Abstract
Background
Maternal depression is associated with negative outcomes for offspring, including increased incidence of child psychopathology. Quality of mother-child relationships can be compromised among affectively ill dyads, such as those characterized by maternal depression and child psychopathology, and negatively impact outcomes bidirectionally. Little is known about the neural mechanisms that may modulate depressed mothers’ responses to their psychiatrically ill children during middle childhood and adolescence, partially because of a need for ecologically valid personally relevant fMRI tasks that might most effectively elicit these neural mechanisms.
Methods
The current project evaluated maternal response to child positive and negative affective video clips in 19 depressed mothers with psychiatrically ill offspring using a novel fMRI task.
Results
The task elicited activation in the ventral striatum when mothers viewed positive clips and insula when mothers viewed negative clips of their own (versus unfamiliar) children. Both types of clips elicited activation in regions associated with affect regulation and self-related and social processing. Greater lifetime number of depressive episodes, comorbid anxiety, and poor mother-child relationship quality all emerged as predictors of maternal response to child affect.
Limitations
Findings may be specific to dyads with psychiatrically ill children.
Conclusions
Altered neural response to child affect may be an important characteristic of chronic maternal depression and may impact mother-child relationships negatively. Existing interventions for depression may be improved by helping mothers respond to their children’s affect more adaptively.
Keywords: Maternal Depression, Affect, Reward, Threat
1. Introduction
Maternal depression is associated with negative outcomes for offspring, including higher rates of child psychiatric illness (Goodman et al., 2011). Conceptual models (Hammen et al., 2004) and empirical findings from behavioral studies (Lovejoy et al., 2000) suggest that one putative mechanism for disease transmission across generations is maladaptive parent-child relationships in dyads with a depressed parent. Altered responses to child affect and maladaptive relationship patterns may be especially apparent in depressed mothers with psychiatrically ill children (Zalewski et al., 2013). Specifically, maternal neural function in emotional processing circuits is altered in depression (Barrett and Fleming, 2011) and may be associated with disruptions in parent-child relationships previously observed in depressed mother-child dyads.
In particular, anomalies in neural processing of reward and threat have been associated with depression (Zhang et al., 2013), suggesting that these biologic processes may constitute key elements in depression transmission across generations. Parental capacity to enjoy the rewarding aspects and manage the difficult aspects of parenting may be attenuated by parental depression (Zalewski et al., 2013). Depressed individuals show blunted ventral striatal (VS) activation in response to positive stimuli (Zhang et al., 2013), suggesting diminished pleasure in response to reward, and heightened response in the amygdala and insula to negative stimuli (Hamilton et al., 2012), indicating heightened negative affect in response to threatening stimuli. These findings demonstrate that depressed individuals engage these neural reward and threat systems differently than healthy individuals, and that such neural disruptions may be involved in the clinical course of the disorder.
Disruptions in affect regulation may also explain why depressed individuals respond differently to rewarding and threatening stimuli. Depressed adults show altered activation in regions implicated in affect regulation, such as the dorsolateral and ventrolateral and dorsomedial and ventromedial prefrontal cortex (dlPFC, vlPFC, dmPFC, vmPFC) to both positive and negative stimuli (Phillips et al., 2008). Specifically, depressed adults show heightened mPFC response to monetary rewards (Zhang et al., 2013), suggesting over-engagement of regulatory regions to dampen positive affect. Other work has demonstrated that depression is also associated with heightened dorsal anterior cingulate cortex (ACC) and diminished dlPFC response to threatening stimuli (Hamilton et al., 2012), suggesting that depressed adults not only place greater salience on threatening stimuli, but they also may have difficulty regulating negative affect elicited by these stimuli.
Because social function is clearly disrupted in depressed individuals as evidenced by maladaptive emotional behavior when interacting with family members (Hammen et al., 2004) and impaired interpersonal functioning in general (Petty et al., 2004), depression may be associated with differential response to their own offspring in brain regions associated with social processing. Also, there is some evidence to suggest that individuals with depression attribute their experience of negative events to personal failings more frequently than do healthy individuals (Grimm et al., 2011). Because children are kin, it is possible that depressed parents may identify closely with their children’s experiences, viewing their own child’s negative emotions as highly related to self. Thus, evaluating neural response to personally relevant positive and negative stimuli in depressed parents of psychiatrically ill children could give us important information in self-related and social processing circuitry, including regions such as the dmPFC, vmPFC, posterior cingulate (PCC), thalamus, and precuneus ((Northoff et al., 2006). Function in this network of regions has been shown to be altered in depression (Grimm et al, 2009).
Given the aforementioned interpersonal disruptions, affective displays by close family members could be particularly effective in eliciting differences in affective processing. Measurement of parental neural response to their offspring’s affect (rather than responses to more general stimuli) may be a more meaningful way to evaluate the neural systems involved in depression, especially because disrupted parent-child interactions are present for many depressed parents and because children’s affect can be a potent stimulus for affect regulation in parents (Lovejoy et al., 2000). However, investigations of depression-related disruptions in neural functioning have largely focused on non-personally relevant paradigms that measure general reward and threat processing (i.e., using money and/or unfamiliar faces as stimuli). In contrast, there is a dearth of personally relevant neuroimaging paradigms that incorporate family context. Some work has evaluated adolescent neural response to maternal stimuli using video clips of maternal affect (Whittle et al., 2012) or audio clips of maternal criticism (Lee et al., 2014), and found that altered adolescent response to maternal stimuli is associated with greater depressive symptoms (Whittle et al., 2012). Other work has evaluated maternal response to infant stimuli, using images of one’s own baby or audio clips of infant cry, and provided evidence for maternal response circuits (Barrett and Fleming, 2011), that include the thalamus, striatum, and ACC, and that are altered in depressed mothers of infants. However, to our knowledge, no study has evaluated mothers’ response to their own children during middle childhood and adolescence, when the parent-child relationship has changed substantially from infancy (Laursen et al., 1998) and when youth may have their own affective problems (Paus et al., 2008). Altered neural responses to child affect could explain the lower levels of parental warmth and higher parental hostility reported by child offspring of depressed mothers (Lovejoy et al., 2000).
Aberrant responding may be even more likely in dyads in which both parent and child suffer from emotional problems. These families face even greater difficulties relative to dyads in which only one member is psychiatrically ill (Weissman et al., 2005). In particular, because of the increased burden of caring for a psychiatrically ill child, in addition to coping with one’s own depression, it is possible that parents within these very high risk dyads may respond to their offspring’s affective displays in different ways than other parents. This altered responding could contribute to a cycle of dysfunction in the parent-child relationship, including greater levels of hostility and lower levels of warmth.
