Abstract
Background
Bipolar disorder carries a substantive morbidity and mortality burden, particularly related to cardiovascular disease. Abnormalities in peripheral inflammatory markers, which have been commonly reported in case-control studies, potentially link these co-morbidities. However, it is not clear whether inflammatory markers change episodically in response to mood states or are indicative of chronic pro-inflammatory activity, regardless of mood, in bipolar disorder.
Methods
Investigations focused on comparing concentrations of specific inflammatory cytokines associated with immune activation status (primary outcome = tumor necrosis factor alpha (TNF-α)) in 37 participants with bipolar disorder across 3 mood states (mania N=15, depression N=9, normal mood N=13) and 29 controls without a psychiatric disorder (total N=66). Cytokine levels were also compared to T1ρ, a potential neuroimaging marker for inflammation, in select brain regions in a subsample (N=39).
Results
Participants with bipolar disorder and healthy controls did not differ significantly in inflammatory cytokine concentrations. However, compared to cases with normal mood, cases with abnormal mood states (mania and depression) had significantly elevated levels of TNF-α, its soluble receptors (sTNFR1/sTNFR2), other macrophage-derived cytokines (interleukin 1β (IL-1β), IL-6, IL-10, IL-18) in addition to IL-4, interferon-γ, monocyte chemotactic protein-1, fibroblast growth factor β, and vascular endothelial growth factor. Cytokine levels were not correlated with signals from T1ρ imaging in selected structures (amygdalae, hippocampi, hypothalamus, anterior cingulate gyrus, middle frontal gyrus).
Limitations
Participants were not followed prospectively across mood states.
Conclusion
Activation of inflammatory markers was found in abnormal mood states of bipolar disorder. Longitudinal study of individuals with mood disorders is needed to confirm these findings and to elucidate the time course of any such changes.
Keywords: affect, bipolar disorder, inflammation, neuroimaging
INTRODUCTION
Bipolar disorder is characterized by episodic fluctuations in mood, activity, and sleep typically lasting weeks to months and conveys a considerable morbidity and mortality burden. The disorder is also highly hereditable, yet there remains limited mechanistic insight into factors that contribute to its etiology and the pathogenesis of mood episodes. The most consistent neuroimaging finding in bipolar disorder involves a greater prevalence of white matter hyperintensities (WMH) on T2-weighted magnetic resonance images (Marlinge et al., 2014). These WMH are seen 2.5 times as often as would be expected from healthy controls and it has been suggested that these lesions may occur secondary to inflammation (Ahn et al., 2004; Mahon et al., 2010). Abnormalities in peripheral inflammatory markers with bipolar disorder have been commonly reported in case control studies (do Prado et al., 2013; Tsai et al., 2012) although it is not clear from these studies whether these markers change in response to mood state or are trait markers elevated in a subset of individuals with bipolar disorder.
In a meta-analysis of case-control studies of inflammation in bipolar disorder, including 761 cases and 919 healthy controls, those with bipolar disorder were found to have higher concentrations of soluble interleukin-2 receptor (sIL-2R), interleukin-6R receptor (sIL-6R), tumor necrosis factor alpha (TNF-α), soluble tumor necrosis factor receptor type 1 (sTNFR1), and interleukin-4 (IL-4) (Munkholm et al., 2013). Of these cytokines, TNF-α has been studied the most and was associated with an effect size of 1.5 SD in the meta-analysis (Munkholm et al., 2013). It was hypothesized that these findings are suggestive of a macrophage-T-lymphocyte process, involving the three macrophage derived cytokines (TNF-α, sTNFR1, sIL-6R) and T-cell derived sIL-2R. The majority of these studies, however, assumed trait differences in measures by simply comparing cases with bipolar disorder to controls. A few studies have assessed differences in inflammatory cytokines across mood states by either following participants from an acute mania to remission (Liu et al., 2004; Tsai et al., 2001) or comparing individuals with bipolar disorder who were euthymic to those in a mood episode (Barbosa et al., 2014; Brietzke et al., 2009; Cunha et al., 2008; De Berardis et al., 2008; Fontoura et al., 2012; Hope et al., 2011; O'Brien et al., 2006a; Ortiz-Dominguez et al., 2007; Tsai et al., 2012). These studies identified a variety of differences in cytokines across mood states, generally suggestive of greater inflammation with abnormal mood states. The study of Barbosa et al. is an exception, which found no differences between mania and euthymia in levels of the only cytokines assessed, IL-33, and the soluble receptor through which it exerts its effects (Barbosa et al., 2014).
In a sample of individuals with bipolar disorder and controls, we tested the hypothesis that TNF-α and macrophage-derived cytokines are elevated in bipolar disorder compared with controls and in the setting of acute mood episodes. We also sought to determine if any neuroimaging correlates of circulating cytokines could be identified by a novel whole-brain imaging technology, T1ρ, which is thought to be sensitive to pH and could potentially be influenced by focal areas of inflammation (Johnson et al., 2015a, b; Mangia et al., 2013). In doing so, this is the first study of inflammation across distinct states of bipolar disorder to establish a sole primary hypothesis while simultaneously assessing patterns of inflammation across a variety of markers with neuroimaging correlates.
METHODS
Subjects
In total, 66 unique volunteers participated in this study, consisting of a group of 36 participants with a DSM-IV diagnosis of bipolar I disorder and a group of 30 healthy control participants, balanced for age and sex. Community diagnosis was confirmed by a one hour clinical interview with a study psychiatrist (JGF) using a template that specifically screened for tobacco, alcohol and drug use/abuse/dependence as well as common comorbidities such as psychosis, obsessions, compulsions, and panic attacks. Participants with bipolar I disorder were in either a manic episode (Young Mania Rating Scale (YMRS) ≥ 20), a depressive episode (Montgomery-Asberg Depression Rating Scale (MADRS) > 20), or a euthymic state (YMRS ≤ 12 and MADRS < 10) for the assessment. Healthy control participants had no history of psychiatric illness (either DSM-IV Axis I or Axis II). Participants were recruited through advertisements, referrals from the University of Iowa clinics and inpatient units, and a research registry. Participants were excluded if they had any history of brain damage, neurological problems such as seizure disorder, cardiac or respiratory diseases, alcohol or drug dependence, amphetamine use, or any contraindication to magnetic resonance imaging (MRI). Participants provided written informed consent for this study, which was approved by the University of Iowa Institutional Review Board.
