Abstract
Perception operates on an immense amount of incoming information that greatly exceeds the brain's processing capacity. Because of this fundamental limitation, the ability to suppress irrelevant information is a key determinant of perceptual efficiency. Here, I will review a series of studies investigating suppressive mechanisms in visual motion processing, namely perceptual suppression of large, background-like motions. These spatial suppression mechanisms are adaptive, operating only when sensory inputs are sufficiently robust to guarantee visibility. Converging correlational and causal evidence links these behavioral results with inhibitory center-surround mechanisms, namely those in cortical area MT. Spatial suppression is abnormally weak in several special populations, including the elderly and those with schizophrenia—a deficit that is evidenced by better-than-normal direction discriminations of large moving stimuli. Theoretical work shows that this abnormal weakening of spatial suppression should result in motion segregation deficits, but direct behavioral support of this hypothesis is lacking. Finally, I will argue that the ability to suppress information is a fundamental neural process that applies not only to perception but also to cognition in general. Supporting this argument, I will discuss recent research that shows individual differences in spatial suppression of motion signals strongly predict individual variations in IQ scores.
Keywords: Spatial suppression, visual motion, surround suppression, intelligence, motion segregation, figure-ground segregation
1. Introduction
1.1. Suppressive mechanisms in visual processing
The visual system operates on a large amount of information (Eriksen & Eriksen, 1974; Marois & Ivanoff, 2005). Because incoming sensory inputs greatly exceed neural processing capacity, a critical role of visual processing is to highlight useful and relevant signals while suppressing redundant and less informative signals. These selective processes operate at all stages of visual processing, ranging from attentional selection to retinal receptive fields (Carandini & Heeger, 2012; Kastner & Ungerleider, 2000; Schwartz & Simoncelli, 2001; Vinje & Gallant, 2000). Consequently, only a small portion of incoming visual information reaches our conscious awareness. A ubiquitous neural mechanism for suppressing redundant and less relevant visual information is antagonistic center-surround receptive field organization (Allman et al., 1985a). In general, center-surround receptive fields amplify neural responses to spatially varying visual stimuli while suppressing responses to uniform image regions. This is adaptive as spatially varying visual inputs (e.g., edges) are generally more informative than uniform stimuli (e.g., featureless backgrounds).
Analogous processing constraints characterize visual motion processing. Spatial variations in retinal motion signals carry key visual information about, for example, object motions, 3D object shape and figure-ground segregation (Nakayama, 1985; Regan, 2000; Lappin & Craft, 2000). In contrast, uniform motion fields are generally less informative and are often caused by our own eye and body motions. These characteristics of moving stimuli are paralleled by an important and widespread presence of center-surround mechanisms in motion processing (Section 2). In this paper, I will review both neurophysiological and psychophysical work on suppressive center-surround mechanisms in visual motion processing, focusing on both characterization of underlying mechanisms and their putative functional roles. The concluding argument is that the basic processing demands that underlie the utility of suppressive center-surround mechanisms apply not only to perception but also to cognition in general. In support of this argument, I will review recent results that show a surprisingly strong link between IQ scores and individual differences in spatial suppression of motion signals.
1.2. Integration vs. segmentation
While various capacity limitations in visual processing essentially necessitate involvement of suppressive mechanisms, there are important situations where a different approach is fitting. On one hand, spatial and temporal variations in visual signals carry important information about the relative locations, motions, orientations and shapes of surfaces (Regan, 2000; Lappin & Craft, 2000; Warren, 1995). On the other hand, local visual signals are noisy, requiring spatial and temporal integration by visual mechanisms (Dakin, Mareschal & Bex, 2005). Evidently, visual processing faces two conflicting demands: integration and segmentation. For motion perception, the conflicting roles of integration and segmentation processes were explicitly discussed by Braddick (1993; also see reviews by Nakayama, 1985; Albright & Stoner, 1995). Spatial integration is key to motion perception, both because motion signals are often noisy and because of pervasive local velocity ambiguities (Adelson & Movshon, 1982; Lorenceau & Shiffrar 1992, 1999). However, local velocity differences provide key information for visual segregation (Nakayama, 1985; Sachtler & Zaidi, 1995; Regan, 2000). This inherent conflict between integration and segmentation raises an important question: how does the visual system determine the right balance between integrating and differentiating processes? The answer to this question is important not only for our understanding of motion perception, but also for elucidation of basic principles that underlie visual processing in general. While various types of visual cues can help guide integration and segregation of motion signals (Rivest & Cavanagh, 1996; Croner & Albright, 1997, 1999; Lorenceau & Alais, 2001; Tadin et al., 2002), the quality of sensory signals per se is an important factor determining the appropriate balance between integrating and differentiating processes (Faisal, Selen & Wolpert, 2008; Rubin, Van Hooser & Miller, 2015). Research by the author and other groups (Section 3.2) shows that the nature of spatial integration of motion adapts to visual conditions, with spatial summation giving way to spatial suppression as stimulus saliency increases.
2. Neural mechanisms of motion integration and segmentation
By definition, receptive fields integrate information over space and time. Segmentation of inputs can be accomplished by inhibitory surround regions (Allman et al., 1985a). Such neurons are inhibited when stimulated with spatially uniform motion patterns and respond well when center motion is different from background motion. This simple center-surround mechanism is, in fact, ubiquitous in sensory systems; occurring in vision (Barlow, 1953; Hartline, 1940; Kuffler, 1953; Allman et al., 1985a), audition (Knudsen & Konishi, 1978), touch (Mountcastle & Powell, 1959; Vega-Bermudez & Johnson, 1999), olfaction (Yokoi et al., 1995; Olsen, Bhandawat & Wilson, 2010) and even electroreception (Bastian, 1975). In motion perception, early theoretical work (Nakayama & Loomis, 1974) showed that center-surround mechanisms could be used to extract the spatial structure of moving fields and suppress relatively uninformative uniform motion fields. The existence of center-surround receptive fields in visual motion processing was confirmed by subsequent neurophysiological studies.
In primate motion processing, center-surround receptive fields are found in primary visual cortex (V1, Jones et al., 2001), MST (Eifuku & Wurtz, 1998) superior colliculus (Davidson & Bender, 1991) and are particularly common in the key motion area MT (e.g., Allman et al., 1985b; Tanaka et al., 1986; Born & Tootell, 1992; Bradley & Andersen, 1998; Born et al., 2000; DeAngelis & Uka, 2003; Pack, Hunter & Born, 2005; Perge et al., 2005; Lui et al., 2007; Huang et al., 2007; 2008; Churan et al., 2008; Anton-Erxleben et al., 2009). A typical MT neuron responds strongly if its receptive field center is stimulated by the motion in the preferred direction of the neuron. For center-surround neurons, the preferred-direction response becomes at least partially suppressed when the spatial extent of stimulation is enlarged to include the receptive field surround (Tadin & Lappin, 2005a). When the surround motion is in the anti-preferred direction, its suppressive effect diminishes and, sometimes, becomes facilitatory. The overall result is a poor neural response to large, background motions. These neurons are frequently found in all MT layers with the exception of the input layer IV (Raiguel et al., 1995; Born, 2000). This anatomical observation indicates that surround inhibition observed in the area MT is not inherited from its feedforward inputs.
Of note, area MT also contains cells that are sensitive to large, uniformly moving fields, called ‘wide-field’ neurons (Born & Tootell, 1992; Born et al., 2000). There is ample evidence that center-surround and wide-field neurons have different functional roles, with center-surround neurons signaling object motion and wide-field neurons coding background motion (Born et al., 2000). Moreover, in owl monkey, they are found in anatomically distinct clusters and make different efferent connections (Born & Tootell, 1992; Berezovskii & Born, 2000). The existence of this parallel pathway and relatively late origin of MT center-surround suppression suggests the existence of motion processes that are not affected by suppressive MT interactions (Section 3.3). Determining which motion processes are affected by surround suppression (and which are not) will help constrain its possible functional roles.
Importantly, recent work shows that MT center-surround mechanisms are not fixed but adapt to changing stimulus conditions. Specifically, surround suppression can shift to facilitation at low-contrast (Pack, Hunter & Born, 2005) or when motion in the receptive field center is ambiguous (Huang et al., 2007; 2008)—all conditions where motion integration is beneficial. Consequently, perceptual mechanisms that critically depend on surround suppression should also exhibit analogous stimulus dependency. This hypothesis is explored in Section 3.1.
3. Perceptual correlates of surround suppression
Given the prominent role of surround suppression in neural mechanisms of motion processing, we should to expect to find observable perceptual correlates of surround suppression (see Sections 3.2 and 3.4 for detailed considerations of issues behind this linking hypothesis). Indeed, psychophysical studies have reported results consistent with surround suppression. Sachtler & Zaidi (1995) showed that detection of motion-defined boundaries could be explained by eccentricity-dependent center-surround mechanisms. Verghese & Stone (1996) found that speed discriminations improved when a single large moving stimulus was divided into several smaller stimuli. The authors suggested suppressive surround mechanisms as a possible explanation. Derrington & Goddard (1989) found that direction discriminations of brief, large gratings worsened as the contrast increased. This result is consistent with contrast-dependent surround suppression (Pack, Hunter & Born, 2005), although the authors did not vary stimulus size and did not consider size-dependent explanations. Murakami & Shimojo (1993) investigated induced motion in stationary stimuli presented within a large patch of moving dots. They found that motion induction (i.e., motion contrast) transitioned into motion assimilation when stimulus contrast and size were reduced or when the stimuli were shown in the visual periphery. This finding suggests that motion induction changes to spatial summation under low visibility conditions. Surround suppression is also suggested by findings in several motion aftereffect (MAE) studies in which large, high-contrast adaptation patterns produced attenuated MAEs (Sachtler & Zaidi, 1993; Murakami & Shimojo, 1995; Tadin et al., 2003; 2008; Falkenberg & Bex, 2007).
While the above-described results are consistent with suppressive center-surround mechanisms, further advancement in our understanding of surround suppression and its functional roles requires stronger linking of center-surround antagonism with its behavioral correlates. This raises the following question: what are the direct perceptual consequences of suppressive center-surround mechanisms? A simple prediction is that motion sensitivity should decrease with increasing stimulus size, but this prediction conflicts with established reports of strong spatial summation in motion (Anderson & Burr, 1991; Watson & Turano, 1995). Importantly, these psychophysical results relied on contrast thresholds measurements, which restricted their measurements to low-contrast stimuli. On the other hand, neurophysiological work on surround suppression was typically restricted to high-contrast motion stimuli. This stimulus difference is important because center-surround interactions can vary with contrast, with suppression dominating at high contrast and summation at low contrast (Sceniak et al., 1999; Nauhaus et al., 2009).
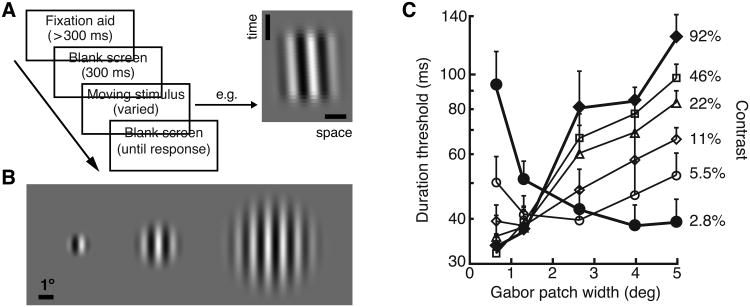
Our results (Tadin et al., 2003) revealed that spatial integration of motion signals indeed critically depends on stimulus contrast (Fig. 1). At low-contrast, duration thresholds improved as stimulus size increased—replicating previous psychophysical results on spatial summation. At high-contrast, however, motion direction discriminations became substantially more difficult as the stimulus size increased. The observed effects were strong: the motion direction of large, high-contrast stimuli was several times less visible than the motion of the same stimuli when (1) shown at low-contrast, (2) embedded in dynamic noise (3) or presented at isoluminance. In order to clearly distinguish these psychophysical results from neurophysiological surround suppression, we use the term spatial suppression as referring to the psychophysical results indicating weakening of motion processing with increasing stimulus size. In a series of psychophysical studies, we and others have investigated spatial suppression using duration thresholds for motion direction discriminations (Tadin et al., 2003, 2005b, 2006a; Lappin et al., 2009; Betts et al., 2005, 2009; Golomb et al., 2009; Glasser & Tadin, 2010; 2011; 2014; Melnick et al., 2013), MAE (Tadin et al., 2003, 2008; Falkenberg & Bex, 2007), reaction times (Tadin et al., 2007), binocular rivalry (Paffen et al., 2004; 2005; 2006) and reverse correlation (Tadin et al., 2006b; Neri & Levi, 2009).
