.Green GM, Taguchi S, Friestman J, Chey WY, Liddle RA. Plasma secretin, CCK, and pancreatic secretion in response to dietary fat in the rat. Am. J. Physiol. 1989;256:G1016–G1021. doi: 10.1152/ajpgi.1989.256.6.G1016. [DOI] [PubMed] [Google Scholar]
- 4.Raybould HE, Meyer JH, Tabrizi Y, Liddle RA, Tso P. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am. J. Physiol. 1998;274:R1834–R1838. doi: 10.1152/ajpregu.1998.274.6.R1834. [DOI] [PubMed] [Google Scholar]
- 5.Harper AA, Raper HS. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J. Physiol. 1943;102:115–125. doi: 10.1113/jphysiol.1943.sp004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DENTON RW, GERSHBEIN LL, IVY AC. Response of human and canine gall bladder to cholecystokinin. J. Appl. Physiol. 1950;2:671–679. [PubMed] [Google Scholar]
- 7.Dreiling DA. Mechanisms of pancreatic exocrine secretion. Am. J. Gastroenterol. 1969;52:17–24. [PubMed] [Google Scholar]
- 8.Ahren B, Hedner P, Lundquist I. Interaction of gastric inhibitory polypeptide (GIP) and cholecystokinin (CCK-8) with basal and stimulated insulin secretion in mice. Acta Endocrinol. (Copenh) 1983;102:96–102. doi: 10.1530/acta.0.1020096. [DOI] [PubMed] [Google Scholar]
- 9.Moran TH, Sawyer TK, Seeb DH, Ameglio PJ, Lombard MA, McHugh PR. Potent and sustained satiety actions of a cholecystokinin octapeptide analogue. Am. J. Clin. Nutr. 1992;55:286S–290S. doi: 10.1093/ajcn/55.1.286s. [DOI] [PubMed] [Google Scholar]
- 10.Rushakoff RA, Goldfine ID, Beccaria LJ, Mathur A, Brand RJ, Liddle RA. Reduced postprandial cholecystokinin (CCK) secretion in patients with noninsulin-dependent diabetes mellitus: evidence for a role for CCK in regulating postprandial hyperglycemia. J. Clin. Endocrinol. Metab. 1993;76:489–493. doi: 10.1210/jcem.76.2.8432795. [DOI] [PubMed] [Google Scholar]
- 11.Lo CM, Obici S, Dong HH, Haas M, Lou D, Kim DH, Liu M, D'Alessio D, Woods SC, Tso P. Impaired insulin secretion and enhanced insulin sensitivity in cholecystokinin-deficient mice. Diabetes. 2011;60:2000–2007. doi: 10.2337/db10-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J. Comp Physiol Psychol. 1973;84:488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 13.Morley JE, Levine AS, Lindblad S. Intraventricular cholecystokinin- octapeptide produces hypothermia in rats. Eur. J. Pharmacol. 1981;74:249–251. doi: 10.1016/0014-2999(81)90538-0. [DOI] [PubMed] [Google Scholar]
- 14.Zetler G. Cholecystokinin octapeptide, caerulein and caerulein analogues: effects on thermoregulation in the mouse. Neuropharmacology. 1982;21:795–801. doi: 10.1016/0028-3908(82)90067-3. [DOI] [PubMed] [Google Scholar]
- 15.Kapas L, Obal F, Jr, Alfoldi P, Rubicsek G, Penke B, Obal F. Effects of nocturnal intraperitoneal administration of cholecystokinin in rats: simultaneous increase in sleep, increase in EEG slow-wave activity, reduction of motor activity, suppression of eating, and decrease in brain temperature. Brain Res. 1988;438:155–164. doi: 10.1016/0006-8993(88)91334-0. [DOI] [PubMed] [Google Scholar]
- 16.Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 17.Mueller K, Hsiao S. Specificity of cholecystokinin satiety effect: reduction of food but not water intake. Pharmacol. Biochem. Behav. 1977;6:643–646. doi: 10.1016/0091-3057(77)90089-2. [DOI] [PubMed] [Google Scholar]
- 18.Kraly FS, Carty WJ, Resnick S, Smith GP. Effect of cholecystokinin on meal size and intermeal interval in the sham-feeding rat. J. Comp Physiol Psychol. 1978;92:697–707. doi: 10.1037/h0077501. [DOI] [PubMed] [Google Scholar]
- 19.Moran TH, Robinson PH, Goldrich MS, McHugh PR. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986;362:175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- 20.Corwin RL, Gibbs J, Smith GP. Increased food intake after type A but not type B cholecystokinin receptor blockade. Physiol Behav. 1991;50:255–258. doi: 10.1016/0031-9384(91)90529-w. [DOI] [PubMed] [Google Scholar]
