Abstract
Global motion processing depends on a network of brain regions that includes extrastriate area V5 in the dorsal visual stream. For this reason, psychophysical measures of global motion perception have been used to provide a behavioural measure of dorsal stream function. This approach assumes that global motion is relatively independent of visual functions that arise earlier in the visual processing hierarchy such as contrast sensitivity and visual acuity. We tested this assumption by assessing the relationships between global motion perception, contrast sensitivity for coherent motion direction discrimination (henceforth referred to as contrast sensitivity) and habitual visual acuity in a large group of 4.5-year-old children (n = 117). The children were born at risk of abnormal neurodevelopment because of prenatal drug exposure or risk factors for neonatal hypoglycaemia. Motion coherence thresholds, a measure of global motion perception, were assessed using random dot kinematograms. The contrast of the stimuli was fixed at 100% and coherence was varied. Contrast sensitivity was measured using the same stimuli by fixing motion coherence at 100% and varying dot contrast. Stereoacuity was also measured. Motion coherence thresholds were not correlated with contrast sensitivity or visual acuity. However, lower (better) motion coherence thresholds were correlated with finer stereoacuity (rho=0.38, p=0.004). Contrast sensitivity and visual acuity were also correlated (rho= −0.26, p=0.004) with each other. These results indicate that global motion perception for high contrast stimuli is independent of contrast sensitivity and visual acuity and can be used to assess motion integration mechanisms in children.
Keywords: Visual development, extrastriate visual cortex, preschool vision assessment, at risk infant
Introduction
A well-established theory of functional organization across visual brain areas suggests that visual information is processed within two distinct pathways: the ventral stream and the dorsal stream (Goodale & Milner, 1992). The ventral stream receives parvocellular input and includes V2, V4, and the inferior temporal cortex. The dorsal stream, on the other hand, receives magnocellular input and includes V2, V3a, V5 (the homologue of the macaque middle temporal area; MT), and the posterior parietal lobe (de Haan & Cowey, 2011; Goodale & Milner, 1992; Goodale, 2013; Grinter, Maybery, & Badcock, 2010). Functionally, the ventral stream has been shown to underpin object recognition, whereas the dorsal stream supports object localization and visuomotor control (Almeida, Mahon, & Alfonso, 2010; Goodale, 2013; Johnson & Grafton, 2003; Rizzolatti & Matelli, 2003), although there is significant cross-talk between the two pathways (Cloutman, 2013; Himmelbach & Karnath, 2005; Zanon, Busan, Monti, Pizzolato, & Battaglini, 2010).
The dorsal stream vulnerability hypothesis proposes that neurodevelopmental problems have a greater impact on dorsal than ventral stream development (Braddick, Atkinson, & Wattam-Bell, 2003; Spencer et al., 2000). Much of the evidence for this hypothesis comes from the measurement of global motion perception, which involves the integration of local motion signals. Global motion perception is measured typically using random dot kinematograms (RDKs), which consist of two populations of moving dots; a signal population that move in the same direction and a noise population that move randomly. The observer identifies the direction of the signal dots and the relative proportion of signal to noise in the stimulus is varied to measure a psychophysical ‘motion coherence’ threshold (Newsome & Pare, 1988). Neurophysiological (Andersen, 1997; Edwards & Badcock, 1994), neuroimaging (Braddick et al., 2001; Klaver et al., 2008), lesion (Newsome & Pare, 1988; Rudolph & Pasternak, 1999) and brain stimulation studies (Cai, Chen, Zhou, Thompson, & Fang, 2014; Kaderali, Kim, Reynaud, & Mullen, 2015; Salzman, Britten, & Newsome, 1990) have shown that the perception of global motion in RDKs involves dorsal stream extrastriate area MT/V5 in macaques and humans, although a range of other brain areas may also be involved (Braddick et al., 2001).
Impairments in global motion perception due to abnormal visual cortex development have been reported in adults with strabismic and anisometropic amblyopia (Simmers & Bex, 2004; Simmers, Ledgeway, Hess, & McGraw, 2003; Simmers, Ledgeway, Mansouri, Hutchinson, & Hess, 2006) and children with deprivation amblyopia (Ellemberg, Lewis, Maurer, Brar, & Brent, 2002). Furthermore, in support of the dorsal stream vulnerability hypothesis, impaired global motion perception has been observed in children with William’s syndrome (Atkinson et al., 1997), dyslexia (Raymond & Sorensen, 1998), autism (Brieber et al., 2010; Manning, Charman, & Pellicano, 2013; Manning & Charman, 2015) a history of preterm birth (Taylor, Jakobson, Maurer, & Lewis, 2009), and fetal alcohol syndrome (Gummel, Ygge, Benassi, & Bolzani, 2012). However, not all neurodevelopmental studies report visual deficits that are consistent with the dorsal stream vulnerability hypothesis (Bertone & Faubert, 2006; Bertone, Mottron, Jelenic, & Faubert, 2003).
In addition to motion integration, global motion perception also relies on accurate processing of local motion signals (Simoncelli & Heeger, 1998). Therefore, global motion deficits may originate from abnormal processing of local motion, abnormal motion integration or both. One technique for separating these possibilities is to measure motion coherence thresholds for RDKs presented at a range of different contrast levels (Simmers, Ledgeway, & Hess, 2005; Simmers, Ledgeway, Hess, & McGraw, 2003). This approach is based on psychophysical data indicating that contrast thresholds for the detection of global motion in RDKs are limited by local mechanisms that are sensitive to motion direction (Morrone, Burr, & Vaina, 1995). Specifically, contrast thresholds for direction discrimination of coherent RDKs do not exhibit spatial summation, whereas motion coherence thresholds benefit from spatial summation. In macaques, cells in V1 that project to MT are tuned for motion direction, have high contrast sensitivity and may support the processing of local motion signals in global motion stimuli (Movshon & Newsome, 1996). By extension, human V1 may be involved in the local processing of motion in RDKs. In adult observers with normal vision, motion coherence thresholds remain stable over a broad range of dot contrasts and then rapidly increase for contrasts that are sufficiently low to impair local motion processing (Hess, Hutchinson, Ledgeway, & Mansouri, 2007). Using the same technique of measuring motion coherence thresholds at different stimulus contrasts, Allen et al. (2010) investigated global motion perception in elderly observers. A deficit in global motion perception was observed in the older observers. Reduced contrast sensitivity rather than impaired motion integration was found to be the key factor. Similarly, Blumenthal et al. (2013) found no difference in motion coherence thresholds for 3 and 7 month old infants when the stimulus dots were presented at a fixed multiple of their contrast threshold for coherent motion direction discrimination. The authors suggest that previous reports of global motion development in infancy (Banton & Bertenthal, 1996; Mason, Braddick, & Wattam-Bell, 2003; Wattam-Bell, 1994; Wattam-bell, 1996) reflect changes in local motion processing rather than motion integration.