The goal of the current project was to evaluate how the association between parental response to child affect and maternal depression course and mother-child relationship quality in very high risk dyads, defined as mothers and children both diagnosed with syndromal psychiatric disorders. The study objective was to utilize video-recordings of “real” parent-child interactions to develop ecologically valid probes for social reward and threat, similar to methods used by Whittle et al. (2012). We hypothesized that this task, the Positive and Negative Social Interaction Task- Mother-Child (PANSIT-MC), would activate regions previously implicated in reward (VS), threat (amygdala, insula), self-related and social processing (thalamus, precuneus), and affect regulation (dlPFC, vlPFC, mPFC). We further hypothesized that these neural differences in responding to their own child’s positive and negative affect would be associated with important clinical correlates of depression, namely chronicity of depression and comorbid anxiety, and with depression-related parenting difficulties, specifically lower child-rated maternal warmth and higher child-rated maternal hostility.
2. Method
2.1 Participants
The participants were 32 mothers with a recent history of depression recruited from a clinical trial (N=168) conducted at the University of Pittsburgh for depressed mothers of children diagnosed with internalizing disorders (Grant R01 MH083647). Mothers were recruited from the larger study at any point during their treatment, during the 12-month follow-up phase, or after completing the trial.
The final sample included 19 participants; 11 of the original 32 did not complete the fMRI scan due to ineligibility (n=6 metal in body; n=5 chest circumference), and two did not complete the PANSITMC in the scanner. All 19 participants had adequate coverage (>90% of VS/amygdala coverage) and <2 mm of movement across the task. The participants were mostly white (n=17 White/Caucasian, n=2 Black/African American), had at least some college education (n=16 Some College, n=3 High School Diploma), and had a mean age of 43.9 years (SD=8.70). Of these 19, 7 participants reported psychiatric medication use (n=6 taking an antidepressant medication, n=2 taking a benzodiazepine) and 3 participants reported daily nicotine use. Children of these participating mothers ranged from 10 to 18 years old (M age=14.78, SD =3.09) and were 63% Female. All children met criteria for at least one current internalizing disorder (n=7 with MDD, n=7 Generalized Anxiety Disorder (GAD), n=2 Anxiety Disorder NOS, n=2 Bipolar NOS, n=1 Depressive Disorder NOS, n=1 Social Phobia, n=1 Panic Disorder, n=1 Obsessive Compulsive Disorder, n=1 Specific Phobia). Some children had comorbid externalizing disorders (n=5 with Attention Deficit Hyperactivity Disorder, n=1 Oppositional Defiant Disorder). There were no significant differences in mothers’ ethnicity, education level, or age for the 19 participants with usable fMRI data compared to the 13 other recruited participants or compared to the participants from the larger clinical trial (ts =.39-.52, ns for mothers’ age; χ2s=1.47–14.72, ns, for ethnicity and education level).
All participants completed self-report questionnaires as well as the Family Interaction Task and PANSIT-MC Task described below. The University of Pittsburgh Institutional Review Board approved all research procedures, and written informed consent was obtained from all participants.
2.2 Measures
Maternal clinical characteristics
Mothers reported number of lifetime episodes of depression using the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1995) with masters’ level clinicians trained to reliability (Kappa=1.00 for primary diagnoses). Mothers also reported on other lifetime Axis I disorders during this interview. Based on this interview, 68% of mothers reported multiple episodes of depression in their lifetime (M=5.00, SD=5.01, Range of 1 to 15 lifetime episodes). In addition, 68% of mothers met criteria for a lifetime anxiety disorder (n=4 GAD, n=4 Post-Traumatic Stress Disorder, n=3 Social Phobia, n=3 Panic Disorder, and n=2 Specific Phobia). Given that these mothers were not necessarily the same mothers with multiple episodes of depression, we also compared how mothers with and without comorbid anxiety differed on neural response to child affect.
Maternal warmth and hostility
Children rated parent-child relationship quality using the Parent-Child Relationship Quality Questionnaire, a measure adapted from the Getting Along with My Parent questionnaire from the NICHD study of early child care (Conger et al., 2002). This 19-item measure evaluates the level of warmth and hostility in the mother-child relationship in the past year. Sample items include, “How often does your mother act loving toward you, hug, or kiss you?” from the warmth subscale and “How often does your mother shout or yell at you because she is mad at you?” from the hostility subscale. Internal consistency for this measure was good to excellent (α= .97 for Warmth subscale; α=.86 for Hostility subscale).
Family interaction task
Mother-child pairs were asked to engage in three video recorded interaction tasks: two pleasant event discussions followed by a disagreement discussion. Personally relevant topics for discussions were chosen based on participants’ responses to modified versions of the Pleasant Event Checklist (PEC) (MacPhillamy and Lewinsohn, 1976) and the Issues Checklist (IC) (Prinz et al., 1979). The PEC includes a list of activities that families may enjoy doing together (e.g., taking a trip). Similarly, the IC contains a list of topics on which parents and children often disagree (e.g., household chores). Both checklists have been used in prior work and have strong psychometric properties, including high stability and good concurrent, predictive, discriminative, and construct validity (MacPhillamy & Lewinsohn, 1982; Prinz et al., 1979). For the two three-minute pleasant event discussions, participants were asked to 1) discuss an event from the PEC that they enjoyed together in the past, and 2) plan a pleasant activity from the PEC that they would like to do together in the future. For the disagreement discussion, participants were asked to talk together for six minutes about a mutual topic of disagreement from the IC that engendered mild to moderate conflict. They were also asked to discuss why they were upset about the topic and how they might solve the issue. The experimenter remained in the room behind a curtain for the pleasant events discussions and left the room for the disagreement discussion. These tasks have been used successfully in healthy and depressed samples (Sheeber et al., 2007).