Fig. 1.
Psychophysical spatial suppression: experimental task, stimuli and typical results. A. The experimental task is to identify motion direction (left vs. right) of a briefly presented moving stimulus. The space-time plot depicts a rightward moving stimulus (full-width at half-height duration = 53 ms). Vertical and horizontal scale bars are 10 ms and 1°. B. A range of stimulus sizes suitable to demonstrate spatial suppression (here shown as sizes used in Melnick et al. (2013)). Only one stimulus is shown on each trial. C. The main results from Tadin et al (2003). At low contrast, thresholds improve with increasing size, motion perception exhibits spatial summation. However, at high contrast, increasing size results in worsening of motion perception, i.e., spatial suppression. Adapted from Tadin et al. (2003) and Melnick et al., (2013) with permission from Nature Publishing Group and Elsevier.
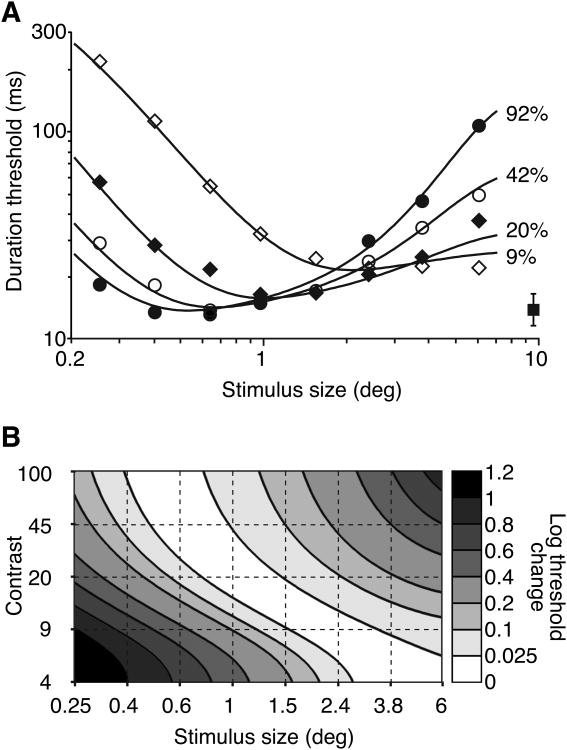
In general, these studies show strong spatial suppression for large, high-contrast moving stimuli. Moreover, this effect generalizes to a range of tasks, stimuli and psychophysical measurements. Simply stated, larger is not always better for motion perception. Instead, the optimal moving stimulus size depends on contrast: as contrast increases the most discriminable stimulus size decreases (Fig. 2; Tadin & Lappin, 2005b). I speculate that this decreasing optimal stimulus size with increasing contrast reflects changes in the point where inhibitory surround mechanisms overcome excitatory summation processes.
Fig. 2.
Optimal stimulus size decreases with increasing contrast. A. Motion direction discriminations as a function of stimulus size at different contrasts. For clarity, only the average between-subject SEM is shown (filled square). B. Threshold change relative to the optimal size at different contrasts. The diagonally oriented white region indicates that the optimal size increases with decreasing contrast. Adapted from Tadin and Lappin (2005b) with permission from Elsevier.
3.1. Adaptive integration and segregation of motion signals
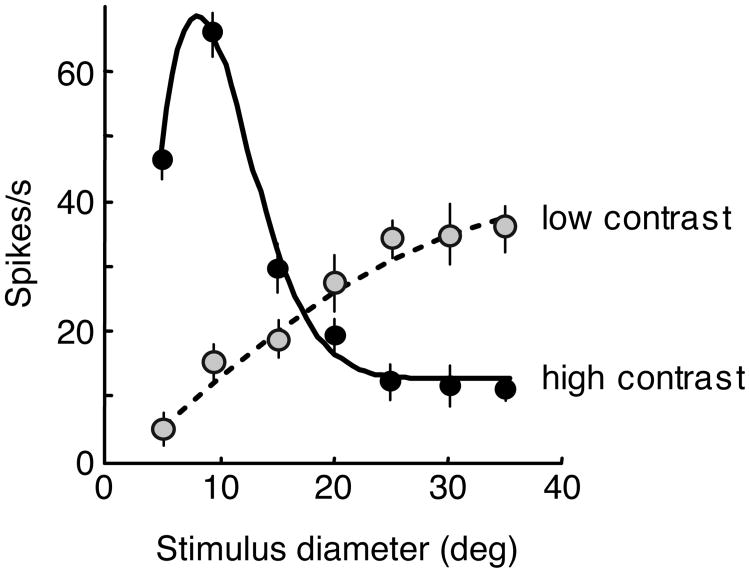
The key observation about spatial suppression is that it depends on stimulus strength. Spatial suppression weakens or disappears when stimulus strength is lowered by, for example, decreasing contrast or adding noise (Tadin et al., 2003). From these results, we hypothesized that center-surround interactions in area MT will also depend on stimulus salience. Indeed, it was subsequently shown that MT surround suppression can shift to facilitation at low-contrast (Fig. 3, Pack, Hunter & Born, 2005; Hunter & Born, 2011) or when center motion is ambiguous (Huang et al., 2007, 2008). This adaptive integration of motion signals over space might be vision's way of dealing with conflicting demands of integration and segmentation (Braddick, 1993; Section 1.2), where functionally useful suppressive mechanisms (see Section 5) operate only when the sensory input is sufficiently strong to guarantee visibility (Rubin, Van Hooser & Miller, 2015). On the other hand, at low contrast or high noise, sensitivity becomes more important (Faisal, Selen & Wolpert, 2008). Under such conditions, velocity and direction differences may be caused by low signal-to-noise ratios, and, consequently, spatial segregation of local motion signals may lead to spurious percepts. Moreover, processing of low signal-to-noise ratio stimuli can benefit from averaging. Thus, it is likely functionally beneficial that receptive field organization changes from surround suppression to spatial summation as stimulus visibility decreases.
Fig. 3.
Contrast-dependent size tuning in cortical area MT. An example neuron's responses for increasing stimulus sizes at low (dashed line) and high (solid line) contrasts. Adapted from Pack, Hunter & Born (2005) with permission from The American Physiological Society.
Additional evidence for stimulus-dependent spatial integration of motion signals comes from studies investigating perception of moving objects seen though multiple apertures (Lorenceau & Shiffrar 1992, 1999; Shiffrar & Lorenceau, 1996; Alais et al. 1998, Takeuchi 1998; Lorenceau & Zago, 1999). To perceive such stimuli as rigid moving objects, local motion information should be integrated across apertures. Without spatial integration, these stimuli are perceived as collections of independently small moving objects. The results showed that spatial integration was more likely to occur at low-contrast, high stimulus noise, eccentric viewing and at isoluminance. Notably, these are the same conditions where we found strong spatial summation and weak spatial suppression (Tadin et al., 2003). Takeuchi (1998) showed that the transition from spatial grouping to spatial segregation occurred around 5% stimulus contrast— approximately the same contrast level where we found the transition from spatial summation to spatial suppression for grating stimuli (Fig. 1).
Evidently, one way in which vision deals with motion signals that are characterized by widely varying reliabilities is to utilize flexible spatial integration mechanisms that adapt to fluctuating stimulus conditions. While it can be argued that this flexible spatial processing is both necessary and beneficial, it is also a pragmatic compromise that can fail under certain conditions. The above-described work by Lorenceau and Shiffrar shows that vision can make the error of excessive segregation under high visibility conditions. On the other hand, vision can make the complimentary error of failing to segregate distinct moving stimuli when the motion signal is degraded (Regan and Beverley, 1984; Regan, 1989).
3.2. A link with area MT?
Based on the converging evidence from a series of experiments, we showed that psychophysical spatial suppression has characteristics analogous to suppressive center-surround receptive fields, namely those in cortical area MT (Tadin et al., 2003). (1) The “critical size” where strong spatial suppression is first found matches foveal MT receptive fields in the macaque monkey (Raiguel et al., 1995). (2) This critical size increases with increasing eccentricity—a result consistent with increasing receptive field sizes in visual periphery (Raiguel et al., 1995). (3) Contrast dependency of spatial suppression (Fig. 1,2) matches contrast dependency of a sub-population of MT neurons (Pack, Hunter & Born, 2005; but see Section 3.4 for limitations of this link). (4) MAE, a perceptual aftereffect linked with MT mechanisms (Huk et al., 2001), is attenuated for large, high-contrast stimuli. This finding may be caused by inhibition of MT neurons whose adaptation normally causes MAE. (5) We found no evidence of spatial suppression for isoluminant moving stimuli, a finding consistent with weak MT responses to isoluminant gratings (Gegenfurtner et al., 1994). (6) Spatial suppression is absent for stimulus speeds slower than 1°/s (Lappin et al., 2009). This finding is consistent with results from MT neurons showing preferred speeds well over 1°/s and an absence of direction sensitivity for speeds slower than 0.5°/s (Lagae et al., 1993; Priebe et al., 2006). In sum, these findings provide converging behavioral evidence that spatial suppression is, at least in part, a behavioral correlate of surround suppression in cortical area MT.
However, several issues need to be considered when making this linking hypothesis. First, we must consider the population response and not only single neurons. Certainly, large, moving stimuli will suppress neurons whose both center and inhibitory surround regions of the receptive field are covered by the stimulus. But, neurons with receptive fields along the stimulus border would be only partially suppressed. Moreover, increasing stimulus size increases the size of the neural population that might potentially signal stimulus motion direction. So, why the behavioral outcome is a decrease in motion discriminability? Here, I argue that the use of gradual spatial envelopes in behavioral studies becomes critical (e.g., Gaussian or raised cosine). The speculation is that the blurred stimulus border would minimize contribution of less suppressed units to the population response. Indeed, when the gradual envelope is replaced with a rectangular spatial profile, increasing stimulus size does not result in decreased performance (personal observation).
Second, we also have to consider ‘wide-field’ MT neurons that respond strongly to large moving stimuli (Born & Tootell, 1992). As such, the unsuppressed neurons could, in principle, provide motion direction information for large stimuli. Namely, a simple decoding mechanism that relies on the most informative neurons (e.g., Pitkow et al., 2015) should be able to use responses of wide-field neurons to extract motion direction. The fact that this does not occur requires an explanation. I suggest two non-exclusive possibilities. Firstly, neurophysiological results indicate that surround suppressed and wide-field neurons likely have distinct functional roles, with surround suppressed neurons directly involved in perceiving object motion, while wide-field neurons represent background motion (Born et al., 2000). In fact, while the responses of surround suppressed neurons generally correlate with perceptual motion discriminations, wide-field neurons can outperform behavioral performance for large, high-contrast stimuli (Liu & Pack, 2014). This dissociation further argues for a distinct functional role of wide-field neurons. Moreover, it is also consistent with our behavioral results showing that while motion direction of briefly presented, large, moving stimuli is perceptually below threshold, these same stimuli can drive other visual processes (Glasser et al., 2011; Glasser & Tadin, 2014; Section 3.3). Secondly, the majority of spatial suppression studies measured duration thresholds and, consequently, utilized brief motion stimuli (see Section 3.4 for an additional discussion). It can be argued that these studies unknowingly exploited a recently discovered property of MT neurons. For briefly presented stimuli, surround-suppressed neurons dominate motion processing in MT (Churan et al., 2008). For 40 ms stimulus durations, surround-suppressed neurons exhibit strong directional tuning, as long the stimulus size is not too large to evoke the inhibitory surround responses. In contrast, wide-field neurons—neurons that are well suited to represent large moving stimuli—show poor selectivity for briefly presented stimuli regardless of their size. Studies measuring threshold exposure duration required for correct motion direction perception (i.e., duration thresholds) essentially capitalize on this result by relying on stimuli that are too brief to be processed by wide-field MT mechanisms.