However, deficits in motion integration that are not due to impaired or underdeveloped local motion processing have also been reported (Raymond & Sorensen, 1998; Schellekens, Van Wezel, Petridou, Ramsey, & Raemaekers, 2013). For example, adults with amblyopia exhibit global motion deficits that are independent from stimulus contrast and therefore likely reflect abnormal development of extrastriate areas such as V5 (Simmers, Ledgeway, Mansouri, Hutchinson, & Hess, 2006; Simmers & Bex, 2004; Simmers et al., 2005, 2003). Global motion impairments in studies of dorsal stream vulnerability are also interpreted typically in the context of abnormal motion integration (Atkinson et al., 1997; Brieber et al., 2010; Palomares & Shannon, 2013). Many of these studies were conducted with preschool or school aged children and used high contrast stimuli in order to minimize any effects of reduced acuity or contrast sensitivity deficits on task performance (Gummel et al., 2012; Manning & Charman, 2015). However, the influence of contrast dependent local motion processing on global motion perception is largely unknown in this population. A number of studies have measured both contrast sensitivity and global motion perception in children with neurodevelopmental disorders, but different stimuli were used for each type of task (Cornelissen, Richardson, Mason, Fowler, & Stein, 1995; Pellicano, Gibson, Maybery, Durkin, & Badcock, 2005; Pellicano & Gibson, 2008). This is because the studies were designed to target different stages of dorsal stream processing and not explore the relationship between local and global motion processing. For example, Pellicano et al., (2005) and Pellicano & Gibson (2008) tested global motion perception with RDKs and contrast sensitivity by measuring detection thresholds for a low spatial frequency, high temporal frequency stimulus that was designed to target early magnocellular processing. Tasks designed to target different stages of dorsal stream processing do not correlate well with one another (Dakin & Frith, 2005; Goodbourn et al., 2012), and therefore current data do not directly address the question of whether contrast sensitivity impacts global motion perception in children.
The relationships between motion coherence thresholds and clinical measures of vision such as visual acuity and stereopsis have also been investigated. Visual acuity, which involves processing in V1 (Duncan & Boynton, 2003) and relies on parvocellular function (Merigan, Katz, & Maunsell, 1991), was not significantly correlated with motion coherence thresholds in children (Ho et al., 2005) or adults (Simmers et al., 2003) with strabismic, anisometropic or mixed amblyopia. Similarly, Ellemberg et al. reported a dissociation between acuity deficits and global motion deficits in a group of children and young adults with deprivation amblyopia (Ellemberg et al., 2002). More recently, Giaschi et al. have reported deficits for a form-from-motion task designed to target global motion processing in a group of children with amblyopia that persisted despite visual acuity improvements following occlusion therapy (Giaschi, Chapman, Meier, Narasimhan, & Regan, 2015).
Results that are consistent with the dissociation between visual acuity and motion coherence thresholds in patients with amblyopia have also been found in observers with normal vision. For example, motion coherence thresholds are unaffected by stimulus manipulations that significantly impair visual acuity such as low lighting conditions (Grossman & Blake, 1999) and optical defocus (Trick & Silverman, 1991; Trick, Steinman, & Amyot, 1995). Furthermore, no relationship between visual acuity and motion coherence thresholds was found in a group of 2-year old children born at risk of neonatal hypoglycaemia (Yu et al., 2013). Therefore the available data suggest that global motion processing is largely independent of visual acuity.
Stereoacuity has been linked to processing in both the dorsal and ventral streams (Anzai, Chowdhury, & DeAngelis, 2011; Neri, 2005; Parker, 2007; Uka & DeAngelis, 2004; Umeda, Tanabe, & Fujita, 2007). Within the dorsal stream, areas that are sensitive to global motion such as V3A and MT/V5 have also been found to exhibit sensitivity to retinal disparity (Anzai et al., 2011; Cottereau, McKee, & Norcia, 2012; DeAngelis & Uka, 2003; DeAngelis, 1998; Rokers, Cormack, & Huk, 2009). This may provide the basis for the correlations between finer stereoacuity and lower motion coherence thresholds that have been reported in a number of populations such as young children born at risk of neonatal hypoglycaemia (Yu et al., 2013) and children with a low birth weight (MacKay et al., 2005). However, the inverse relationship has also been reported whereby poorer stereopsis was related to lower (better) motion coherence thresholds in children with amblyopia (Ho et al., 2005).
Building on this previous work we investigated the relationship between contrast sensitivity for direction discrimination with fully coherent RDKs and motion coherence thresholds for high contrast RDKs in a group of one hundred and twenty five 4.5-year-old children born at risk of abnormal neurodevelopment. The children were enrolled in one of two longitudinal follow up studies that included optometric screening at 4.5 years of age. Therefore, we were also able to assess the relationship between motion coherence thresholds and both visual acuity and stereoacuity.
2. Materials and methods
2.1 Subjects
One hundred and twenty five children aged 54 (±2) months took part in the study. Of these, one hundred and seventeen (94%; 59 boys, 58 girls) were able to complete all psychophysical and clinical tests and were therefore included in the final analyses. The children were participants in one of two large-scale, multidisciplinary follow-up studies; the Children with Hypoglycemia and their Later Development (CHYLD) study or the Infant, Development, Environment and Lifestyle (IDEAL) study. Both study protocols included a comprehensive developmental assessment at 4.5 years of age. Our data were collected as part of this assessment. The Northern Y Regional Health and Disability Ethics Committee approved both study protocols. The IDEAL study was also approved by Auckland and Waitemata District Health Boards and their Māori ethics committees. All caregivers gave informed consent and the study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
The CHYLD study was designed to assess the neurodevelopmental outcomes of children who were born with one or more of the following risk factors for neonatal hypoglycemia; child of a diabetic mother, being small (< 2.5 Kg or < 10th centile) or large (> 4.5 Kg or > 90th centile) at birth or late preterm birth (≥ 32 weeks’ gestation) (McKinlay et al., 2015). The IDEAL study participants included children who were exposed prenatally to methamphetamine and controls matched for birth weight, socio-economic status, ethnicity and level of maternal education (LaGasse et al., 2011; Wouldes et al., 2013, 2014). Although children were recruited into the IDEAL study on the basis of methamphetamine exposure, all children were exposed to socioeconomic risk factors and the majority of the children, including controls, experienced prenatal exposure to a range of drugs including alcohol, marijuana and nicotine.
Both neonatal hypoglycaemia and prenatal drug exposure can affect the visual cortex. Diffusion-weighted imaging has revealed restricted diffusion in the occipital lobes of infants with neonatal hypoglycaemia, possibility indicating myelin edema (Tam et al., 2008). Abnormal visual evoked potentials were also reported (Tam et al., 2008). Furthermore, severe neonatal hypoglycaemia (not present in our study cohort) can cause occipital lobe injury and cortical visual impairment (Burns, Rutherford, Boardman, & Cowan, 2008; Yalnizoglu, Haliloglu, Turanli, Cila, & Topcu, 2007). Prenatal drug exposure has also been linked to visual evoked potentials that indicate impaired cortical processing of visual information (Hamilton et al., 2010; McGlone et al., 2013). Although the specific effects of neonatal hypoglycaemia and prenatal drug exposure were not the focus of our research question in this study, we anticipated that the children from these two cohorts would vary sufficiently in their contrast sensitivity for coherent motion direction discrimination and global motion perception to allow for any relationships between these two factors to be detected.
2.2. Stimuli
Random dot kinematograms consisted of 100 circular dots (dot diameter 0.24°, dot density 1.27 dot/deg2) presented within a circular aperture (10° diameter) at a viewing distance of 60 cm. Dots were displaced by 0.1° every 17ms to achieve a speed of 6°/second. The stimuli were presented for 1 second. These parameters were chosen on the basis of previous studies that have investigated global motion perception in children (Narasimhan & Giaschi, 2012; Gunn et al., 2002; Lewis & Maurer, 2005). Dots had a limited lifetime, whereby each dot had a 5% chance of disappearing on each frame and being redrawn in a random location. The mean lifetime was 300 msec. Bright dots were presented on a grey background (45 cd/m2) and dot contrast was defined using the Michelson equation: (Ldots − Lbackground) / (Ldots + Lbackground).
Motion coherence thresholds were measured using a RDK constructed from dots presented at maximum brightness (137 cd/m2; Michelson contrast of 0.51). Signal dots moved coherently upwards or downwards and noise dots moved in random directions. Contrast thresholds for coherent motion direction discrimination were measured using a fully coherent (signal dots only) RDK with variable dot contrast.