Children’s positive, negative, and neutral affect during these discussions were coded in 5-second epochs by a team of trained observers using an adaptation of the Living in Family Environments coding system (Hops et al., 1995), an observational coding system used to generate affect codes (e.g., happy, anger). Based on this system, positive and negative affect were dichotomously coded as “present” (code of 2) or “absent” (code of 1). If both were “absent,” the segment was coded “neutral.” Affective codes took into account facial, vocal, and bodily expressions. Positive affect included expressions such as wide smiles, laughter, and positive statements made with an excited tone. Negative affect included expressions of anger (e.g., furrowed brows, abrupt word usage with one word or syllable being stressed), contempt (e.g., rolling eyes, sarcastic tone), anxiety (e.g., stuttering, nervous laughter), and dysphoria (e.g., tearfulness, slow speech). Neutral affect was coded only in the absence of positive or negative affect and included expressions such as matter-of-fact questions and exchanges made with a relaxed tone of voice. Mean ICCs for the coders on the 20-second clips were considered to be good (positive: ICC=.87; negative: ICC=.87; neutral: ICC=.86). Final codes for each epoch were determined by a consensus agreement between two trained raters and total positive and negative emotion codes for each fMRI clip were created by summing across epochs (range of 4–8). Based on these codes and the content of the segments, six unique 20-second clips were selected for viewing as fMRI stimuli for periods in which the child displayed positive, negative, or neutral affect (two clips for each affect condition). Stimulus clips included the head and shoulders of the children and audio of both mothers and children. We made efforts to ensure that video clips were equivalent in lighting and size.
PANSIT-MC
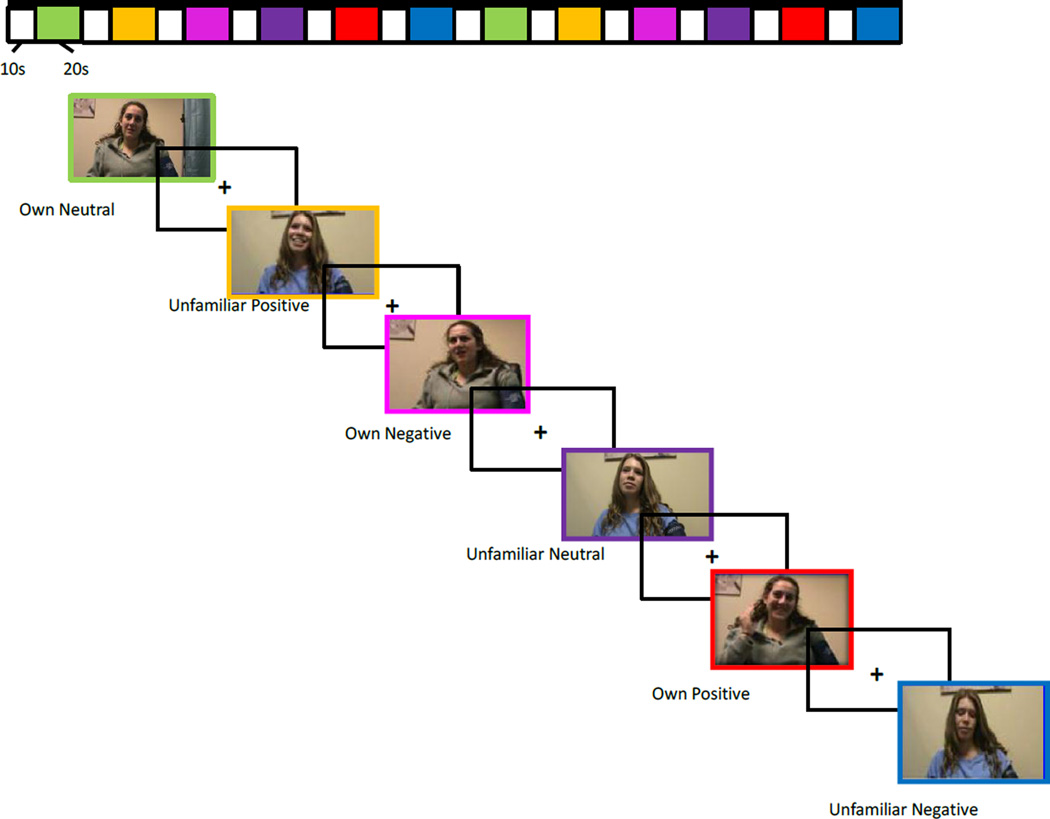
Similar to methods used in Whittle et al. (2012), unfamiliar child clips were created using actors and using the same procedure as described above. Four sets of unfamiliar child clips were created using pre and post-puberty boys and girls so that clips of the participant’s child could be matched for gender and approximate age (pre/post puberty) with clips of an unfamiliar child. Twenty-second clips of the participant’s child or an unfamiliar child were presented in a block design, interspersed with 10-second fixation cross-hair displays, during a 6-minute run. Clips were presented in a predetermined pseudorandom order. Positive, negative, and neutral clips were alternated with participant’s own child and the unfamiliar child’s clips (i.e., own-neutral, unfamiliar-positive, etc., see Figure 1). Because we were interested in evaluating maternal neural response to their own child relative to their response to an unfamiliar child, which isolates response to relationship while holding valence constant, the contrasts of interest generated from task data were own child’s positive affect versus stranger child’s positive affect and own child’s negative affect versus stranger child’s negative affect. Following the fMRI scan, each mother rated how happy, sad, and angry she felt when viewing her own child’s positive and negative clips and the unfamiliar child’s positive and negative clips on a 10-point Likert scale.
Figure 1.
PANSIT-MC fMRI Task
fMRI acquisition and preprocessing
Each participant was scanned using a Siemens 3T Trio scanner. BOLD functional images were acquired with a gradient echo planar imaging (EPI) sequence and covered 39 axial slices, 3.1 mm thick, beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (TR/TE=2000/25 ms, FOV=20 cm, matrix=64 × 64, flip angle=90°). All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired a reference EPI scan that we visually inspected for artifacts (e.g., ghosting) and for good signal across the entire volume of acquisition. The fMRI data from all included participants were cleared of such problems.
Preprocessing and image analysis were completed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). For every scan, images for each participant were segmented and then realigned to correct for head motion. Data sets were then selected for quality based on our standard small-motion correction (<3 mm). Realigned images were spatially normalized into standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model. Normalized images were smoothed with a 6 mm full-width at half-maximum Gaussian filter. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. Voxels were resampled during preprocessing to be 2 mm3.