Finally, to provide causal evidence for a link between spatial suppression and cortical area MT, we used transcranial magnetic stimulation (TMS) to temporarily attenuate MT processing (Tadin et al., 2011). The study was motivated by an observation that surround-suppressed neurons are absent from the MT's input layer, which indicates that surround suppression observed in MT is not inherited from feedforward inputs (Raiguel et al., 1995; Born, 2000). Behavioral spatial suppression, as defined, is essentially a reflection of brain mechanisms that prevent conscious perception of motion direction of large, high-contrast moving stimuli. Our hypothesis was that if area MT is indeed the neural site where this “brake” on motion perception is implemented, then interfering with MT processing may lead to conscious perception of these normally suppressed motion signals. Indeed, TMS targeting of MT resulted in weaker spatial suppression and better-than-normal motion perception of large, high-contrast moving stimuli—causally linking human area MT and spatial suppression.
3.3. What survives spatial suppression?
Evidence for strong perceptual suppression of large, high-contrast moving stimuli (Tadin et al., 2003) does not indicate that the suppressed motion information is discarded. In fact, there are numerous instances where perceptually suppressed stimuli are processed by other brain mechanisms (e.g., He et al., 1996; Blake et al., 2006; Maruya et al., 2008). Elucidating which brain processes are affected by spatial suppression and which are not will further constrain its neural correlates. For example, if spatial suppression is indeed a perceptual manifestation of surround suppression in area MT (Section 3.2) then it might not be reflected in motion processes that derive from earlier stages of processing and those that are in parallel. In fact, there is strong neurophysiological evidence that surround suppression mechanisms are a part of a parallel pathway. As detailed above, MT surround suppressed neurons are found in distinct clusters and make different efferent connections than wide-field neurons (Born & Tootell, 1992; Berezovskii & Born, 2000). These two types of neurons are also believed to have different functional roles (see Section 5).
A starting point for determining what survives spatial suppression is the use of large, high-contrast stimuli that are sufficiently brief to yield at-chance motion direction discrimination (25-67 ms). Next, we asked whether there are aspects of visual motion processing that evade complete spatial suppression and found that fully suppressed moving stimuli can still induce static MAEs (Glasser et al., 2011) and measurable ocular following responses (OFR, Glasser & Tadin, 2014).
Behavioral and neurophysiological evidence in the MAE study (Glasser et al., 2011) indicated a pre-MT locus (or loci) of static motion adaptation, which in turn provides additional evidence that neural correlates of spatial suppression are at later stages of processing. The argument here is that spatial suppression occurs subsequent to neural mechanisms responsible for the buildup of static MAE. It is currently unknown whether spatial suppression also affects the buildup of dynamic MAE, an aftereffect that reflects higher levels of motion processing (Nishida et al., 1997; Blake et al., 2006; Maruya et al., 2008). Given the links between spatial suppression and MT mechanisms (Section 3.2), I hypothesize that the dynamic MAE would be strongly affected by spatial suppression.
Dissociation between OFRs and spatial suppression is best explained by a parallel oculomotor pathway that is unaffected by suppressive mechanisms that operate at the spatial scale of MT receptive fields (Glasser & Tadin, 2014). This is consistent with results that OFRs are likely driven by wide-field motion mechanisms exhibiting a foveal summation area of ∼30° in diameter (Barthélemy et al., 2006) and adds to the evidence that the occulomotor system can be driven by motion information not reflected in motion perception per se (Masson et al., 2002; Churchland et al., 2003; Chen et al., 2005; Sheliga et al., 2005; 2006; Spering & Carrasco, 2012; Spering, Pomplun & Carrasco, 2011; Rothkirch, Stein, Sekutowicz & Sterzer, 2012). On the other hand, Glasser and Tadin's (2014) findings support the characterization of spatial suppression as a mechanism predominantly affecting perceptual representation of motion—a characterization that offers cues about its functional roles (see Section 5 for more details on different functional roles of surround suppressed and wide-field mechanisms).
3.4. Limitations
With most spatial suppression studies measuring temporal duration thresholds (e.g., Tadin et al., 2003, 2005b; Betts et al., 2005, 2009), spatial suppression is usually characterized using very brief stimuli, typically shorter than 100 ms and sometimes shorter than 10 ms (Tadin et al., 2006b; Lappin et al., 2009). The assumption behind this method is the following: if the neural response to a given stimulus is noisy, weak or actively suppressed, then the longer exposure duration will be necessary for the stimulus to be correctly perceived (cf. Roitman & Shadlen, 2002; Kiani et al., 2008). This method has an inherent advantage: Brief stimuli are better matched to natural motion stimuli: because of both saccadic eye movements and motions in the world, environmental moving stimuli usually activate motion selective neurons only for a fraction of a second. However, the use of duration threshold measurements also requires the use of transient motions, stimuli that affect the quality of local motion information (Derrington & Goddard, 1989; Zhang et al., 2013). Despite the ecological relevance of brief moving stimuli, there is limited neurophysiological evidence about how neural responses change when the motion stimulation is transient (for exceptions see Buracas et al., 1998; Churan et al., 2008; Glasser et al., 2011). There are also concerns that spatial suppression may be actually caused by stimulus onset transients. Churan and colleagues (2009) showed that elimination of onset transients dramatically reduces spatial suppression strength, as measured by motion-step thresholds (i.e., phase shift thresholds). The stimulus onset transients were eliminated by presenting a stationary stimulus for at least 120 ms before it started moving. The addition of this stationary phase, however, also made the task considerably easier as evident by phase-shift thresholds around 2 arcmin. Such small displacements effectively correspond to very slow stimulus speeds. This is notable because both first-order motion perception (Tsujimura & Zaidi, 2002) and MT neurons show poor selectivity for very slow motions. Indeed, spatial suppression strength decreases with decreasing speed and is absent for speeds slower than 1°/s (Lappin et al., 2009).
Recent evidence shows that stimulus dependency of MT surround suppression is much more complex than that of behavioral spatial suppression. While, on average, the strength of MT surround suppression decreases with decreasing contrast (Pack, Hunter & Born, 2005; Hunter & Born, 2011), the pattern of contrast-dependency of MT surround suppression is considerably more complex than that of behavioral spatial suppression (Tsui & Pack, 2011). For example, for many MT neurons, the strongest surround suppression is observed at intermediate contrasts. This result is at odds with psychophysical findings (Fig. 1,2), but it does match a pattern of results found in older adults (Betts et al., 2009). An additional difference between neurophysiology and behavior is in the context of changes in suppression strength with changes in signal-to-noise ratio. While both spatial suppression and MT surround suppression, on average, weaken with decreasing contrast, MT surround suppression actually strengthens with decreasing stimulus coherences (Hunter & Born, 2011). This result is inconsistent with a weakening of spatial suppression with increasing random pixel noise (Tadin et al., 2003). It is possible, however, that differences in the type of stimulus noise account for this discrepancy, with decreasing coherence strengthening surround suppression by better stimulating broadly tuned suppressive surrounds in MT (Hunter & Born, 2011). MT receptive field surrounds also exhibit highly diverse spatial and directional anisotropies (Xiao et al., 1995; Cui et al., 2013). These complex and variable receptive field properties are unlikely to be directly observable in psychophysical results, although their putative functional roles (e.g., optic flow estimation; Cui et al., 2013) are certainly open for investigation.
The majority of the above-described psychophysical work used moving gratings or moving texture patterns. This leaves open the question to what degree these findings generalize to other types of moving stimuli, particularly to the often-used class of motion coherence stimuli. There are neurophysiological and psychophysical indications that the spatial tuning of such stimuli may be different than that of gratings. As noted above, Hunter & Born (2011) found strong MT suppression with weakly coherent motions, a low signal-to-noise stimulus. Psychophysically, stimulus size seems to have small effects on the discriminability of moving dot stimuli (Watamaniuk, 1993; Dakin, Mareschal & Bex, 2005).
Finally, Aaen-Stockdale et al. (2009) raised an issue that is potentially of relevance to all studies that change stimulus size at a fixed contrast: given that contrast sensitivity improves with increasing size, larger stimuli have higher effective contrasts (Anderson & Burr, 1991). In their study, Aaen-Stockdale and colleagues estimated the amount of contrast imbalance required to reliably bias a pair of counterphasing gratings into one direction. At high stimulus contrast, the results revealed higher thresholds with increasing stimulus size—seemingly revealing spatial suppression in this task. However, this size-dependent effect disappeared when the effective stimulus contrast was equalized across stimulus sizes (i.e., stimuli were set at a fixed contrast relative to their contrast threshold). While these results may not necessarily generalize to direction discrimination measurements, the issue of relative contrast is worth considering as it may provide a parsimonious account of spatial suppression. Therefore, we measured duration thresholds for motion direction discriminations of large and small high-contrast gratings (Glasser & Tadin, 2010). As expected (Fig. 1,2), motion discriminations were substantially better for small stimuli. We then equalized effective stimulus contrasts by setting all contrasts as a fixed multiple of corresponding contrast thresholds. Importantly, even with the effective contrast equalized, motion of large stimuli was considerably harder to discriminate. It should be noted, however, that because of the evidence that distinct spatial processes operate at different contrasts (e.g., Lorenceau & Shiffrar, 1992, 1999; Tadin et al., 2003), it can be argued that it is not appropriate to normalize high-contrast stimuli using measurements obtained at contrast threshold.
3.5. Spatial suppression and summation across visual sub-modalities
Suppressive center-surround mechanisms are a ubiquitous property of visual information processing (Allman et al., 1985a), and, in addition to motion processing, are also found in orientation (Jones et al., 2002) and color processing (Solomon et al., 2004). This raises the question of whether contrast-dependent center-surround mechanisms analogous to those found in motion perception generalize to color and orientation processing. Answering this question is complicated by the fact that many experimental approaches (e.g., motion direction discriminations) are specific to certain visual sub-modalities. To circumvent this problem, we utilized binocular rivalry as a modality-independent way to measure suppression strength, using the relative predominance of rival stimuli as a proxy for stimulus strength (Levelt, 1965). An additional motivation to utilize binocular rivalry as a methodological tool are results showing that binocular rivalry is affected by the surrounding visual context in a manner that is generally consistent with known neurophysiology (Fukuda & Blake, 1992; Sobel & Blake, 2002; Paffen et al., 2004).
For motion, orientation and color processing, the addition of a binocular surround that matched one of the rival stimuli strongly altered rivalry dynamics (Paffen et al., 2006). At high contrast, predominance of the rival stimulus matched to the surround was significantly reduced. This result suggests spatial suppression mechanisms. At low contrast, however, predominance of the rival stimulus matched to the surround was boosted, a finding consistent with spatial summation. Evidently, contrast-dependency of center-surround mechanisms appears to be a general property of visual processing.
