Abstract
Septins are a highly conserved family of proteins in eukaryotes that is recognized as a novel component of the cytoskeleton. Septin 9 (SEPT9) interacts directly with actin filaments and functions as an actin stress fiber cross-linking protein that promotes the maturation of nascent focal adhesions and cell migration. However, the molecular details of how SEPT9 interacts with F-actin remain unknown. Here, we use electron microscopy and image analysis to show that SEPT9 binds to F-actin in a highly polymorphic fashion. We demonstrate that the basic domain (B-domain) of the N-terminal tail of SEPT9 is responsible for actin cross-linking, while the GTP-binding domain (G-domain) does not bundle F-actin. We show that the B-domain of SEPT9 binds to three sites on F-actin, and the two of these sites overlap with the binding regions of myosin and cofilin. SEPT9 inhibits actin-dependent ATPase activity of myosin and competes with the weakly-bound state of myosin for binding to F-actin. At the same time, SEPT9 significantly reduces the extent of F-actin depolymerization by cofilin. Taken together, these data suggest that SEPT9 protects actin filaments from depolymerization by cofilin and myosin, and indicate a mechanism by which SEPT9 could maintain the integrity of growing and contracting actin filaments.
Graphical abstract
Introduction
Septins were first discovered in the budding yeast as genes that are crucial for cell division1. Since the discovery of septins in yeast, proteins with homologous sequences have been found in almost all eukaryotic cells. The number of septin genes per organism is variable and ranges from two isoforms in Caenorhabditis elegans to 13 isoforms in humans2. Based on sequence similarity, human septins are classified into four homology groups named after their founding members: SEPT2, SEPT3, SEPT6 and SEPT7. In this manuscript we focus on SEPT9 which belongs to the SEPT3 subgroup and has a unique N-terminal domain (NTD) consisting of a basic domain (B-domain) and an acidic domain (A-domain) (Fig. 1a)3. All septins have a GTP-binding domain (G-domain), which is evolutionarily and structurally related to the Ras GTPases4. Unlike the monomeric small GTPases, septins form polymers that consist of non-polar oligomeric complexes5,6.
Figure 1.
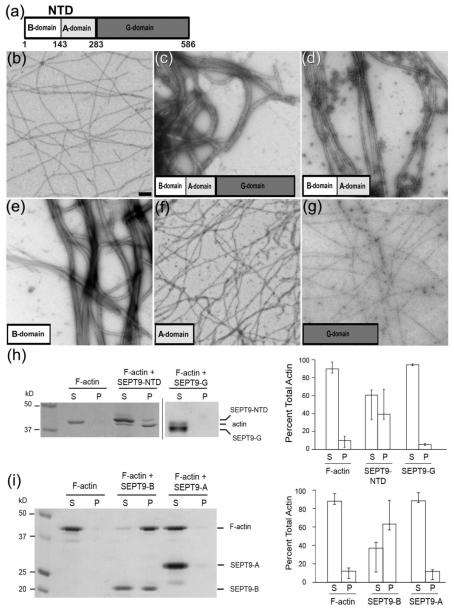
SEPT9 bundles F-actin through the B-domain of its NTD. (a) - Schematic representation of SEPT9 domains. In addition to the G-domain found in all septin paralogs, SEPT9 has a unique N-terminal domain (NTD) consisting of a basic domain (B-domain) and an acidic domain (A-domain). (b) - Pure F-actin, scale bar: 1 nm. (c) - Full length SEPT9 efficiently bundles actin filaments within 2 minutes of incubation. (d) - NTD consisting of the B- and A-domains packs F-actin into tight bundles. (e) – The B-domain bundles F-actin into tight bundles morphologically similar to those shown in (d). (f) - The A-domain does not bind or bundle F-actin. (g) – The G-domain does not bundle F-actin. (h-i) – Low-speed sedimentation of F-actin in the presence of full length SEPT9 and its domains. Coomassie-stained gels with the corresponding bar graphs show that F-actin is recovered in the pellet in the presence of the NTD (h, SEPT9-NTD) and the B-domain (i, SEPT9-B). Error bars correspond to the maximum and minimum values from the independent experiments. In the presence of the G-domain (h, SEPT9-G) and the A-domain (i, SEPT9-A) most of the actin is recovered in the supernatant.
Septins have been recognized as the fourth component of the cytoskeleton because of their filamentous appearance and their interdependence with actin filaments and microtubules. The N-terminal domain of SEPT9 has been shown to bind and bundle microtubules by interacting with the acidic C-terminal tails of β-tubulin3. Septins have been shown to bind actin filaments indirectly via anillin7 or the motor protein myosin II8 Recent data, however, demonstrated that Drosophila septin complexes (Sep1-Sep2-pnut) can directly interact with actin9 and cross-link actin filaments into curved bundles, which are critical for the organization and function of contractile actin rings during Drosophila embryonic cleavage9. Moreover, we recently found that human SEPT9 directly binds and bundles F-actin, and that cross-linking of actin filaments by SEPT9 promotes focal adhesion maturation and epithelial cell motility10.
Despite the new evidence that septins can directly interact with the actin cytoskeleton, the molecular details of these interactions and how septins interact with the surface of the actin filament remain unknown. Here we use electron microscopy, image analysis and co-sedimentation assays to identify how SEPT9 interacts with F-actin. Our data indicate that the B-domain of SEPT9 is responsible for its F-actin bundling activity, while the G-domain of SEPT9 does not bundle actin filaments. We demonstrate that SEPT9 binds to three sites on the surface of F-actin that are commonly bound by other actin binding proteins. Importantly, two sites overlap with the regions of the actin molecule involved in the binding of myosin and cofilin. As predicted by the structural data, SEPT9 inhibits F-actin depolymerization by cofilin, which severs actin filaments, and reduces the actin-dependent ATPase activity of myosin. Our results suggest that SEPT9 could protect growing actin filaments from the combined depolymerizing activity of cofilin and myosin during actin filament assembly and contraction.