2.3 Data Analytic Strategy
First, we examined neural response to child affect using within-sample t-tests. Next, we investigated associations with mothers’ number of depressive episodes and comorbid anxiety and with child-rated maternal warmth and maternal hostility using multiple regression models conducted in SPM8. Separate regression models were run to evaluate the effect of our predictors on neural response in order to reduce multi-collinearity, given that our predictor variables were highly correlated or conceptually similar (albeit still distinct). All models included daily nicotine use and psychiatric medication use, entered as binary variables, and child age and sex as covariates. Because care was taken to choose video segments with high levels of positive or negative emotion for our fMRI clips across participants, positive and negative emotion clip codes were not associated with parental warmth or hostility (rs =.11–.36, ps=.13 – .68) and were not included as covariates in the model. Given that we hypothesized that this task would activate multiple neural regions across networks (including reward circuitry, threat circuitry, self-referential processing circuits, regulatory regions), we used whole brain analyses to test our models. All analyses were corrected for multiple comparisons using Monte Carlo simulations that estimated the number of contiguous voxels required to avoid Type I error (Alpha Sim,(Ward, 2000)) using a p< .01 threshold (144 voxels).
3. Results
Maternal warmth and hostility were negatively correlated with one another (r=−.78, p<.001). Number of depressive episodes was not significantly related to either maternal warmth (r=−.38, p=.11) or maternal hostility (r=.28, p=.34).
3.1 Main effect of own child condition
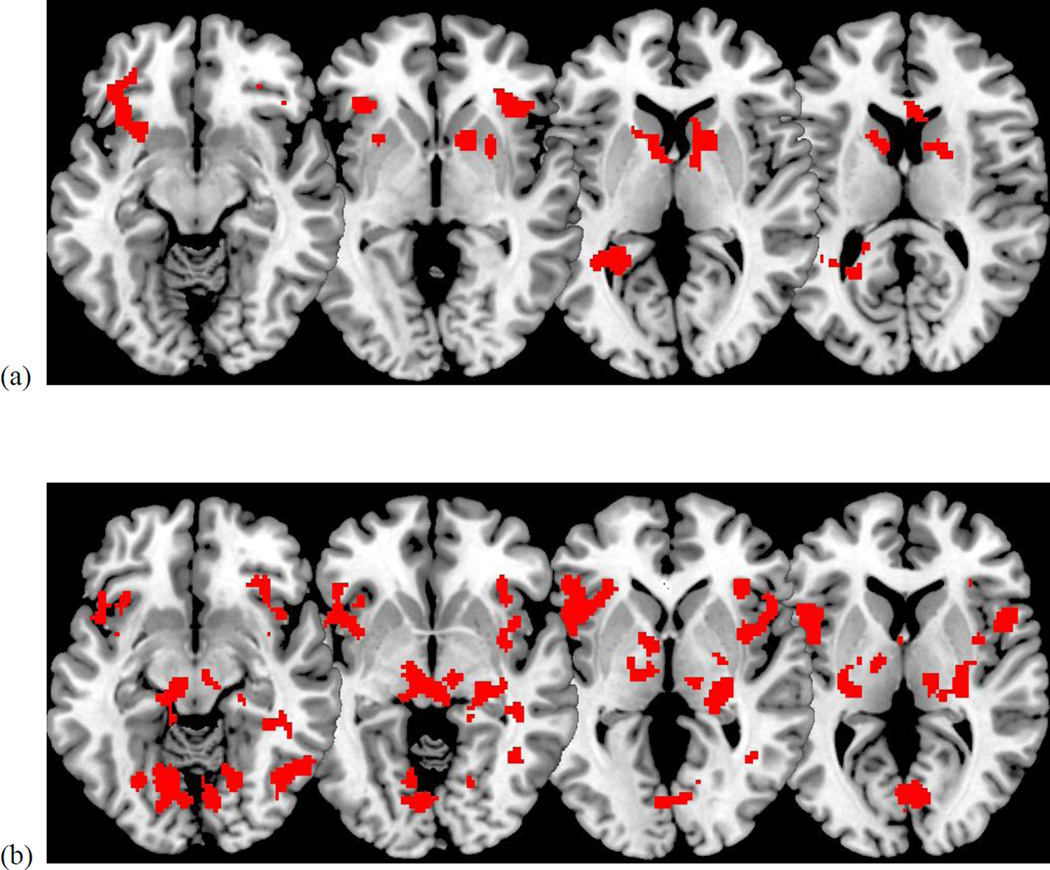
There was a significant main effect of the task for both the positive (Own Positive>Stranger Positive) conditions and negative (Own Negative>Stranger Negative) conditions (Table 1). During the positive condition, mothers showed greater activation in the VS, vlPFC, and temporal lobe. During the negative condition, mothers showed greater activation in the ACC, ventral PFC and insula, thalamus, and occipital lobe.
Table 1.
Effect of Task on Brain Response
| Contrast | Region | x y z | t | Cluster size | p |
|---|---|---|---|---|---|
| Own Positive vs. Stranger Positive | Effect of Task | ||||
| VS, Left | −14, 10, 14 | 4.92 | 156 | .032 | |
| VS, Right | 28, 8, −4 | 4.95 | 394 | .002 | |
| vlPFC, Left | −38, 30, −6 | 10.16 | 368 | .002 | |
| vlPFC, Right | 32, 32, −4 | 4.77 | 184 | .021 | |
| Temporal Lobe | −36, −48, 6 | 5.09 | 332 | .003 | |
| Own Negative vs. Stranger Negative | Effect of Task | ||||
| ACC | 4, 26, 16 | 5.62 | 456 | .002 | |
| Ventral PFC/Insula, Left | −46, 14, 4 | 6.01 | 852 | .000 | |
| Ventral PFC/Insula, Right | 48, 10, 6 | 6.83 | 607 | .000 | |
| Thalamus | 28, −22, 4 | 5.01 | 1323 | .000 | |
| Occipital Lobe | 44, −66, −12 | 3.83 | 272 | .011 |
Note. Coordinates are in Talairach space. Findings are significant at p < .01, using whole brain analyses and corrected for multiple comparisons using AlphaSim (144 voxel threshold).