Laboratory
Whole blood samples were obtained in the early afternoon and then, immediately following venipuncture, centrifuged at 4G for 12 minutes at which time plasma was extracted, aliquotted, and stored at −80 degrees C. Subsequent laboratory procedures were conducted at the University of Michigan Comprehensive Depression Center and the University of Michigan Comprehensive Cancer Center (ARP) with individual enzyme linked immune-sorbent assays (ELISA) completed for each inflammatory protein of interest. ELISA assay plates were developed in-house using manufacturer provided reagents. Sample absorbancies were detected and compared against a standard curve of known concentrations to determine sample concentration for a given inflammatory protein. All samples were run in duplicate with the final data calculated as the average of individual duplicate pairs. Based on prior evidence and/or preliminary data, the following inflammatory proteins were quantified using standard ELISA techniques (upper limit of detection): tumor necrosis factor-α (TNF-α, 7,500 pg/mL), soluble TNF receptor 1 and 2 (sTNF-R1, sTNF-R2, 6,250 pg/mL), Interleukin (IL)-1β (3,750 pg/mL), IL-1 receptor antagonist (IL-1RA, 3,000 pg/mL), IL-4 (3,750 pg/mL), IL-6 (3,750 pg/mL), IL-10 (7,500 pg/mL), IL-18 (16,875 pg/mL), IL-17, IL-18 binding protein (IL-18-BP, 180,000 pg/mL), interferon(IFN)-γ (3,750 pg/mL), monocyte chemotactic protein-1 (MCP-1, 3,750 pg/mL), fibroblast growth factor β (FGF-β, 5,000 pg/mL), transforming growth factor (TGF-β), brain-derived neurotrophic factor (BDNF), and vascular endothelial growth factor (VEGF, 6,000 pg/mL). Values were truncated at the maximal value for any assay and values below the minimally detected threshold were recorded as zero. CRP was also assessed but results did not meet quality control standards and were subsequently not used in any analyses.
Imaging
Brain images were acquired using a 3 T Siemens Tim Trio MRI system (Magnetom; Siemens Healthcare, Erlangen, Germany) and a vendor-provided 12-channel receiver head coil. Anatomical T1- and T2-weighted images with 1.0 mm isotropic spatial resolution and a coronal whole-brain quantitative T1ρ map with 1.7×1.7×5.0 mm spatial resolution were acquired and processed using previously described methods (Johnson et al., 2015a). Briefly, transformations were calculated to spatially align the anatomical images of the individual participants to a common brain atlas with brain tissue segmentation labels.(Halle et al., 2013) The transformations were then use to transform the T1ρ maps from the coordinate system of the individuals to the common coordinate system. Imaging transformations were made using a combination of BRAINS AutoWorkup (Pierson et al., 2011), Advanced Normalization Tools (Avants et al., 2011), and Analysis of Functional NeuroImages (Cox, 1996). The T1ρ values, averaged over a given brain region, were extracted for use in subsequent analyses.
Data Analysis
Data analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC). All statistical tests were two-tailed with an α=0.05. We primarily hypothesized the macrophage-derived TNF-α (sole a priori primary hypothesis) and secondarily hypothesized its soluble receptors (sTNF-R1, sTNF-R2), and other macrophage-derived cytokines (IL-1β, IL-6, IL-10, IL-18) would be elevated in bipolar disorder, particularly the abnormal mood states of mania and depression. Depression and mania groups were combined prior to any analyses because of the limited number of participants in these mood states and the existing literature outlining inflammatory perturbations in manic and depressive episodes. All other inflammatory proteins and related biomarkers were assessed in exploratory analyses. Univariate analyses compared concentrations of inflammatory proteins between diagnostic groups (bipolar disorder vs. healthy controls) as well as cases with abnormal mood states (i.e., mania and depression) and euthymia using the Wilcoxon Rank Sum test. Due to extreme skews of the laboratory data, variables were rank transformed for use in linear regression in models that adjusted for the potential confounding variables of age (linear effect), sex, smoking (dichotomous based on current use), and body mass index. To avoid over-fitting, medication use was subsequently controlled for in a sensitivity analysis by individually adding indicator variables for lithium, anticonvulsant, antipsychotic, antidepressant, and sedative/hypnotic use to the multivariate models. Regression diagnostics tested whether any statistical assumptions were violated.
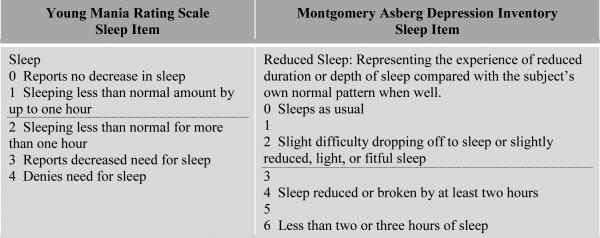
To assess the potential role of changes in sleep with abnormal mood states on inflammatory and related biomarkers, we identified participants with reduced sleep based on a score of ≥2 on the YMRS or ≥3 on the MDRS sleep items as illustrated in Figure 1. Given multicollinearity, the parameter estimates for reduced sleep were compared to parameter estimates for abnormal mood state in analogous models (not modeled simultaneously given collinearity) to determine whether the strength of the association was stronger or weaker.
Figure 1.
Thresholds for Reduced Sleep. The dotted line illustrates the thresholds that were selected to identify individuals with reduced sleep, which involved a score of ≥2 on the YMRS sleep item or a score of ≥3 on the MADRS sleep item.