4. Impairments of spatial suppression in special populations
A major perceptual consequence of spatial suppression in motion processing is impaired perception of large, high-contrast moving stimuli (Tadin et al., 2003). Consequently, abnormal weakening of spatial suppression should be manifested as improved motion perception of large, high-contrast stimuli. The prediction that an underlying suppression abnormality should result in better-than-normal performance makes the spatial suppression paradigm very appealing for use in special populations. When testing special populations (e.g., psychiatric disorders), perceptual performance impairments are typically harder to interpret than performance improvements. The former can often be caused by extraneous factors such as differences in motivation and attention. Additionally, spatial suppression is also of interest because of its presumed links with inhibitory neural mechanisms (Sections 2 and 3.2). Abnormalities in cortical inhibition and/or excitatory-inhibitory balance are implicated in a wide range of conditions (Rubenstein & Merzenich, 2003; Leventhal et al., 2003; Wassef et al., 2003; Aurora & Wilkinson, 2007).
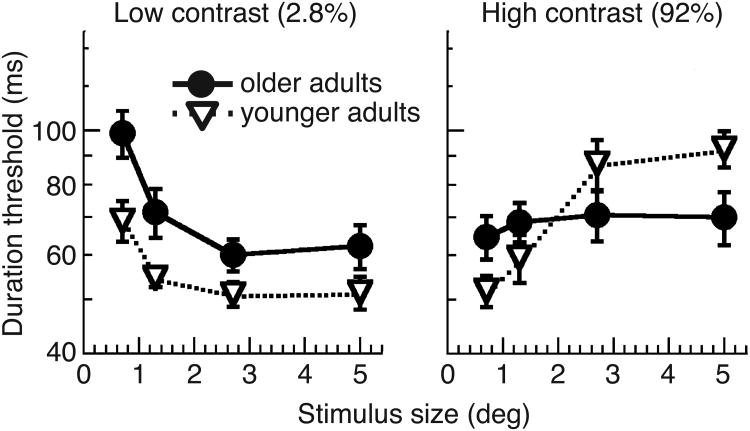
Betts and colleagues (2005) were the first to test spatial suppression in a special population, focusing on older adults. The results revealed markedly reduced spatial suppression; older adults had better-than-normal thresholds for perceiving motion direction of large, high contrast moving stimuli (Fig. 4; Betts et al., 2005; 2009; but see Karas & McKendrick, 2011). The authors hypothesized that this age-dependent improvement in motion perception is caused by a reduction in the efficacy of cortical inhibition and a related weakening of suppressive center-surround interactions. In a subsequent study, we found that individuals with schizophrenia also exhibit reduced spatial suppression, particularly those patients with strong negative symptoms (Tadin et al., 2006a). This finding adds to the similarities between schizophrenia and aging. In addition to exhibiting similar deficits in other aspects of motion processing (Bidwell et al., 2006), both schizophrenia and aging are associated with GABAergic impairments (Schmolesky et al., 2000; Leventhal et al., 2003; Wassef et al., 2003).
Fig. 4.
Effects of aging on spatial suppression. At low contrast, both young and older adults exhibit spatial summation, with older adults showing a small, but a consistent threshold elevation. At high contrast, only young adults exhibit spatial suppression. Consequently, older adults outperform young adults at discriminating motion direction of large, high-contrast stimuli. Adapted from Betts et al. (2005) with permission from Elsevier.
Weaker spatial suppression is also found in patients with a history of major depression (Golomb et al., 2009) and in children (Lewis, Sekuler & Bennett, 2008)—both populations linked with reduced cortical inhibition. Studying spatial suppression in children with autism, another population associated with excitatory-inhibitory imbalance, we found no group differences in spatial suppression at high contrast (Foss-Feig et al., 2013). However, autism was associated with a two-fold enhancement of motion direction discriminations across all stimulus sizes, raising the possibility that this large group difference in performance might have masked true group differences in spatial suppression. At a lower contrast, where we found no overall differences in performance, children with autism did exhibit weaker spatial suppression.
Yet, the link between spatial suppression and inhibitory dysfunction is not as clean as suggested by above described studies. Given the links between migraine and cortical hyperexcitability (Aurora & Wilkinson, 2007), abnormally weak spatial suppression can be hypothesized for individuals with migraines (Battista et al., 2010). However, migraine is associated not with weaker, rather with increased spatial suppression for moving stimuli (Battista et al., 2010, 2011). Moreover, a recent study found that acute alcohol intoxication, and thus potentiation of GABAergic transmission, has essentially no effect on spatial suppression strength (Read et al., 2015). In order adults, Karas & McKendrick (2011) found increased spatial suppression in a contrast perception task conducted with moving stimuli—a result opposite to the Betts et al. results shown in Fig. 4. Neurophysiological evidence also questions the assumed link between cortical inhibition and surround suppression. In primate MT, a local blockade of GABA receptors does not directly cause a reduction of surround suppression (Liu & Pack, 2014). Evidently, the link between neural excitatory-inhibitory imbalance and spatial suppression is not as direct as implied by earlier studies. One possible reason for this complex pattern of results is that surround suppression processes can be affected by a wide range of inhibitory and excitatory factors (Ozeki et al., 2009; Rubin, Van Hooser & Miller, 2015).
The simple interpretation of links between suppression and inhibition has also been questioned in other visual domains. In cat V1, orientation-dependent surround suppression is actually associated with a decrease in inhibition received by neurons (Ozeki et al., 2009). Work with schizophrenia showed that abnormally weak center-surround interactions in one perceptual domain do not predict abnormalities in analogous center-surround tasks in other domains (Yang et al., 2013a,b). More recent work (Tibber et al., 2015) found a similar lack of a generalized schizophrenia deficit in a variety of tasks that require spatial pooling of local information. In aging, a number of researchers have examined tasks that are considered to reflect inhibitory neural mechanisms and found evidence for ranging from intact processing (Delahunt et al., 2008; Govenlock et al., 2009; 2010) to weaker suppression (Karas & McKendrick, 2009, 2015; Melnick, Dieter & Tadin, 2014) to stronger suppression (Melnick, Dieter & Tadin, 2014).
In sum, where there is ample evidence for abnormal spatial suppression in various special population that are linked with excitatory-inhibitory imbalances, a number of studies caution about drawing general conclusions from these results. Of note, better-than-normal motion discriminations of large moving stimuli observed in several special populations can be mimicked by interfering with MT processing in typical subjects (Tadin et al., 2011). This suggests that an abnormality in MT processing may be sufficient to cause the spatial suppression impairments seen in special populations.
5. Functional role of spatial suppression
Unusual perceptual phenomena (e.g., illusions) generally fall into one of two categories: they either have a direct functional role (e.g., color constancy illusions; Eagleman, 2001) or are largely a side effect of a different neural process (e.g., color afterimages). The fundamental question about spatial suppression is whether a considerable perceptual insensitivity to brief, large, high-contrast moving stimuli actually serves a functional role in visual perception.
A defining property of neural surround suppression is a stronger response to small, moving objects than to large, uniform motions. This basic response property has motivated the hypothesis that center-surround mechanisms play a direct role in segmenting moving objects from their visual backgrounds (e.g., Allman et al., 1985a, 1985b; Born & Tootell, 1992; Jones et al., 2001). The plausibility of this hypothesis is supported by theoretical and modeling studies (Nakayama & Loomis, 1974; Buracas & Albright, 1994, 1996; Nowlan & Sejnowski, 1995; Sachtler & Zaidi, 1995; Liu & Van Hulle, 1998; Gautama & Van Hulle, 2001, Loffler & Orbach, 2003; Petkov & Subramanian, 2007; Gao et al., 2008). However, with a few exceptions, neurophysiological and direct behavioral support for this hypothesis is limited. For example, Sachtler & Zaidi (1995) showed that a center-surround model could describe detection thresholds of motion boundaries defined by spatial velocity distributions. The spatial scale of model parameters, however, was considerably smaller than MT receptive fields, indicating an earlier mechanism.
Arguably, the best neurophysiological link between surround suppression and motion segmentation is reported by Born et al. (2000). Born and colleagues microstimulated small clusters of MT neurons just before monkeys made saccades to a moving target in their visual periphery, a type of microstimulation that usually causes a small shift in the ensuing post-saccadic pursuit eye movements. When stimulation was delivered to surround suppressed neurons, these shifts were in the neurons' preferred direction—an expected result reflecting the established role of MT in coding motion direction. However, the stimulation of wide-field neurons shifted pursuit eye movements in the neurons' anti-preferred direction. One explanation of this result is that the brain interprets the activation of wide-field neurons as signaling the presence of background motion. Indeed, when the authors replaced microstimulation with a moving background stimulus, the outcome matched findings from stimulation of wide-field neurons. The exact function of this background motion coding in MT is unclear, although it may serve to encode perceptual consequences of self-motion, which is a notable source of wide-field motion (Born et al., 2000).
Using the MAE as an experimental tool, we examined how spatial suppression strength is modulated by context manipulations that change the figure-ground relationship but keep local motion signals constant (Tadin et al., 2008). We found strong spatial suppression when the visual context suggested a large background motion. However, when the visual context suggested the presence of several small moving objects, spatial suppression strength decreased. This pattern of results indicates a link between center-surround mechanisms and figure-ground segmentation.
Further suggestive evidence for this hypothesis is given by results showing no spatial suppression for second-order moving stimuli (Glasser and Tadin, 2011), stimuli that do not support efficient motion segregation (Ashida, Seiffert & Osaka, 2001). Moreover, older adults and individuals with schizophrenia, two populations that exhibit abnormally weak spatial suppression (Tadin et al., 2006a; Betts at al., 2005), are also linked with defects in tasks that require motion segregation (Wist et al., 2000; Schwartz et al., 1999). This is again consistent with a functional link between these two visual mechanisms (Tadin & Blake, 2005). However, none of these studies make a strong case for a link between spatial suppression and motion segregation.
In sum, there is a clear disparity between the amount of theoretical and empirical work on the links between spatial suppression and figure-ground segregation in motion processing, revealing the need for further experimental work. Given the omnipresence of center-surround mechanisms in visual processing, it is entirely likely that the functional role of spatial suppression is to support other essential processes and functions in motion perception. For example, center-surround interactions have been associated with a range of important visual processes, such as redundancy reduction, sparse coding, input normalization, estimation of optic flow, heading direction, shape-from-motion representation and detection of contours and edge discontinuities (Heeger, 1992; Field et al., 1993; Sillito et al., 1995; Xiao et al., 1995; Buracas and Albright, 1996; Vinje & Gallant, 2000; Schwartz & Simoncelli, 2001; Royden, 2002; Cui et al., 2013). Moreover, motion-based figure-ground segregation might be accomplished by mechanisms selective for the orientation and position of motion-defined edges such as those found in V2 (Marcar et al., 2000).
6. What can perceptual suppression tell us about intelligence?
The rationale for spatial suppression is that it provides an effective way of suppressing large, background-like motions, consequently enhancing relative neural responses to smaller moving stimuli. The important advantage of this mechanism is that it concentrates perceptual motion processing on stimulus inputs that are more likely to be caused by object motion (Section 1.1), while other brain mechanisms still retain ability to process background motion (Section 3.3). In other words, spatial suppression can be construed as a strategy for effectively dealing with a vast amount of motion signals faced by the visual system. My argument here is that processing demands indexed by spatial suppression—rapid processing of relevant information and suppression of redundant and less informative signals—are applicable not only to motion perception, but to any brain system that operates on information that exceeds its processing capacity, including “processes” as complex as intelligence.