- 21.Yox DP, Brenner L, Ritter RC. CCK-receptor antagonists attenuate suppression of sham feeding by intestinal nutrients. Am. J. Physiol. 1992;262:R554–R561. doi: 10.1152/ajpregu.1992.262.4.R554. [DOI] [PubMed] [Google Scholar]
- 22.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am. J. Physiol. 1997;272:R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- 23.Blouet C, Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS. One. 2012;7:51898. doi: 10.1371/journal.pone.0051898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapas L, Obal F, Jr, Penke B, Obal F. Cholecystokinin-octapeptide- induced hypothermia in rats: dose-effect and structure-effect relationships, effect of ambient temperature, pharmacological interactions and tolerance. Neuropharmacology. 1987;26:131–137. doi: 10.1016/0028-3908(87)90200-0. [DOI] [PubMed] [Google Scholar]
- 25.South EH. Cholecystokinin reduces body temperature in vehicle- but not capsaicin-pretreated rats. Am. J. Physiol. 1992;263:R1215–R1221. doi: 10.1152/ajpregu.1992.263.6.R1215. [DOI] [PubMed] [Google Scholar]
- 26.Szelenyi Z, Bartho L, Szekely M, Romanovsky AA. Cholecystokinin octapeptide (CCK-8) injected into a cerebral ventricle induces a fever-like thermoregulatory response mediated by type B CCK-receptors in the rat. Brain Res. 1994;638:69–77. doi: 10.1016/0006-8993(94)90634-3. [DOI] [PubMed] [Google Scholar]
- 27.Rezayat M, Ravandeh N, Zarrindast MR. Cholecystokinin and morphine-induced hypothermia. Eur. Neuropsychopharmacol. 1999;9:219–225. doi: 10.1016/s0924-977x(98)00029-7. [DOI] [PubMed] [Google Scholar]
- 28.Balasko M, Rostas I, Furedi N, Miko A, Tenk J, Cseplo P, Koncsecsko-Gaspar M, Soos S, Szekely M, Petervari E. Age and nutritional state influence the effects of cholecystokinin on energy balance. Exp. Gerontol. 2013;48:1180–1188. doi: 10.1016/j.exger.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Liu HJ, Lin MT. Cholecystokinin-induced hypothermia: possible involvement of serotoninergic mechanisms in the rat hypothalamus. Pharmacology. 1985;31:108–114. doi: 10.1159/000138105. [DOI] [PubMed] [Google Scholar]
- 30.Shian LR, Lin MT. Effects of cholecystokinin octapeptide on thermoregulatory responses and hypothalamic neuronal activity in the rat. Naunyn Schmiedebergs Arch. Pharmacol. 1985;328:363–367. doi: 10.1007/BF00692901. [DOI] [PubMed] [Google Scholar]
- 31.Shido O, Yoneda Y, Nagasaka T. Changes in brown adipose tissue metabolism following intraventricular vasoactive intestinal peptide and other gastrointestinal peptides in rats. Jpn. J. Physiol. 1989;39:359–369. doi: 10.2170/jjphysiol.39.359. [DOI] [PubMed] [Google Scholar]
- 32.Spannagel AW, Nakano I, Tawil T, Chey WY, Liddle RA, Green GM. Adaptation to fat markedly increases pancreatic secretory response to intraduodenal fat in rats. Am. J. Physiol. 1996;270:G128–G135. doi: 10.1152/ajpgi.1996.270.1.G128. [DOI] [PubMed] [Google Scholar]
- 33.Little TJ, Feltrin KL, Horowitz M, Meyer JH, Wishart J, Chapman IM, Feinle-Bisset C. A high-fat diet raises fasting plasma CCK but does not affect upper gut motility, PYY, and ghrelin, or energy intake during CCK-8 infusion in lean men. Am. J. Physiol Regul. Integr. Comp Physiol. 2008;294:R45–R51. doi: 10.1152/ajpregu.00597.2007. [DOI] [PubMed] [Google Scholar]
- 34.Matters GL, Cooper TK, McGovern CO, Gilius EL, Liao J, Barth BM, Kester M, Smith JP. Cholecystokinin mediates progression and metastasis of pancreatic cancer associated with dietary fat. Dig. Dis. Sci. 2014;59:1180–1191. doi: 10.1007/s10620-014-3201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyd KA, O'Donovan DG, Doran S, Wishart J, Chapman IM, Horowitz M, Feinle C. High-fat diet effects on gut motility, hormone appetite responses to duodenal lipid in healthy men. Am. J. Physiol Gastrointest. Liver Physiol. 2003;284:G188–G196. doi: 10.1152/ajpgi.00375.2002. [DOI] [PubMed] [Google Scholar]