Correlations were assessed between TNF-α and the average T1ρ signal bilaterally across specific brain regions of interest (i.e., amygdalae, hippocampus, and hypothalamus) as identified by the brain atlas segmentation labels (Halle et al., 2013). The T1ρ within these three specific brain regions was hypothesized a priori to be correlated with specific inflammatory protein concentrations based on the extant literature identifying dysregulated processing of emotionally salient and stressful events as well as hypothesized sensitivity to cytokine-induced depression (Dantzer et al., 2008) and human evidence of neuro-immune interactions (Prossin et al., 2011; Prossin et al., 2015). Exploratory analyses assessed correlations between other inflammatory proteins and these regions as well as two other specific regions of interest component to (or with significant projections to) cortico-limbic circuitry, the anterior cingulate and middle frontal gyrus.
RESULTS
The sociodemographic and clinical characteristics of the study sample are noted in Table 1. Controls were significantly more educated than those with bipolar disorder and less likely to receive psychotropic medications. In linear regression models, those with mania had higher Young Mania Rating Scale (YMRS) scores (p<0.0001), higher Montgomery Asberg Depression Rating Scale (MADRS) scores (p<0.0001), and a marginally higher body mass index (BMI) (p=0.06) while those with depression had higher MADRS scores (p<0.0001) and a higher BMI (p=0.03). Smoking did not significantly differ across the four groups (p=0.18).
Table 1.
Sociodemographic and clinical characteristics of the sample.
| Variable | Total Sample N=66 | Controls N=29 | Bipolar Disorder N=37 | ||
|---|---|---|---|---|---|
| Mania N=15 | Depression N=9 | Euthymia N=13 | |||
| Mean (SD) | |||||
| Age, years | 41 (13) | 43 (13) | 36 (13) | 44 (13) | 41 (14) |
| Education, years‡ | 15 (3) | 17 (2) | 13 (2) | 14 (2) | 14 (2) |
| Body Mass Index, kg/m2 | 27.7 (5.8) | 26.1 (4.2) | 29.4 (7.9) | 31.0 (6.1) | 26.8 (4.7) |
| MADRS‡ | 7.2 (10.9) | 0.4 (0.7) | 9 (7.9) | 30.4 (7.3) | 4.2 (2.2) |
| YMRS‡ | 5.9 (10.1) | 0.1 (0.4) | 22.9 (7.1) | 2.1 (3.3) | 1.8 (2.8) |
| N (%) | |||||
| Male gender | 43 (65%) | 19 (66%) | 11 (73%) | 5 (56%) | 8 (62%) |
| Married/partnered† | 29 (44%) | 18 (62%) | 3 (20%) | 2 (25%) | 6 (46%) |
| Unemployed‡ | 20 (30%) | 2 (7%) | 7 (47%) | 7 (78%) | 4 (31%) |
| Current Smoker | 15 (23%) | 4 (14%) | 6 (40%) | 3 (33%) | 2 (15%) |
| Medication Use | |||||
| Lithium‡ | 13 (20%) | 0 (0%) | 5 (33%) | 3 (33%) | 5 (38%) |
| Anticonvulsant‡ | 16 (24%) | 1 (3%) | 5 (33%) | 3 (33%) | 7 (54%) |
| Antidepressant† | 15 (23%) | 2 (7%) | 2 (13%) | 6 (67%) | 5 (42%) |
| Antipsychotic‡ | 18 (27%) | 0 (0%) | 9 (60%) | 6 (67%) | 3 (23%) |
| Sedative/hypnotic‡ | 16 (24%) | 2 (7%) | 4 (27%) | 4 (44%) | 6 (46%) |
Significant differences between cases with bipolar disorder and controls are indicated by the variable name.
p<0.05
p<0.01.
None of the 17 inflammatory proteins and related biomarkers were significantly elevated in cases with bipolar disorder relative to controls, when including those in euthymic states. As illustrated in Table 2, fifteen were elevated in abnormal mood states when compared to the presence of euthymia (univariate Wilcoxon Rank Sum p-values): TNF-α (p=0.003, primary outcome), sTNF-R1 (p=0.002), sTNF-R2 (p=0.0007), IL-1β (p=0.006), IL-6 (p=0.003), IL-10 (p=0.008), IL-18 (p=0.0006) (all 6 secondary outcomes have false discovery rates <0.01), IL-1RA (p=0.047), IL-17 (p=0.04), IFN-γ (p=0.009), IL-4 (p=0.003), IL-18-BP (p=0.047), MCP-1 (p=0.002), FGFβ (p=0.001), and VEGF (p=0.002). Following rank transformation and adjusting for the confounding effects of age, sex. smoking, and BMI in multivariate linear regression models, the primary model (TNF-α (p=0.007)) remained significant as did the following eleven variables: sTNF-R1 (p=0.005), sTNF-R2 (p=0.0008), IL-1β (p=0.01), IL-6 (p=0.01), IL-10 (p=0.03), IL-18 (p=0.003) (all 6 secondary outcomes have false discovery rates <0.03), IL-4 (p=0.02), IFN-γ (p=0.04), MCP-1 (p=0.003), FGFβ (p=0.006), VEGF (p=0.007). Parameter estimates were not substantively altered and all findings retrained significance after adjusting for lithium, anticonvulsant, antidepressant, antipsychotic, or sedative/hypnotic use.
Table 2.