RESULTS
The B-domain of SEPT9 promotes F-actin bundling
Using negative stain EM and low-speed pelleting assays, we sought to understand how SEPT9 interacts with F-actin by expressing and purifying each of the three SEPT9 domains; the domain composition of SEPT9 is shown in Fig. 1a. Full-length SEPT9 (isoform 1) as well as the N-terminal domain (NTD) of SEPT9 effectively assemble F-actin into bundles (Fig. 1c and d, respectively). Strikingly, the B-domain alone promotes formation of ordered thick bundles (Fig. 1e), while the A-domain does not possess any bundling activity (Fig. 1f). Similarly to the A-domain, the G-domain of SEPT9 does not possess any bundling activity (Fig. 1g). Our results indicate that the B-domain of SEPT9 is responsible for the SEPT9 F-actin bundling activity. To independently corroborate these EM observations, we examined the ability of individual SEPT9 domains to cross-link actin filaments using a low speed actin sedimentation assay (Fig. 1h and i). In agreement with the EM data, the NTD (Fig. 1h) and B-domain (Fig. 1i) of SEPT9 pelleted F-actin at low speed, while the G-domain (Fig. 1h) and the A-domain (Fig. 1h) did not. Taken together, these data indicate that the B-domain of the NTD of SEPT9 is responsible for the actin-bundling activity of SEPT9.
SEPT9 binds to three sites on the surface of F-actin
To identify the binding site of SEPT9 on the surface of F-actin we performed a 3D-reconstruction of actin filaments decorated with the two SEPT9 actin bundling fragments – the NTD and the B-domain (Fig. 2). In order to obtain single actin filaments suitable for image analysis, the NTD or B-domain were incubated with actin filaments that were pre-bound to a carbon film to reduce bundle formation. The overall 3D-reconstruction of F-actin segments (each segment contained ~14 actin subunits) decorated with the NTD of SEPT9 revealed three sites of interaction of the NTD with the actin filament (Fig. S1). We used a modeling approach and cross-correlation sorting (see Methods) to compute individual 3D-reconstructions for each of the three modes of binding of the NTD to F-actin revealed in the overall reconstruction (Fig. 2a). Since model-based sorting may be biased, we used two independent approaches to confirm that our 3D-reconstructions reflected the intrinsic properties of the images. First, we computed two independent 3D-reconstructions for each of the three structural modes starting the IHRSR algorithm from a featureless solid cylinder, or from a 3D-reconstruction of a different structural mode (Fig. S2). This approach proved that the resultant EM map was independent from the starting model and hence all its features reflected the intrinsic properties of the raw images (Fig. S2). Second, we calculated reference free 2D-averages for each class of NTD-decorated F-actin segments to confirm that all three 2D averages were different from each other and hence, the differences in the 3D reconstructions reflected the differences in the raw images (Fig. S3).
Figure 2.
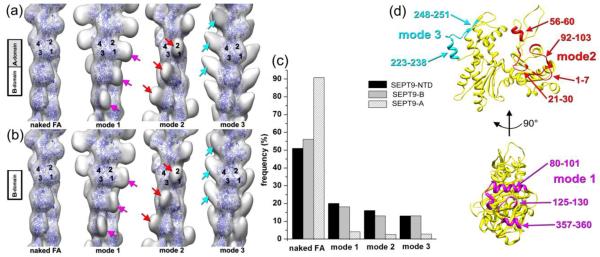
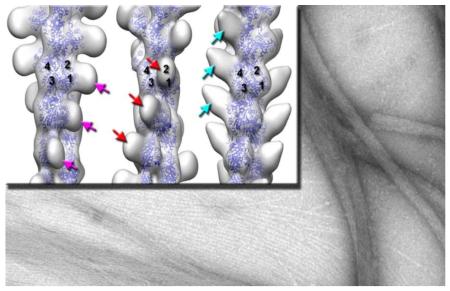
The NTD and B-domain of SEPT9 bind to three sites on the surface of F-actin. (a-b) – Three dimensional reconstructions show the three modes of binding of the NTD (a) and B-domain (b) to F-actin. Three sites on the surface of the actin filament are shown: side of actins SD1/SD2 (magenta arrows), front portion of the same subdomains (red arrows), and the top of SD4 (cyan arrows). Actin molecules are shown as blue ribbons while non-filled empty envelopes represent additional mass on the surface of the actin filament that corresponds to the sites of binding of SEPT9 constructs to F-actin. (c) - Cross-correlation sorting of segments of actin filaments decorated with SEPT9 domains shows that the NTD and B-domain bind to the three sites on F-actin with similar frequency, while the A-domain does not bind to F-actin. (d) – Actin residues that are likely to be involved in the interaction with SEPT9 are marked on the actin monomer (yellow ribbons) for mode 1 in magenta, mode 2 in red, and mode 3 in cyan.
The NTD of SEPT9 binds to three distinct sites on the surface of F-actin. In mode 1 (Fig 2a, magenta arrows), the NTD binds to the side of the SD1/SD2 interface of the actin molecule which includes actin residues 80-101, 125-130, and 357-360 (Fig. 2d, shown in magenta). In mode 2 (Fig. 2a, red arrows), the NTD is bound to the front portion of the SD1/2 interface, which involves residues 1-7, 21-30, 56-60, and 92-103 (Fig. 2d, shown in red). In mode 3 (Fig. 2a, cyan arrows), actin residues 223-238 and 248-251, which belong to the SD4 of F-actin (Fig. 2d, shown in cyan), are located at the interface with the NTD. Using the same set of references, we found that these three sites are also bound by the B-domain (Fig. 2b).
We applied the same sorting procedure to F-actin incubated with the A-domain of SEPT9 and compared the frequency of decorated and naked actin segments between the three SEPT9 domains (Fig. 2c). In contrast to the NTD and the B-domain, more than 90% of actin segments incubated with the A-domain yielded a best match with naked F-actin. The remaining 10% generated an uninterpretable 3D-reconstruction (data not shown). To exclude any possibility that the A-domain binds to F-actin, we used the same set of references to run cross-correlation sorting of pure F-actin segments (Fig. S4). Not surprisingly, the sorting showed a very similar distribution of the pure F-actin segments between the four classes which reflected the margin of error in the sorting procedure.