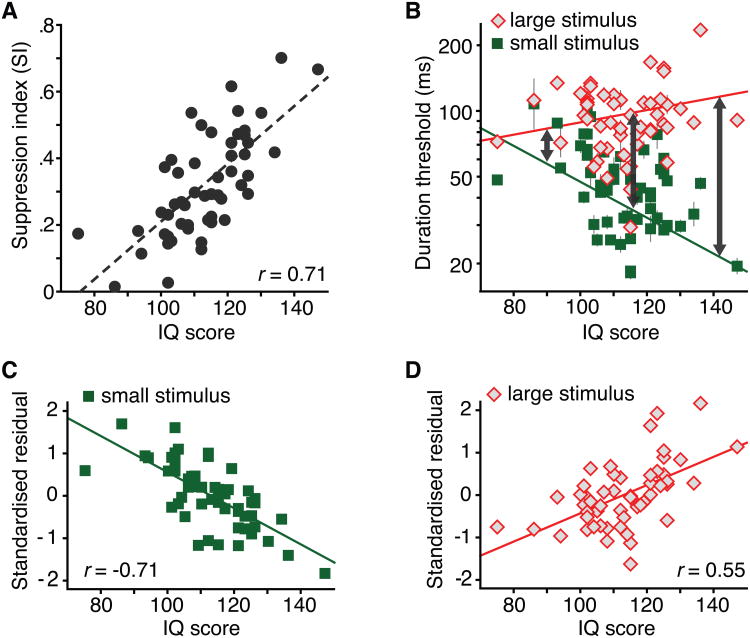
This idea about the broad relevance of processing demands that support the utility of spatial suppression, motivated me to examine the link between spatial suppression strength and IQ scores in the control subjects that participated in the schizophrenia study by Tadin et al. (2006b). Suppression strength strongly correlated with IQ (r = 0.64). Given that most perceptual tasks only weakly correlate with IQ (usually between 0.2 and 0.4; Deary, 2012; Jensen, 2006), this was a surprising result, but possibly attributable to the relatively small sample size in that study. Next, we designed a larger study to explicitly test the link between suppression strength and IQ (Melnick et al., 2013) and replicated the strong link between SI and IQ (Fig. 5A, r = 0.71, P = 10−9, N = 53). Specifically, we found that high IQ individuals had lower duration thresholds for perceiving small moving stimuli but had higher thresholds for discriminating large motions (Fig. 5B-D). In other words, high IQ was associated with more selective motion processing (arrows in Fig. 5B). While low IQ individuals performed about equally for small and large stimuli (resulting in small SI), high IQ individuals exhibited a large difference in performance between two sizes. In sum, we showed that individual variations in IQ scores are predicted by the relative inability to quickly perceive large moving stimuli (i.e., spatial suppression strength; Fig. 5A). For a detailed consideration of alternative explanations of this finding see Melnick et al. (2013).
Fig. 5.
A strong link between spatial suppression and intelligence. A. The correlation between spatial suppression strength and IQ. B. The relationships between IQ and duration thresholds for small and large moving stimuli. Error bars are ±SEM. C. The relationship between IQ and standardized residuals after regressing thresholds for the small stimulus on large stimulus thresholds. D. Same as panel C, except that large stimulus thresholds were regressed on the thresholds for the small stimulus. Adapted from Melnick et al. (2013) with permission form Elsevier.
Notably, spatial suppression strength predicted broad intellectual ability, with significant and approximately equal correlations with Verbal Comprehension, Processing Speed, Working Memory and Perceptual Reasoning indices (r = 0.69, 0.50, 0.47 and 0.47, respectively; all P < 0.001). Additionally, suppression strength was strongly linked with the General Ability Index (r = 0.69; P = 10−8), a measure of general intellectual ability.
6.1. Why are spatial suppression and IQ are related?
The key question is why spatial suppression is a better predictor of IQ than other perceptual measures, which typically exhibit much lower correlations and are usually selectively linked with performance aspects of IQ (Deary, 2012; Jensen, 2006). Dating back to Sir Francis Galton, James McKeen Cattell and Charles Spearman, scientists have searched for links between intelligence and perception, largely focusing on processing speed measures (e.g., reaction times) with the rationale that faster neural processing is important for both perception and intelligence (Deary, 1994). The benefits of faster neural processing are undeniable. Fast information processing in high IQ individuals may also reflect a higher degree of axon myelination, which has a wide range of beneficial effects on the efficiency of neural computations (Miller, 1994). Nevertheless, the utility of fast processing is limited in systems where incoming inputs greatly exceed processing capacity. As outlined below, such systems also require suppression of less relevant information. Spatial suppression paradigm demands both (a) rapid stimulus processing (inherent in duration threshold measurements) and (b) is defined by suppression of large, background-like moving stimuli. I believe that these two task demands account for the surprisingly robust link between spatial suppression strength and IQ.
While Melnick et al. (2013) is the first study linking intelligence and spatial suppression, the key role of suppression in perception and cognition is well established. Both perception and intelligent cognition operate on an immense amount of incoming information (Eriksen & Eriksen, 1974; Marois & Ivanoff, 2005). Because of this fundamental and ubiquitous brain limitation, neural efficiency is constrained not only by processing speed but also by the ability to suppress irrelevant information. In fact, suppressive processes play a key role in a wide range of neural functions (intelligence: Burgess et al., 2011; Conway, Kane, & Engle, 2003; Dempster, 1991; Gray, Chabris, & Braver, 2003; Jung & Haier, 2007; cognition and perception: Carandini & Heeger, 2012; Gazzaley et al., 2005; Kastner & Ungerleider, 2000; Schwartz & Simoncelli, 2001; Vinje & Gallant, 2000; Zanto & Gazzaley, 2009). For example, working memory ability is predicted not by a neural boost of task-relevant targets, but rather by individual variability in distracter suppression (Gazzaley et al., 2005; Zanto & Gazzaley, 2009). Moreover, the ability to ignore working memory distracters both correlates with variations in IQ (Burgess et al., 2011) and can account for brain activity differences between low and high IQ individuals (Gray, Chabris, & Braver, 2003). Suppressive mechanisms also play critical roles in low-level sensory processing, where they enable our perceptual systems to efficiently process the enormous amount of incoming sensory information (Carandini & Heeger, 2012; Schwartz & Simoncelli, 2001; Vinje & Gallant, 2000).
It is worth noting that there are good indications that the underlying relationship between sensory discriminations and IQ is likely stronger than suggested by bivariate correlations in past studies. Structural equation modeling has revealed remarkably strong links (0.68 < r < 0.92) between two latent traits: general intelligence and general sensory discrimination (Deary et al., 2004; Meyer et al., 2010). Additionally, reaction times (RTs) correlate better with IQ if the number of response alternatives is higher (Deary, Derb & Ford, 2001); choice RTs for choosing among four options better correlate with IQ than RTs for just two options; simple RTs show the weakest link. Evidently, adding processing demands to a simple speeded perceptual task increases its link with IQ. The suppression component in Melnick et al. (2013) can be viewed as one such processing demand—one that appears to be particularly effective at increasing the strength of the link between perception and intelligence.
6.2. Limitations
While the fundamental and ubiquitous role of suppression in neural processing provides an appealing context for explaining the strong link between spatial suppression and IQ, this account comes with important caveats. Neural suppression is not a unitary mechanism but includes a broad range of inhibitory processes (Friedman & Miyake, 2004; Priebe & Ferster, 2012). As detailed in Section 4, even the seemingly straightforward link between suppression and cortical inhibition is considerably more complex.
If there is indeed a general suppression factor in neural processing, suppressive processes in cognition and perception should be related. While that is often the case (see Section 6.1), many such processes are only weakly related with one another and only some predict IQ scores (e.g., Eriksen flanker, Strop, antisaccade and stop-signal tasks; Friedman & Miyake, 2004; Friedman et al., 2006). Moreover, we found that spatial suppression in motion perception does not correlate with other visual tasks that implicate suppressive processes (motion and orientation repulsion, brightness induction, Chubb contrast illusion and Ebbinghaus size illusion; Melnick & Tadin, 2012).
Thus, the explanation for the link between spatial suppression and IQ that is proposed here should be considered a good starting point. More research will be needed to elucidate the observed link and to better understand relationships between various neural processes that involve different forms of suppression.
7. Key outstanding questions
Beyond continuing general psychophysical investigations of spatial suppression, there are three specific future directions that hold promise for the most informative advances, both for our understanding of visual mechanisms and for brain processing in general.
What is the functional role, if any, of neural processes reflected in spatial suppression? Although there are theoretical links between spatial suppression and motion segregation (Section 5), behavioral evidence for this link is lacking.
What underlies the strong link between spatial suppression and IQ? While the argument about the broad importance of suppressive processes is appealing (Sections 1 and 6), more empirical work is needed to test this relatively general hypothesis and address concerns and outstanding questions raised in Sections 6.1 and 6.2. Aside from explaining the link between spatial suppression and intelligence, the high degree of individual variability in spatial suppression strength (e.g. Fig. 5A) requires an explanation of its own.
Spatial suppression is impaired in a number of special populations, often resulting in better-than-normal task performance. Such atypical enhancements are of both scientific and clinical interest because cases where a condition leads to better-than-normal performance provide a more direct index of underlying biological mechanisms. However, more recent studies challenge the assumed link between diminished cortical inhibition and hyperexcitability on one side and weaker spatial suppression on the other (Section 4). Elucidating links between observed spatial suppression deficits and underlying brain abnormalities will be essential for drawing valid conclusions from special population studies. One promising approach is to pair behavioral measurements with measurements of neurotransmitter concentration levels using magnetic resonance spectroscopy (Yoon et al., 2010).
8. Conclusion
Work on spatial suppression in motion processing started with a counterintuitive discovery that motion of large objects can be considerably harder to perceive than motion of small objects (Tadin et al., 2003). This study was directly inspired by a known property of visual neurons, which are inhibited when exposed to large moving stimuli and provided new evidence linking single neurons to our conscious perceptual experience. Spatial suppression can be conceptualized as a strategy the visual system uses to suppress background motion, freeing up neural resources for detecting foreground objects. Ultimately, I believe that the brain's ability to suppress less relevant information is a key not only to visual perception, but also for other brain processes, including intelligence. Supporting this argument, we recently found that individuals who have difficulty seeing large moving stimuli also have high IQ scores (Melnick et al., 2013). This result highlights the central importance of suppression in neural processing—a broad conclusion from a line of work that started with a discovery of a simple but counterintuitive visual phenomenon.
Highlights.