Levels of inflammatory proteins and related biomarkers by participant group.
| Measure (pg/mL) | Controls | Bipolar Disorder | Bipolar Disorder vs. Controls (p-value) | Mania/Depression vs. Euthymia (p-value) | ||
|---|---|---|---|---|---|---|
| Mania | Depression | Euthymia | ||||
| Primary Outcome | ||||||
| TNF-α | 1532 (317; 2526) | 2610 (699; 3245) | 2807 (859; 3563) | 224 (0; 453) | 0.96 | 0.0029 |
| Secondary Outcomes | ||||||
| sTNF-R1 | 1781 (1186; 1376) | 2237 (1549; 1439) | 2945 (1892; 2159) | 1214 (1142; 313) | 0.20 | 0.0019 |
| sTNF-R2 | 3811 (3463; 1433) | 4631 (4502; 1368) | 5039 (5056; 1193) | 3170 (2992; 882) | 0.22 | 0.0007 |
| IL-1β | 457 (47; 1031) | 913 (45; 1407) | 1283 (73; 1851) | 60 (1; 126) | 0.94 | 0.0058 |
| IL-6 | 586 (104; 1177) | 1005 (113; 1504) | 1319 (225; 1827) | 61 (10; 113) | 0.80 | 0.0033 |
| IL-10 | 1428 (269; 2607) | 2511 (365; 3260) | 2693 (617; 3613) | 219 (100; 310) | 0.78 | 0.0082 |
| IL-18 | 2230 (541; 3735) | 3125 (932; 3683) | 5525 (890; 6232) | 604 (260; 682) | 0.75 | 0.0006 |
| Exploratory Outcomes | ||||||
| IL-4 | 327 (102; 682) | 687 (247; 995) | 814 (304; 1038) | 84 (41; 105) | 0.35 | 0.0029 |
| IL-17 | 195 (0; 476) | 352 (0; 608) | 408 (0; 597) | 16 (0; 52) | 0.83 | 0.040 |
| IFN-γ | 519 (92; 994) | 1203 (561; 1371) | 1274 (775; 1427) | 153 (22; 261) | 0.44 | 0.0087 |
| IL-1RA | 11,259 (0; 34,103) | 10,285 (0; 22,031) | 37,763 (0; 89,482) | 2809 (0; 10,129) | 0.32 | 0.047 |
| IL-18-BP | 30,270 (22,603; 20,970) | 41,098 (27,982; 44,268) | 41,724 (25,529; 23,660) | 24,336 (19,942; 10,176) | 0.36 | 0.047 |
| MCP-1 | 446 (303; 520) | 578 (401; 354) | 850 (392; 1138) | 230 (227; 160) | 0.43 | 0.0017 |
| FGF-β | 489 (250; 771) | 696 (332; 722) | 825 (712; 857) | 118 (48; 151) | 0.94 | 0.0013 |
| VEGF | 442 (325; 449) | 501 (518; 183) | 1099 (542; 1797) | 249 (200; 198) | 0.51 | 0.0021 |
| BDNF | 9346 (9058; 2663) | 9173 (9868; 3091) | 9046 (7968; 3223) | 8017 (8895; 3718) | 0.65 | 0.33 |
| TGF-β | 22,102 (22,020; 9188) | 26,908 (23,173; 12,214) | 23,683 (22,573; 8371) | 20,225 (21,411; 7409) | 0.66 | 0.16 |
The table presents the mean (median; SD) values in pg/mL in each of the assessed measures. Zero indicates non-detectable level. On univariate contrasts (Wilcoxon Rank Sum), no significant differences were found between healthy controls compared to all participants with bipolar disorder regardless of mood state (trait contrast). Significant differences for abnormal mood states (depression and mania) vs. euthymia (state contrast) are italicized.
Reduced sleep as previously defined was highly related to the presence of an abnormal mood state – all with reduced sleep were in an abnormal mood state and 75% of those in an abnormal mood states had reduced sleep (χ2=19.0, df=1, p<0.0001). Given multi-collinearity, we replaced our group of interest indicator variable (depressed/manic vs. euthymic) with the reduced sleep variable in multivariate models adjusting for age, sex, smoking, and BMI. In such models, reduced sleep was significantly associated with sTNF-R2 (p=0.05), IL-1β (p=0.04), IL-6 (p=0.04), IL-18 (p=0.04), MCP-1 (p=0.0003), FGFβ (p=0.03), and VEGF(p=0.045) (7 instead of the previous 12 variables). The magnitude of the parameter estimates from these multivariate models was lower for reduced sleep compared to abnormal mood in 16/17 models as illustrated in Table 3.
Table 3.
Parameter estimates from regression models for abnormal mood state versus reduced sleep.
| Abnormal Mood State | Reduced Sleep | % | |
|---|---|---|---|
| TNF-α | 18.5 | 13.0 | 70% |
| sTNF-R1 | 18.4 | 11.1 | 61% |
| sTNF-R2 | 22.2 | 13.9 | 63% |
| IL-1β | 17.7 | 14.8 | 84% |
| IL-6 | 18.3 | 15.2 | 83% |
| IL-10 | 16.0 | 12.5 | 78% |
| IL-18 | 19.2 | 13.9 | 73% |
| IL-4 | 16.9 | 10.4 | 62 % |
| IL-17 | 10.2 | 8.2 | 81% |
| IFN-β | 15.1 | 13.0 | 86% |
| IL-1RA | 9.5 | 7.1 | 75% |
| IL-18-BP | 14.0 | 9.6 | 68% |
| MCP-1 | 20.9 | 21.1 | 101% |
| FGF-β | 19.4 | 16.2 | 83% |
| VEGF | 19.2 | 14.8 | 77% |
| BDNF | 9.7 | 3.2 | 33% |
| TGF-β | 13.4 | 10.4 | 77% |
All models were adjusted for age, gender, current smoking, and BMI and include either the original indicator variable for an abnormal mood state or an indicator for reduced sleep. In all but one model, the parameter estimates for the reduced sleep indicator were of lower magnitude.
In the sub-sample with available imaging (N=39), 21 controls and 18 with bipolar disorder, 8 of whom were in a euthymic state, 7 with mania, and 3 with depression), there was no correlation between TNF-α levels (rank-transformed) and average T1ρ signal across the amygdalae, hippocampi, and hypothalamus (Pearson r=0.048, p=0.77). TNF-α levels were also not associated with T1ρ signal in the anterior cingulate gyrus (r=−0.080, p=0.63) or middle frontal gyrus (r=−0.14, p=0.40). In exploratory analyses assessing correlations involving the 16 remaining inflammatory proteins and T1ρ signals in five brain regions, there were no significant correlations apart from a single significant correlation between VEGF and T1ρ signal in the middle frontal gyrus (r=−0.34, p=0.03).