We did not find a predominant mode of NTD or B-domain binding to F-actin as the frequencies of the three structural modes were quite similar (Fig. 2c). Because we did not find any segments with all three sites occupied simultaneously, individual binding modes of SEPT9 appear to be highly cooperative and mutually exclusive; note that full length SEPT9 exhibited the same three modes of actin binding as the NTD and B-domains (data not shown). It is therefore likely that if a SEPT9 molecule is bound to an actin protomer in either mode 1, 2 or 3, adjacent SEPT9 molecules would also bind actin protomers in the same mode. Such a cooperativity has been reported for many actin binding proteins11,12 and relies on cooperative structural transitions that can propagate along the actin filament13. In addition to the structural cooperative transitions within the actin filament, the spatial arrangement of the SEPT9 domains on the surface of F-actin could result in mutually exclusive modes of SEPT9 binding to F-actin. Binding modes 1 and 2 have an overlapping interface on the surface of F-actin (Fig. 2d, 80-101 helix in mode 1 and 92-103 in mode 2), and modes 2 and 3 would clash spatially if present simultaneously within the same actin filament. Taken together, our results indicate that SEPT9 interacts with F-actin via the B-domain of its NTD in a polymorphic fashion. Importantly, this interaction involves actin-binding sites, which are commonly bound by actin-binding proteins including myosin14 and cofilin15.
SEPT9 inhibits actomyosin activity
The localization of myosin S1-fragment on the surface of F-actin in the absence of ATP (rigor state) has been previously resolved14. While the position of myosin on the surface of the actin filament in the presence of ATP (weak binding mode) has not been visualized, modeling studies based on the X-ray diffraction from permeabilized muscles suggest that the N-terminus of F-actin is at the interface with the weakly bound myosin head16. Our data suggest that the position of myosin in a rigor state would clash with the position of the NTD of SEPT9 in two of its three binding modes (Fig. 3a-b, red arrows); the N-terminus of F-actin (denoted with red asterisk in Fig. 3a-c) is also at the interface of NTD with F-actin. Thus, binding of SEPT9 to F-actin in modes 1 or 2 could interfere with the actin binding of myosin in both rigor and weak states (Fig. 3a-b), while binding of SEPT9 to F-actin in mode 3 may not interfere with the acto-myosin interaction (Fig. 3c).
Figure 3.
SEPT9 inhibits the activity and interaction of myosin with actin. (a-c) – An atomic model of the actomyosin complex in rigor state14 was docked onto the 3D-reconstructions of the three modes of binding of the NTD to F-actin (Fig 2a) using the best fit of the actin molecules. Actin molecules are shown as blue ribbons and myosin head is depicted as solid surface. The clashes between the myosin head and the NTD density (red arrows) in modes 1 and 2 is marked with red arrows (a and b). There are no such clashes in mode 3 (c). The position of the N-terminus of F-actin which resides at the interface between the NTD and F-actin is denoted with red asterisks. (d) – Plot shows the ATPase activity of the S1 fragment of myosin V in the absence and presence of full length SEPT9 and the A- and B-domains of SEPT9. As predicted by the structural data, full length SEPT9 and the B-domain inhibit the ATPase activity of myosin, while the A-domain does not have a significant effect. (e) - Coomassie-stained gels show the pellet (P; top) and supernatant (S; bottom) fractions from a competitive high-speed actin pelleting assay in the presence of ATP (1.25 μM). F-actin (2 μM) was incubated with increasing concentrations of recombinant full length SEPT9 and the myosin S1 fragment was added at a constant concentration (0.75 μM). (f) - Bar graphs show the fraction of total S1 fragment in the pellet and supernatant. Error bars correspond to the minimum and maximum values obtained from three independent experiments. (g) - Coomassie-stained gels show the pellet (P; top) and supernatant (S; bottom) fractions from a competitive high-speed actin pelleting assay in the absence of ATP. F-actin (2 μM) was incubated with increasing concentrations of recombinant full length SEPT9 and the myosin S1 fragment was added at a constant concentration (1 μM). (f) - Bar graphs show the fraction of total S1 fragment in the pellet and supernatant. Error bars correspond to the minimum and maximum values obtained from three independent experiments.
To test these structural predictions, we first used an actin-activated steady-state ATPase assay. In this assay, myosin ATP hydrolysis is coupled with its interaction with F-actin. Thus, any protein that interferes with the actomyosin interaction will reduce the ATPase activity of the myosin head. To evaluate the effect of SEPT9 on the actomyosin cross-bridge cycle, we carried out the actin activated steady-state ATPase assay using the NADH coupled method (Fig 3d). The S1 fragment of myosin V and F-actin were mixed with increasing concentrations of full length SEPT9, or its individual B- and A-domains domains (see Methods for details). The full length SEPT9 exhibited the largest inhibition with an apparent Ki of 0.46 μM followed by the B-domain with a Ki of 2.44 μM. The A-domain had almost no effect on myosin ATPase activity with an apparent Ki of 185 μM.
Since bundling would reduce the number of F-actin sites that are available for myosin binding, we tested whether the reduction in myosin ATPase activity correlated with an increase in F-actin bundling. Low-speed actin sedimentation assays showed that both the full length SEPT9 and SEPT9 B-domain did not significantly affect F-actin bundling over concentration ranges that caused a drastic reduction in myosin activity (Fig. S5). Hence, the bundling activity of SEPT9 by itself could not account for full inhibition of the acto-myosin ATPase activity. Taken together with our structural data that predict a mutually exclusive binding of myosin and SEPT9 in modes 1 and 2 (Fig. 3a,b), our results indicate that SEPT9 inhibits actin-dependent myosin ATPase activity by competing with myosin for binding to the same sites of F-actin.
ATP hydrolysis and subsequent dissociation of the nucleotide from the myosin head changes acto-myosin interaction from the initial weakly-bound state to a rigor-bound state. This transition in the acto-myosin interaction is an essential part of myosin’s ATPase cross-bridge cycle. To test how SEPT9 inhibits acto-myosin activity, we performed competitive high-speed actin pelleting assays of myosin V S1-fragment with the full length SEPT9 in the presence or absence of ATP, so we can distinguish between the weakly-bound (ATP) and rigor-bound (no ATP) states of myosin. In the presence of ATP, full length SEPT9 efficiently competed with the myosin S1-fragment for binding to F-actin (Fig. 3e). Interestingly, in the absence of ATP (only ADP was present in solution) SEPT9 co-sedimented with myosin S1-fragment (Fig. 3f). The affinity of the ADP-bound myosin V to F-actin (~7.6 nM)17 is ~1000 fold higher than its affinity to F-actin in the presence of ATP (~13 μM)18. Taking into account that the affinity of septins to F-actin is in the micromolar range9 (Fig. S6), SEPT9 molecules that are bound to F-actin in modes 1 and 2 are likely to be displaced by ADP-bound myosin, which has a high affinity for F-actin. Cosedimentation of SEPT9 with rigor-bound myosin V suggests that SEPT9 is bound to F-actin in mode 3 (Fig. 3c), which does not clash with the position of rigor-bound myosin. In the presence of ATP, however, SEPT9 is likely to compete with myosin for the binding modes 1 and 2, which account for ~70% of the binding of SEPT9 to F-actin (Fig. 2c), and thereby, SEPT9 can inhibit the interaction of myosin with F-actin. To summarize, our data indicate that SEPT9 inhibits the weak acto-myosin interaction through its B-domain, while it can bind to F-actin in the presence of rigor-bound myosin.