Suppressive spatial mechanisms play a key role in motion perception
Spatial integration of motion adapts to visual conditions
Spatial suppression mechanisms are impaired is several special populations
Motion segregation is a hypothesized functional role of spatial suppression
Spatial suppression strength strongly predicts individual variations in IQ
Acknowledgments
I would like to thank the Vision Sciences Society and Elsevier for the 2014 Young Investigator Award. I am grateful for the outstanding mentoring of Joe Lappin and Randolph Blake, under whose guidance much of this work was accomplished. I also thank a large group of colleagues and students who significantly contributed to the research described in this paper (Sara Agosta, Lorella Battelli, Loisa Bennetto, Carissa Casico, Kevin Dieter, Jen Foss-Feig, Davis Glasser, Oh-Sang Kwon, Michael Melnick, Chris Pack, Chris Paffen, Sohee Park, Woon Ju Park, Alvaro Pascaul-Leone, Kim Schauder, Frans Verstraten and Ruyuan Zhang). Finally, I thank Molly Tadin and Joe Lappin for comments on the manuscript. This work was supported by NIH R01-EY019295 and Provost's Multidisciplinary Award (to D. T.), NIH P30 EY001319, P30-EY08126, and T32 EY007125.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaen-Stockdale CR, Thompson B, Huang PC, Hess RF. Low-level mechanisms may contribute to paradoxical motion percepts. Journal of Vision. 2009;9(5):9, 1–14. doi: 10.1167/9.5.9. [DOI] [PubMed] [Google Scholar]
- Adelson EH, Movshon JA. Phenomenal coherence of moving visual patterns. Nature. 1982;300:523–5. doi: 10.1038/300523a0. [DOI] [PubMed] [Google Scholar]
- Alais D, van der Smagt MJ, van den Berg AV, van de Grind WA. Local and global factors affecting the coherent motion of gratings presented in multiple apertures. Vision Research. 1998;38:1581–1591. doi: 10.1016/s0042-6989(97)00331-3. [DOI] [PubMed] [Google Scholar]
- Albright TD, Stoner GR. Visual motion perception. Proceedings of the National Academy of Sciences. 1995;92(7):2433–2440. doi: 10.1073/pnas.92.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local-global comparisons in visual neurons. Annual Review of Neuroscience. 1985;8:407–430. doi: 10.1146/annurev.ne.08.030185.002203. [DOI] [PubMed] [Google Scholar]
- Allman J, Miezin F, McGuinness E. Direction- and velocity-specific responses from beyond the classical receptive field in the middle temporal visual area (MT) Perception. 1985;14(2):105–126. doi: 10.1068/p140105. [DOI] [PubMed] [Google Scholar]
- Anderson SJ, Burr DC. Spatial summation properties of directionally selective mechanisms in human vision. Journal of the Optical Society of America A. 1991;8(8):1330–1339. doi: 10.1364/josaa.8.001330. [DOI] [PubMed] [Google Scholar]
- Anton-Erxleben K, Stephan VM, Treue S. Attention Reshapes Center-Surround Receptive Field Structure in Macaque Cortical Area MT. Cerebral Cortex. 2009;19:2466–2478. doi: 10.1093/cercor/bhp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora SK, Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia. 2007;27(12):1442–1453. doi: 10.1111/j.1468-2982.2007.01502.x. [DOI] [PubMed] [Google Scholar]
- Ashida H, Osaka N. Difference of spatial frequency selectivity between static and flicker motion aftereffects. Perception. 1994;23:1313–1320. doi: 10.1068/p231313. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Summation and inhibition in the frog's retina. Journal of Physiology. 1953;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy FV, Vanzetta I, Masson GS. Behavioral receptive field for ocular following in humans: dynamics of spatial summation and center-surround interactions. Journal of Neurophysiology. 2006;95(6):3712–3726. doi: 10.1152/jn.00112.2006. [DOI] [PubMed] [Google Scholar]
- Bastian J. Electrolocation. Journal of comparative physiology. 1981;144(4):465–479. [Google Scholar]
- Battista J, Badcock DR, McKendrick AM. Center-surround visual motion processing in migraine. Investigative ophthalmology & visual science. 2010;51(11):6070–6076. doi: 10.1167/iovs.10-5290. [DOI] [PubMed] [Google Scholar]
- Battista J, Badcock DR, McKendrick AM. Migraine increases centre-surround suppression for drifting visual stimuli. PloS one. 2011;6(4):e18211. doi: 10.1371/journal.pone.0018211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezovskii VK, Born RT. Specificity of projections from wide-field and local motion-processing regions within the middle temporal visual area of the owl monkey. Journal of Neuroscience. 2000;20(3):1157–1169. doi: 10.1523/JNEUROSCI.20-03-01157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts LR, Sekuler AB, Bennett PJ. Spatial characteristics of center-surround antagonism in younger and older adults. Journal of Vision. 2009;9(1):25, 1–15. doi: 10.1167/9.1.25. [DOI] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging reduces center-surround antagonism in visual motion processing. Neuron. 2005;45(3):361–366. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Holzman PS, Chen Y. Aging and visual motion discrimination in normal adults and schizophrenia patients. Psychiatry Research. 2006;145(1):1–8. doi: 10.1016/j.psychres.2005.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R, Tadin D, Sobel KV, Raissian TA, Chong SC. Strength of early visual adaptation depends on visual awareness. Proceedings of the National Academy of Sciences of the U S A. 2006;103(12):4783–4788. doi: 10.1073/pnas.0509634103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born RT. Center-surround interactions in the middle temporal visual area of the owl monkey. Journal of Neurophysiology. 2000;84(5):2658–2669. doi: 10.1152/jn.2000.84.5.2658. [DOI] [PubMed] [Google Scholar]
- Born RT, Groh JM, Zhao R, Lukasewycz SJ. Segregation of object and background motion in visual area MT: effects of microstimulation on eye movements. Neuron. 2000;26(3):725–734. doi: 10.1016/s0896-6273(00)81208-8. [DOI] [PubMed] [Google Scholar]
- Born RT, Tootell RB. Segregation of global and local motion processing in primate middle temporal visual area. Nature. 1992;357(6378):497–499. doi: 10.1038/357497a0. [DOI] [PubMed] [Google Scholar]
- Braddick O. Segmentation versus integration in visual motion processing. Trends in Neuroscience. 1993;16(7):263–268. doi: 10.1016/0166-2236(93)90179-p. [DOI] [PubMed] [Google Scholar]
- Bradley DC, Andersen RA. Center-surround antagonism based on disparity in primate area MT. Journal of Neuroscience. 1998;18(18):7552–7565. doi: 10.1523/JNEUROSCI.18-18-07552.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain and Behavior. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buracas GT, Albright TD. The role of MT neuron receptive field surrounds in computing object shape from velocity fields. Advances in Neural Information Processing Systems. 1994;6:969–976. [Google Scholar]
- Buracas GT, Albright TD. Contribution of area MT to perception of three-dimensional shape: a computational study. Vision Research. 1996;36(6):869–887. doi: 10.1016/0042-6989(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Buracas GT, Zador AM, DeWeese MR, Albright TD. Efficient discrimination of temporal patterns by motion-sensitive neurons in primate visual cortex. Neuron. 1998;20(5):959–969. doi: 10.1016/s0896-6273(00)80477-8. [DOI] [PubMed] [Google Scholar]
- Burgess GC, Gray JR, Conway AR, Braver TS. Neural mechanisms of interference control underlie the relationship between fluid intelligence and working memory span. Journal of Experimental Psychology: General. 2011;140:674–692. doi: 10.1037/a0024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Liu LD, Khawaja FA, Pack CC, Butts DA. Diverse suppressive influences in area mt and selectivity to complex motion features. Journal of Neuroscience. 2013;33(42):16715–16728. doi: 10.1523/JNEUROSCI.0203-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2012;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KJ, Sheliga BM, Fitzgibbon EJ, Miles FA. Initial ocular following in humans depends critically on the fourier components of the motion stimulus. Annals of the New York Academy of Sciences. 2005;1039:260–271. doi: 10.1196/annals.1325.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churan J, Khawaja FA, Tsui JM, Pack CC. Brief motion stimuli preferentially activate surround-suppressed neurons in macaque visual area MT. Current Biology. 2008;18:R1051–2. doi: 10.1016/j.cub.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Churan J, Richard AG, Pack CC. Interaction of spatial and temporal factors in psychophysical estimates of surround suppression. Journal of Vision. 2009a;9(4):15, 1–15. doi: 10.1167/9.4.15. [DOI] [PubMed] [Google Scholar]
- Churchland A, Gardner JL, Chou IH, Priebe N, Lisberger SG. Directional anisotropies reveal a functional segregation of visual motion processing for perception and action. Neuron. 2003;37:1001–11. doi: 10.1016/s0896-6273(03)00145-4. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences. 2003;7(12):547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Croner LJ, Albright TD. Image segmentation enhances discrimination of motion in visual noise. Vision Research. 1997;37:1415–1427. doi: 10.1016/s0042-6989(96)00299-4. [DOI] [PubMed] [Google Scholar]
- Croner LJ, Albright TD. Seeing the big picture: integration of image cues in the primate visual system. Neuron. 1999;24:777–789. doi: 10.1016/s0896-6273(00)81026-0. [DOI] [PubMed] [Google Scholar]
- Dakin SC, Mareschal I, Bex PJ. Local and global limitations on direction integration assessed using equivalent noise analysis. Vision Research. 2005;45(24):3027–3049. doi: 10.1016/j.visres.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Davidson RM, Bender DB. Selectivity for relative motion in the monkey superior colliculus. Journal of Neurophysiology. 1991;65(5):1115–1133. doi: 10.1152/jn.1991.65.5.1115. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Uka T. Coding of horizontal disparity and velocity by MT neurons in the alert macaque. Journal of Neurophysiology. 2003;89(2):1094–1111. doi: 10.1152/jn.00717.2002. [DOI] [PubMed] [Google Scholar]
- Delahunt PB, Hardy JL, Werner JS. The effect of senescence on orientation discrimination and mechanism tuning. Journal of Vision. 2008;8(3):5. doi: 10.1167/8.3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Goddard PA. Failure of motion discrimination at high contrasts: evidence for saturation. Vision Research. 1989;29(12):1767–1776. doi: 10.1016/0042-6989(89)90159-4. [DOI] [PubMed] [Google Scholar]
- Deary IJ. Sensory discrimination and intelligence: postmortem or resurrection. Am J Psychol. 1994;107:95–115. [PubMed] [Google Scholar]
- Deary IJ. Intelligence. Annu Rev Psychol. 2012;63:453–482. doi: 10.1146/annurev-psych-120710-100353. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Bell PJ, Bell AJ, Campbell ML, Fazal ND. Sensory discrimination and intelligence: testing Spearman's other hypothesis. American Journal of Psychology. 2004;117:1–18. [PubMed] [Google Scholar]
- Deary IJ, Der G, Ford G. Reaction times and intelligence differences: A population-based cohort study. Intelligence. 2001;29:389–399. [Google Scholar]
- Dempster FN. Inhibitory processes: A neglected dimension of intelligence. Intelligence. 1991;15:157–173. [Google Scholar]
- Eagleman DM. Visual illusions and neurobiology. Nature Reviews Neuroscience. 2001;2(12):920–926. doi: 10.1038/35104092. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nature Reviews Neuroscience. 2008;9(4):292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: evidence for a local “association field”. Vision Research. 1993;33(2):173–193. doi: 10.1016/0042-6989(93)90156-q. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. J Neurosci. 2013;33:8243–8249. doi: 10.1523/JNEUROSCI.1608-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: a latent-variable analysis. J Exp Psychol Gen. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, Hewitt JK. Not all executive functions are related to intelligence. Psych Sci. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Fredericksen RE, Bex PJ, Verstraten FA. How big is a Gabor patch, and why should we care? Journal of the Optical Society of America A. 1997;14(1):1–12. doi: 10.1364/josaa.14.000001. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Blake R. Spatial interactions in binocular rivalry. Journal of experimental psychology Human perception and performance. 1992;18(2):362–370. doi: 10.1037//0096-1523.18.2.362. [DOI] [PubMed] [Google Scholar]