- 36.Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides. 1998;19:1407–1415. doi: 10.1016/s0196-9781(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 37.Covasa M, Marcuson JK, Ritter RC. Diminished satiation in rats exposed to elevated levels of endogenous or exogenous cholecystokinin. Am. J. Physiol Regul. Integr. Comp Physiol. 2001;280:R331–R337. doi: 10.1152/ajpregu.2001.280.2.R331. [DOI] [PubMed] [Google Scholar]
- 38.Savastano DM, Covasa M. Adaptation to a high-fat diet leads to hyperphagia, diminished sensitivity to cholecystokinin in rats. J. Nutr. 2005;135:1953–1959. doi: 10.1093/jn/135.8.1953. [DOI] [PubMed] [Google Scholar]
- 39.Lo CM, King A, Samuelson LC, Kindel TL, Rider T, Jandacek RJ, Raybould HE, Woods SC, Tso P. Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity. Gastroenterology. 2010;138:1997–2005. doi: 10.1053/j.gastro.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, Samuelson LC. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am. J. Physiol. 1999;276:G1302–G1309. doi: 10.1152/ajpgi.1999.276.5.G1302. [DOI] [PubMed] [Google Scholar]
- 41.Lo CM, Samuelson LC, Chambers JB, King A, Heiman J, Jandacek RJ, Sakai RR, Benoit SC, Raybould HE, Woods SC, Tso P. Characterization of mice lacking the gene for cholecystokinin. Am. J. Physiol Regul. Integr. Comp Physiol. 2008;294:R803–R810. doi: 10.1152/ajpregu.00682.2007. [DOI] [PubMed] [Google Scholar]
- 42.FOLCH J, LEES M, SLOANE STANLEY GH. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 43.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology. 2004;127:139–144. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J. Lipid Res. 1990;31:1613–1625. [PubMed] [Google Scholar]
- 45.Lo CM, Nordskog BK, Nauli AM, Zheng S, Vonlehmden SB, Yang Q, Lee D, Swift LL, Davidson NO, Tso P. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am. J. Physiol Gastrointest. Liver Physiol. 2008;294:G344–G352. doi: 10.1152/ajpgi.00123.2007. [DOI] [PubMed] [Google Scholar]
- 46.Hansen LM, Hardie BS, Hidalgo J. Fat emulsion for intravenous administration: clinical experience with intralipid 10% Ann. Surg. 1976;184:80–88. doi: 10.1097/00000658-197607000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jandacek RJ, Zheng S, Yang Q, Tso P. Rapid clearance of hexachlorobenzene from chylomicrons. Lipids. 2004;39:993–995. doi: 10.1007/s11745-004-1321-4. [DOI] [PubMed] [Google Scholar]
- 48.Kohan AB, Wang F, Li X, Bradshaw S, Yang Q, Caldwell JL, Bullock TM, Tso P. Apolipoprotein A-IV regulates chylomicron metabolism-mechanism and function. Am. J. Physiol Gastrointest. Liver Physiol. 2012;302:G628–G636. doi: 10.1152/ajpgi.00225.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am. J. Physiol. 1986;250:G715–G726. doi: 10.1152/ajpgi.1986.250.6.G715. [DOI] [PubMed] [Google Scholar]
- 50.Liddle RA. Cholecystokinin cells. Annu. Rev. Physiol. 1997;59:221–242. doi: 10.1146/annurev.physiol.59.1.221. [DOI] [PubMed] [Google Scholar]
- 51.Buffa R, Solcia E, Go VL. Immunohistochemical identification of the cholecystokinin cell in the intestinal mucosa. Gastroenterology. 1976;70:528–532. [PubMed] [Google Scholar]
- 52.Larsson LI, Rehfeld JF. Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res. 1979;165:201–218. doi: 10.1016/0006-8993(79)90554-7. [DOI] [PubMed] [Google Scholar]
- 53.Yamada J, Krause WJ. An immunohistochemical survey of endocrine cells and nerves in the proximal small intestine of the platypus, Ornithorhynchus anatinus. Cell Tissue Res. 1983;234:153–164. doi: 10.1007/BF00217409. [DOI] [PubMed] [Google Scholar]
- 54.Patterson LM, Zheng H, Ward SM, Berthoud HR. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res. 2001;305:11–23. doi: 10.1007/s004410100402. [DOI] [PubMed] [Google Scholar]
- 55.Gutierrez JG, Chey WY, Dinoso VP. Actions of cholecystokinin and secretin on the motor activity of the small intestine in man. Gastroenterology. 1974;67:35–41. [PubMed] [Google Scholar]