SEPT9 reduces cofilin-driven F-actin depolymerization
Superimposition of the 3D reconstructions of SEPT9-decorated F-actin in mode 1 (Fig. 4a, top) and mode 2 (Fig. 4a, bottom) revealed significant clashes between the SEPT9 density (Fig. 4a, black arrows) and cofilin (Fig. 4a, shown as solid red surface). Similar to our results with myosin, we hypothesized that SEPT9 may inhibit cofilin-actin binding. Using electron microscopy and high-speed pelleting assays, we sought to test whether SEPT9 affects cofilin-driven F-actin depolymerization.
Figure 4.
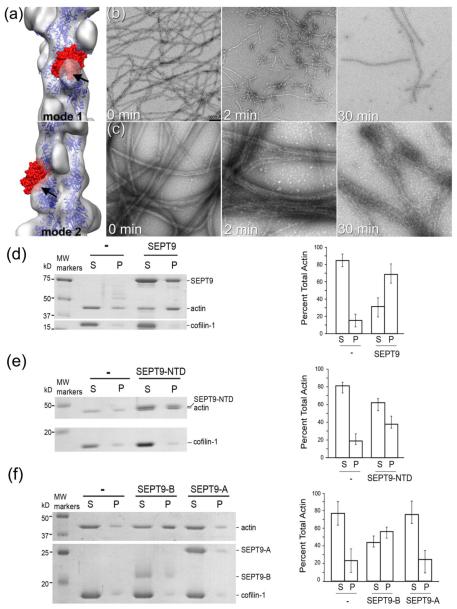
SEPT9 reduces the extent of cofilin-driven F-actin depolymerization. (a) – Atomic model of actin-cofilin complex15 docked into the 3D-reconstructions of mode 1 and 2 (transparent grey surfaces) shows a clash between the cofilin molecule (red surface) and SEPT9 density (marked with black arrows). (b) - Long F-actin filaments formed after 2 h of polymerization (b; 0 min) are disrupted into short segments within 2 min of incubation with cofilin-1 (b; 2 min); after a 30 min incubation with cofilin (b; 30 min), only few filaments which are heavily decorated with cofilin remain. (c) F-actin forms tight bundles in the presence of SEPT9 (c; 0 min). After 2 min of incubation with cofilin-1, SEPT9-induced F-actin bundles still remain intact (c; 2 min) and even after 30 min of incubation F-actin bundles still persist (c; 30 min). (d-f) High-speed sedimentation assay of F-actin in the presence of SEPT9 domains and cofilin-1. Actual gels with the corresponding bar graphs (d-f) show that in the presence of cofilin-1 most of the actin is found in the supernatant (d), while in the presence of full size SEPT9 (d), NTD (e) or the B-domain (f) more than half of the actin is recovered in the pellet. In the presence of the A-domain most of the actin remains in the supernatant (f).
EM imaging of actin filaments showed that within 2 min of incubation, cofilin disrupts the meshwork of naked actin filaments, which are disintegrated into short twisted filaments (Fig. 4b, 2 min). After 30 min, only a few cofilin-decorated actin filaments remain (Fig. 4b, 30 min). In the presence of full length SEPT9, F-actin bundles (Fig. 4c, 0 min) remain intact after 2 min of incubation with equimolar concentration of cofilin-1 (Fig. 4c, 2 min). Even after 30 min of incubation with cofilin-1, F-actin bundles are still present (Fig. 4c, 30 min). To further test whether SEPT9 protects against actin depolymerization by cofilin, we performed high-speed sedimentation assays in the presence of full length SEPT9 or its domains and cofilin-1 (Fig. 4,d-f). In our experiments we used equimolar concentrations of SEPT9 and cofilin, because the affinity of human ADF/cofilin to ADP-F-actin19 is similar to septins9 (Fig. S6). After 30 min incubation of F-actin with cofilin-1, ~90% of actin is recovered in the supernatant (Fig. 4d). Pre-incubation of F-actin with SEPT9 reduced the amount of actin in the supernatant to ~30% (Fig. 4d,). The NTD (Fig. 4e) and the B-domain (Fig. 4f) of SEPT9 also reduced the amount of depolymerized actin in the supernatant to ~50-60% of total actin, while the A-domain had no effect (Fig. 4f). Thus, similar to the inhibition of myosin binding to F-actin, SEPT9 appears to inhibit cofilin binding, and thereby, protect actin filaments from cofilin-mediated depolymerization.
DISCUSSION
Drosophila septins have been recently reported to directly bind and bundle F-actin9. Mammalian septins (SEPT2/6/7 oligomers) have been proposed to interact with F-actin indirectly via anillin7 and myosin II8, but it was recently shown that the mammalian SEPT9 can bind and bundle actin filaments10. Our data show that the actin bundling activity of SEPT9 resides in the NTD – a unique domain that is not present in other septin paralogs. SEPT9 interacts with F-actin in a highly polymorphic fashion (Fig. 2a-b). Such multiplicity in binding modes in not unique to SEPT9 and has been reported for other actin binding proteins (ABPs) such as Arg/Abl kinase12, drebrin20, myosin binding protein C21, utrophin22, and a giant muscle protein nebulin23. Since, the N-terminal domain of SEPT9 does not possess any detectable amino acid identity with these ABPs, it suggests that multiplicity of actin-binding modes is a common feature of ABPs independently of their actin-binding sequences and domains.
Recently we showed that F-actin can exist in multiple structural states and that the structural transitions in the mobile regions of the actin protomers are coupled13. SEPT9 binds to the two mobile regions of actin molecule including residues 1-7 of the N-terminus and residues 56-60 of the SD2 (Fig. 2d). It is plausible that in the cell the structural mode of SEPT9 binding to F-actin is determined by the structural state of the actin filament and the presence of other actin-bound ABPs. Our results suggest that SEPT9 may switch from mode 1 and 2, where it interferes with the actin-myosin interaction (Fig. 3a-b), to mode 3 where both rigor-bound myosin and SEPT9 can simultaneously interact with F-actin. By switching between these structural modes of binding, SEPT9 may alter acto-myosin interactions in migrating cells, where tensile forces are tightly controlled and are linked to focal adhesion turnover 24,25 and cell-ECM adhesion 25,26. Thus, SEPT9 may influence actomyosin activity at stress fibers and focal adhesions.