3.2 Mother’s depression recurrence
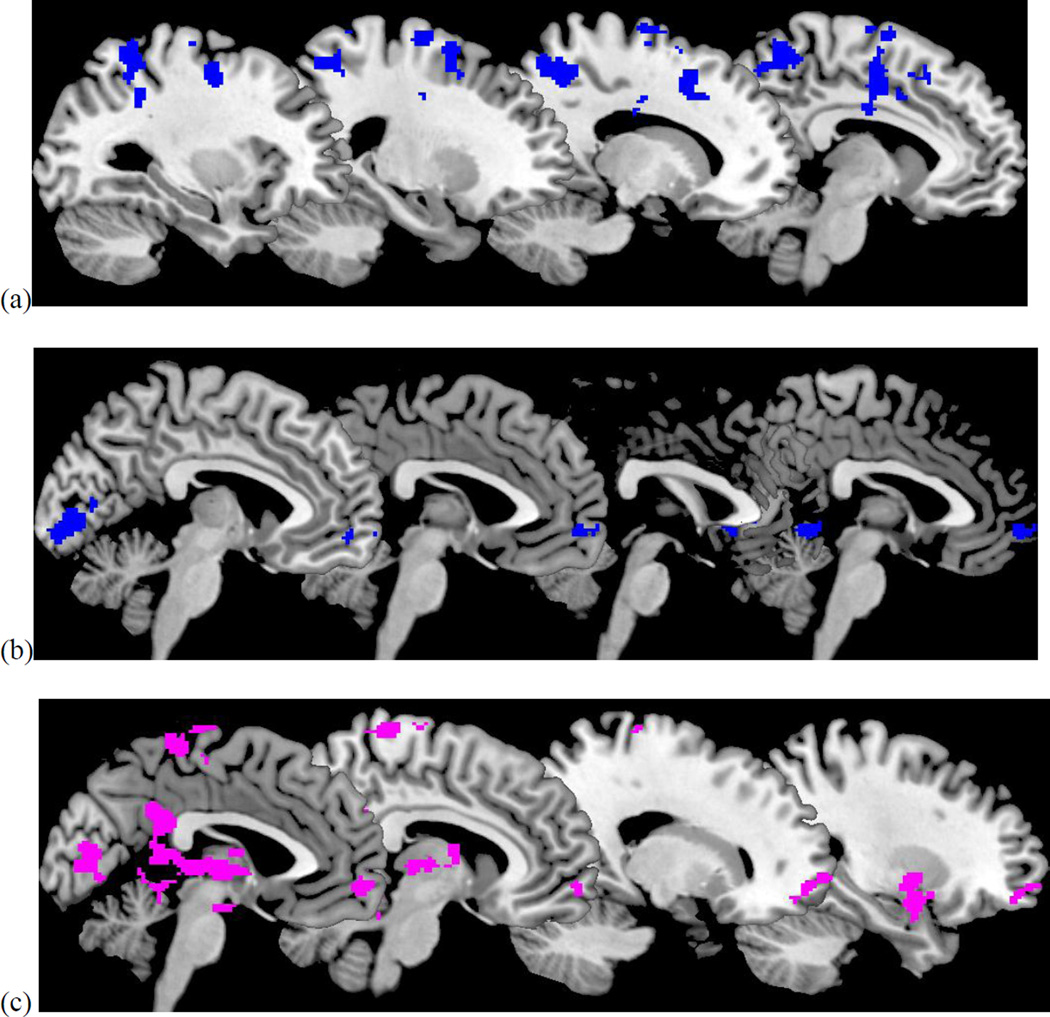
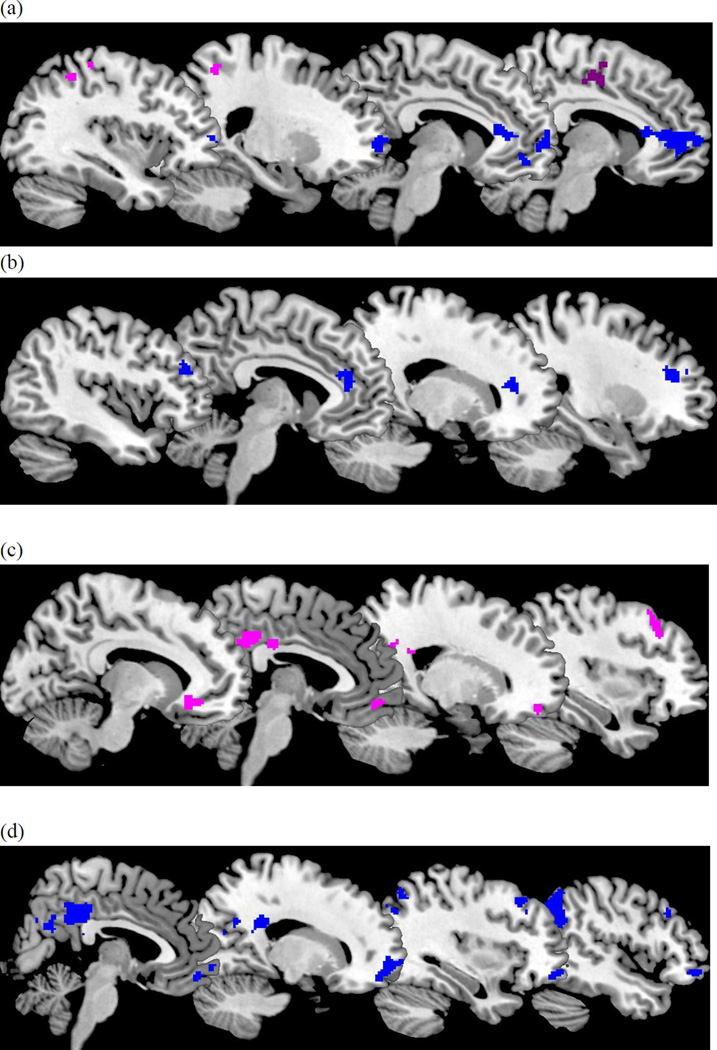
During the positive condition, greater lifetime number of depressive episodes was associated with less response in self-related regions (precuneus, superior and middle frontal gyrus) and parietal lobe1 (Table 2). In contrast, during the negative condition, greater lifetime number of depressive episodes was associated with greater response in self-related regions (precuneus and precentral gyrus) and less activation in the vmPFC when mothers viewed clips of their own children expressing negative emotions.
Table 2.