All stimuli were presented on a 15” Dell cathode ray tube (CRT) monitor (model: E771p) with a 120 Hz refresh rate and 1024×768 resolution. Stimuli used for motion coherence threshold measurement were generated using MATLAB 2013a and psychtoolbox-3 (Pelli, 1997). Stimuli used to measure contrast thresholds for coherent motion direction discrimination were generated using Psykinematix software (Beaudot, 2009) which allows for a 10.8-bit contrast resolution by using a bit-stealing algorithm (Kontsevich & Tyler, 1999). The stimuli were matched for all parameters described above. All RDK stimuli were viewed binocularly.
2.3. Procedure
Observers viewed the stimuli using their habitual vision corrections. Motion coherence thresholds were measured first, followed by an optometric screening and then contrast thresholds for coherent motion direction discrimination were measured.
2.3.1. Motion coherence thresholds
Prior to threshold measurement, children were familiarized with the stimuli and task. First, the children were presented with 100% coherent (all signal dots), high contrast RDKs moving up or down. After 4 successive correct responses at the 100% coherence level, the experimenter manually varied the direction and coherence of the RDK to demonstrate the appearance of RDKs with different coherence levels. Once the child was familiar with the stimulus and task, a 2-down-1-up adaptive staircase test was used to vary the coherence of the RDK to measure a motion coherence threshold (contrast was fixed at 100% of maximum). The staircase began at 100% coherence and had a proportional step size of 50% until the first reversal and 25% thereafter. The staircase was terminated after 5 reversals and the threshold was calculated by averaging the last 4 reversals.
2.3.2. Contrast thresholds for coherent motion direction discrimination
A second familiarization session was conducted prior to the measurement of contrast thresholds for coherent motion direction discrimination. Participants viewed a fully coherent RDK at 70% of maximum contrast. Motion direction was varied (up/down) until the participant was able to correctly identify the motion direction on four consecutive trials. Dot contrast was then manually varied to demonstrate the appearance of the stimulus at different contrast levels. A contrast threshold for coherent motion direction discrimination was then measured using a 2-down-1-up adaptive staircase that varied dot contrast (RDK coherence was fixed at 100%). The starting contrast was 70% of maximum and the staircase employed a proportional step size of 50% before the first reversal and 25% thereafter. The staircase ran for 5 reversals and the threshold was calculated as the mean of the last 4 reversals.
2.3.3. Optometric examination
A comprehensive vision screening was conducted to rule out any significant ocular pathology. Monocular and binocular habitual visual acuities were tested using the crowded linear Lea symbols test in the CHYLD cohort and the crowded Keeler logMAR test in the IDEAL cohort. Subsequent references to visual acuity should be interpreted as the better eye’s monocular visual acuity. Stereoacuity was measured using the graded circles portion of the stereo fly test. Ocular motility was assessed using a cover test, a broad H-test, a 20-prism base out test and near point of convergence. Ocular health was assessed using the red reflex test, external inspection and pupillary evaluation. Children were tested with their habitual refractive correction, if any.
2.4. Statistical Analysis
Contrast thresholds for coherent motion direction discrimination were converted to log contrast sensitivity. Data were analyzed using SPSS v22 (IBM Corp, Armonk, NY, USA). The Shapiro-Wilk test was used to assess whether data were normally distributed and parametric (ANOVA) or nonparametric (Kruskal Wallis, Mann-Whiney U and Spearman’s rank correlation coefficients) statistical tests were chosen accordingly. Data are reported as mean and standard deviation or median and range.
3. Results
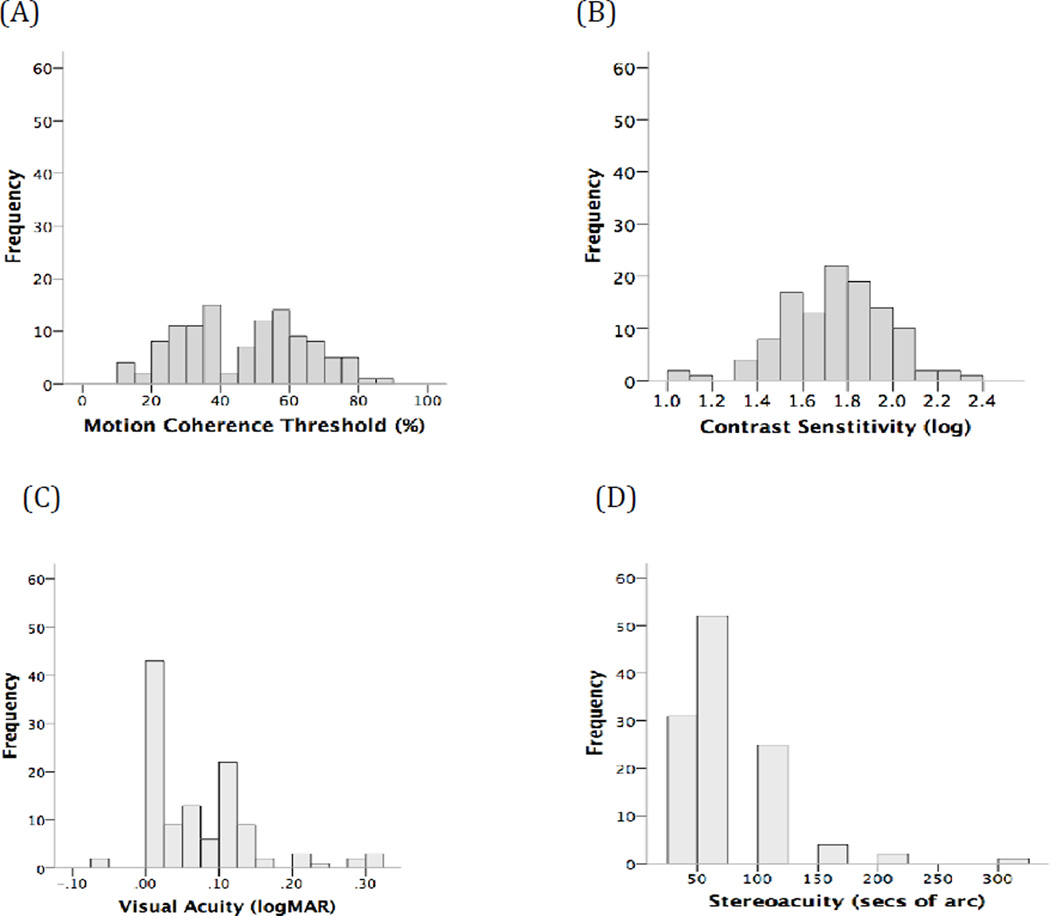
Distributions for motion coherence thresholds, log contrast sensitivity for motion direction discrimination, better eye visual acuity and stereoacuity are shown in Fig 1. Motion coherence thresholds (Fig 1A, median 49%, range 11% to 86%), better eye visual acuity (Fig 1C, median 0.06 logMAR, range −0.06 logMAR to 0.30 logMAR) and stereoacuity (Fig 1D, median 63” of arc, range 25” to 300” of arc) were not normally distributed. Log contrast sensitivity (Fig 1B) was normally distributed with a mean of 1.7 + 0.2 logCS.
Figure 1.
Distribution (n = 117) of (A) motion coherence thresholds (B) log contrast sensitivity for coherent motion direction discrimination (C) visual acuity and (D) stereoacuity.