SEPT9-dependent actin bundling activity is required for the maintenance of a functional stress fiber network in the leading edge of migratory epithelial cells10. Interestingly, our results indicate that SEPT9 protects actin filaments from depolymerization by cofilin. Actin cross-linking proteins have been shown to protect actin filaments from depolymerization in vitro27-29. In living cells, varying levels of expression of actin bundling proteins have been shown to alter the rates of actin filaments turnover30,31 and myosin has been shown to regulate the turnover of actin bundles in neuronal growth cones32,33.
In addition, recent studies show that actin depolymerization by ADF/cofilin is differentially regulated by actin motors and actin-cross-linking proteins34. While myosin II enhances the disintegration of F-actin bundles, cross-linking proteins inhibit the disassembly of actin bundles by cofilin34. Notably, cross-linking proteins such as fascin may affect the severing activity of cofilin by reducing the flexibility of bundled F-actin, and thereby, limit the torsional twist that cofilin triggers on F-actin35. Because the efficiency of cofilin severing is the highest between decorated and non-decorated segments of F-actin, the severing activity of cofilin will be the highest on more rigid bundled actin filaments, which do not allow for a uniform, cooperative binding that is enabled by a torsional twist35. Thus, depending on the ABP composition of F-actin bundles and their flexibility, cross-linking proteins such as fascin may either increase or decrease the activity of cofilin 35,36.
Our results indicate that SEPT9 inhibits both myosin and cofilin interaction with F-actin, and hence may work as a protective factor for growing actin bundles. Given that both myosin37 and cofilin38 are involved in the regulation of actin organization and contractility in a diversity of cellular processes and structures, our results suggest that the ubiquitously expressed SEPT939 may be a key player in the regulation of F-actin organization and function.
METHODS
Protein expression and purification
G-Mg2+-actin was prepared as described41. SEPT9 constructs were prepared as follows. E. Coli BL21 (DE3) cells were transformed with SEPT9_i1, SEPT9-NTD, SEPT9-G, SEPT9-A, or SEPT9-B in kanamycin-resistant pET-28a42. Plasmids expressing His-tagged SEPT9-N (aa 1–283 of SEPT9_i1), SEPT9-B (aa 1–142 of SEPT9_i1), SEPT9-A (aa 143–283 of SEPT9_i1), SEPT9-G (aa 284–586 of SEPT9_i1) were made with the QuikChange II Site-Directed Mutagenesis kit (Agilent Technologies) using the pET-SEPT9_v1 and pET-SEPT9_v3 plasmids. Bacterial cultures of OD600 were induced with 0.5 mM IPTG for 16h at 18°C, collected by centrifugation and resuspended in lysis buffer containing 50 mM Tris, pH 8.0, 1% Triton X-100, 150 mM NaCl, 10% glycerol, and 10 mM imidazole. Bacteria were lysed using a French pressure cell at 1,280 psi, and lysates were cleared using centrifugation (14,000 g) for 30 min at 4°C. Columns containing Ni-NTA beads were equilibrated with lysis buffer prior to passing supernatants through by gravity flow. The beads were washed with buffer containing 50mM Tris, pH 8.0, 300 mM NaCl, 10% glycerol, and 10 mM imidazole, and proteins were eluted with buffer containing 50mM Tris, pH 8.0, 300 mM NaCl, 10% glycerol, and 250 mM imidazole.
MyosinV-S1 heavy chain, 907 amino acid residues including 6IQ domains was expressed in a baculovirus/Sf9 cell system. The proteins were purified using FLAG-affinity chromatography and then concentrated and fractionated on a MonoQ ion-exchange column with a linear gradient of 0.1–0.5 M NaCl in 10 mM MOPS, 0.1 mM EGTA, 3 mM NaN3, and 1 mM DTT, pH 7.2, using HPLC. The concentration of myosinV-S1 was determined from the A280 after correction for light scattering (A280–1.5A320). The extinction coefficient was calculated to be 1.1 × 105 M-1 cm-1 from the number of tyrosine and tryptophan residues and their molar extinction coefficients: ε = (n = 10)trp × 5690 + (n = 42)tyr × 1280. All preparations were analyzed by SDS protein gel electrophoresis and by active site titration with deac-aminoATP. Cofilin-1 was a generous gift from Dr. Emil Reisler (UCLA).
Electron microscopy of negatively stained SEPT9 domains
G-actin was polymerized for 1–2 hours in F-buffer (10mM HEPES, pH 7.4, 100mM KCl, 1mM MgCl2, 0.5mM ATP). For EM samples shown in Fig. 1c-g, 2 μM F-actin was incubated for 2 min in tube with 34 μM NTD, B-domain, A-domain, or G-domain. Samples were blotted and negatively stained with 2% (wt/vol) uranyl acetate. To obtain single actin filaments decorated with SEPT9 domains was 2 μM F-actin was incubated for 1 min on carbon coated glow discharged grids in a humidified chamber, gently blotted and incubated again with 34 μM NTD, B-domain, A-domain, or G-domain. Previous work has shown that decoration of actin filaments with cofilin/ADF results in the same binding modes independently of whether it is performed on carbon grid or in test tube43,44. Samples were negatively stained with 2% (wt/vol) uranyl acetate. A JEM-2100F (JEOL) Field Emission Transmission Electron Microscope, equipped with 11-megapixel Gatan SC1000 ORIUS CCD camera, was used at an accelerating voltage of 200 kV, and a nominal magnification of 60,000x to record micrographs at a raster of 1.58 Å/pixel.
Electron microscopy of SEPT9 in presence of cofilin-1 and SEPT9
F-actin (2 μM) was mixed with 10 μM of cofilin-1, or preincubated with 10 μM of SEPT9 before addition of 10 μM of cofilin-1. Samples were left at room temperature and analyzed by TEM at 2 and 30 min time points.