Regression of Maternal Characteristics on Neural Response to Task
| Contrast | Variable/ Region | x y z | t | Cluster | p |
|---|---|---|---|---|---|
| Own Positive vs. Stranger Positive | Number of Depressive Episodes | ||||
| Precuneus, Left (−) | −46, −38, 40 | 5.69 | 1230 | .000 | |
| Precuneus, Right (−) | 18, −70, 50 | 5.32 | 448 | .001 | |
| Middle Frontal Gyrus (−) | −32, −6, −52 | 4.62 | 147 | .034 | |
| Middle Frontal Gyrus (−) | −22, −8, 72 | 4.76 | 217 | .012 | |
| Superior Frontal Gyrus (−) | −16, 14, 48 | 4.56 | 282 | .005 | |
| Parietal Lobe (−) | 50, −26, 42 | 7.37 | 168 | .025 | |
| Comorbid Anxiety | |||||
| -- | -- | -- | -- | -- | |
| Maternal Warmth | |||||
| vmPFC (−) | −18, 64, −8 | 6.43 | 237 | .010 | |
| Occipital Lobe (−) | −6, −96, −8 | 5.55 | 586 | .000 | |
| Maternal Hostility | |||||
| Amygdala/Parahippocampal Gyrus, Left (+) | −14, 0, −20 | 6.18 | 416 | .001 | |
| Amygdala/Parahippocampal Gyrus, Right (+) | 26, 0, −2 | 6.61 | 237 | .009 | |
| vmPFC (+) | −14, 64, −8 | 6.19 | 443 | .001 | |
| Posterior Cingulate/Thalamus (+) | −10, −4, 6 | 5.32 | 1773 | .000 | |
| Medial Frontal Gyrus/Precuneus (+) | 6, −36, 76 | 4.78 | 498 | .000 | |
| Middle Frontal Gyrus (+) | 44, −62, 0 | 4.74 | 204 | .014 | |
| Cuneus (+) | 0, −78, 10 | 3.90 | 209 | .013 | |
| Insula (+) | −48, −24, 14 | 3.61 | 157 | .027 | |
| Occipital Lobe (+) | −8, −94, −8 | 4.74 | 155 | .028 | |
| Temporal Lobe (+) | −56, −18, −10 | 4.50 | 154 | .029 | |
| Own Negative vs. Stranger Negative | Number of Depressive Episodes | ||||
| Precentral Gyrus (+) | 32, −22, 68 | 5.28 | 691 | .000 | |
| Precuneus/Inferior Parietal (+) | −40, −44, 50 | 5.07 | 216 | .020 | |
| vmPFC/ACC/sgACC (−) | 26, 62, 6 | 6.73 | 1645 | .000 | |
| Comorbid Anxiety | |||||
| pgACC (−) | −4, 39, 9 | 4.14 | 191 | .027 | |
| mPFC (−) | 32, 28, 17 | 4.53 | 209 | .022 | |
| dlPFC (−) | −32, 32, 21 | 4.91 | 162 | .040 | |
| Maternal Warmth | |||||
| dlPFC (+) | 38, 20, 54 | 4.97 | 147 | .049 | |
| vmPFC (+) | 16, 50, −16 | 4.80 | 145 | .050 | |
| sgACC (+) | −10, 30, −8 | 4.78 | 208 | .022 | |
| Posterior Cingulate (+) | 6, 22, 32 | 3.96 | 336 | .005 | |
| Maternal Hostility | |||||
| vmPFC/vlPFC (−) | 20, 50, −14 | 6.57 | 613 | .000 | |
| dlPFC(−) | 46, 24, 36 | 4.22 | 162 | .038 | |
| Precuneus (−) | 10, −62, 26 | 3.50 | 211 | .021 | |
| PCC/Precuneus (−) | 14, −38, 26 | 4.62 | 666 | .000 | |
| Angular/Inferior Parietal Lobe (−) | 42, −66, 50 | 4.53 | 667 | .000 | |
| Temporal Lobe (−) | −40, −12, −18 | 4.91 | 144 | .049 |
Coordinates are in Talairach space. Findings are significant at p < .01, using whole brain analyses and corrected for multiple comparisons using AlphaSim (144 voxel threshold). (+) refers to positive effects; (−) refers to negative effects.
3.3 Mother’s comorbid anxiety
During the negative condition, mothers with comorbid anxiety showed less activation in the perigenual ACC, dlPFC, and mPFC relative to those without anxiety. There was no difference between mothers with and without comorbid anxiety for neural response during the positive condition.
3.4 Maternal warmth
Lower levels of maternal warmth were associated with greater activation in the vmPFC and occipital lobe during the positive condition. During the negative condition, lower levels of maternal warmth were also associated with less response in regulatory regions (vmPFC and dlPFC) and less response in self-referential processing regions (PCC).
3.5 Maternal hostility
Higher levels of maternal hostility were associated with greater activation in threat (amygdala and insula), affect regulatory (vmPFC), and self-related (thalamus, PCC, precuneus, temporal lobe) regions during the positive condition. Greater maternal hostility was also associated with greater activation in the cuneus and occipital lobe during the positive condition. During the negative condition, higher levels of maternal hostility were associated with less activation in affect regulatory regions (vmPFC, vlPFC, dlPFC), and self-related processing regions (precuneus, PCC, angular/inferior parietal lobe, and temporal lobe.)
3.6 Post-scan ratings
Mothers’ post-scan ratings of anger when viewing own child positive affect were negatively associated with parental warmth (r=−.47, p<.05) and approached significance in positively relating to parental hostility (r=.41, p=.08). None of the other post-scan ratings were associated with parental warmth or hostility or with number of depressive episodes or comorbid anxiety.
4. Discussion
We demonstrated that mothers respond to images of their own child, relative to an unfamiliar child, in key regions associated with reward processing (VS) and threat processing (insula) for positive and negative clips, respectively. Both types of stimuli also elicited activation in regions associated with affect regulation (vlPFC, ACC) and self-related processing (temporal lobe, thalamus). These findings mirror maternal neural networks (VS, thalamus, ACC, insula) previously identified in work with mothers of infants and toddlers (Barrett & Fleming, 2011), and suggest that these regions continue to be relevant to parenting even later in development once the parent-child relationship has changed substantially (Laursen et al., 1998).
Furthermore, brain-behavior associations demonstrated that neural response to child affect is associated with depression course, comorbid anxiety, and mother-child relationship quality. Specifically, when viewing positive clips of their children, relative to positive clips of an unfamiliar child, mothers with more recurrent episodes of depression showed less response in regions implicated in self-referential processing, including the precuneus, and in autobiographical memory, including the medial frontal regions (Northoff et al., 2006). In contrast, they showed greater response in self-referential regions (precuneus and precentral gyrus) when viewing negative clips of their children, relative to negative clips of an unfamiliar child. These findings corroborate prior behavioral findings that depression is associated with biased autobiographical memory for negative relative to positive events (Bergouignan et al., 2008), and extends these findings to autobiographical events involving parent-child interactions. This finding may also suggest a neural basis for depressed parents’ predisposition to internalize negative experiences related to their children—e.g., feeling overly sensitive to their children’s failures. Additionally, recurrent depression was associated with less activation in regulatory regions of the prefrontal cortex (vmPFC and ACC), when viewing negative clips, suggesting that more recurrently depressed mothers may have diminished ability to regulate their affect in response to their offspring’s negative affect.