3.1. Correlations involving motion coherence thresholds
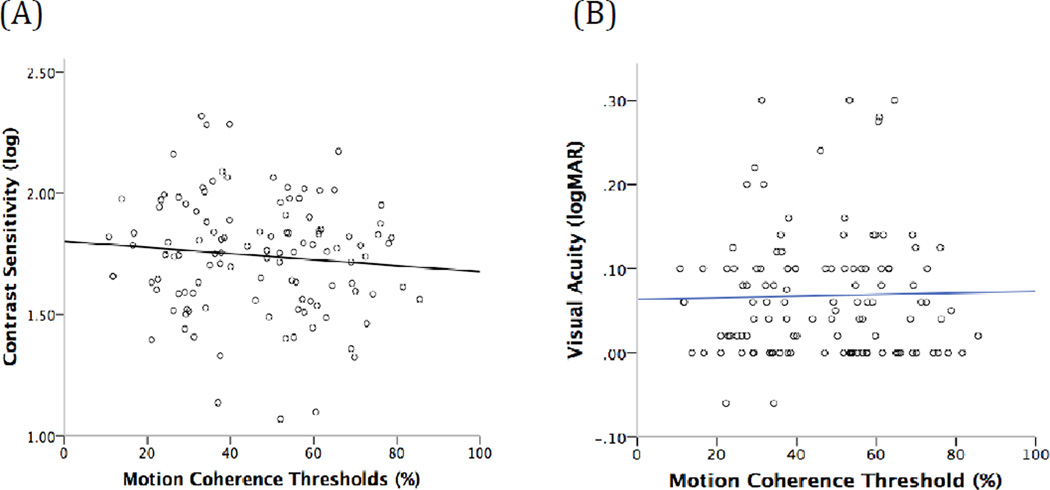
Motion coherence thresholds and log contrast sensitivities were not correlated significantly (rho= −0.06, p= 0.52; Fig 2A). Motion coherence thresholds were also not correlated with visual acuities (rho=0.005, p=0.96, Fig 2B).
Figure 2.
Relationships between motion coherence thresholds and (A) contrast sensitivity for coherent motion direction discrimination and (B) visual acuity.
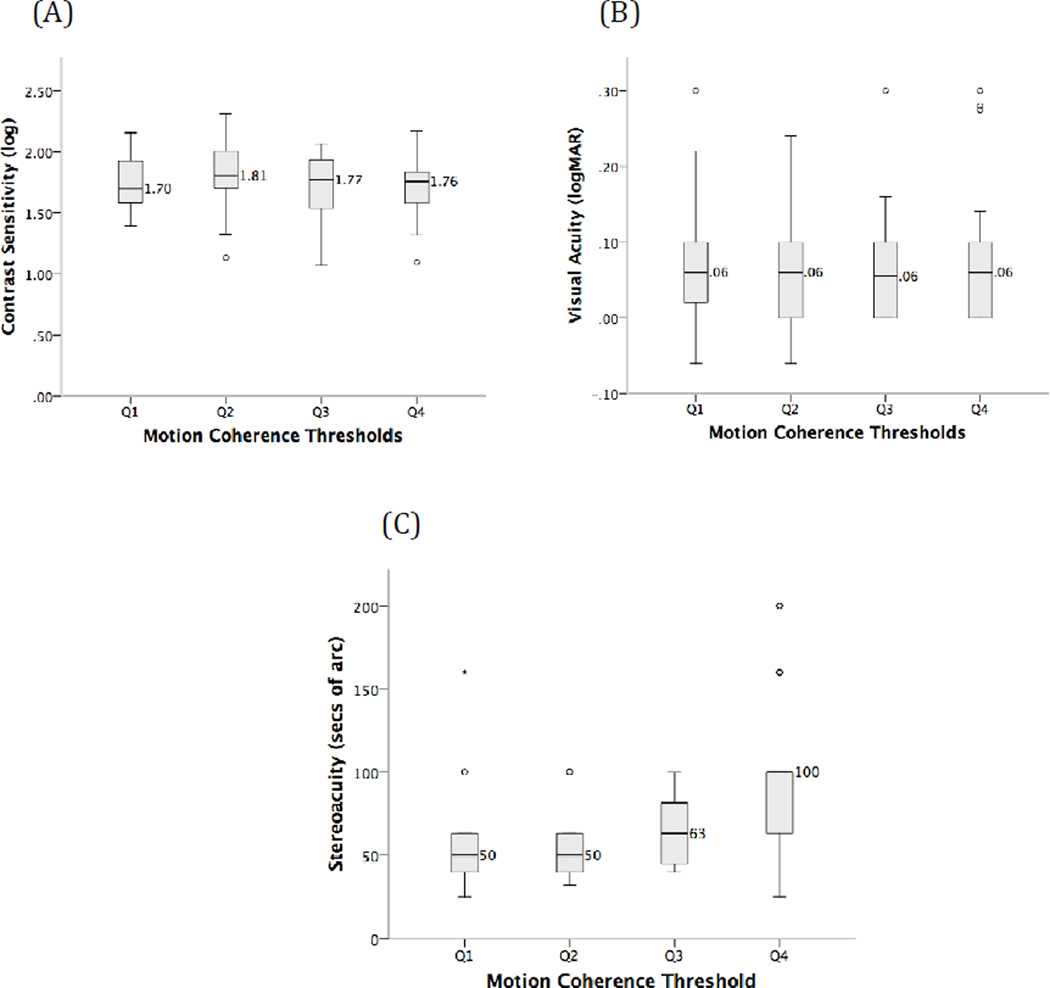
To further test for any relationships between motion coherence threshold and contrast sensitivity for coherent motion direction discrimination, motion coherence thresholds (MCT) were divided into quartiles (Q) (Q1=MCT < 31.75%, Q2=MCT ≥ 31.75% to < 48.75%, Q3=MCT ≥ 48.75% to < 59.75% and Q4=MCT ≥ 59.75%; Fig 3A). Contrast sensitivity did not vary significantly across motion coherence threshold quartiles (ANOVA, F3,113=1.42, p=0.24) (Fig 3A). Similarly, visual acuity (Fig 3B) did not vary significantly across each of the motion coherence threshold quartiles (Kruskal Wallis χ2(3) = 0.81, p = 0.85).
Figure 3.
Comparison of (A) log contrast sensitivity for coherent motion direction discrimination, (B) visual acuity, and (C) stereoacuity across four quartiles of motion coherence threshold. In panel A, horizontal lines indicate the mean log contrast sensitivity for each quartile of motion coherence threshold. Mean log contrast sensitivity values are given to the right of each data point. The error bars show standard deviation and open circles indicate outliers (values larger or smaller than 1.5 times the interquartile range). In panels B and C, horizontal lines indicate medians with the median value given to the right of each data point. In these panels, error bars show the range and open circles indicate outliers. Absent error bars indicate that no data points (excluding outliers) fell outside of the relevant box plot boundary.
Motion coherence thresholds were correlated moderately and statistically significantly with stereoacuity (rho= 0.38, p=0.004) whereby lower (better) motion coherence thresholds were associated with lower (better) stereoacuity scores. In agreement with this correlation, stereoacuity varied significantly across the four quartiles of motion coherence threshold [Kruskal Wallis, χ2(3) = 16.5, p = 0.001; Fig 3C], with a significant difference between the stereoacuity scores for children in the first and fourth quartiles of motion coherence thresholds (post hoc Mann-Whitney, U=193.5, p<0.001).