- 56.Wang DQ, Schmitz F, Kopin AS, Carey MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J. Clin. Invest. 2004;114:521–528. doi: 10.1172/JCI16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang HH, Portincasa P, Liu M, Tso P, Samuelson LC, Wang DQ. Effect of gallbladder hypomotility on cholesterol crystallization, growth in CCK-deficient mice. Biochim. Biophys. Acta. 2010;1801:138–146. doi: 10.1016/j.bbalip.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayashi H, Sato Y, Kanai S, Ichikawa M, Funakoshi A, Miyasaka K. Increased lymphatic lipid transport in genetically diabetic obese rats. Am J. Physiol Gastrointest. Liver Physiol. 2002;282:G69–G76. doi: 10.1152/ajpgi.2002.282.1.G69. [DOI] [PubMed] [Google Scholar]
- 59.Phillips ML, Pullinger C, Kroes I, Kroes J, Hardman DA, Chen G, Curtiss LK, Gutierrez MM, Kane JP, Schumaker VN. A single copy of apolipoprotein B-48 is present on the human chylomicron remnant. J. Lipid Res. 1997;38:1170–1177. [PubMed] [Google Scholar]
- 60.Elovson J, Chatterton JE, Bell GT, Schumaker VN, Reuben MA, Puppione DL, Reeve JR, Jr, Young NL. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J. Lipid Res. 1988;29:1461–1473. [PubMed] [Google Scholar]
- 61.Miles JM, Jensen MD. Counterpoint: visceral adiposity is not causally related to insulin resistance. Diabetes Care. 2005;28:2326–2328. doi: 10.2337/diacare.28.9.2326. [DOI] [PubMed] [Google Scholar]
- 62.Harris RB, Leibel RL. Location, location, location. Cell Metab. 2008;7:359–361. doi: 10.1016/j.cmet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Scow RO, Blanchette-Mackie EJ, Smith LC. Transport of lipid across capillary endothelium. Fed. Proc. 1980;39:2610–2617. [PubMed] [Google Scholar]
- 64.Olivecrona G, Olivecrona T. Triglyceride lipases and atherosclerosis. Curr. Opin. Lipidol. 1995;6:291–305. doi: 10.1097/00041433-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 66.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 67.Davis RA. Cell and molecular biology of the assembly and secretion of apolipoprotein B-containing lipoproteins by the liver. Biochim. Biophys. Acta. 1999;1440:1–31. doi: 10.1016/s1388-1981(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 68.Olofsson SO, Stillemark-Billton P, Asp L. Intracellular assembly of VLDL: two major steps in separate cell compartments. Trends Cardiovasc. Med. 2000;10:338–345. doi: 10.1016/s1050-1738(01)00071-8. [DOI] [PubMed] [Google Scholar]
- 69.Xiao C, Hsieh J, Adeli K, Lewis GF. Gut-liver interaction in triglyceride-rich lipoprotein metabolism. Am. J. Physiol Endocrinol. Metab. 2011;301:E429–E446. doi: 10.1152/ajpendo.00178.2011. [DOI] [PubMed] [Google Scholar]
- 70.Ohlsson B, Axelson J, Rehfeld JF, Ihse I. Devazepide-induced hyperplasia in the rat liver and bile ducts. Eur. Surg. Res. 1996;28:299–305. doi: 10.1159/000129470. [DOI] [PubMed] [Google Scholar]
- 71.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 73.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 74.Dulloo AG. A role for suppressed skeletal muscle thermogenesis in pathways from weight fluctuations to the insulin resistance syndrome. Acta Physiol Scand. 2005;184:295–307. doi: 10.1111/j.1365-201X.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- 75.Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am. J. Physiol Gastrointest. Liver Physiol. 2007;293:G963–G971. doi: 10.1152/ajpgi.00146.2007. [DOI] [PubMed] [Google Scholar]
- 76.Ohlsson L, Kohan AB, Tso P, Ahren B. GLP-1 released to the mesenteric lymph duct in mice: effects of glucose and fat. Regul. Pept. 2014;189:40–45. doi: 10.1016/j.regpep.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyasaka K, Ichikawa M, Ohta M, Kanai S, Yoshida Y, Masuda M, Nagata A, Matsui T, Noda T, Takiguchi S, Takata Y, Kawanami T, Funakoshi A. Energy metabolism and turnover are increased in mice lacking the cholecystokinin-B receptor. J. Nutr. 2002;132:739–741. doi: 10.1093/jn/132.4.739. [DOI] [PubMed] [Google Scholar]