Image analysis
The SPIDER software package45 was used for most image processing, while the EMAN package46 was used to extract filament images from micrographs. Images were corrected for the contrast transfer function (CTF) using theoretical CTF obtained with the CTFIND software47 and decimated to 3.16 Å/pixel scale. From these CTF-corrected images segments (each 120 pixels long) of F-actin decorated with the NTD (n=8,193), B-domain (n=2,708), A-domain (n=5,435) constructs were extracted. Segments of filament decorated with the NTD were reconstructed using IHRSR approach48. The resultant reconstruction showed three additional densities bound to F-actin - one at the front interface between SD1 and SD2 of two adjacent actin protomers, the other one at the side of SD1, and one on the top of SD4 (Fig. S1). Three model volumes were created by using pseudo-atomic model of F-actin49 having model density attached to the first site, second site, or third site. These volumes, along with a naked F-actin volume, were projected onto 120 × 120-pixel images with an azimuthal rotational increment of 4°, and the resultant 360 reference projections (4 × 90) were cross-correlated with the 8,193 NTD-decorated actin filament segments. Each of the four classes was reconstructed starting from the two independent starting models (Fig. S2). The naked class (n=4,222) yielded a symmetry of 166.6°/27.3 Å, the mode1 class (n=1,590) yielded a symmetry of 166.7°/27.2 Å, the mode 2 class (n=1,305) converged to 166.1°/27.2 Å, while a set of images classified as mode 3 (n=1,079) reached a stable solution having 167.0°/27.2 Å symmetry values. The same sorting procedure was applied to actin filaments decorated with the B-domain. The naked segments extracted from the filaments decorated with the B-domain (n=1,571) converged to a 166.6°/27.2 Å solution, the mode1 class from that set (n=473) yielded 166.3°/27.1 Å, the mode 2 class (n=332) converged to a symmetry of 166.1°/27.3 Å, while the mode 3 class (n=332) yielded a symmetry of 166.5°/27.2 Å. Segments of filaments incubated with the A-domain were mostly naked actin (n=4,935) with only 218 segments assigned to mode 1 class, 135 segments assigned to the mode 2 class, and 147 segments assigned to the mode 3 class. Neither of these classes yielded reasonable 3D reconstruction (data not shown). The naked class converged to the stable solution of 166.5°/27.1 Å. Fourier Shell Correlation approach was used to determine the resolution of the 3D-reconstructions. Each image set was split into two non-overlapping subsets, and these subsets were reconstructed independently. Resultant volumes were used for resolution determination. Using 0.5 criterion all maps yielded 27-28 Å resolution (Fig. S7). All the 3D-reconstructions shown in the paper were filtered to the measured resolution. The Chimera software50 was used to dock a protomer from the atomic model of F-actin49 into the reconstruction manually. Figures 2, 3 and 4 were produced using the Chimera software50.
Actin activated steady-state ATPase experiments
Actin activated steady-state experiments were done by the NADH coupled assay at 25 °C. 20 nM Myosin V-S16IQ was mixed with 10 μM actin and increasing concentrations of SEPT9 A- domain , B- domain or full length SEPT9 in a buffer containing 10 mM MOPS (pH 7.0), 2 mM MgCl2, 25 mM KCl, 0.15 mM EGTA, 2 mM ATP, 40 units/ml lactate dehydrogenase, 200 units/ml pyruvate kinase, 1 mM phosphoenolpyruvate, and 200 μM NADH51. Changes in NADH absorption at 340 nm (ε340 = 6220 M-1cm-1) were followed in a Beckman DU640 spectrophotometer. The ATPase activity of blanks containing actin but no myosin was subtracted from the actomyosin data. The data was fit to the equation F (x) = Vmax/1+x/Ki .
Low-speed actin pelleting assays
G-actin in G buffer (5 mM Tris-HCl and 0.2 mM CaCl2) was polymerized in buffer containing 20 mM Hepes, pH 7.4, 100 mM KCl, and 1 mM MgCl2 supplemented with 0.2 mM ATP and 4 mM DTT for 1 h at 25°C. In Figure 1, 8 μM of recombinant SEPT9-N, SEPT9-G, SEPT9-A, or SEPT9-B were incubated with 2 μM F-actin for 1 h at 25°C. In Supplemental Figure 6, increasing concentrations of full-length SEPT9 or SEPT9-B were incubated with 2 μM F-actin for 1 h at 25°C. Reactions were subsequently centrifuged on top of a glycerol cushion (20 mM Hepes, 100 mM NaCl, 1 mM MgCl2, and 10% glycerol) at 8,000 g for 20 min at 25°C. Supernatant and pellet fractions were collected in SDS sample buffer, and equal volumes of each fraction were resolved with a 10% or 6-12% gradient SDS-PAGE gel and stained with Coomassie brilliant blue. Protein band densities from scanned gels were quantified using the Odyssey infrared scanning system (LI-COR Biosciences) and analyzed in excel. All experiments were repeated 3 times.
High-speed actin pelleting assays
Competition between SEPT9 and the myosin V-S1 heavy chain for F-actin binding were performed in buffer containing 10mM MOPS, pH 7.0, 100 mM KCl, 2 mM MgCl2, 0.5 mM EGTA, and 2 mM DTT with 1.25 mM ATP or without ATP. For experiments without ATP, the F-actin stock was dialyzed in F-buffer without ATP. F-actin (2 μM) was pre-incubated with increasing concentrations of SEPT9 for 30 minutes at 25°C. Reactions were incubated with myosin V-S1 heavy chain (0.75 – 1 μM) for an additional 30 minutes at 25°C, placed on a glycerol cushion (10mM MOPS, pH 7.0, 100 mM KCl, 2 mM MgCl2, 0.5 mM EGTA, 20% glycerol) and centrifuged at 200,000 g for 30 minutes at 21°C. Equal volumes of supernatant and pellet fractions were collected in SDS sample buffer, and equal volumes of each fraction were resolved using a 10% SDS-PAGE gel and stained with Coomassie brilliant blue. Protein band densities were quantified using the Odyssey infrared scanning system and analyzed in excel. Each experiment was repeated 3 times.