3.2. Correlations among contrast sensitivity for direction discrimination, visual acuity and stereoacuity
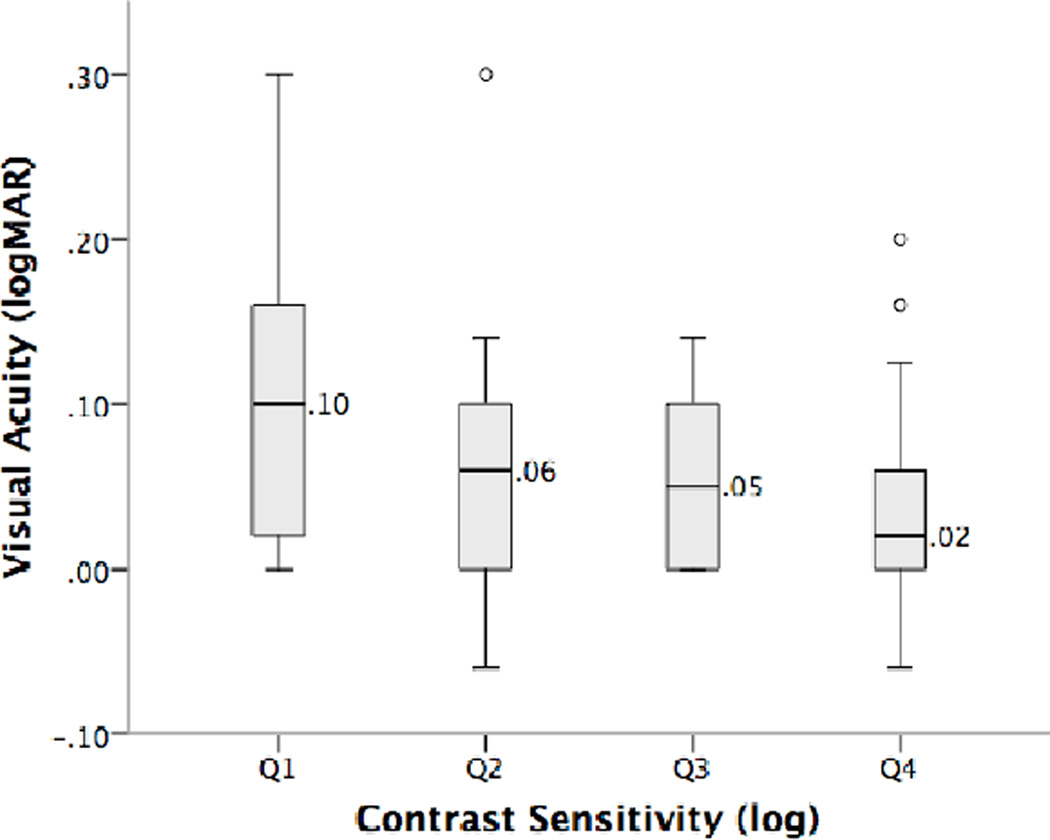
A statistically significant positive correlation was observed between better eye visual acuity and contrast sensitivity for coherent motion direction discrimination (rho= −0.26, p=0.004). Furthermore, visual acuity varied significantly across the four quartiles of contrast sensitivity (Q1=logCS < 1.58, Q2= logCS ≥ 1.58 to < 1.76, Q3= logCS ≥ 1.76 to < 1.88 and Q4= logCS ≥ 1.88, χ2(3) = 9.67, p=0.022; Fig 4), with a statistically significant (but clinically small) difference between the visual acuities of children in the first and fourth quartiles of contrast sensitivity (U=239, p=0.004, difference = 0.08 logMAR). No significant relationships were found between visual acuity and stereopsis or contrast sensitivity and stereopsis.
Figure 4.
Variation in visual acuity as a function of log contrast sensitivity for coherent motion direction discrimination quartile. Data are shown as in Figure 3B.
4. Discussion
The aim of this study was to assess whether motion coherence thresholds in preschool age children were independent from contrast thresholds for coherent motion direction discrimination measured using the same stimuli. This question is important as studies investigating the dorsal stream vulnerability hypothesis often interpret elevated motion coherence thresholds as evidence for abnormal motion integration in extrastriate visual areas (Raymond & Sorensen, 1998; Schellekens et al., 2013), but elevated thresholds could be related to impairments at earlier stages of visual processing.
In our large group of 4.5-year-old children born at risk of abnormal neurodevelopment, we found no evidence for a relationship between contrast thresholds for coherent motion direction discrimination and motion coherence thresholds for the same RDK stimuli. Motion coherence thresholds were also unrelated to visual acuity, as has previously been reported for patients with amblyopia (Ellemberg et al., 2002; Simmers et al., 2003). This suggests that motion coherence thresholds for high contrast RDKs are independent of normal variations in contrast sensitivity for direction discrimination and acuity in children. Therefore, elevated motion coherences thresholds for high contrast RDK stimuli are likely to reflect abnormal motion integration, a visual function that has been linked to dorsal stream extrastriate visual areas such as V5. This is in agreement with recent work using equivalent noise techniques indicating that integration processes limit global motion perception in 5–11 year old children and not local motion processing (Manning, Dakin, Tibber, & Pellicano, 2014). Our results are also consistent with previous work demonstrating that, in adults, motion coherence thresholds are constant across a wide range of stimulus contrasts (Hess et al., 2007).
The absence of a relationship between contrast thresholds for coherent motion direction discrimination and motion coherence thresholds in our group of 4.5-year-old children is not consistent with studies that have reported a link between contrast sensitivity and global motion perception. Blumenthal et al. (2013) found that motion coherence thresholds were dependent on contrast thresholds for direction discrimination in infants 3–7 months of age. Furthermore, they argued that motion integration mechanisms function at adult levels by 3 months of age and that apparent maturation of global motion perception is primarily a result of contrast sensitivity development. The discrepancy between the results of Blumenthal et al., (2013) and those we report here could result from a number of factors. Contrast sensitivity develops significantly between early infancy (3–7 months as assessed by Blumenthal et al., 2013) and childhood (4.5 years of age, as assessed in the current study). Therefore it is possible that stimulus contrast has a more pronounced effect on global motion processing for young infants than for 4.5-year-old children whose contrast sensitivity is closer to adult levels (reviewed in Daw, 2003). In addition, Blumenthal et al., (2013) used an eye movement based measure of global motion perception, which may involve both cortical and subcortical mechanisms in children below the age of 2 years (Lewis, Maurer, Chung, Holmes-Shannon, & Van Schaik, 2000).
Although motion coherence thresholds were not correlated with contrast sensitivity for coherent motion direction discrimination or acuity, there was a moderate and statistically significant correlation with stereoacuity. This is consistent with a previous study of the CHYLD study cohort at 2 years of age, which reported a moderate and significant correlation between an eye-movement-based motion coherence threshold measure and stereo acuity (Yu et al., 2013). Neurophysiological recordings from macaques have identified cells in MT that encode both global motion and retinal disparity (DeAngelis & Uka, 2003; Felleman & Essen, 1987; DeAngelis, 1998). If human V5 also supports both global motion perception and disparity processing, these correlations may reflect parallel development of these two important visual functions. We also observed a weak but statistically significant correlation between better eye visual acuity and contrast sensitivity for coherent motion direction discrimination suggesting a partial relationship between these two measurements of spatial (visual acuity) and spatio-temporal (RDK contrast sensitivity) vision. Although both visual acuity (Duncan & Boynton, 2003) and motion direction encoding (Movshon & Newsome, 1996) involve V1, these two processes primarily rely on parvocellular and magnocellular inputs from the LGN respectively, and therefore the neural basis for this relationship is unclear.
In summary, motion coherence thresholds for high contrast RDK stimuli were independent from contrast sensitivity for coherent motion direction discrimination and visual acuity in our sample of 4.5-year-old children. This suggests that motion coherence thresholds measured with high contrast RDK stimuli reflect the function of motion integration mechanisms within extrastriate areas of the visual cortex. In practical terms, the results indicate that RDK stimuli can be used to investigate motion integration in studies of dorsal stream development and function.
Highlights.