- 78.Nomoto S, Ohta M, Kanai S, Yoshida Y, Takiguchi S, Funakoshi A, Miyasaka K. Absence of the cholecystokinin-A receptor deteriorates homeostasis of body temperature in response to changes in ambient temperature. Am. J. Physiol Regul. Integr. Comp Physiol. 2004;287:R556–R561. doi: 10.1152/ajpregu.00542.2003. [DOI] [PubMed] [Google Scholar]
- 79.Weiland TJ, Voudouris NJ, Kent S. The role of CCK2 receptors in energy homeostasis: insights from the CCK2 receptor-deficient mouse. Physiol Behav. 2004;82:471–476. doi: 10.1016/j.physbeh.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 80.Attoub S, Levasseur S, Buyse M, Goiot H, Laigneau JP, Moizo L, Hervatin F, Le Marchand-Brustel Y, Lewin JM, Bado A. Physiological role of cholecystokinin B/gastrin receptor in leptin secretion. Endocrinology. 1999;140:4406–4410. doi: 10.1210/endo.140.10.7079. [DOI] [PubMed] [Google Scholar]
- 81.Chen H, Kent S, Morris MJ. Is the CCK2 receptor essential for normal regulation of body weight and adiposity? Eur. J. Neurosci. 2006;24:1427–1433. doi: 10.1111/j.1460-9568.2006.05016.x. [DOI] [PubMed] [Google Scholar]
- 82.Flatt JP. Body composition, respiratory quotient, and weight maintenance. Am. J. Clin. Nutr. 1995;62:1107S–1117S. doi: 10.1093/ajcn/62.5.1107S. [DOI] [PubMed] [Google Scholar]
- 83.Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J. Clin. Invest. 1999;103:383–391. doi: 10.1172/JCI4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bi S, Chen J, Behles RR, Hyun J, Kopin AS, Moran TH. Differential body weight and feeding responses to high-fat diets in rats and mice lacking cholecystokinin 1 receptors. Am. J. Physiol Regul. Integr. Comp Physiol. 2007;293:R55–R63. doi: 10.1152/ajpregu.00002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.French SJ, Murray B, Rumsey RD, Sepple CP, Read NW. Preliminary studies on the gastrointestinal responses to fatty meals in obese people. J. Obes. Relat Metab Disord. 1993;17:295–300. [PubMed] [Google Scholar]
- 86.Hamilton BS, Paglia D, Kwan AY, Deitel M. Increased obese mRNA expression in omental fat cells from massively obese humans. Nat. Med. 1995;1:953–956. doi: 10.1038/nm0995-953. [DOI] [PubMed] [Google Scholar]
- 87.Lonnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat. Med. 1995;1:950–953. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- 88.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 89.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 90.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 91.Kahn BB, Flier JS. Obesity and insulin resistance. J. Clin. Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghibaudi L, Cook J, Farley C, Van HM, Hwa JJ. Fat intake affects adiposity, comorbidity factors, and energy metabolism of sprague-dawley rats. Obes. Res. 2002;10:956–963. doi: 10.1038/oby.2002.130. [DOI] [PubMed] [Google Scholar]
- 93.Woods SC, D'Alessio DA, Tso P, Rushing PA, Clegg DJ, Benoit SC, Gotoh K, Liu M, Seeley RJ. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83:573–578. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 94.Buse JB, Polonsky KS, Burant CF. Type 2 Diabetes Mellitus. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, editors. Williams Textbook of Endocrinology. 11 ed. Piladephia, PA: Saunders Elsevier; 2008. pp. 1329–1389. [Google Scholar]
- 95.Herberg L, Doppen W, Major E, Gries FA. Dietary-induced hypertrophic--hyperplastic obesity in mice. J. Lipid Res. 1974;15:580–585. [PubMed] [Google Scholar]
- 96.Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism. 1993;42:1405–1409. doi: 10.1016/0026-0495(93)90190-y. [DOI] [PubMed] [Google Scholar]
- 97.Donovan MJ, Paulino G, Raybould HE. CCK(1) receptor is essential for normal meal patterning in mice fed high fat diet. Physiol Behav. 2007;92:969–974. doi: 10.1016/j.physbeh.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]