CONCLUSION
In this case-control study which sampled individuals with bipolar disorder in three distinct mood states, elevated inflammatory biomarkers were present in those with bipolar disorder in abnormal mood states, but not euthymia. The pattern of inflammatory activation, consistent with the prior literature and as subsequently hypothesized, appeared consistent with macrophage activation with marginal elevations in TNF-α, and significant elevations in its soluble receptors, IL-18, IL-18BP, and monocyte chemotactic protein-1. While the findings were strongest for these inflammatory biomarkers, specificity was limited as other inflammatory biomarkers were also elevated in those with abnormal mood states. Apart from a single, likely spurious, correlation, levels of inflammatory markers did not appear related to a novel imaging methodology, T1ρ, in the selected regions of interest. Perhaps other new imaging methods could be useful, such as positron emission tomography of translocator protein density in activated microglia as has recently been evidenced during major depressive episodes (Setiawan et al., 2015).
Prior studies assessing inflammatory markers across mood states in bipolar disorder have either been exploratory in design or made corrections for multiple comparisons of up to 7 inflammatory markers, as illustrated in Table 4 (Bai et al., 2014; Barbosa et al., 2014; Cunha et al., 2008; De Berardis et al., 2008; Dickerson et al., 2007; Fontoura et al., 2012; Hope et al., 2011; Liu et al., 2004; O'Brien et al., 2006b; Ortiz-Dominguez et al., 2007; Tsai et al., 2012; Tsai et al., 2001). Informed by these studies, our work identified a sole primary hypothesis for testing while assembling a panel of 17 makers to explore for potential patterns. Apart from two studies following inpatients with mania to remission (Liu et al., 2004; Tsai et al., 2001), studies of inflammatory involvement across mood states in bipolar disorder, including the current analysis, have not been prospective, rendering it difficult to discern whether any such changes persist beyond mood episodes and if so for how long and in what pattern. Consistent with our data, previous reports have shown that certain pro-inflammatory cytokines are not elevated during euthymia in research participants with a diagnosis of bipolar disorder (Guloksuz et al., 2010). In a small prospective study, increased levels of IL-6 and IL-8 have been seen with mania (Munkholm et al., 2015). Cross-sectional data from NHANES III, which assessed CRP as compared to time since last major depressive episode in participants with depression, suggest that CRP may remain elevated for 1-6 months beyond the last episode before normalizing (Danner et al., 2003). If these findings are true for the cytokines assayed in the current analysis and in those with bipolar disorder, this could explain some of the inconsistencies in the existing literature. Several papers have reported higher levels of proinflammatory cytokines in those with bipolar I disorder during euthymic states (Barbosa et al., 2013a; do Prado et al., 2013; Modabbernia et al., 2013) and assessment of the duration of euthymia in future work may potentially add clarity to divergent findings. State-related inflammation changes may also explain the observed dose response between the persistence of mood symptoms, cardiovascular mortality and vascular dysfunction (Fiedorowicz, 2014; Fiedorowicz et al., 2012; Fiedorowicz et al., 2014; Fiedorowicz et al., 2009; Sodhi et al., 2012).
Table 4.
Studies of state related changes in inflammatory markers in persons with mood disorders.
| Author, Year | Sample | Number of Inflammatory and Related Markers | State-Related Findings | Type I Error Management |
|---|---|---|---|---|
| Tsai SY et al., 2001 | 31 inpatients with mania (mean YMRS 34) and during subsequent remission (mean YMRS 4) and 31 healthy controls | 2: sIL-2R, sIL-6R | Higher sIL-2R in mania than remission. YMRS scores correlated with sIL-2R levels. | No single primary hypothesis or control for multiple comparisons. |
| Liu H-C et al., 2004 | 52 persons with mania (mean YMRS 35) followed to remission (mean YMRS 5) and matched healthy controls (expanded Su KP et al., 2002 sample). | 7: IL-1RA, sCD4, sCD8, IFN-γ, IL-2, IL-4, IL-10 | Not statistically assessed although values of IL-1RA and IL-4 decreased by at least 25%. | Hypothesized pattern of cell-mediated immunity impairment with mania. No single primary hypothesis or control for multiple comparisons. |
| O'Brien SM et al., 2006 | 21 persons with bipolar disorder (12 with mania and 9 with depression) and 21 controls | 5: IL-6, sIL-6R, IL-8, IL-10, TNF-α | Higher TNF-α with mania than depression. IL-8 greater with depression than mania. | No primary hypothesis or control for multiple comparisons. |
| Dickerson F et al., 2007 | 122 outpatients with bipolar disorder in various mood states and 165 controls | 1: CRP | CRP associated with YMRS score (mania) and YMRS threshold of > 6. | No primary hypothesis or control for multiple comparisons across continuoius and dichotomized psychopathology measures. |
| Ortiz-Dominguez A et al., 2007 | 20 patients with bipolar 1 (10 with mania and mean YMRS of 30 and 10 with depression and mean HDRS of 23) and 33 controls | 5: TNF-α, IL-1β, IL-2, IL-4, IL-6 | Higher IL-4 with mania compared to depression, higher IL-1β and IL-6 with depression compared to mania. | No primary hypothesis or control for multiple comparisons |
| Cunha AB et al., 2008 | 80 patients with bipolar disorder (30 with mania and mean YMRS of 35, 30 with euthymia, and 20 with depression and mean HDRS of 23) and 32 controls | 1: CRP | Higher hsCRP with mania compared to depression or euthymia. | N/A |
| De Berardis D et al. 2008 | 90 patients with bipolar I disorder (30 with mania and mean YMRS of 32, 30 euthymic, 30 with depression and mean HDRS of 25) and 30 controls | 1: CRP | CRP levels higher with mania and depression compared to euthymia. CRP correlated with YMRS in mania and HDRS in depression. | No correction for two hypotheses (CRP and total cholesterol). |