Global motion was unrelated to contrast sensitivity and acuity in preschool children
Global motion was correlated with stereoacuity
High contrast stimuli assess motion integration in at-risk pediatric populations
Acknowledgements
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R01HD0692201, National Institutes on Drug Abuse grants 2RO1DA014948 and RO1DA021757 and the Auckland Medical Research Foundation. We acknowledge the contribution of all members of the CHYLD Study team: Coila Bevan, Jessica Brosnahan, Ellen Campbell, Tineke Crawford, Kelly Fredell, Karen Frost, Claire Hahnhaussen, Safayet Hossin, Greg Gamble, Anna Gsell, Yannan Jiang, Kelly Jones, Sapphire Martin, Neil Micklewood, Chris McKinlay, Grace McKnight, Christina McQuoid, Janine Paynter, Raquel O. Rodrigues, Jenny Rogers, Kate Sommers, Heather Stewart, Anna Timmings, Jess Wilson, Rebecca Young, from the Liggins Institute, University of Auckland, New Zealand; Jo Arthur, Susanne Bruder, Gillian Matheson, Tzu-Ying (Sandy) Yu from the School of Optometry and Vision Science, University of Auckland, New Zealand Nataliia Burakevych, Department of Paediatrics; Child and Youth Health, University of Auckland, New Zealand. Judith Ansell, Ryan San Diego, Department of Psychological Medicine, University of Auckland, New Zealand Matthew Signal, Aaron Le Compte, Department of Engineering, University of Canterbury, New Zealand. Max Berry, Arun Nair, Ailsa Tuck, Alexandra Wallace, Phil Weston from the Department of Paediatrics, Waikato Hospital, Hamilton, New Zealand. The CHYLD Steering Group: Jane Alsweiler, Department of Paediatrics; Child and Youth Health, University of Auckland, J. Geoffery Chase, Department of Engineering, University of Canterbury, Jane Harding, Liggins Institute, University of Auckland, Deborah Harris, Newborn Intensive Care Unit, Waikato District Health Board, Benjamin Thompson, Department of Optometry and Vision Science, University of Auckland, Trecia Ann Wouldes, Department of Psychological Medicine, University of Auckland, Auckland, New Zealand. International Advisory Group: Heidi Feldman, Stanford University School of Medicine, USA; William Hay, University of Colorado School of Medicine, USA; Darrell Wilson, Stanford University School of Medicine, USA; Robert Hess, McGill Vision Research Unit, Department of Ophthalmology, McGill University, USA. We also acknowledge the members of NZ IDEAL study team: Jenny Rogers, Josephine Cliffe, Suzanne Cumming, and Heather Stewart.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen Ha, Hutchinson CV, Gayle P. The role of contrast sensitivity in global motion processing deficits in the elderly. Journal of Vision. 2010;10(2010):1–10. doi: 10.1167/10.10.15. [DOI] [PubMed] [Google Scholar]
- Almeida J, Mahon ZB, Alfonso C. The role of the dorsal visual processing stream in tool identification. Pyschol Sci. 2010;21(6):772–778. doi: 10.1177/0956797610371343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen Ra. Neural mechanisms of visual motion perception in primates. Neuron. 1997;18(6):865–872. doi: 10.1016/s0896-6273(00)80326-8. [DOI] [PubMed] [Google Scholar]
- Anzai A, Chowdhury Sa, DeAngelis GC. Coding of stereoscopic depth information in visual areas V3 and V3A. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2011;31(28):10270–10282. doi: 10.1523/JNEUROSCI.5956-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams’ syndrome. Neuroreport. 1997;8(8):1919–1922. doi: 10.1097/00001756-199705260-00025. [DOI] [PubMed] [Google Scholar]
- Banton T, Bertenthal BI. Infants’ sensitivity to uniform motion. Vision Research. 1996;36(11):1633–1640. doi: 10.1016/0042-6989(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Beaudot WHA. Psykinematix : A New Psychophysical Tool for Investigating Visual Impairment due to Neural Dysfunctions. Journal of the Vision Society of Japan. 2009;21(1):19–32. [Google Scholar]
- Bertone A, Faubert J. Demonstrations of decreased sensitivity to complex motion information not enough to propose an autism-specific neural etiology. Journal of Autism and Developmental Disorders. 2006;36(1):55–64. doi: 10.1007/s10803-005-0042-5. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: a “complex” issue. Journal of Cognitive Neuroscience. 2003;15(2):218–225. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- Blumenthal EJ, Bosworth RG, Dobkins KR. Fast development of global motion processing in human infants. Journal of Vision. 2013;13(13) doi: 10.1167/13.13.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: motion coherence and “dorsalstream vulnerability”. Neuropsychologia. 2003;41(13):1769–1784. doi: 10.1016/s0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O’Brien JMD, Wattam-Bell J, Atkinson J, Hartley T, Turner R. Brain areas sensitive to coherent visual motion. Perception. 2001;30(1):61–72. doi: 10.1068/p3048. [DOI] [PubMed] [Google Scholar]
- Brieber S, Herpertz-Dahlmann B, Fink GR, Kamp-Becker I, Remschmidt H, Konrad K. Coherent motion processing in autism spectrum disorder (ASD): an fMRI study. Neuropsychologia. 2010;48(6):1644–1651. doi: 10.1016/j.neuropsychologia.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Burns CM, Rutherford Ma, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122(1):65–74. doi: 10.1542/peds.2007-2822. [DOI] [PubMed] [Google Scholar]
- Cai P, Chen N, Zhou T, Thompson B, Fang F. Global versus local: double dissociation between MT+ and V3A in motion processing revealed using continuous theta burst transcranial magnetic stimulation. Experimental Brain Research. 2014 doi: 10.1007/s00221-014-4084-9. (Braddick 1993). [DOI] [PubMed] [Google Scholar]
- Cloutman LL. Interaction between dorsal and ventral processing streams: Where, when and how? Brain and Language. 2013;127(2):251–263. doi: 10.1016/j.bandl.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Cornelissen P, Richardson a, Mason a, Fowler S, Stein J. Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls. Vision Research. 1995;35(10):1483–1494. doi: 10.1016/0042-6989(95)98728-r. [DOI] [PubMed] [Google Scholar]
- Cottereau BR, McKee SP, Norcia AM. Bridging the gap: global disparity processing in the human visual cortex. Journal of Neurophysiology. 2012;107(9):2421–2429. doi: 10.1152/jn.01051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48(3):497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Daw NW. Neurobiology of Infant Vision. 2003rd ed. Westport, CT: Praeger; 2003. Critical Periods in the Visual System; pp. 43–103. [Google Scholar]
- De Haan EHF, Cowey A. On the usefulness of “what” and “where” pathways in vision. Trends in Cognitive Sciences. 2011;15(10):460–466. doi: 10.1016/j.tics.2011.08.005. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Uka T. Coding of horizontal disparity and velocity by MT neurons in the alert macaque. Journal of Neurophysiology. 2003;89(2):1094–1111. doi: 10.1152/jn.00717.2002. [DOI] [PubMed] [Google Scholar]
- Duncan RO, Boynton GM. Cortical magnification within human primary visual cortex correlates with acuity thresholds. Neuron. 2003;38(4):659–671. doi: 10.1016/s0896-6273(03)00265-4. [DOI] [PubMed] [Google Scholar]