Likewise, comorbid anxiety was associated with less activation in multiple regulatory regions, including the pgACC, mPFC, and dlPFC when viewing clips of own child’s negative affect. These findings provide more evidence that more severely impaired mothers (comorbid anxiety and/or chronic depression) may have difficulty regulating their response to their children’s negative affect. Prior work with mothers of infants and toddlers has demonstrated that mothers with comorbid depression and anxiety show the greatest disruptions in maternal care (Carter et al., 2001) and our findings extend this pattern to neural disruptions in comorbidly depressed and anxious mothers of older youth. Interestingly, comorbid anxiety was not associated with aberrant responding to positive clips, as would be suggested by the tripartite model of depression and anxiety which theorizes that depression, but not anxiety, is associated with diminished positive affectivity (Clark and Watson, 1991). This suggests some specificity of depression in neural response to positive affect, despite the frequent co-occurrence of depression and anxiety.
Child ratings of mother-child relationship quality were also associated with mothers’ neural responses to viewing affectively-charged clips of their children. Greater levels of child-reported maternal hostility were related to more activation in threat-related regions, including the amygdala and insula, when viewing positive clips of own child relative to unfamiliar child. This finding suggests that hostile parental behavior may be associated with responding to positive situations as if they are aversive. Indeed, prior behavioral work demonstrated that displaying negativity toward their adolescent offspring in contexts that typically elicit positive emotion distinguished depressed mothers from healthy mothers (McMakin et al., 2011), whereas this negativity pattern in response to child negative affect did not. Maladaptive response to child positive affect (rather than child negative affect) may be a better indicator of a hostile emotional style, possibly because it elicits depression-specific negativity that is extended inappropriately across contexts (Rottenberg et al., 2005). Furthermore, mothers’ post-scan ratings of anger during child positive clips were negatively associated with warmth and approached significance in positively relating to hostility, providing greater support that heightened amygdala response to child positive stimuli for more hostile mothers in our study may be related to emotional valence (i.e., negative emotion) rather than emotional salience, another important function of these regions (e.g., see Barrett et al., 2012). More hostility was also associated with greater activation in self-related regions, including the precuneus, thalamus, and PCC (Grimm et al., 2009), and greater activation in visual processing regions including the occipital lobe and cuneus, when viewing clips of their child’s positive affect. Coupled with our findings of higher amygdala and insula activity, it may be that more hostile mothers have attribution biases that contribute to heightened vigilance toward their children’s positive displays and a tendency to interpret these displays as not only annoying, but also self-targeted.
In regard to negative clips, greater maternal hostility was associated with less activation in selfreferential regions (precuneus, PCC, inferior parietal lobe) and regulatory regions (vmPFC/vlPFC, dlPFC) when viewing clips of offspring displaying negative emotions. This finding may indicate that hostility in the mother-child relationship may be associated with greater difficulty empathizing with their child’s negative affect and regulating their response to these displays.
Lower maternal warmth was associated with less activation in regions implicated in emotional pain (subgenual ACC; Rotge et al., 2014), in affect regulation (dlPFC, vmPFC), and in self- and other-related processing (PCC) in response to negative clips. Warm parenting is characterized by high levels of empathic concern and sensitive responding to child affect and thus requires the ability to regulate one’s own affect, feel the child’s emotional pain, and use theory of mind skills to evaluate how to respond (Darling and Steinberg, 1993). Given the putative functions of the subgenual ACC for empathy (Rotge et al., 2014), this finding may provide neural evidence that less maternal warmth is associated with lower levels of empathy and responsiveness to child displays of negative affect. Surprisingly, with regard to positive clips, lower maternal warmth was associated with greater activation in the vmPFC and occipital lobe. Although we did not expect to find this association, it may be that less warm mothers need to engage in more visual processing when their child is expressing positive, exuberant emotions, perhaps because of this being incongruent with the mother’s usual perception of her child, compared to warmer mothers for whom this process may be more efficient.
Given that depression is a disorder associated with impaired family functioning (Hammen et al., 2004), there is a need for personally relevant family context fMRI tasks to understand how differential neural responses to family stimuli might underlie these depression characteristics. We demonstrated that our task is associated with measures of clinical course, comorbidity, and parenting. Future work using our novel task should compare neural response to child stimuli in depressed mothers relative to healthy mothers.
A limitation to our study is our small sample size, which necessarily renders our findings preliminary. More work is needed to replicate our findings in a larger sample. Unexpectedly, in our sample, number of depressive episodes was not associated with parenting quality, although this seemed to be a power issue since these variables were inter-related in a larger sample (n=32) which included dyads assessed but not scanned. Nevertheless, because of this lack of significant effect, we were unable to evaluate a mediation model in which altered neural response served as a mediator of parental depression and compromised caregiving. Future work with larger samples should evaluate these potential mediation models.
More work is also needed to compare findings from a depressed sample to a healthy sample. Also, given that participants in our study all had children with at least one internalizing disorder, our findings may not generalize to mothers with psychiatrically healthy children or children with other psychiatric disorders. Although we propose that our findings are related to the combined effect of a depressed parent caring for a psychiatrically ill child, it is possible that our findings may be specific to the burden of caring for a child with emotional difficulties. In order to clarify this, future work should compare how depressed parents with healthy children may differ from depressed parents with psychiatrically ill children in responding to their children’s affect. We also were not able to evaluate how current depressive severity was associated with altered neural responding to child affect. Recent work has shown that improvements in maternal functioning are associated with improvements in caregiving, particularly in how parents respond to their children (Weissman et al., 2015), thus current depressive severity would have likely been an important correlate of neural response to child affect. Future work should consider how current symptoms affect these processes.
Overall, our study provides evidence that maternal neural response to child affect is associated with depression course, comorbid anxiety, and impaired mother-child relationship quality. Given the devastating consequences of psychopathology within families, including the intergenerational transmission of depression from parent to child and the emotional burden of caring for a psychiatrically ill child, greater knowledge of mechanisms underlying this process is greatly needed to create effective treatment strategies. Existing interventions for depression may be improved by focusing on the parent-child relationship, including helping mothers process and respond to their children’s affect more effectively and adaptively.
Figure 2.
Effect of task on VS, vlPFC, and temporal lobe activation when viewing positive child clips (a) and of task on anterior cingulate, ventral PFC/insula, thalamus, and occipital lobe when viewing negative child clips (b).