Co-sedimentation assays (Fig. 4) that tested for cofilin-mediated depolymerization were performed by pre-incubation of aged F-actin with recombinant SEPT9_i1 (5 μM) or 5 μM SEPT9 fragments (NTD, B-domain or A-domain) in buffer containing 10 mM Hepes, pH 7.6, 100 mM KCl, 1 mM MgCl2, and 2 mM DTT for 30 min at 25°C. Reactions were incubated with 5 μM recombinant human cofilin-1 for an additional 30 min at 25°C, placed on a glycerol cushion (20 mM HEPES, pH 7.6, 100 mM NaCl, 1 mM MgCl2, and 10% glycerol) and centrifuged at 200,000 g for 30 min at 21°C in an ultracentrifuge (Optima TL100, Beckman Coulter). Supernatant and pellet fractions were collected in SDS sample buffer, and equal volumes of each fraction were resolved using a 8-15% gradient SDS-PAGE gel and stained with Coomassie brilliant blue. Protein band densities from scanned gels were quantified using the Odyssey infrared scanning system (LI-COR Biosciences) and analyzed in excel. Each experiment was repeated 3 times.
High-speed pelleting assays between SEPT9 and F-actin (Fig. S5) were performed by incubating full-length SEPT9 (1 μM) with increasing concentrations of F-actin for 30 minutes at 25°C in buffer containing 20 mM Hepes, pH 7.6, 100 mM KCl, and 1 mM MgCl2 supplemented with 0.2 mM ATP and 4 mM DTT. Reactions were placed on a glycerol cushion (20 mM HEPES, pH 7.6, 100 mM NaCl, 1 mM MgCl2, and 20% glycerol) and centrifuged at 200,000 g for 30 min at 21°C. Supernatant and pellet fractions were collected in SDS sample buffer, and equal volumes were resolved using a 10% SDS-PAGE gel. Gels were stained with Coomassie brilliant blue, and protein band densities from scanned gels were quantified using the Odyssey infrared scanning system. The fraction of SEPT9 protein in each pellet fraction was plotted against the concentration of actin in GraphPad and fitted with a one-site specific binding curve to determine the dissociation constant.
Supplementary Material
Highlights.
Septins bind F-actin, but the molecular details of this interaction are unknown.
Septin 9 (SEPT9) binds and bundles F-actin through its N-terminal basic domain.
SEPT9 interacts with three sites on the surface of F-actin in a polymorphic fashion
SEPT9 inhibits actomyosin activity and actin depolymerization by cofilin
SEPT9 may protect F-actin from myosin- and cofilin-induced depolymerization
Acknowledgments
We thank Dr. Emil Reisler (UCLA) for his generous gift of cofilin-1. This work was supported by startup funds from EVMS (to V.E.G.), and NIH grants CA176910 (to L.D.) and GM097664 (to E.T.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hartwell LH. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp. Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 2.Russell SE, Hall PA. Septin genomics: a road less travelled. Biol. Chem. 2011;392:763–767. doi: 10.1515/BC.2011.079. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 3.Bai X, Bowen JR, Knox TK, Zhou K, Pendziwiat M, Kuhlenbaumer G, Sindelar CV, Spiliotis ET. Novel septin 9 repeat motifs altered in neuralgic amyotrophy bind and bundle microtubules. J. Cell Biol. 2013;203:895–905. doi: 10.1083/jcb.201308068. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leipe DD, Wolf YI, Koonin EV, Aravind L. Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 2002;317:41–72. doi: 10.1006/jmbi.2001.5378. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 5.Bertin A, McMurray MA, Grob P, Park SS, Garcia G, III, Patanwala I, Ng HL, Alber T, Thorner J, Nogales E. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8274–8279. doi: 10.1073/pnas.0803330105. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311–315. doi: 10.1038/nature06052. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of Mammalian septins. Dev. Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 8.Joo E, Surka MC, Trimble WS. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev. Cell. 2007;13:677–690. doi: 10.1016/j.devcel.2007.09.001. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 9.Mavrakis M, zou-Gros Y, Tsai FC, Alvarado J, Bertin A, Iv F, Kress A, Brasselet S, Koenderink GH, Lecuit T. Septins promote F-actin ring formation by crosslinking actin filaments into curved bundles. Nat. Cell Biol. 2014;16:322–334. doi: 10.1038/ncb2921. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 10.Dolat L, Hunyara JL, Bowen JR, Karasmanis EP, Elgawly M, Galkin VE, Spiliotis ET. Septins promote stress fiber-mediated maturation of focal adhesions and renal epithelial motility. J. Cell Biol. 2014;207:225–235. doi: 10.1083/jcb.201405050. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlova A, Egelman EH. Cooperative rigor binding of myosin to actin is a function of F-Actin structure. J. Mol. Biol. 1997;265:469–474. doi: 10.1006/jmbi.1996.0761. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 12.Galkin VE, Orlova A, Koleske AJ, Egelman EH. The Arg non-receptor tyrosine kinase modifies F-actin structure. J. Mol. Biol. 2005;346:565–575. doi: 10.1016/j.jmb.2004.11.078. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 13.Galkin VE, Orlova A, Schroder GF, Egelman EH. Structural polymorphism in F-actin. Nat. Struct. Mol. Biol. 2010;17:1318–1323. doi: 10.1038/nsmb.1930. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrmann E, Muller M, Penczek PA, Mannherz HG, Manstein DJ, Raunser S. Structure of the rigor actin-tropomyosin-myosin complex. Cell. 2012;150:327–338. doi: 10.1016/j.cell.2012.05.037. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galkin VE, Orlova A, Kudryashov DS, Solodukhin A, Reisler E, Schroder GF, Egelman EH. Remodeling of actin filaments by ADF/cofilin proteins. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20568–20572. doi: 10.1073/pnas.1110109108. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu J, Xu S, Yu LC. A model of cross-bridge attachment to actin in the A*M*ATP state based on x-ray diffraction from permeabilized rabbit psoas muscle. Biophys. J. 2002;82:2123–2133. doi: 10.1016/S0006-3495(02)75559-8. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yengo CM, De La Cruz EM, Safer D, Ostap EM, Sweeney HL. Kinetic characterization of the weak binding states of myosin V. Biochemistry. 2002;41:8508–8517. doi: 10.1021/bi015969u. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 18.De La Cruz EM, Wells AL, Rosenfeld SS, Ostap EM, Sweeney HL. The kinetic mechanism of myosin V. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13726–13731. doi: 10.1073/pnas.96.24.13726. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J. Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grintsevich EE, Galkin VE, Orlova A, Ytterberg AJ, Mikati MM, Kudryashov DS, Loo JA, Egelman EH, Reisler E. Mapping of drebrin binding site on F-actin. J. Mol. Biol. 2010;398:542–554. doi: 10.1016/j.jmb.2010.03.039. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orlova A, Galkin VE, Jeffries CM, Egelman EH, Trewhella J. The N-terminal domains of myosin binding protein C can bind polymorphically to F-actin. J. Mol. Biol. 2011;412:379–386. doi: 10.1016/j.jmb.2011.07.056. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galkin VE, Orlova A, VanLoock MS, Rybakova IN, Ervasti JM, Egelman EH. The Utrophin Actin-Binding Domain Binds F-Actin in Two Different Modes: Implications for the Spectrin Superfamily of Proteins. J. Cell Biol. 2002;157:243–251. doi: 10.1083/jcb.200111097. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukoyanova N, VanLoock MS, Orlova A, Galkin VE, Wang K, Egelman EH. Each actin subunit has three nebulin binding sites: implications for steric blocking. Curr. Biol. 2002;12:383–388. doi: 10.1016/s0960-9822(02)00678-4. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 24.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peacock JG, Miller AL, Bradley WD, Rodriguez OC, Webb DJ, Koleske AJ. The Abl-related gene tyrosine kinase acts through p190RhoGAP to inhibit actomyosin contractility and regulate focal adhesion dynamics upon adhesion to fibronectin. Mol. Biol. Cell. 2007;18:3860–3872. doi: 10.1091/mbc.E07-01-0075. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebart MC, Hubert F, Boiteau C, Venteo S, Roustan C, Benyamin Y. Biochemical characterization of the L-plastin-actin interaction shows a resemblance with that of alpha-actinin and allows a distinction to be made between the two actin-binding domains of the molecule. Biochemistry. 2004;43:2428–2437. doi: 10.1021/bi030151p. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 28.Cano ML, Cassimeris L, Fechheimer M, Zigmond SH. Mechanisms responsible for F-actin stabilization after lysis of polymorphonuclear leukocytes. J. Cell Biol. 1992;116:1123–1134. doi: 10.1083/jcb.116.5.1123. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zigmond SH, Furukawa R, Fechheimer M. Inhibition of actin filament depolymerization by the Dictyostelium 30,000-D actin-bundling protein. J. Cell Biol. 1992;119:559–567. doi: 10.1083/jcb.119.3.559. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilney LG, Connelly PS, Ruggiero L, Vranich KA, Guild GM. Actin filament turnover regulated by cross-linking accounts for the size, shape, location, and number of actin bundles in Drosophila bristles. Mol. Biol. Cell. 2003;14:3953–3966. doi: 10.1091/mbc.E03-03-0158. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhina S, Wang YL, Murata-Hori M. Alpha-actinin is required for tightly regulated remodeling of the actin cortical network during cytokinesis. Dev. Cell. 2007;13:554–565. doi: 10.1016/j.devcel.2007.08.003. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa R, Sakamoto T, Ando T, Higashi-Fujime S, Kohama K. Polarized actin bundles formed by human fascin-1: their sliding and disassembly on myosin II and myosin V in vitro. J. Neurochem. 2003;87:676–685. doi: 10.1046/j.1471-4159.2003.02058.x. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 34.Schmoller KM, Semmrich C, Bausch AR. Slow down of actin depolymerization by cross-linking molecules. J. Struct. Biol. 2011;173:350–357. doi: 10.1016/j.jsb.2010.09.003. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 35.Breitsprecher D, Koestler SA, Chizhov I, Nemethova M, Mueller J, Goode BL, Small JV, Rottner K, Faix J. Cofilin cooperates with fascin to disassemble filopodial actin filaments. J. Cell Sci. 2011;124:3305–3318. doi: 10.1242/jcs.086934. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkhatib N, Neu MB, Zensen C, Schmoller KM, Louvard D, Bausch AR, Betz T, Vignjevic DM. Fascin plays a role in stress fiber organization and focal adhesion disassembly. Curr. Biol. 2014;24:1492–1499. doi: 10.1016/j.cub.2014.05.023. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 37.guilar-Cuenca R, Juanes-Garcia A, Vicente-Manzanares M. Myosin II in mechanotransduction: master and commander of cell migration, morphogenesis, and cancer. Cell Mol. Life Sci. 2014;71:479–492. doi: 10.1007/s00018-013-1439-5. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolat L, Hu Q, Spiliotis ET. Septin functions in organ system physiology and pathology. Biol. Chem. 2013 doi: 10.1515/hsz-2013-0233. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haviv L, Gillo D, Backouche F, Bernheim-Groswasser A. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J. Mol. Biol. 2008;375:325–330. doi: 10.1016/j.jmb.2007.09.066. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 41.Orlova A, Egelman EH. Structural dynamics of F-actin. I. Changes in the C-terminus. J. Mol. Biol. 1995;245:582–597. doi: 10.1006/jmbi.1994.0048. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 42.Bowen JR, Hwang D, Bai X, Roy D, Spiliotis ET. Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia. J. Cell Biol. 2011;194:187–197. doi: 10.1083/jcb.201102076. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galkin VE, Orlova A, Lukoyanova N, Wriggers W, Egelman EH. Actin Depolymerizing Factor Stabilizes an Existing State of F-Actin and Can Change the Tilt of F-Actin Subunits. J. Cell Biol. 2001;153:75–86. doi: 10.1083/jcb.153.1.75. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galkin VE, Orlova A, VanLoock MS, Shvetsov A, Reisler E, Egelman EH. ADF/cofilin use an intrinsic mode of F-actin instability to disrupt actin filaments. J. Cell Biol. 2003;163:1057–1066. doi: 10.1083/jcb.200308144. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank J, Shimkin B, Dowse H. SPIDER - A modular software system for electron image processing. Ultramicroscopy. 1981;6:343–358. Ref Type: Journal. [Google Scholar]
- 46.Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 47.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 2003;142:334–347. doi: 10.1016/s1047-8477(03)00069-8. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 48.Egelman EH. A robust algorithm for the reconstruction of helical filaments using single-particle methods. Ultramicroscopy. 2000;85:225–234. doi: 10.1016/s0304-3991(00)00062-0. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 49.Fujii T, Iwane AH, Yanagida T, Namba K. Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature. 2010;467:724–728. doi: 10.1038/nature09372. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 50.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 51.Trentham DR, Bardsley RG, Eccleston JF, Weeds AG. Elementary processes of the magnesium ion-dependent adenosine triphosphatase activity of heavy meromyosin. A transient kinetic approach to the study of kinases and adenosine triphosphatases and a colorimetric inorganic phosphate assay in situ. Biochem. J. 1972;126:635–644. doi: 10.1042/bj1260635. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.