- Edwards M, Badcock DR. Global motion perception: Interaction of the ON and OFF pathways. Vision Research. 1994;34(21):2849–2858. doi: 10.1016/0042-6989(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Maurer D, Brar S, Brent HP. Better perception of global motion after monocular than after binocular deprivation. Vision Research. 2002;42(2):169–179. doi: 10.1016/s0042-6989(01)00278-4. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Essen DC Van. Receptive field properties of neurons in area V3 of macaque monkey extrastriate cortex Receptive Field Properties of Neurons in Area V3 of Macaque Monkey Extrastriate Cortex. Journal of Neurophysiology. 1987;57(4):889–920. doi: 10.1152/jn.1987.57.4.889. [DOI] [PubMed] [Google Scholar]
- Giaschi D, Chapman C, Meier K, Narasimhan S, Regan D. The effect of occlusion therapy on motion perception deficits in amblyopia. Vision Research. 2015 doi: 10.1016/j.visres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Goodale Ma. Separate visual systems for perception and action: a framework for understanding cortical visual impairment. Developmental Medicine and Child Neurology. 2013;55(Suppl 4):9–12. doi: 10.1111/dmcn.12299. [DOI] [PubMed] [Google Scholar]
- Goodale MA. Separate visual systems for perception and action: a framework for understanding cortical visual impairment. Developmental Medicine & Child Neurology. 2013;55:9–12. doi: 10.1111/dmcn.12299. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner aD. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15(I):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodbourn PT, Bosten JM, Hogg RE, Bargary G, Lawrance-Owen AJ, Mollon JD. Do different “magnocellular tasks” probe the same neural substrate? Proceedings of the Royal Society B: Biological Sciences. 2012 doi: 10.1098/rspb.2012.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C, DeAngelis BGC, W TN. Cortical area MT and the perception of stereoscopic depth. Nature. 1998 Aug;394:677–680. doi: 10.1038/29299. [DOI] [PubMed] [Google Scholar]
- Grinter EJ, Maybery MT, Badcock DR. Vision in developmental disorders: is there a dorsal stream deficit? Brain Research Bulletin. 2010;82(3–4):147–160. doi: 10.1016/j.brainresbull.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Perception of coherent motion, biological motion and form-from-motion under dim-light conditions. Vision Research. 1999;39(22):3721–3727. doi: 10.1016/s0042-6989(99)00084-x. [DOI] [PubMed] [Google Scholar]
- Gummel K, Ygge J, Benassi M, Bolzani R. Motion perception in children with foetal alcohol syndrome. Acta Paediatrica (Oslo, Norway : 1992) 2012;101(8):e327–e332. doi: 10.1111/j.1651-2227.2012.02700.x. [DOI] [PubMed] [Google Scholar]
- Gunn A, Cory E, Atkinson J, Braddick O, Wattam-Bell J, Guzzetta A, Cioni G. Dorsal and ventral stream sensitivity in normal development and hemiplegia. Neuroreport. 2002;13(6):843–847. doi: 10.1097/00001756-200205070-00021. [DOI] [PubMed] [Google Scholar]
- Hamilton R, McGlone L, MacKinnon JR, Russell HC, Bradnam MS, Mactier H. Ophthalmic, clinical and visual electrophysiological findings in children born to mothers prescribed substitute methadone in pregnancy. The British Journal of Ophthalmology. 2010;94(6):696–700. doi: 10.1136/bjo.2009.169284. [DOI] [PubMed] [Google Scholar]
- Hess RF, Hutchinson CV, Ledgeway T, Mansouri B. Binocular influences on global motion processing in the human visual system. Vision Research. 2007;47(12):1682–1692. doi: 10.1016/j.visres.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Himmelbach M, Karnath H-O. Dorsal and ventral stream interaction: contributions from optic ataxia. Journal of Cognitive Neuroscience. 2005;17(4):632–640. doi: 10.1162/0898929053467514. [DOI] [PubMed] [Google Scholar]
- Ho CS, Giaschi DE, Boden C, Dougherty R, Cline R, Lyons C. Deficient motion perception in the fellow eye of amblyopic children. Vision Research. 2005;45(12):1615–1627. doi: 10.1016/j.visres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Grafton ST. From “acting on” to “acting with”: the functional anatomy of object-oriented action schemata. Progress in Brain Research. 2003;142:127–139. doi: 10.1016/S0079-6123(03)42010-4. [DOI] [PubMed] [Google Scholar]
- Kaderali S, Kim YJ, Reynaud A, Mullen KT. The Role of Human Brain Area hMT+ in the Perception of Global Motion Investigated With Repetitive Transcranial Magnetic Stimulation (rTMS) Brain Stimulation. 2015;8(2):200–207. doi: 10.1016/j.brs.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Klaver P, Lichtensteiger J, Bucher K, Dietrich T, Loenneker T, Martin E. Dorsal stream development in motion and structure-from-motion perception. NeuroImage. 2008;39(4):1815–1823. doi: 10.1016/j.neuroimage.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Kontsevich LL, Tyler CW. Nonlinearities of near-threshold contrast transduction. Vision Research. 1999;39:1869–1880. doi: 10.1016/s0042-6989(98)00286-7. (April 1998) [DOI] [PubMed] [Google Scholar]
- LaGasse LL, Wouldes T, Newman E, Smith LM, Shah RZ, Derauf C, Lester BM. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicology and Teratology. 2011;33(1):166–175. doi: 10.1016/j.ntt.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Maurer D. Multiple sensitive periods in human visual development: Evidence from visually deprived children. Developmental Psychobiology. 2005;46:163–183. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Maurer D, Chung JY, Holmes-Shannon R, Van Schaik CS. The development of symmetrical OKN in infants: quantification based on OKN acuity for nasalward versus temporalward motion. Vision Research. 2000;40(4):445–453. doi: 10.1016/s0042-6989(99)00190-x. [DOI] [PubMed] [Google Scholar]
- MacKay TL, Jakobson LS, Ellemberg D, Lewis TL, Maurer D, Casiro O. Deficits in the processing of local and global motion in very low birthweight children. Neuropsychologia. 2005;43(12):1738–1748. doi: 10.1016/j.neuropsychologia.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Manning C, Charman T. Brief Report : Coherent Motion Processing in Autism : Is Dot Lifetime an Important Parameter ? J Autism Dev Disord. 2015 doi: 10.1007/s10803-015-2365-1. [DOI] [PubMed] [Google Scholar]
- Manning C, Charman T, Pellicano E. Processing Slow and Fast Motion in Children With Autism Spectrum Conditions. Autism Research. 2013;6(6):531–541. doi: 10.1002/aur.1309. [DOI] [PubMed] [Google Scholar]
- Manning C, Dakin SC, Tibber MS, Pellicano E. Averaging, not internal noise, limits the development of coherent motion processing. Developmental Cognitive Neuroscience. 2014;10:1–13. doi: 10.1016/j.dcn.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason aJS, Braddick OJ, Wattam-Bell J. Motion coherence thresholds in infants––different tasks identify at least two distinct motion systems. Vision Research. 2003;43(10):1149–1157. doi: 10.1016/s0042-6989(03)00077-4. [DOI] [PubMed] [Google Scholar]
- McGlone L, Hamilton R, McCulloch DL, Boulton R, Bradnam MS, Weaver LT, Mactier H. Neonatal visual evoked potentials in infants born to mothers prescribed methadone. Pediatrics. 2013;131(3):e857–e863. doi: 10.1542/peds.2012-2113. [DOI] [PubMed] [Google Scholar]
- McKinlay CJD, Alsweiler JM, Ansell JM, Anstice NS, Chase JG, Gamble GD, Harris DL, Jacobs JJ, Jiang Y, Paudel N, Signal M, Thompson B, Wouldes TA, Yu TY, Harding JE. Neonatal Glycemia and Neurodevelopmental Outcomes in Term and Late-Preterm Infants. New England Journal of Medicine. 2015 In Press. [Google Scholar]
- Merigan WH, Katz LM, Maunsell JH. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. The Journal of Neuroscience. 1991;11(4):994–1001. doi: 10.1523/JNEUROSCI.11-04-00994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner A, Goodale M. The visual brain in action. 27. New York: Oxford; 1995. [Google Scholar]