Figure 3.
Effect of number of depressive episodes (a), maternal warmth (b), and maternal hostility (c) when viewing positive clips. Positive effects are in violet. Negative effects are in blue.
Figure 4.
Effect of number of depressive episodes (a), comorbid anxiety (b), maternal warmth (c), and maternal hostility (d) when viewing negative clips. Positive effects in violet. Negative effects in blue.
Highlights.
We evaluated how maternal neural response to child affect is related to depression.
Affective clips of one’s own child elicited activation in multiple neural networks.
Altered maternal neural response to child affect predicted depression chronicity.
These maternal neural alterations also related to poor mother-child dyadic quality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For all negative effects, follow-up post hoc-analyses that masked identified clusters on effects from own child affect > baseline and unfamiliar child > baseline conditions confirmed that the negative associations signified correlation with a reduced response to own child in the identified region, rather than a greater response to the unfamiliar child in that region.
References
- Barrett J, Fleming AS. Annual research review: All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Bergouignan L, Lemogne C, Foucher A, Longin E, Vistoli D, Allilaire J-F, Fossati P. Field perspective deficit for positive memories characterizes autobiographical memory in euthymic depressed patients. Behaviour research and therapy. 2008;46:322–333. doi: 10.1016/j.brat.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Carter AS, Garrity-Rokous FE, Chazan-Cohen R, Little C, Briggs-Gowan MJ. Maternal depression and comorbidity: predicting early parenting, attachment security, and toddler social-emotional problems and competencies. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:18–26. doi: 10.1097/00004583-200101000-00012. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Conger RD, Wallace LE, Sun Y, Simons RL, McLoyd VC, Brody GH. Economic pressure in African American families: a replication and extension of the family stress model. Developmental Psychology. 2002;38:179. [PubMed] [Google Scholar]
- Darling N, Steinberg L. Parenting style as context: An integrative model. Psychological bulletin. 1993;113:487. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part I: Description. Journal of Personality disorders. 1995;9:83–91. [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clinical Child and Family Psychology Review. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Boeker H, Northoff G. Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. World Journal of Biological Psychiatry. 2011;12:627–637. doi: 10.3109/15622975.2010.545145. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Shih JH, Brennan PA. Intergenerational transmission of depression: test of an interpersonal stress model in a community sample. Journal of Consulting and Clinical Psychology. 2004;72:511. doi: 10.1037/0022-006X.72.3.511. [DOI] [PubMed] [Google Scholar]
- Hops H, Biglan A, Tolman A, Arthur J, Longoria N. Living in Family Environments (LIFE) coding system: Reference manual for coders. Eugene, OR.: Oregon Research Institute; 1995. [Google Scholar]
- Laursen B, Coy KC, Collins WA. Reconsidering Changes in Parent-Child Conflict across Adolescence: A Meta-Analysis. Child Development. 1998;69:817–832. [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ, Dahl RE, Hooley JM, Silk JS. Neural responses to maternal criticism in healthy youth. Social Cognitive and Affective Neuroscience, nsu133. 2014 doi: 10.1093/scan/nsu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical psychology review. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- MacPhillamy DJ, Lewinsohn PM. In: Manual for the pleasant events schedule. MacPhillamy DJ, Lewinsohn PM, editors. 1976. [Google Scholar]
- McMakin DL, Burkhouse KL, Olino TM, Siegle GJ, Dahl RE, Silk JS. Affective functioning among early adolescents at high and low familial risk for depression and their mothers: A focus on individual and transactional processes across contexts. Journal of Abnormal Child Psychology. 2011;39:1213–1225. doi: 10.1007/s10802-011-9540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty SC, Sachs-Ericsson N, Joiner TE. Interpersonal functioning deficits: temporary or stable characteristics of depressed individuals? Journal of Affective Disorders. 2004;81:115–122. doi: 10.1016/S0165-0327(03)00158-7. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz RJ, Foster S, Kent RN, O'Leary KD. Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. Journal of applied behavior analysis. 1979;12:691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotge J-Y, Lemogne C, Hinfray S, Huguet P, Grynszpan O, Tartour E, George N, Fossati P. A meta-analysis of the anterior cingulate contribution to social pain. Social Cognitive and Affective Neuroscience, nsu110. 2014 doi: 10.1093/scan/nsu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114:627. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Sheeber LB, Davis B, Leve C, Hops H, Tildesley E. Adolescents' relationships with their mothers and fathers: associations with depressive disorder and subdiagnostic symptomatology. Journal of Abnormal Psychology. 2007;116:144. doi: 10.1037/0021-843X.116.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HA, Frank E, Zuckoff A, Cyranowski JM, Houck PR, Cheng Y, MA Dana Fleming R, Grote NK, Brent DA, M Katherine Shear M. Brief interpersonal psychotherapy for depressed mothers whose children are receiving psychiatric treatment. The American journal of psychiatry. 2008;165:1155–1162. doi: 10.1176/appi.ajp.2008.07081339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 [Google Scholar]
- Weissman MM, Wickramarante P, Nomura Y, Warner V, Verdell H, Pilowsky DJ, Grillon CP, Bruder G. Families at High and Low Risk for Depression; A 3-Generation Study. Archives of General Psychiatry. 2015;62:29–36. doi: 10.1001/archpsyc.62.1.29. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wickramarante P, Pilowsky DJ, Poh E, Batten LA, Hernandez M, Flament MF, Stewart JA, McGrath P, Blier P, Stewart JW. Treatment of maternal depression in a medication clinical trial and its effect on children. American Journal of Psychiatry. 2015;172:450–459. doi: 10.1176/appi.ajp.2014.13121679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yücel M, Forbes EE, Davey CG, Harding IH, Sheeber L, Yap MB, Allen NB. Adolescents’ depressive symptoms moderate neural responses to their mothers’ positive behavior. Social Cognitive and Affective Neuroscience. 2012;7:23–34. doi: 10.1093/scan/nsr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski M, Cyranowski JM, Cheng Y, Swartz HA. Role of maternal childhood trauma on parenting among depressed mothers of psychiatrically ill children. Depression and anxiety. 2013;30:792–799. doi: 10.1002/da.22116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W-N, Chang S-H, Guo L-Y, Zhang K-L, Wang J. The neural correlates of reward-related processing in major depressive disorder: A meta-analysis of functional magnetic resonance imaging studies. Journal of Affective Disorders. 2013;151:531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]