- Gao D, Mahadevan V, Vasconcelos N. On the plausibility of the discriminant center-surround hypothesis for visual saliency. Journal of Vision. 2008;8(7):13, 1–18. doi: 10.1167/8.7.13. [DOI] [PubMed] [Google Scholar]
- Gautama T, Van Hulle MM. Function of center-surround antagonism for motion in visual area MT/V5: a modeling study. Vision Research. 2001;41(28):3917–3930. doi: 10.1016/s0042-6989(01)00246-2. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR, Kiper DC, Beusmans JM, Carandini M, Zaidi Q, Movshon JA. Chromatic properties of neurons in macaque MT. Visual Neuroscience. 1994;11:455–466. doi: 10.1017/s095252380000239x. [DOI] [PubMed] [Google Scholar]
- Glasser DM, Tadin D. Low-level mechanisms do not explain paradoxical motion percepts. Journal of Vision. 2010;10(4):20, 1–9. doi: 10.1167/10.4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser DM, Tadin D. Increasing stimulus size impairs first- but not second-order motion perception. Journal of Vision. 2011;11(13):22, 1–8. doi: 10.1167/11.13.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser DM, Tadin D. Modularity in the motion system: independent oculomotor and perceptual processing of brief moving stimuli. Journal of Vision. 2014;14(3):28, 1–13. doi: 10.1167/14.3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser DM, Tsui J, Pack CC, Tadin D. Perceptual and neural consequences of rapid motion adaptation. Proceedings of the National Academy of Sciences. 2011;108:E1080–E1088. doi: 10.1073/pnas.1101141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb JD, McDavitt JR, Ruf BM, Chen JI, Saricicek A, Maloney KH, et al. Enhanced visual motion perception in major depressive disorder. Journal of Neuroscience. 2009;29(28):9072–9077. doi: 10.1523/JNEUROSCI.1003-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govenlock SW, Taylor CP, Sekuler AB, Bennett PJ. The effect of aging on the orientational selectivity of the human visual system. Vision Research. 2009;49(1):164–172. doi: 10.1016/j.visres.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Govenlock SW, Taylor CP, Sekuler AB, Bennett PJ. The effect of aging on the spatial frequency selectivity of the human visual system. Vision Research. 2010;50(17):1712–1719. doi: 10.1016/j.visres.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Hartline HK. The receptive fields of optic nerve fibers. Am J Physiol. 1940;130(69):690. [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383(6598):334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- Heeger DJ. Normalization of cell responses in cat striate cortex. Visual Neuroscience. 1992;9(2):181–197. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- Huang X, Albright TD, Stoner GR. Adaptive surround modulation in cortical area MT. Neuron. 2007;53(5):761–70. doi: 10.1016/j.neuron.2007.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Albright TD, Stoner GR. Stimulus dependency and mechanisms of surround modulation in cortical area MT. Journal of Neuroscience. 2008;28:13889–906. doi: 10.1523/JNEUROSCI.1946-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huk AC, Ress D, Heeger DJ. Neuronal basis of the motion aftereffect reconsidered. Neuron. 2001;32(1):161–172. doi: 10.1016/s0896-6273(01)00452-4. [DOI] [PubMed] [Google Scholar]
- Hunter JN, Born RT. Stimulus-dependent modulation of suppressive influences in MT. Journal of Neuroscience. 2011;31(2):678–686. doi: 10.1523/JNEUROSCI.4560-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AR. Clocking the mind: mental chronometry and individual differences. Amsterdam: Elsevier; 2006. [Google Scholar]
- Jones HE, Grieve KL, Wang W, Sillito AM. Surround suppression in primate V1. Journal of Neurophysiology. 2001;86(4):2011–2028. doi: 10.1152/jn.2001.86.4.2011. [DOI] [PubMed] [Google Scholar]
- Jones HE, Wang W, Sillito AM. Spatial organization and magnitude of orientation contrast interactions in primate V1. Journal of Neurophysiology. 2002;88(5):2796–2808. doi: 10.1152/jn.00403.2001. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behavioral and Brain Sciences. 2007;30(2):135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Karas R, McKendrick AM. Aging alters surround modulation of perceived contrast. Journal of Vision. 2009;9(5):11. doi: 10.1167/9.5.11. [DOI] [PubMed] [Google Scholar]
- Karas R, McKendrick AM. Age related changes to perceptual surround suppression of moving stimuli. Seeing and Perceiving. 2012;25(5):409–424. doi: 10.1163/187847611X595873. [DOI] [PubMed] [Google Scholar]
- Karas R, McKendrick AM. Contrast and stimulus duration dependence of perceptual surround suppression in older adults. Vision Research. 2015;110:7–14. doi: 10.1016/j.visres.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kiani R, Hanks TD, Shadlen MN. Bounded integration in parietal cortex underlies decisions even when viewing duration is dictated by the environment. Journal of Neuroscience. 2008;28:3017–29. doi: 10.1523/JNEUROSCI.4761-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Konishi M. Center-surround organization of auditory receptive fields in the owl. Science. 1978;202(4369):778–780. doi: 10.1126/science.715444. [DOI] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. Journal of Neurophysiology. 1953;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Lagae L, Raiguel S, Orban GA. Speed and direction selectivity of macaque middle temporal neurons. Journal of Neurophysiology. 1993;69(1):19–39. doi: 10.1152/jn.1993.69.1.19. [DOI] [PubMed] [Google Scholar]
- Lappin JS, Craft WD. Foundations of spatial vision: from retinal images to perceived shapes. Psychological Review. 2000;107:6–38. doi: 10.1037/0033-295x.107.1.6. [DOI] [PubMed] [Google Scholar]
- Lappin JS, Tadin D, Nyquist JB, Corn AL. Spatial and temporal limits of motion perception across variations in speed, eccentricity, and low vision. Journal of Vision. 2009;9:1–14. doi: 10.1167/9.1.30. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. On binocular rivalry. Soesterberg: Institute for Perception RVO-TNO; 1965. [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300(5620):812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Lewis T, Sekuler A, Bennett P. Psychophysical measurements of surround suppression in 5-year-olds. Journal of Vision. 2008;8(6):388–388. [Google Scholar]
- Liu L, Pack C. Bidirectional manipulation of GABAergic inhibition in MT: A comparison of neuronal and psychophysical performance. Journal of Vision. 2014;14(10):13–13. [Google Scholar]
- Liu L, Van Hulle MM. Modeling the surround of MT cells and their selectivity for surface orientation in depth specified by motion. Neural Computation. 1998;10(2):295–312. doi: 10.1162/089976698300017773. [DOI] [PubMed] [Google Scholar]
- Loffler G, Orbach HS. Modeling the integration of motion signals across space. Journal of the Optical Society of America A. 2003;20(8):1472–1489. doi: 10.1364/josaa.20.001472. [DOI] [PubMed] [Google Scholar]
- Lorenceau J, Alais D. Form constraints in motion binding. Nature Neuroscience. 2001;4:745–751. doi: 10.1038/89543. [DOI] [PubMed] [Google Scholar]
- Lorenceau J, Shiffrar M. The influence of terminators on motion integration across space. Vision Research. 1992;32:263–273. doi: 10.1016/0042-6989(92)90137-8. [DOI] [PubMed] [Google Scholar]
- Lorenceau J, Shiffrar M. The linkage of visual motion signals. Visual Cognition. 1999;6:431–460. [Google Scholar]
- Lorenceau J, Zago L. Cooperative and competitive spatial interactions in motion integration. Visual Neuroscience. 1999;16:755–770. doi: 10.1017/s0952523899164149. [DOI] [PubMed] [Google Scholar]
- Lui LL, Bourne JA, Rosa MG. Spatial summation, end inhibition and side inhibition in the middle temporal visual area (MT) Journal of Neurophysiology. 2007;97(2):1135–1148. doi: 10.1152/jn.01018.2006. [DOI] [PubMed] [Google Scholar]
- Marcar VL, Raiguel SE, Xiao D, Orban GA. Processing of kinetically defined boundaries in areas V1 and V2 of the macaque monkey. Journal of Neurophysiology. 2000;84(6):2786–2798. doi: 10.1152/jn.2000.84.6.2786. [DOI] [PubMed] [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn Sci. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Maruya K, Watanabe H, Watanabe M. Adaptation to invisible motion results in low-level but not high-level aftereffects. Journal of Vision. 2008;8:1–11. doi: 10.1167/8.11.7. [DOI] [PubMed] [Google Scholar]
- Masson GS, Yang DS, Miles FA. Reversed short-latency ocular following. Vision Research. 2002;42(17):2081–2087. doi: 10.1016/s0042-6989(02)00082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick M, Dieter K, Tadin D. Highly abnormal visual context processing in older adults. Journal of Vision. 2014;14(10):215. [Google Scholar]
- Melnick MD, Harrison BR, Park S, Bennetto L, Tadin D. A strong interactive link between sensory discriminations and intelligence. Current Biology. 2013;23:1013–1017. doi: 10.1016/j.cub.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick MD, Tadin D. The strength of contextual modulation does not correlate across visual sub-modalities. Journal of Vision. 2012;12(9):1300. [Google Scholar]
- Meyer CS, Hagmann-von Arx P, Lemola S, Grob A. Correspondence between the general ability to discriminate sensory stimuli and general intelligence. Journal of Individual Differences. 2010;31:46–56. [Google Scholar]
- Miller EM. Intelligence and brain myelination: A hypothesis. Personality and Individual Differences. 1994;17(6):803–832. [Google Scholar]
- Mountcastle VB, Powell TPS. Neural mechanisms subserving cutaneous sensibility with special reference to the role of afferent inhibition in sensory perception and discrimination. Bull Johns Hopkins Hosp. 1959;105:201–232. [PubMed] [Google Scholar]
- Murakami I, Shimojo S. Motion capture changes to induced motion at higher luminance contrasts, smaller eccentricities, and larger inducer sizes. Vision Research. 1993;33(15):2091–2107. doi: 10.1016/0042-6989(93)90008-k. [DOI] [PubMed] [Google Scholar]
- Murakami I, Shimojo S. Modulation of motion aftereffect by surround motion and its dependence on stimulus size and eccentricity. Vision Research. 1995;35(13):1835–1844. doi: 10.1016/0042-6989(94)00269-r. [DOI] [PubMed] [Google Scholar]
- Nakayama K. Biological image motion processing: a review. Vision Research. 1985;25:625–60. doi: 10.1016/0042-6989(85)90171-3. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Loomis JM. Optical velocity patterns, velocity-sensitive neurons, and space perception: a hypothesis. Perception. 1974;3(1):63–80. doi: 10.1068/p030063. [DOI] [PubMed] [Google Scholar]
- Nauhaus I, Busse L, Carandini M, Ringach DL. Stimulus contrast modulates functional connectivity in visual cortex. Nat Neurosci. 2009;12(1):70–6. doi: 10.1038/nn.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri P, Levi D. Surround motion silences signals from same-direction motion. Journal of Neurophysiology. 2009;102:2594–2602. doi: 10.1152/jn.00489.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida S, Ashida H, Sato T. Contrast dependencies of two types of motion aftereffect. Vision Research. 1997;37(5):553–563. doi: 10.1016/s0042-6989(96)00181-2. [DOI] [PubMed] [Google Scholar]
- Nowlan SJ, Sejnowski TJ. A selection model for motion processing in area MT of primates. Journal of Neuroscience. 1995;15(2):1195–1214. doi: 10.1523/JNEUROSCI.15-02-01195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66(2):287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki H, Finn IM, Schaffer ES, Miller KD, Ferster D. Inhibitory stabilization of the cortical network underlies visual surround suppression. Neuron. 2009;62(4):578–592. doi: 10.1016/j.neuron.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack CC, Hunter JN, Born RT. Contrast dependence of suppressive influences in cortical area MT of alert macaque. Journal of Neurophysiology. 2005;93(3):1809–1815. doi: 10.1152/jn.00629.2004. [DOI] [PubMed] [Google Scholar]
- Paffen CL, Alais D, Verstraten FA. Center-surround inhibition deepens binocular rivalry suppression. Vision Research. 2005;45(20):2642–2649. doi: 10.1016/j.visres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Paffen CL, Tadin D, te Pas SF, Blake R, Verstraten FA. Adaptive center-surround interactions in human vision revealed during binocular rivalry. Vision Research. 2006;46(5):599–604. doi: 10.1016/j.visres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Paffen CL, te Pas SF, Kanai R, van der Smagt MJ, Verstraten FA. Center-surround interactions in visual motion processing during binocular rivalry. Vision Research. 2004;44(14):1635–1639. doi: 10.1016/j.visres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Perge JA, Borghuis BG, Bours RJ, Lankheet MJ, van Wezel RJ. Dynamics of directional selectivity in MT receptive field centre and surround. European Journal of Neuroscience. 2005;22(8):2049–2058. doi: 10.1111/j.1460-9568.2005.04363.x. [DOI] [PubMed] [Google Scholar]