- Morrone MC, Burr DC, Vaina LM. Two stages of visual processing for radial and circular motion. Nature. 1995 Aug 10; doi: 10.1038/376507a0. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Newsome WT. Visual Response Properties of Striate Cortical Neurons Projecting to Area MT in Macaque Monkeys. The Journal of Neuroscience. 1996;16(23):7733–7741. doi: 10.1523/JNEUROSCI.16-23-07733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Giaschi D. The effect of dot speed and density on the development of global motion perception. Vision Research. 2012;62:102–107. doi: 10.1016/j.visres.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Neri P. A stereoscopic look at visual cortex. Journal of Neurophysiology. 2005;93(4):1823–1826. doi: 10.1152/jn.01068.2004. [DOI] [PubMed] [Google Scholar]
- Newsome T, Pare EB. A Selective Impairment of Motion Perception the Middle Temporal Visual Area (MT) Following Lesions of. The Journal of Neuroscience. 1988;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomares M, Shannon MT. Global dot integration in typically developing children and in Williams Syndrome. Brain and Cognition. 2013;83(3):262–270. doi: 10.1016/j.bandc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Parker AJ. Binocular depth perception and the cerebral cortex. Nature Reviews. Neuroscience. 2007;8(5):379–391. doi: 10.1038/nrn2131. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: A possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43:1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Gibson LY. Investigating the functional integrity of the dorsal visual pathway in autism and dyslexia. Neuropsychologia. 2008;46:2593–2596. doi: 10.1016/j.neuropsychologia.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Sorensen RE. Visual Motion Perception in Children with Dyslexia : Normal Detection but Abnormal Integration. Visual Cognition. 1998;5(3):389–404. [Google Scholar]
- Rizzolatti RG, Matelli M. Two different streams form the dorsal visual system : anatomy and functions. Experimental Brain Research. 2003;153:146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Rokers B, Cormack LK, Huk AC. Disparity- and velocity-based signals for three-dimensional motion perception in human MT+ Nature Neuroscience. 2009;12(8):1050–1055. doi: 10.1038/nn.2343. [DOI] [PubMed] [Google Scholar]
- Rudolph K, Pasternak T. Transient and permanent deficits in motion perception after lesions of cortical areas MT and MST in the macaque monkey. Cerebral Cortex. 1999;9(1):90–100. doi: 10.1093/cercor/9.1.90. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Britten KH, Newsome WT. Cortical microstimulation influences perceptual judgements of motion direction. Nature. 1990;346(6280):174–177. doi: 10.1038/346174a0. [DOI] [PubMed] [Google Scholar]
- Schellekens W, Van Wezel RJa, Petridou N, Ramsey NF, Raemaekers M. Integration of Motion Responses Underlying Directional Motion Anisotropy in Human Early Visual Cortical Areas. PLoS ONE. 2013;8(6):e67468. doi: 10.1371/journal.pone.0067468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmers AJ, Bex PJ. The representation of global spatial structure in amblyopia. Vision Research. 2004;44(5):523–533. doi: 10.1016/j.visres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Ledgeway T, Hess RF. The influences of visibility and anomalous integration processes on the perception of global spatial form versus motion in human amblyopia. Vision Research. 2005;45(4):449–460. doi: 10.1016/j.visres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Research. 2003;43(6):729–738. doi: 10.1016/s0042-6989(02)00684-3. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Ledgeway T, Mansouri B, Hutchinson CV, Hess RF. The extent of the dorsal extra-striate deficit in amblyopia. Vision Research. 2006;46(16):2571–2580. doi: 10.1016/j.visres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Simoncelli EP, Heeger DJ. A model of neuronal responses in visual area MT. Vision Research. 1998;38(5):743–761. doi: 10.1016/s0042-6989(97)00183-1. [DOI] [PubMed] [Google Scholar]
- Spencer J, O’Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. Neuroreport. 2000;11(12):2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- Tam EWY, Widjaja E, Blaser SI, Macgregor DL, Satodia P, Moore AM. Occipital lobe injury and cortical visual outcomes after neonatal hypoglycemia. Pediatrics. 2008;122(3):507–512. doi: 10.1542/peds.2007-2002. [DOI] [PubMed] [Google Scholar]
- Taylor NM, Jakobson LS, Maurer D, Lewis TL. Differential vulnerability of global motion, global form, and biological motion processing in full-term and preterm children. Neuropsychologia. 2009;47(13):2766–2778. doi: 10.1016/j.neuropsychologia.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Trick GL, Silverman SE. Visual sensitivity to motion Age-related changes and deficits in senile dementia of the Alzheimer type. Neurology. 1991;41(9):1437. doi: 10.1212/wnl.41.9.1437. [DOI] [PubMed] [Google Scholar]
- Trick GL, Steinman SB, Amyot M. Motion perception deficits in glaucomatous optic neuropathy. Vision Research. 1995;35(15):2225–2233. doi: 10.1016/0042-6989(94)00311-4. [DOI] [PubMed] [Google Scholar]
- Uka T, DeAngelis GC. Contribution of area MT to stereoscopic depth perception: choice-related response modulations reflect task strategy. Neuron. 2004;42(2):297–310. doi: 10.1016/s0896-6273(04)00186-2. [DOI] [PubMed] [Google Scholar]
- Umeda K, Tanabe S, Fujita I. Representation of stereoscopic depth based on relative disparity in macaque area V4. Journal of Neurophysiology. 2007;98(1):241–252. doi: 10.1152/jn.01336.2006. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell J. Coherence thresholds for discrimination of motion direction in infants. Vision Research. 1994;34(7):877–883. doi: 10.1016/0042-6989(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Wattam-bell J. Visual Motion Processing in One-month-old Infants : Preferential Looking Experiments. Vision Res. 1996;36(11):1671–1677. doi: 10.1016/0042-6989(95)00236-7. [DOI] [PubMed] [Google Scholar]
- Wouldes TA, LaGasse LL, Derauf C, Newman E, Shah R, Smith LM, Lester BM. Co-morbidity of substance use disorder and psychopathology in women who use methamphetamine during pregnancy in the US and New Zealand. Drug and Alcohol Dependence. 2013;127(1–3):101–107. doi: 10.1016/j.drugalcdep.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouldes TA, Lagasse LL, Huestis Ma, Dellagrotta S, Dansereau LM, Lester BM. Prenatal methamphetamine exposure and neurodevelopmental outcomes in children from 1 to 3 years. Neurotoxicology and Teratology. 2014;42:77–84. doi: 10.1016/j.ntt.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalnizoglu D, Haliloglu G, Turanli G, Cila A, Topcu M. Neurologic outcome in patients with MRI pattern of damage typical for neonatal hypoglycemia. Brain and Development. 2007;29(5):285–292. doi: 10.1016/j.braindev.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Yu T-Y, Jacobs RJ, Anstice NS, Paudel N, Harding JE, Thompson B. Global Motion Perception in 2-Year-Old Children: A Method for Psychophysical Assessment and Relationships With Clinical Measures of Visual Function. Investigative Ophthalmology & Visual Science. 2013;54(13):8408–8419. doi: 10.1167/iovs.13-13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanon M, Busan P, Monti F, Pizzolato G, Battaglini PP. Cortical connections between dorsal and ventral visual streams in humans: Evidence by TMS/EEG co-registration. Brain Topography. 2010;22(4):307–317. doi: 10.1007/s10548-009-0103-8. [DOI] [PubMed] [Google Scholar]