| Brietzke E et al., 2009 | 61 patients with bipolar disorder (23 with mania and mean YMRS 33, 14 euthymic, and 24 with depression and mean HDRS 20) and 25 controls | 6: TNF-α, IL-2, IL-4, IL-6, IL-10, IFN-γ | Elevated IL-2, IL-4, and IL-6 with mania vs. controls. Elevated Il-6 with depression vs. controls. Manic symptoms correlated with IL-6 and IL-2, depressive symptoms correlated with IL-2. | Bonferroni correction for multiple comparisons. |
| Hope S et al., 2011 | 112 patients with bipolar disorder (58 depressed, 26 euthymic, 17 “elevated” with mean YMRS only 7.8), 153 with schizophrenia, and 239 controls | 6: sTNF-R1, IL-1RA, IL-6, hsCRP, OPG, vWf | Lower OPG and IL-6 with depression relative to euthymia. Lower sTNF-R1 and IL-1Ra with depression relative to mania. | No primary hypothesis or control for multiple comparisons. |
| Fontoura PC et al., 2012 | 28 patients with bipolar disorder (9 with mania, 10 with euthymia, and 9 with depression) and 12 controls | 1 inflammatory (several others for nitric oxide signaling and antioxidant activity) | CRP elevated in mania compared with all other groups. Superoxide dismutase activity higher with mania. | Bonferroni correction for multiple comparisons. |
| Tsai SY et al., 2012 | 33 patients with bipolar I and mania (YMRS>26), some followed to partial or full remission, and 33 controls | 3: hsCRP, IL-1RA, sTNF-R1 | hsCRP higher for those in full remission than partial remission, but not mania. IL-1Ra higher with mania (marginally) and partial remission than full remission. | No primary hypothesis or control for multiple comparisons. |
| Barbosa IG et al., 2014 | 46 patients with bipolar disorder (23 with mania and mean YMRS of 26, and 23 with euthymia and mean YMRS of 1) and 23 controls. | 2: IL-33, sST2 | None identified. | No primary hypothesis or control for multiple comparisons. |
| Bai YM et al. 2014 | 130 patients with bipolar disorder (I or II, various states) and 130 normal subjects | 6: sIL-6R, sIL-2R, CRP, sTNF-R1, sP-selectin, MCP-1 | Lower sTNF-R1 with bipolar II vs. bipolar I disorder and depression vs. manic/hypomanic/euthymic. | No primary hypothesis or control for multiple comparisons. |
The following studies were identified from systematic review of PubMed using search terms for major depression or bipolar disorder and inflammation (limit=human) and review of references from selected articles. Studies not designed to assess the impact of specific mood states in a mood disorder (e.g. no comparison to normal mood state in those with mood disorder) are not included. In the case of duplicate publications of the same sample, the most recent sample was selected. Those studies focused on changes in response to a particular treatment (e.g. lithium, sertraline) were also excluded.
Abbreviations: CRP=C-reactive protein, HDRS=Hamilton Depression Rating Scale, hsCRP=highly sensitive CRP, IFN-γ=interferon gamma, IL=interleukin, IL-1RA=interleukin-1 receptor antagonist, MCP-1=monocyte chemotactic protein-1, OPG= osteoprotegerin, sCD4=soluble CD4, sCD8=soluble CD8, sIL-2R=soluble interleukin-2 receptors, sIL-6R=soluble interleukin-6 receptors, sP-selectin=soluble P-selectin receptor, sST2=soluble receptor ST2, sTNF-R1=soluble tumor necrosis factor receptor 1, TNF-α=tumor necrosis factor alpha, vWF=von Willebrand factor, YMRS=Young Mania Rating Scale
The monocyte/macrophage pattern of immune activation observed in the current study is consistent with prior work (Munkholm et al., 2013) and may provide some clue to a biologically plausible potential pathway. Sympathetic nervous system (SNS) related patterns of immune response are designed to steer innate responses away from antiviral and toward natural (non-specific), proinflammatory responses (Irwin and Cole, 2011). During fight-or-flight responses, the SNS release of norepinephrine to primary and secondary lymphoid organs redeploys leukocytes and activates NF-κB-mediated pro-inflammatory responses (Irwin and Cole, 2011). Mood states may hijack these pathways, which are evolutionarily conserved and designed to proactively protect organisms from risk of bacterial infection. This may maladaptively promote a variety of chronic diseases, including vascular disease, which accounts for considerable morbidity and mortality in bipolar disorder (Weiner et al., 2011).
There exist certain limitations in the current study design. Diagnosis was confirmed on a clinical interview, rather than a structured interview, and may have missed some co-occurring psychiatric disorders. The cross-sectional nature of the data allows us to only infer that differences in cytokines are due to mood states. Prospective study is required to confirm that these cytokine levels acutely increase with onset of mood episodes. Based on the existing literature, which suggests overlapping patterns of inflammatory activation with these abnormal mood states (Barbosa et al., 2013b) and with a limited sample size, we grouped those with mania and depression for analyses. Such grouping does not allow us to differentiate any differences that may exist between these states, as have been shown for some, but not most, cytokines in other studies. This assumption would have been expected to, if anything, increase risk of type II error, particular with regard to any non-overlapping portions of cytokine activation. Multivariate models attempted to control for relevant confounding variables although some degree of residual confounding due undetected medical issues is possible. Our panel did not sample all cytokines, perhaps most notably T-cell derived sIL-2R, which has been significantly associated with bipolar disorder (Munkholm et al., 2013). Our plasma assays are also unable to identify the source of cytokines. Cytokines such as TNF-α have been proposed to play a role as “neuromodulators in addition to inflammatory mediators” in the brain (Vitkovic et al., 2000). The fact that T1ρ signal was not found to be correlated with cytokine levels in the selected regions of interest does not rule-out the possibility of brain involvement in neuroimmune interactions involved in mood disorders (Setiawan et al., 2015). T1ρ is sensitive to increased free water and normal T1ρ suggests that measurable shifts or accumulation of free water (or swelling) that may derive from clinically relevant inflammation do not appear to be present.