- Petkov N, Subramanian E. Motion detection, noise reduction, texture suppression, and contour enhancement by spatiotemporal Gabor filters with surround inhibition. Biological Cybernetics. 2007 doi: 10.1007/s00422-007-0182-0. [DOI] [PubMed] [Google Scholar]
- Pitkow X, Liu S, Angelaki DE, DeAngelis GC, Pouget A. How can single sensory neurons predict behavior? Neuron. 2015;87(2):411–423. doi: 10.1016/j.neuron.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Mechanisms of neuronal computation in mammalian visual cortex. Neuron. 2012;75(2):194–208. doi: 10.1016/j.neuron.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Lisberger SG, Movshon JA. Tuning for spatiotemporal frequency and speed in directionally selective neurons of macaque striate cortex. Journal of Neuroscience. 2006;26(11):2941–2950. doi: 10.1523/JNEUROSCI.3936-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiguel S, Van Hulle MM, Xiao DK, Marcar VL, Orban GA. Shape and spatial distribution of receptive fields and antagonistic motion surrounds in the middle temporal area (V5) of the macaque. European Journal of Neuroscience. 1995;7(10):2064–2082. doi: 10.1111/j.1460-9568.1995.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Read JC, Georgiou R, Brash C, Yazdani P, Whittaker R, Trevelyan AJ, Serrano-Pedraza I. Moderate acute alcohol intoxication has minimal effect on surround suppression measured with a motion direction discrimination task. Journal of vision. 2015;15(1):5. doi: 10.1167/15.1.5. [DOI] [PubMed] [Google Scholar]
- Regan D. Orientation discrimination for objects defined by relative motion and objects defined by luminance contrast. Vision Research. 1989;29(10):1389–1400. doi: 10.1016/0042-6989(89)90194-6. [DOI] [PubMed] [Google Scholar]
- Regan D. Human perception of objects. Sinauer Press; Sunderland, MA: 2000. [Google Scholar]
- Regan DM, Beverley KI. Figure-ground segregation by motion contrast and by luminance contrast. Journal of the Optical Society of America A. 1984;1:433–442. doi: 10.1364/josaa.1.000433. [DOI] [PubMed] [Google Scholar]
- Rivest J, Cavanagh P. Localizing contours defined by more than one attribute. Vision Research. 1996;36:53–66. doi: 10.1016/0042-6989(95)00056-6. [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. Journal of neuroscience. 2002;22(21):9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkirch M, Stein T, Sekutowicz M, Sterzer P. A direct oculomotor correlate of unconscious visual processing. Current Biology. 2012;2(13):R514–515. doi: 10.1016/j.cub.2012.04.046. [DOI] [PubMed] [Google Scholar]
- Royden CS. Computing heading in the presence of moving objects: a model that uses motion-opponent operators. Vision research. 2002;42(28):3043–3058. doi: 10.1016/s0042-6989(02)00394-2. [DOI] [PubMed] [Google Scholar]
- Rubin DB, Van Hooser SD, Miller KD. The stabilized supralinear network: A unifying circuit motif underlying multi-input integration in sensory cortex. Neuron. 2015;85:402–417. doi: 10.1016/j.neuron.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachtler WL, Zaidi Q. Effect of spatial configuration on motion aftereffects. Journal of the Optical Society of America A. 1993;10(7):1433–1449. doi: 10.1364/josaa.10.001433. [DOI] [PubMed] [Google Scholar]
- Sachtler WL, Zaidi Q. Visual processing of motion boundaries. Vision Research. 1995;35:807–26. doi: 10.1016/0042-6989(94)00160-n. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast's effect on spatial summation by macaque V1 neurons. Nature Neuroscience. 1999;2(8):733–739. doi: 10.1038/11197. [DOI] [PubMed] [Google Scholar]
- Schwartz BD, Maron BA, Evans WJ, Winstead DK. High velocity transient visual processing deficits diminish ability of patients with schizophrenia to recognize objects. Neuropsychiatry Neuropsychology and Behavioral Neurology. 1999;12(3):170–177. [PubMed] [Google Scholar]
- Schwartz O, Simoncelli EP. Natural signal statistics and sensory gain control. Nature Neuroscience. 2001;4(8):819–825. doi: 10.1038/90526. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Chen KJ, Fitzgibbon EJ, Miles FA. Initial ocular following in humans: a response to first-order motion energy. Vision Research. 2005;45(25-26):3307–3321. doi: 10.1016/j.visres.2005.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Chen KJ, FitzGibbon EJ, Miles FA. The initial ocular following responses elicited by apparent-motion stimuli: reversal by inter-stimulus intervals. Vision Research. 2006;46(6-7):979–992. doi: 10.1016/j.visres.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrar M, Lorenceau J. Increased motion linking across edges with decreased luminance contrast, edge width and duration. Vision Research. 1996;36:2061–2067. doi: 10.1016/0042-6989(95)00283-9. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Grieve KL, Jones HE, Cudeiro J, Davis J. Visual cortical mechanisms detecting focal orientation discontinuities. Nature. 1995;378:492–496. doi: 10.1038/378492a0. [DOI] [PubMed] [Google Scholar]
- Sobel KV, Blake R. How context influences predominance during binocular rivalry. Perception. 2002;31(7):813–824. doi: 10.1068/p3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SG, Peirce JW, Lennie P. The impact of suppressive surrounds on chromatic properties of cortical neurons. Journal of Neuroscience. 2004;24(1):148–160. doi: 10.1523/JNEUROSCI.3036-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spering M, Carrasco M. Similar effects of feature-based attention on motion perception and pursuit eye movements at different levels of awareness. Journal of Neuroscience. 2012;32(22):7594–7601. doi: 10.1523/JNEUROSCI.0355-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spering M, Pomplun M, Carrasco M. Tracking without perceiving: a dissociation between eye movements and motion perception. Psychological Science. 2011;22(2):216–225. doi: 10.1177/0956797610394659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadin D, Blake R. Motion perception getting better with age? Neuron. 2005;45(3):325–327. doi: 10.1016/j.neuron.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Tadin D, Grdinovac KK, Hubert-Wallander BP, Blake R. Both simple and choice reaction times reveal suppressive center-surround interactions in motion perception [Abstract] Journal of Vision. 2007;7(9):97–97a. [Google Scholar]
- Tadin D, Kim J, Doop ML, Gibson C, Lappin JS, Blake R, et al. Weakened center-surround interactions in visual motion processing in schizophrenia. Journal of Neuroscience. 2006;26(44):11403–11412. doi: 10.1523/JNEUROSCI.2592-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadin D, Lappin JS, Blake R, Grossman ED. What constitutes an efficient reference frame for vision? Nature Neuroscience. 2002;5:1010–1015. doi: 10.1038/nn914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadin D, Lappin JS. Linking psychophysics and physiology of center-surround interactions in visual motion processing. In: Jenkin MRM, Harris LR, editors. Seeing spatial form. Oxford University Press; Oxford, UK: 2005a. pp. 279–314. [Google Scholar]
- Tadin D, Lappin JS. Optimal size for perceiving motion decreases with contrast. Vision Research. 2005b;45(16):2059–2064. doi: 10.1016/j.visres.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Tadin D, Lappin JS, Blake R. Fine temporal properties of center-surround interactions in motion revealed by reverse correlation. Journal of Neuroscience. 2006;26(10):2614–2622. doi: 10.1523/JNEUROSCI.4253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadin D, Lappin JS, Gilroy LA, Blake R. Perceptual consequences of centre-surround antagonism in visual motion processing. Nature. 2003;424(6946):312–315. doi: 10.1038/nature01800. [DOI] [PubMed] [Google Scholar]
- Tadin D, Paffen CLE, Blake R, Lappin JS. Contextual modulations of center-surround interactions in motion revealed with the motion aftereffect. Journal of Vision. 2008;8(7):9, 1–11. doi: 10.1167/8.7.9. [DOI] [PubMed] [Google Scholar]
- Tadin D, Silvanto J, Pascual-Leone A, Battelli L. Improved motion perception and impaired spatial suppression following disruption of cortical area MT/V5. J Neurosci. 2011;31:1279–1283. doi: 10.1523/JNEUROSCI.4121-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hikosaka K, Saito H, Yukie M, Fukada Y, Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. Journal of Neuroscience. 1986;6(1):134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T. Effect of contrast on the perception of moving multiple Gabor patterns. Vision Research. 1998;38:3069–3082. doi: 10.1016/s0042-6989(98)00019-4. [DOI] [PubMed] [Google Scholar]
- Tibber MS, Anderson EJ, Bobin T, Carlin P, Shergill SS, Dakin SC. Local and global limits on visual processing in schizophrenia. PLOS One. 2015;10(2):e0117951. doi: 10.1371/journal.pone.0117951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JM, Pack CC. Contrast sensitivity of MT receptive field centers and surrounds. Journal of neurophysiology. 2011;106(4):1888–1900. doi: 10.1152/jn.00165.2011. [DOI] [PubMed] [Google Scholar]
- Tsujimura S, Zaidi Q. Similarities between visual processing of shear and uniform motion. Vision Research. 2002;42:3005–17. doi: 10.1016/s0042-6989(02)00391-7. [DOI] [PubMed] [Google Scholar]
- Vega-Bermudez F, Johnson KO. Surround suppression in the responses of primate SA1 and RA mechanoreceptive afferents mapped with a probe array. Journal of Neurophysiology. 1999;81(6):2711–2719. doi: 10.1152/jn.1999.81.6.2711. [DOI] [PubMed] [Google Scholar]
- Verghese P, Stone LS. Perceived visual speed constrained by image segmentation. Nature. 1996;381(6578):161–163. doi: 10.1038/381161a0. [DOI] [PubMed] [Google Scholar]
- Vinje WE, Gallant JL. Sparse coding and decorrelation in primary visual cortex during natural vision. Science. 2000;287(5456):1273–1276. doi: 10.1126/science.287.5456.1273. [DOI] [PubMed] [Google Scholar]
- Warren WH. Self-motion: Visual perception and visual control. In: Epstein W, Rogers S, editors. Perception of space and motion. 2nd. Academic Press; New York: 1995. pp. 263–325. [Google Scholar]
- Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. Journal of Clinical Psychopharmacology. 2003;23(6):601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- Watson AB, Turano K. The optimal motion stimulus. Vision Research. 1995;35(3):325–336. doi: 10.1016/0042-6989(94)00182-l. [DOI] [PubMed] [Google Scholar]
- Wist ER, Schrauf M, Ehrenstein WH. Dynamic vision based on motion-contrast: changes with age in adults. Experimental Brain Research. 2000;134(3):295–300. doi: 10.1007/s002210000466. [DOI] [PubMed] [Google Scholar]
- Xiao DK, Raiguel S, Marcar V, Koenderink J, Orban GA. Spatial heterogeneity of inhibitory surrounds in the middle temporal visual area. Proceedings of the National Academy of Sciences of the U S A. 1995;92(24):11303–11306. doi: 10.1073/pnas.92.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Tadin D, Glasser DM, Hong SW, Blake R, Park S. Visual context processing in bipolar disorder: a comparison with schizophrenia. Frontiers in Psychology. 2013;4:569. doi: 10.3389/fpsyg.2013.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watamaniuk SN. Ideal observer for discrimination of the global direction of dynamic random-dot stimuli. Journal of the Optical Society of America A: Optics and image science. 1993;10(1):16–28. doi: 10.1364/josaa.10.000016. [DOI] [PubMed] [Google Scholar]
- Yang E, Tadin D, Glasser DM, Hong SW, Blake R, Park S. Visual context processing in schizophrenia. Clinical Psychological Science. 2013;1:5–15. doi: 10.1177/2167702612464618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. Journal of Neuroscience. 2010;30(10):3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. Journal of Neuroscience. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Kwon OS, Tadin D. Illusory motion of stationary stimuli in the visual periphery: evidence for a strong centrifugal prior in motion processing. Journal of Neuroscience. 2013;33:4415–4423. doi: 10.1523/JNEUROSCI.4744-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]