Despite several case-control studies, little is known about whether pro-inflammatory states in bipolar disorder are a function of disease (trait), acute episodes (state), or both. The data we present identify the presence of “pro-inflammatory states” within those bipolar volunteers identifying signs/symptoms consistent with acute mood episodes. While this data suggests the presence of inflammatory activation within acute mood episodes, the cross-sectional assessment limits the extent that such conclusions can be justified. A longitudinal study design that is capable of testing temporal variation within both healthy control individuals and bipolar individuals could differentiate between standard temporal/daily inflammatory variations (as have been reported) from true mood state variation in plasma inflammatory profiles. Future prospective study is also greatly needed to better discern the pattern of plasma inflammatory changes over time and their relation to mood state in this clinical group. Prospective study may also discern key mediators such as changes in sleep, the autonomic nervous system, or the hypothalamic-pituitary-adrenal axis. Future studies utilizing emerging neuro-imaging modalities that facilitate direct identification of neuroimmune interactions may be useful to understand any contribution of neuro-immune interactions to the episodic presentation of mood symptoms in bipolar disorder.
Highlights.
Elevations in TNF-α, its soluble and other macrophage-derived cytokines appear most prominent in abnormal mood states of bipolar disorder.
Magnetic resonance imaging, using T1ρ, does not identify changes in the brain associated with these cytokines.
Longitudinal study is needed to better understand the relationship between cytokines and mood states in bipolar disorder.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahn KH, Lyoo IK, Lee HK, Song IC, Oh JS, Hwang J, Kwon J, Kim MJ, Kim M, Renshaw PF. White matter hyperintensities in subjects with bipolar disorder. Psychiatry Clin Neurosci. 2004;58:516–521. doi: 10.1111/j.1440-1819.2004.01294.x. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai YM, Su TP, Tsai SJ, Wen-Fei C, Li CT, Pei-Chi T, Mu-Hong C. Comparison of inflammatory cytokine levels among type I/type II and manic/hypomanic/euthymic/depressive states of bipolar disorder. J Affect Disord. 2014;166:187–192. doi: 10.1016/j.jad.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Barbosa IG, Morato IB, de Miranda AS, Bauer ME, Soares JC, Teixeira AL. A preliminary report of increased plasma levels of IL-33 in bipolar disorder: further evidence of pro-inflammatory status. J Affect Disord. 2014;157:41–44. doi: 10.1016/j.jad.2013.12.042. [DOI] [PubMed] [Google Scholar]
- Barbosa IG, Nogueira CR, Rocha NP, Queiroz AL, Vago JP, Tavares LP, Assis F, Fagundes CT, Huguet RB, Bauer ME, Teixeira AL, de Sousa LP. Altered intracellular signaling cascades in peripheral blood mononuclear cells from BD patients. J Psychiatr Res. 2013a;47:1949–1954. doi: 10.1016/j.jpsychires.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Barbosa IG, Rocha NP, Bauer ME, de Miranda AS, Huguet RB, Reis HJ, Zunszain PA, Horowitz MA, Pariante CM, Teixeira AL. Chemokines in bipolar disorder: trait or state? Eur Arch Psychiatry Clin Neurosci. 2013b;263:159–165. doi: 10.1007/s00406-012-0327-6. [DOI] [PubMed] [Google Scholar]
- Brietzke E, Stertz L, Fernandes BS, Kauer-Sant'anna M, Mascarenhas M, Escosteguy Vargas A, Chies JA, Kapczinski F. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord. 2009;116:214–217. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cunha AB, Andreazza AC, Gomes FA, Frey BN, da Silveira LE, Goncalves CA, Kapczinski F. Investigation of serum high-sensitive C-reactive protein levels across all mood states in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2008;258:300–304. doi: 10.1007/s00406-007-0797-0. [DOI] [PubMed] [Google Scholar]
- Danner M, Kasl SV, Abramson JL, Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom Med. 2003;65:347–356. doi: 10.1097/01.psy.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Berardis D, Conti CM, Campanella D, Carano A, Scali M, Valchera A, Serroni N, Pizzorno AM, D'Albenzio A, Fulcheri M, Gambi F, La Rovere R, Cotellessa C, Salerno RM, Ferro FM. Evaluation of C-reactive protein and total serum cholesterol in adult patients with bipolar disorder. Int J Immunopathol Pharmacol. 2008;21:319–324. doi: 10.1177/039463200802100208. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. Elevated serum levels of C-reactive protein are associated with mania symptoms in outpatients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:952–955. doi: 10.1016/j.pnpbp.2007.02.018. [DOI] [PubMed] [Google Scholar]
- do Prado CH, Rizzo LB, Wieck A, Lopes RP, Teixeira AL, Grassi-Oliveira R, Bauer ME. Reduced regulatory T cells are associated with higher levels of Th1/TH17 cytokines and activated MAPK in type 1 bipolar disorder. Psychoneuroendocrinology. 2013;38:667–676. doi: 10.1016/j.psyneuen.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz JG. Depression and cardiovascular disease: an update on how course of illness may influence risk. Curr Psychiatry Rep. 2014;16:492. doi: 10.1007/s11920-014-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedorowicz JG, Coryell WH, Rice JP, Warren LL, Haynes WG. Vasculopathy related to manic/hypomanic symptom burden and first-generation antipsychotics in a sub-sample from the collaborative depression study. Psychother Psychosom. 2012;81:235–243. doi: 10.1159/000334779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedorowicz JG, Jancic D, Potash JB, Butcher B, Coryell WH. Vascular Mortality in Participants of a Bipolar Genomics Study. Psychosomatics. 2014 doi: 10.1016/j.psym.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedorowicz JG, Solomon DA, Endicott J, Leon AC, Li C, Rice JP, Coryell WH. Manic/hypomanic symptom burden and cardiovascular mortality in bipolar disorder. Psychosom Med. 2009;71:598–606. doi: 10.1097/PSY.0b013e3181acee26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontoura PC, Pinto VL, Matsuura C, Resende Ade C, de Bem GF, Ferraz MR, Cheniaux E, Brunini TM, Mendes-Ribeiro AC. Defective nitric oxide-cyclic guanosine monophosphate signaling in patients with bipolar disorder: a potential role for platelet dysfunction. Psychosom Med. 2012;74:873–877. doi: 10.1097/PSY.0b013e3182689460. [DOI] [PubMed] [Google Scholar]
- Guloksuz S, Cetin EA, Cetin T, Deniz G, Oral ET, Nutt DJ. Cytokine levels in euthymic bipolar patients. J Affect Disord. 2010;126:458–462. doi: 10.1016/j.jad.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Halle M, Talos I-F, Jakab M, Makris N, Meier D, Wald L. Multi-modality MRIbased atlas of the brain. SPL; Boston, MA.: 2013. [Google Scholar]
- Hope S, Dieset I, Agartz I, Steen NE, Ueland T, Melle I, Aukrust P, Andreassen OA. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res. 2011;45:1608–1616. doi: 10.1016/j.jpsychires.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Follmer RL, Oguz I, Warren LA, Christensen GE, Fiedorowicz JG, Magnotta VA, Wemmie JA. Brain abnormalities in bipolar disorder detected by quantitative T1rho mapping. Mol Psychiatry. 2015a;20:201–206. doi: 10.1038/mp.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Follmer RL, Oguz I, Warren LA, Christensen GE, Fiedorowicz JG, Magnotta VA, Wemmie JA. Quantiative T1rho mapping links the cerebellum and lithium use in bipolar disorder. Mol Psychiatry. 2015b;20:149. doi: 10.1038/mp.2015.10. [DOI] [PubMed] [Google Scholar]
- Liu HC, Yang YY, Chou YM, Chen KP, Shen WW, Leu SJ. Immunologic variables in acute mania of bipolar disorder. J Neuroimmunol. 2004;150:116–122. doi: 10.1016/j.jneuroim.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev. 2010;34:533–554. doi: 10.1016/j.neubiorev.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Carpenter AF, Tyan AE, Eberly LE, Garwood M, Michaeli S. Magnetization transfer and adiabatic T1rho MRI reveal abnormalities in normal-appearing white matter of subjects with multiple sclerosis. Mult Scler. 2013;20:1066–1073. doi: 10.1177/1352458513515084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinge E, Bellivier F, Houenou J. White matter alterations in bipolar disorder: potential for drug discovery and development. Bipolar Disord. 2014;16:97–112. doi: 10.1111/bdi.12135. [DOI] [PubMed] [Google Scholar]
- Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Brauner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013;47:1119–1133. doi: 10.1016/j.jpsychires.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Weikop P, Kessing LV, Vinberg M. Elevated levels of IL-6 and IL-18 in manic and hypomanic states in rapid cycling bipolar disorder patients. Brain Behav Immun. 2015;43:205–213. doi: 10.1016/j.bbi.2014.09.021. [DOI] [PubMed] [Google Scholar]
- O'Brien SM, Scott LV, Dinan TG. Antidepressant therapy and C-reactive protein levels. Br J Psychiatry. 2006a;188:449–452. doi: 10.1192/bjp.bp.105.011015. [DOI] [PubMed] [Google Scholar]
- O'Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. J Affect Disord. 2006b;90:263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Ortiz-Dominguez A, Hernandez ME, Berlanga C, Gutierrez-Mora D, Moreno J, Heinze G, Pavon L. Immune variations in bipolar disorder: phasic differences. Bipolar Disord. 2007;9:596–602. doi: 10.1111/j.1399-5618.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- Pierson R, Johnson H, Harris G, Keefe H, Paulsen JS, Andreasen NC, Magnotta VA. Fully automated analysis using BRAINS: AutoWorkup. Neuroimage. 2011;54:328–336. doi: 10.1016/j.neuroimage.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossin AR, Koch AE, Campbell PL, McInnis MG, Zalcman SS, Zubieta JK. Association of plasma interleukin-18 levels with emotion regulation and mu-opioid neurotransmitter function in major depression and healthy volunteers. Biol Psychiatry. 2011;69:808–812. doi: 10.1016/j.biopsych.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Prossin AR, Zalcman SS, Heitzeg MM, Koch AE, Campbell PL, Phan KL, Stohler CS, Zubieta JK. Dynamic Interactions Between Plasma IL-1 Family Cytokines and Central Endogenous Opioid Neurotransmitter Function in Humans. Neuropsychopharmacology. 2015;40:554–565. doi: 10.1038/npp.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH. Role of Translocator Protein Density, a Marker of Neuroinflammation, in the Brain During Major Depressive Episodes. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi SK, Linder J, Chenard CA, Miller del D, Haynes WG, Fiedorowicz JG. Evidence for accelerated vascular aging in bipolar disorder. J Psychosom Res. 2012;73:175–179. doi: 10.1016/j.jpsychores.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Chung KH, Wu JY, Kuo CJ, Lee HC, Huang SH. Inflammatory markers and their relationships with leptin and insulin from acute mania to full remission in bipolar disorder. J Affect Disord. 2012;136:110–116. doi: 10.1016/j.jad.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Yang YY, Kuo CJ, Chen CC, Leu SJ. Effects of symptomatic severity on elevation of plasma soluble interleukin-2 receptor in bipolar mania. J Affect Disord. 2001;64:185–193. doi: 10.1016/s0165-0327(00)00252-4. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, Bockaert J, Jacque C. “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry. 2011;23:40–47. [PMC free article] [PubMed] [Google Scholar]